An End-to-End Deep Learning Approach for Quantitative Microwave Breast Imaging in Real-Time Applications
Abstract
1. Introduction
1.1. Motivation
1.2. Prototypes
1.3. Algorithms
1.4. Machine Learning for Quantitative Microwave Imaging
- The use of fully-connected neural networks to perform quantitative imaging of the breast tissues;
- The use of a direct inversion scheme to obtain the permittivity and conductivity maps of breast tissues;
- The realistic in-house numerical phantom generator and the corresponding dataset for an overall population of 120,000 elements, which is paramount for a proper training of the neural networks to perform a certain task, and therefore an important element of novelty.
2. Problem Statement
3. Methodology
3.1. Breast Database
3.2. Neural Network Design and Training
3.3. Quality Performance Indicator on Testing Population
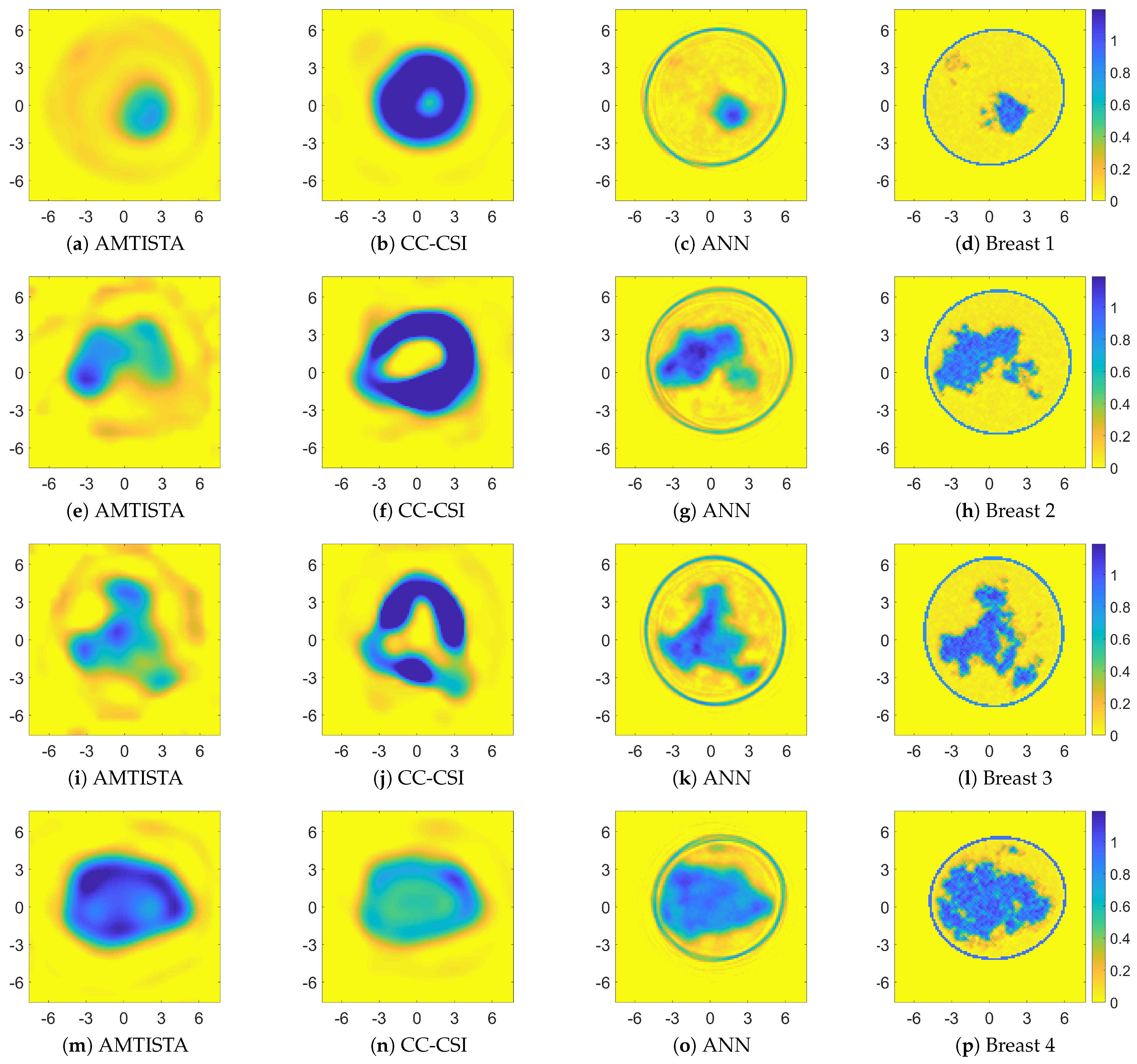
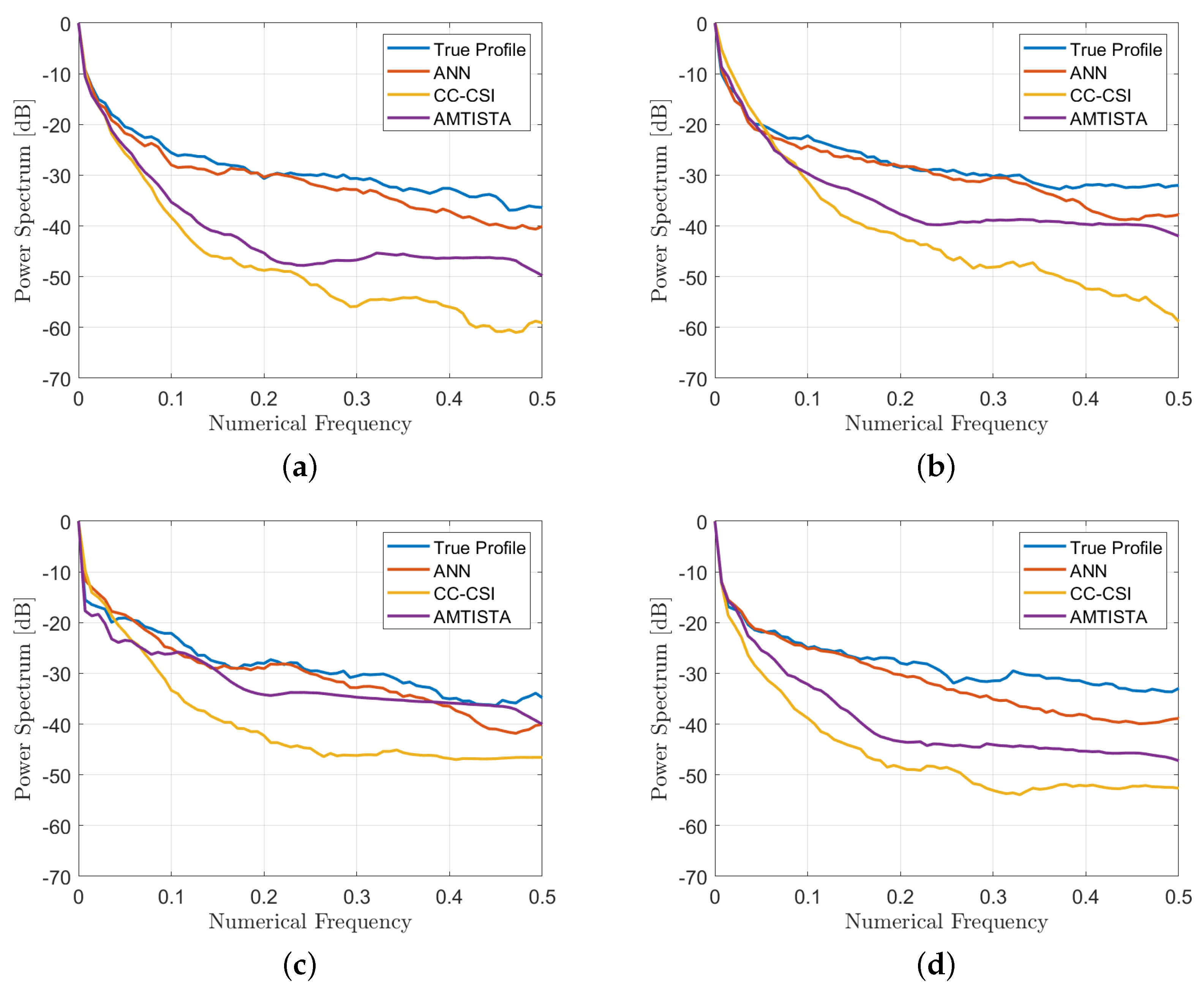
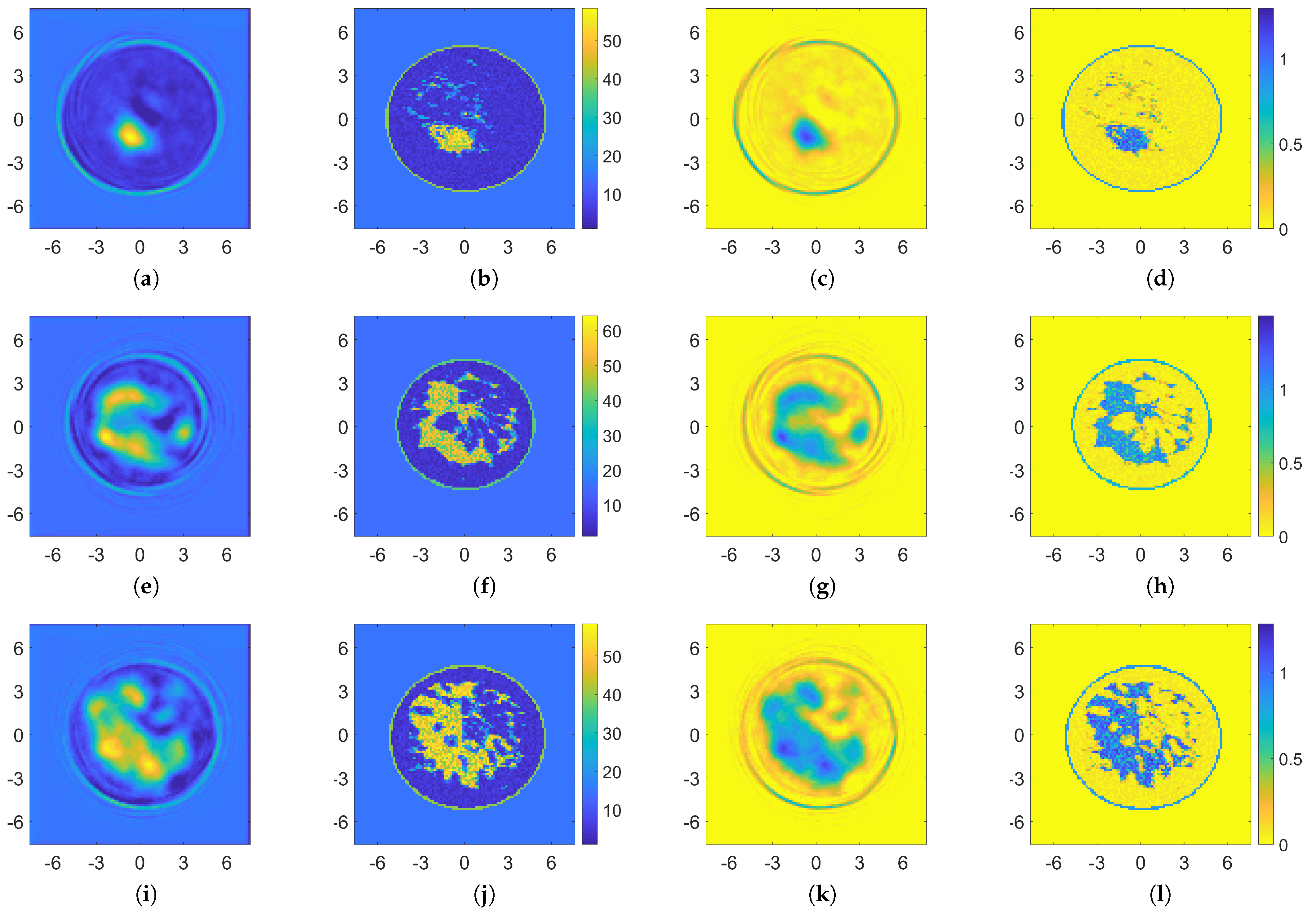
4. Results
4.1. Performance Assessment
4.2. Comparison with Other Nonlinear Approaches
5. Conclusions
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Data Availability Statement
Conflicts of Interest
Abbreviations
| MRI | Magnetic Resonance Imaging |
| SNR | Signal to Noise Ratio |
| EIS | Electromagnetic Inverse Scattering |
| CNN | Convolutional Neural Network |
| ANN | Artificial Neural Network |
| UAT | Universal Approximation Theorem |
| AWGN | Additive, White, Gaussian Noise |
| MoM | Method of Moments |
| FFT-CG | Fast Fourier Transform-Conjugate Gradient |
| NRMSE | Normalised Root mean Square Error |
| SSIM | Structural Similarity Index Measure |
| DBIM | Distorted Born Iterative Method |
| CSI | Contrast Source Inversion |
| CC-CSI | Cross Correlated-Contrast Source Inversion |
References
- Fitzmaurice, C.; Dicker, D.; Pain, A.; Hamavid, H.; Moradi-Lakeh, M.; MacIntyre, M.F.; Allen, C.; Hansen, G.; Woodbrook, R.; Wolfe, C.; et al. The global burden of cancer 2013. JAMA Oncol. 2015, 1, 505–527. [Google Scholar] [CrossRef] [PubMed]
- Siegel, R.L.; Miller, K.D.; Jemal, A. Cancer statistics. CA Cancer J. Clin. 2015, 65, 5. [Google Scholar] [CrossRef]
- Zebari, D.; Ibrahim, D.; Zeebaree, D.; Haron, H.; Salih, M.; Damaševičius, R.; Mohammed, M. Systematic review of computing approaches for breast cancer detection based computer aided diagnosis using mammogram images. Appl. Artif. Intell. 2021, 35, 2157–2203. [Google Scholar] [CrossRef]
- Jaglan, P.; Dass, R.; Duhan, M. Breast cancer detection techniques: Issues and challenges. J. Inst. Eng. Ser. 2019, 1–8. [Google Scholar] [CrossRef]
- Herranz, M.; Ruibal, A. Optical imaging in breast cancer diagnosis: The next evolution. J. Oncol. 2012, 2012. [Google Scholar] [CrossRef] [PubMed]
- Sehgal, C.M.; Weinstein, S.P.; Arger, P.H.; Conant, E.F. A review of breast ultrasound. J. Mammary Gland. Biol. Neoplasia 2006, 11, 113–123. [Google Scholar] [CrossRef]
- Kapur, A.; Carson, P.L.; Eberhard, J.; Goodsitt, M.M.; Thomenius, K.; Lokh, W.M.; Buckley, D.; Roubidoux, M.A.; Helvie, M.A.; Booi, R.C.; et al. Combination of digital mammography with semi-automated 3D breast ultrasound. Technol. Cancer Res. Treat. 2004, 3, 325–334. [Google Scholar] [CrossRef]
- James, A.P.; Dasarathy, B.V. Medical image fusion: A survey of the state of the art. Inf. Fusion 2014, 19, 4–19. [Google Scholar] [CrossRef]
- Nikolova, N.K. Microwave imaging for breast cancer. IEEE Microw. Mag. 2011, 12, 78–94. [Google Scholar] [CrossRef]
- Aldhaeebi, M.A.; Alzoubi, K.; Almoneef, T.S.; Bamatraf, S.M.; Attia, H.; Ramahi, O.M. Review of microwaves techniques for breast cancer detection. Sensors 2020, 20, 2390. [Google Scholar] [CrossRef]
- O’Loughlin, D.; O’Halloran, M.; Moloney, B.M.; Glavin, M.; Jones, E.; Elahi, M.A. Microwave Breast Imaging: Clinical Advances and Remaining Challenges. IEEE Trans. Biomed. Eng. 2018, 65, 2580–2590. [Google Scholar] [CrossRef] [PubMed]
- Lazebnik, M.; Popovic, D.; McCartney, L.; Watkins, C.B.; Lindstrom, M.J.; Harter, J.; Sewall, S.; Ogilvie, T.; Magliocco, A.; Breslin, T.M.; et al. A large-scale study of the ultrawideband microwave dielectric properties of normal, benign and malignant breast tissues obtained from cancer surgeries. Phys. Med. Biol. 2007, 52, 6093. [Google Scholar] [CrossRef] [PubMed]
- Fear, E.C.; Hagness, S.C.; Meaney, P.M.; Okoniewski, M.; Stuchly, M.A. Enhancing breast tumor detection with near-field imaging. IEEE Microw. Mag. 2002, 3, 48–56. [Google Scholar] [CrossRef]
- Conceição, R.C.; Mohr, J.J.; O’Halloran, M. An Introduction to Microwave Imaging for Breast Cancer Detection; Springer: Berlin/Heidelberg, Germany, 2016. [Google Scholar]
- Chandra, R.; Zhou, H.; Balasingham, I.; Narayanan, R.M. On the Opportunities and Challenges in Microwave Medical Sensing and Imaging. IEEE Trans. Biomed. Eng. 2015, 62, 1667–1682. [Google Scholar] [CrossRef] [PubMed]
- Colton, D.L.; Kress, R.; Kress, R. Inverse Acoustic and Electromagnetic Scattering Theory; Springer Nature: Berlin/Heidelberg, Germany, 2019; Volume 93. [Google Scholar]
- Qin, Y.; Rodet, T.; Lambert, M.; Lesselier, D. Joint Inversion of Electromagnetic and Acoustic Data With Edge-Preserving Regularization for Breast Imaging. IEEE Trans. Comput. Imaging 2021, 7, 349–360. [Google Scholar] [CrossRef]
- Qin, Y.; Rodet, T.; Lambert, M.; Lesselier, D. Microwave breast imaging with prior ultrasound information. IEEE Open J. Antennas Propag. 2020, 1, 472–482. [Google Scholar] [CrossRef]
- Bevacqua, M.T.; Isernia, T. Shape reconstruction via equivalence principles, constrained inverse source problems and sparsity promotion. Prog. Electromagn. Res. 2017, 158, 37–48. [Google Scholar] [CrossRef]
- Tobon Vasquez, J.A.; Scapaticci, R.; Turvani, G.; Bellizzi, G.; Joachimowicz, N.; Duchêne, B.; Tedeschi, E.; Casu, M.R.; Crocco, L.; Vipiana, F. Design and experimental assessment of a 2d microwave imaging system for brain stroke monitoring. Int. J. Antennas Propag. 2019, 2019. [Google Scholar] [CrossRef]
- Porter, E.; Kirshin, E.; Santorelli, A.; Coates, M.; Popović, M. Time-domain multistatic radar system for microwave breast screening. IEEE Antennas Wirel. Propag. Lett. 2013, 12, 229–232. [Google Scholar] [CrossRef]
- Meaney, P.; Hartov, A.; Bulumulla, S.; Raynolds, T.; Davis, C.; Schoenberger, F.; Richter, S.; Paulsen, K. A 4-channel, vector network analyzer microwave imaging prototype based on software defined radio technology. Rev. Sci. Instrum. 2019, 90, 044708. [Google Scholar] [CrossRef]
- Duchesne, L.; Fasoula, A.; Kaverine, E.; Robin, G.; Bernard, J.G. Wavelia microwave breast imaging: Identification and mitigation of possible sources of measurement uncertainty. In Proceedings of the 2019 13th European Conference on Antennas and Propagation (EuCAP), Krakow, Poland, 31 March–5 April 2019; pp. 1–5. [Google Scholar]
- Kimura, K.; Kimura, N. Scattering Tomography Method and Scattering Tomography Device. U.S. Patent 10,101,282, 16 October 2018. [Google Scholar]
- Fedeli, A.; Maffongelli, M.; Monleone, R.; Pagnamenta, C.; Pastorino, M.; Poretti, S.; Randazzo, A.; Salvadè, A. A tomograph prototype for quantitative microwave imaging: Preliminary experimental results. J. Imaging 2018, 4, 139. [Google Scholar] [CrossRef]
- O’Loughlin, D.; Oliveira, B.L.; Glavin, M.; Jones, E.; O’Halloran, M. Advantages and disadvantages of parameter search algorithms for permittivity estimation for microwave breast imaging. In Proceedings of the 2019 13th European Conference on Antennas and Propagation (EuCAP), Krakow, Poland, 31 March–5 April 2019; pp. 1–5. [Google Scholar]
- Fear, E.C.; Bourqui, J.; Curtis, C.; Mew, D.; Docktor, B.; Romano, C. Microwave breast imaging with a monostatic radar-based system: A study of application to patients. IEEE Trans. Microw. Theory Tech. 2013, 61, 2119–2128. [Google Scholar] [CrossRef]
- Jeon, S.I.; Kim, B.R.; Son, S.H. Clinical trial of microwave tomography imaging. In Proceedings of the 2016 URSI Asia-Pacific Radio Science Conference (URSI AP-RASC), Seoul, Korea, 21–25 August 2016; pp. 1–2. [Google Scholar]
- Porter, E.; Coates, M.; Popović, M. An early clinical study of time-domain microwave radar for breast health monitoring. IEEE Trans. Biomed. Eng. 2015, 63, 530–539. [Google Scholar] [CrossRef]
- Yang, F.; Sun, L.; Hu, Z.; Wang, H.; Pan, D.; Wu, R.; Zhang, X.; Chen, Y.; Zhang, Q. A large-scale clinical trial of radar-based microwave breast imaging for asian women: Phase I. In Proceedings of the 2017 IEEE International Symposium on Antennas and Propagation & USNC/URSI National Radio Science Meeting, San Diego, CA, USA, 9–14 July 2017; pp. 781–783. [Google Scholar]
- Bourqui, J.; Fear, E.C. Systems for ultra-wideband microwave sensing and imaging of biological tissues. In Proceedings of the 2013 7th European Conference on Antennas and Propagation (EuCAP), Gothenburg, Sweden, 8–12 April 2013; pp. 834–835. [Google Scholar]
- Sani, L.; Ghavami, N.; Vispa, A.; Paoli, M.; Raspa, G.; Ghavami, M.; Sacchetti, F.; Vannini, E.; Ercolani, S.; Saracini, A.; et al. Novel microwave apparatus for breast lesions detection: Preliminary clinical results. Biomed. Signal Process. Control 2019, 52, 257–263. [Google Scholar] [CrossRef]
- Semenov, S.Y.; Svenson, R.H.; Bulyshev, A.E.; Souvorov, A.E.; Nazarov, A.G.; Sizov, Y.E.; Pavlovsky, A.V.; Borisov, V.Y.; Voinov, B.A.; Simonova, G.I.; et al. Three-dimensional microwave tomography: Experimental prototype of the system and vector Born reconstruction method. IEEE Trans. Biomed. Eng. 1999, 46, 937–946. [Google Scholar] [CrossRef]
- Joisel, A.; Mallorqui, J.; Broquetas, A.; Geffrin, J.M.; Joachimowicz, N.; Lossera, M.V.; Joire, L.; Bolomey, J.C. Microwave imaging techniques for biomedical applications. In Proceedings of the 16th IEEE Instrumentation and Measurement Technology Conference, Venice, Italy, 24–26 May 1999; Volume 3, pp. 1591–1596. [Google Scholar]
- Meaney, P.M.; Fanning, M.W.; Li, D.; Poplack, S.P.; Paulsen, K.D. A clinical prototype for active microwave imaging of the breast. IEEE Trans. Microw. Theory Tech. 2000, 48, 1841–1853. [Google Scholar]
- Meaney, P.M.; Fanning, M.W.; Zhou, T.; Golnabi, A.; Geimer, S.D.; Paulsen, K.D. Clinical microwave breast imaging—2D results and the evolution to 3D. In Proceedings of the 2009 International Conference on Electromagnetics in Advanced Applications, Torino, Italy, 14–18 September 2009; pp. 881–884. [Google Scholar]
- Grzegorczyk, T.M.; Meaney, P.M.; Kaufman, P.A.; Paulsen, K.D. Fast 3-D tomographic microwave imaging for breast cancer detection. IEEE Trans. Med. Imag. 2012, 31, 1584–1592. [Google Scholar] [CrossRef]
- Son, S.H.; Simonov, N.; Kim, H.J.; Lee, J.M.; Jeon, S.I. Preclinical prototype development of a microwave tomography system for breast cancer detection. ETRI J. 2010, 32, 901–910. [Google Scholar] [CrossRef]
- Simonov, N.A.; Jeon, S.I.; Son, S.H.; Lee, J.M.; Kim, H.J. 3D microwave breast imaging based on multistatic radar concept system. In Proceedings of the 2011 3rd International Asia-Pacific Conference on Synthetic Aperture Radar (APSAR), Seoul, Korea, 26–30 September 2011; pp. 1–4. [Google Scholar]
- Klemm, M.; Craddock, I.; Leendertz, J.; Preece, A.; Benjamin, R. Experimental and clinical results of breast cancer detection using UWB microwave radar. In Proceedings of the 2008 IEEE Antennas and Propag. Society International Symposium, San Diego, CA, USA, 5–11 July 2008; pp. 1–4. [Google Scholar]
- Preece, A.W.; Craddock, I.; Shere, M.; Jones, L.; Winton, H.L. Maria m4: Clinical evaluation of a prototype ultrawideband radar scanner for breast cancer detection. J. Med. Imaging 2016, 3, 033502. [Google Scholar] [CrossRef]
- Benny, R.; Anjit, T.A.; Mythili, P. An overview of microwave imaging for breast tumor detection. Prog. Electromagn. Res. 2020, 87, 61–91. [Google Scholar] [CrossRef]
- Bertero, M.; Boccacci, P. Introduction to Inverse Problems in Imaging; CRC Press: Boca Raton, FL, USA, 1998. [Google Scholar]
- Pastorino, M. Microwave Imaging; John Wiley & Sons: Hoboken, NJ, USA, 2010; Volume 208. [Google Scholar]
- Salman, A.O.; Gavrilov, S.P.; Vertiy, A.A. Microwave imaging of immersed dielectric bodies: An experimental survey. Electromagnetics 2005, 25, 567–585. [Google Scholar] [CrossRef]
- Bolomey, J.C.; Izadnegahdar, A.; Jofre, R.L.; Du, P.; Mezeray, C.; Peronnet, G. Microwave diffraction tomography for biomedical applications. IEEE Trans. Microw. Theory Tech. 1982, 30, 1998–2000. [Google Scholar] [CrossRef]
- Colton, D.; Haddar, H.; Piana, M. The linear sampling method in inverse electromagnetic scattering theory. Inverse Probl. 2003, 19, S105. [Google Scholar] [CrossRef]
- Cui, T.J.; Chew, W.C.; Aydiner, A.A.; Chen, S. Inverse scattering of two-dimensional dielectric objects buried in a lossy earth using the distorted born iterative method. IEEE Trans. Geosci. Remote Sens. 2001, 39, 339–346. [Google Scholar]
- Crocco, L.; Catapano, I.; Di Donato, L.; Isernia, T. The linear sampling method as a way to quantitative inverse scattering. IEEE Trans. Antennas Propag. 2012, 60, 1844–1853. [Google Scholar] [CrossRef]
- Cui, T.J.; Qin, Y.; Wang, G.L.; Chew, W.C. Low-frequency detection of two-dimensional buried objects using high-order extended born approximations. Inverse Probl. 2004, 20, S41. [Google Scholar] [CrossRef]
- Belkebir, K.; Elissalt, J.M.; Geffrin, J.M.; Pichot, C. Newton-kantorovich and modified gradient-inversion algorithms applied to ipswich data. IEEE Antennas Propag. Mag. 1996, 38, 41. [Google Scholar] [CrossRef][Green Version]
- Nocedal, J.; Wright, S. Numerical Optimization; Springer Science & Business Media: Berlin/Heidelberg, Germany, 2006. [Google Scholar]
- Pastorino, M.; Caorsi, S.; Massa, A. A global optimization technique for microwave nondestructive evaluation. IEEE Trans. Instrum. Meas. 2002, 51, 666–673. [Google Scholar] [CrossRef]
- Caorsi, S.; Gamba, P. Electromagnetic detection of dielectric cylinders by a neural network approach. IEEE Trans. Geosci. Remote Sens. 1999, 37, 820–827. [Google Scholar] [CrossRef]
- Rekanos, I.T. Neural-network-based inverse-scattering technique for online microwave medical imaging. IEEE Trans. Magn. 2002, 38, 1061–1064. [Google Scholar] [CrossRef]
- Xu, K.; Wu, L.; Ye, X.; Chen, X. Deep learning-based inversion methods for solving inverse scattering problems with phaseless data. IEEE Trans. Antennas Propag. 2020. [Google Scholar] [CrossRef]
- Li, L.; Wang, L.G.; Teixeira, F.L.; Liu, C.; Nehorai, A.; Cui, T.J. Deepnis: Deep neural network for nonlinear electromagnetic inverse scattering. IEEE Trans. Antennas Propag. 2018, 67, 1819–1825. [Google Scholar] [CrossRef]
- Wei, Z.; Chen, X. Deep-learning schemes for full-wave nonlinear inverse scattering problems. IEEE Trans. Geosci. Remote Sens. 2018, 57, 1849–1860. [Google Scholar] [CrossRef]
- Sanghvi, Y.; Kalepu, Y.; Khankhoje, U.K. Embedding deep learning in inverse scattering problems. IEEE Trans. Comput. Imaging 2019, 6, 46–56. [Google Scholar] [CrossRef]
- Guo, R.; Song, X.; Li, M.; Yang, F.; Xu, S.; Abubakar, A. Supervised descent learning technique for 2-D microwave imaging. IEEE Trans. Antennas Propag. 2019, 67, 3550–3554. [Google Scholar] [CrossRef]
- Massa, A.; Marcantonio, D.; Chen, X.; Li, M.; Salucci, M. DNNS as applied to electromagnetics, antennas, and propagation—A review. IEEE Antennas Wirel. Propag. Lett. 2019, 18, 2225–2229. [Google Scholar] [CrossRef]
- Aggarwal, H.K.; Mani, M.P.; Jacob, M. Modl: Model-based deep learning architecture for inverse problems. IEEE Trans. Med. Imag. 2018, 38, 394–405. [Google Scholar] [CrossRef]
- Lucas, A.; Iliadis, M.; Molina, R.; Katsaggelos, A.K. Using deep neural networks for inverse problems in imaging: Beyond analytical methods. IEEE Signal Process. Mag 2018, 35, 20–36. [Google Scholar] [CrossRef]
- Ashtari, A.; Noghanian, S.; Sabouni, A.; Aronsson, J.; Thomas, G.; Pistorius, S. Using a priori information for regularization in breast microwave image reconstruction. IEEE Trans. Biomed. Eng. 2010, 57, 2197–2208. [Google Scholar] [CrossRef]
- Khoshdel, V.; Asefi, M.; Ashraf, A.; LoVetri, J. Full 3D microwave breast imaging using a deep-learning technique. J. Imaging 2020, 6, 80. [Google Scholar] [CrossRef]
- Shah, P.; Moghaddam, M. Super resolution for microwave imaging: A deep learning approach. In Proceedings of the International Symposium on Antennas and Propagation & USNC/URSI National Radio Science Meeting, San Diego, CA, USA, 9–14 July 2017; pp. 849–850. [Google Scholar]
- Hornik, K.; Stinchcombe, M.; White, H. Universal approximation of an unknown mapping and its derivatives using multilayer feedforward networks. Neural Netw. 1990, 3, 551–560. [Google Scholar] [CrossRef]
- Isernia, T.; Pascazio, V.; Pierri, R. A nonlinear estimation method in tomographic imaging. IEEE Trans. Geosci. Remote Sens. 1997, 35, 910–923. [Google Scholar] [CrossRef]
- Bucci, O.M.; Isernia, T. Electromagnetic inverse scattering: Retrievable information and measurement strategies. Radio Sci. 1997, 32, 2123–2137. [Google Scholar] [CrossRef]
- Ambrosanio, M.; Kosmas, P.; Pascazio, V. A multithreshold iterative dbim-based algorithm for the imaging of heterogeneous breast tissues. IEEE Trans. Biomed. Eng. 2018, 66, 509–520. [Google Scholar] [CrossRef]
- Bevacqua, M.T.; Bellizzi, G.G.; Crocco, L.; Isernia, T. A method for quantitative imaging of electrical properties of human tissues from only amplitude electromagnetic data. Inverse Probl. 2019, 35, 025006. [Google Scholar] [CrossRef]
- Mojabi, P.; LoVetri, J. Enhancement of the krylov subspace regularization for microwave biomedical imaging. IEEE Trans. Med. Imag. 2009, 28, 2015–2019. [Google Scholar] [CrossRef]
- Shea, J.D.; Kosmas, P.; Van Veen, B.D.; Hagness, S.C. Contrast-enhanced microwave imaging of breast tumors: A computational study using 3D realistic numerical phantoms. Inverse Probl. 2010, 26, 074009. [Google Scholar] [CrossRef]
- Rubæk, T.; Meaney, P.M.; Meincke, P.; Paulsen, K.D. Nonlinear microwave imaging for breast-cancer screening using gauss–newton’s method and the cgls inversion algorithm. IEEE Trans. Antennas Propag. 2007, 55, 2320–2331. [Google Scholar] [CrossRef]
- Burfeindt, M.J.; Colgan, T.J.; Mays, R.O.; Shea, J.D.; Behdad, N.; Van Veen, B.D.; Hagness, S.C. MRI-derived 3-D-printed breast phantom for microwave breast imaging validation. IEEE Antennas Wirel. Propag. Lett. 2012, 11, 1610–1613. [Google Scholar] [CrossRef]
- Reimer, T.; Krenkevich, J.; Pistorius, S. An open-access experimental dataset for breast microwave imaging. In Proceedings of the 2020 14th European Conference on Antennas and Propagation (EuCAP), Copenhagen, Denmark, 15–20 March 2020; pp. 1–5. [Google Scholar]
- Schertzer, D.; Lovejoy, S. Nonlinear variability in geophysics: Multifractal simulations and analysis. In Fractals’ Physical Origin and Properties; Springer: Berlin/Heidelberg, Germany, 1989; pp. 49–79. [Google Scholar]
- Richmond, J. Scattering by a dielectric cylinder of arbitrary cross section shape. IEEE Trans. Antennas Propag. 1965, 13, 334–341. [Google Scholar] [CrossRef]
- Ambrosanio, M.; Franceschini, S.; Baselice, F.; Pascazio, V. Artificial Neural Networks for Quantitative Microwave Breast Imaging. Bioimaging 2020, 204–208. [Google Scholar]
- Franceschini, S.; Ambrosanio, M.; Baselice, F.; Pascazio, V. Neural Networks for Inverse Problems: The Microwave Imaging Case. In Proceedings of the 2021 15th European Conference on Antennas and Propagation (EuCAP), Düsseldorf, Germany, 22–26 March 2021; pp. 1–5. [Google Scholar]
- Ambrosanio, M.; Franceschini, S.; Baselice, F.; Pascazio, V. Machine learning for microwave imaging. In Proceedings of the 2020 14th European Conference on Antennas and Propagation (EuCAP), Copenhagen, Denmark, 15–20 March 2020; pp. 1–4. [Google Scholar]
- Zhang, K.; Zuo, W.; Chen, Y.; Meng, D.; Zhang, L. Beyond a gaussian denoiser: Residual learning of deep cnn for image denoising. IEEE Trans. Image Process. 2017, 26, 3142–3155. [Google Scholar] [CrossRef] [PubMed]
- Vitale, S.; Ferraioli, G.; Pascazio, V. Multi-objective cnn-based algorithm for sar despeckling. IEEE Trans. Geosci. Remote Sens. 2020, 1–14. [Google Scholar] [CrossRef]
- Chew, W.C.; Wang, Y. Reconstruction of two-dimensional permittivity distribution using the distorted born iterative method. IEEE Trans. Med. Imag. 1990, 9, 218–225. [Google Scholar] [CrossRef] [PubMed]
- Chew, W.C. Waves and Fields in Inhomogeneous Media; IEEE Press: Piscataway, NJ, USA, 1995. [Google Scholar]
- den Berg, P.M.V.; Abubakar, A. Contrast source inversion method: State of art. Prog. Electromagn. Res. 2001, 34, 189–218. [Google Scholar] [CrossRef]
- Sun, S.; Kooij, B.J.; Jin, T.; Yarovoy, A.G. Cross-correlated contrast source inversion. IEEE Trans. Antennas Propag. 2017, 65, 2592–2603. [Google Scholar] [CrossRef]

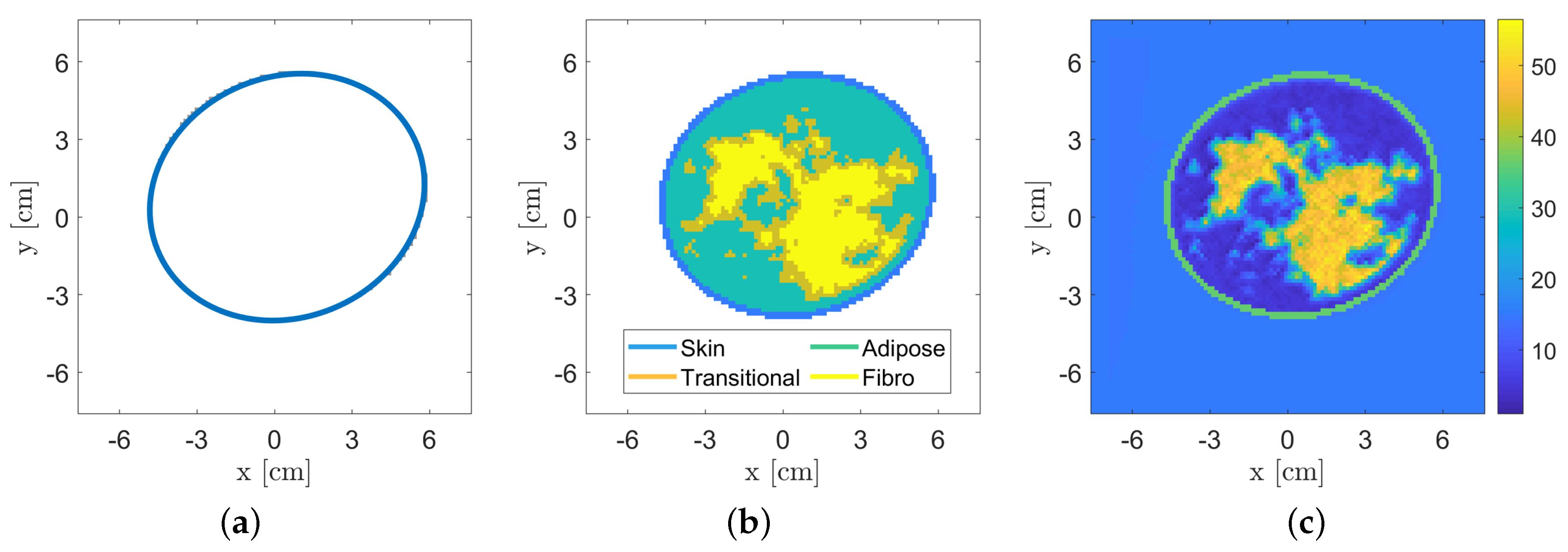
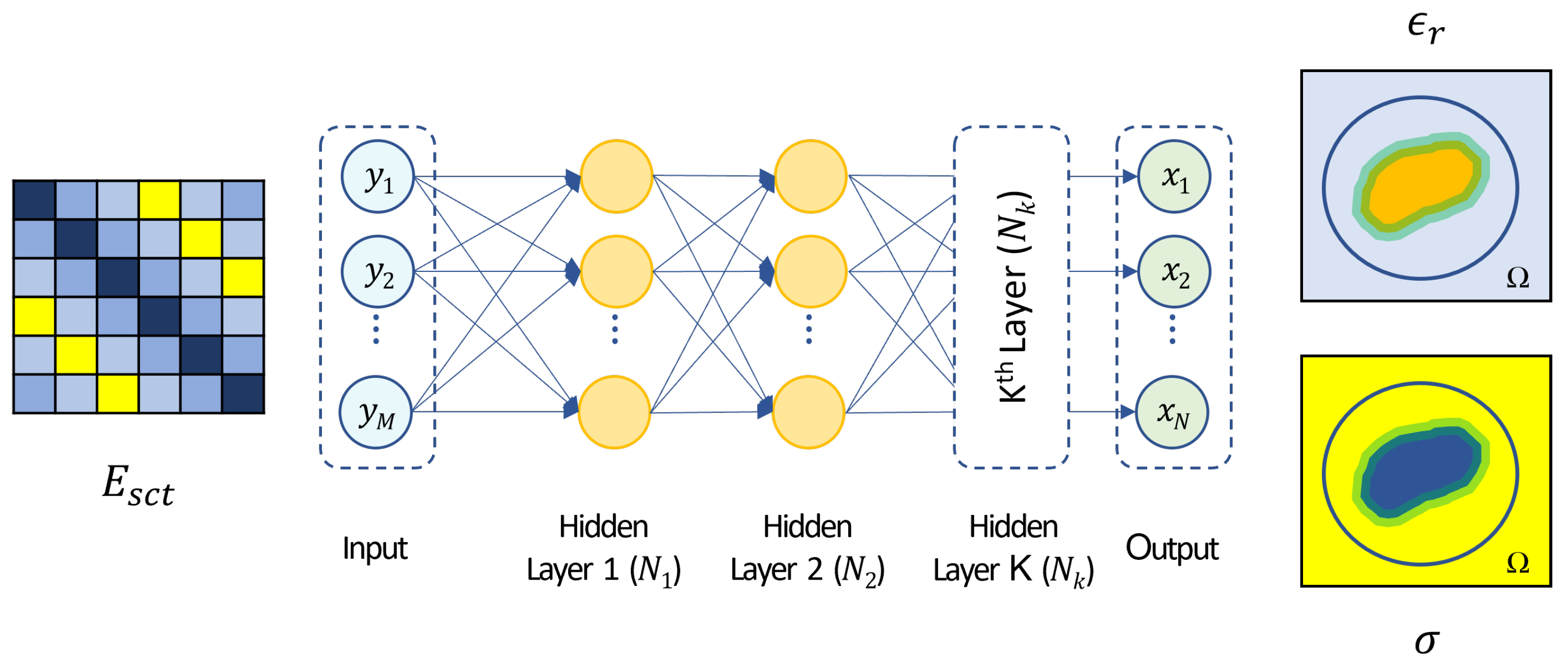
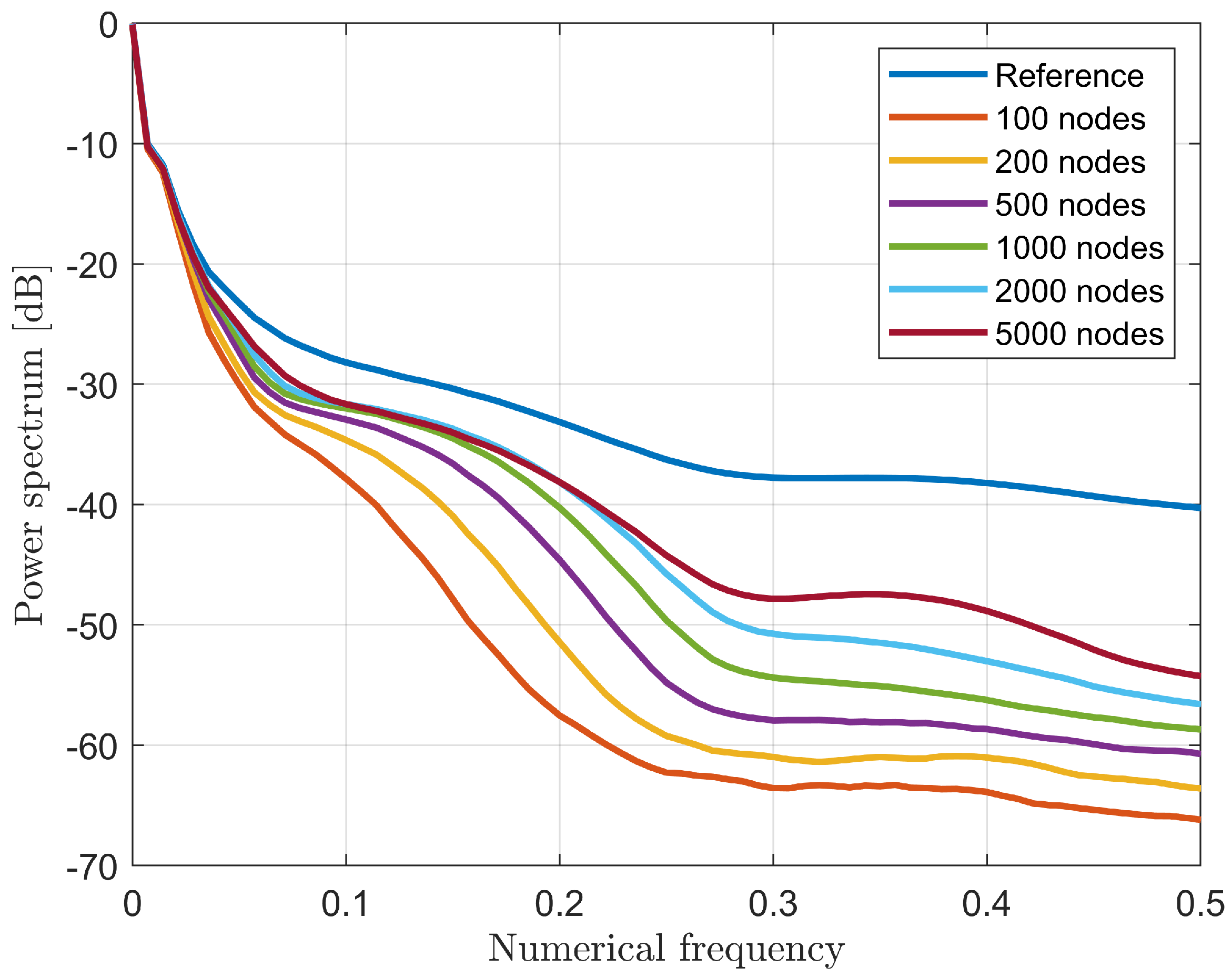

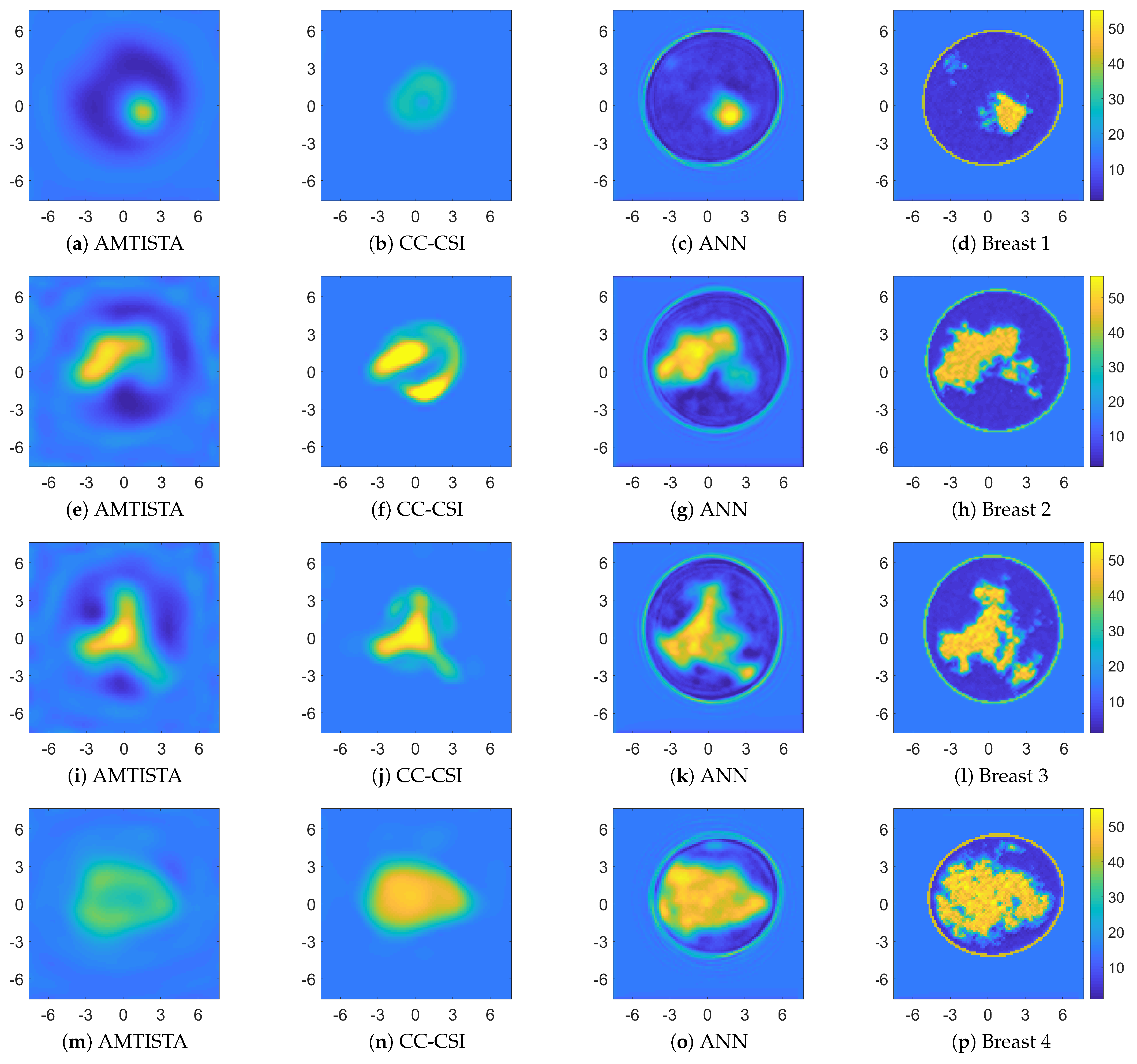


| System | Position | Processing | Frequency [GHz] | Antenna | Scan Time | Reference |
|---|---|---|---|---|---|---|
| Bristol University | prone | radar | 3–8 | slot | 30 s | [26] |
| Dartmouth College | prone | tomographic | 0.7–1.7 | monopole | 5 min | [22] |
| ETRI (Korea) | prone | tomographic | 3–6 | monopole | 15 s/slice | [28] |
| McGill University—table | prone | radar | 2–4 | TWTLTLA | 18 min | [29] |
| McGill University—wearable | wearable | radar | 2–4 | microstrip | 5 min | [29] |
| Southern University of China | prone | radar | 4–8 | horn | 4 min | [30] |
| Calgary University | prone | radar | 1.3–7.6 | vivaldi | 30 min | [31] |
| Microwave Vision | prone | radar | 1–4 | vivaldi | 10 min | [23] |
| Mammowave | prone | Huygens | 1–9 | PulsON P200 | 10 min | [32] |
| Kobe University | supine | tomographic | 0.05–12 | UWB | 30 min | [24] |
| Class | Percentage of Tissue (%) | ||
|---|---|---|---|
| Fibro-glandular | Transitional | Adipose | |
| A | 5–20 | 5–15 | 65–90 |
| B | 20–30 | 10–20 | 50–70 |
| C | 30–40 | 15–20 | 40–55 |
| D | 40–65 | 20–25 | 10–40 |
| Neural Network Details | |
|---|---|
| Input size | 1800 |
| Output size | 23,328 |
| Optimization method | ADAM, initial learning rate: |
| Training epochs | 30 |
| Mini-batch size | 64 |
| Architecture | 3 layers, 2000 nodes per each |
| Population | 120,000 (training: 85%, validation: 10%, testing: 5%) |
| Breast Phantom | Quality Metric | AMTISTA | CC-CSI | ANN | |||
|---|---|---|---|---|---|---|---|
| Breast 1 | SSIM | 0.10 | 0.02 | 0.20 | 0.16 | 0.45 | 0.24 |
| NRMSE | 0.15 | 0.62 | 0.35 | 7.00 | 0.06 | 0.22 | |
| CORR | 0.70 | 0.56 | 0.06 | 0.23 | 0.89 | 0.86 | |
| Breast 2 | SSIM | 0.05 | 0.22 | 0.17 | 0.24 | 0.46 | 0.21 |
| NRMSE | 0.14 | 0.36 | 0.35 | 3.44 | 0.10 | 0.21 | |
| CORR | 0.78 | 0.75 | 0.44 | 0.18 | 0.85 | 0.86 | |
| Breast 3 | SSIM | 0.06 | 0.22 | 0.19 | 0.18 | 0.45 | 0.27 |
| NRMSE | 0.14 | 0.36 | 0.19 | 1.70 | 0.08 | 0.17 | |
| CORR | 0.76 | 0.74 | 0.66 | 0.17 | 0.86 | 0.89 | |
| Breast 4 | SSIM | 0.06 | 0.04 | 0.25 | 0.30 | 0.40 | 0.21 |
| NRMSE | 0.14 | 0.46 | 0.10 | 0.34 | 0.07 | 0.17 | |
| CORR | 0.79 | 0.79 | 0.81 | 0.74 | 0.87 | 0.88 | |
| Breast Class | Quality Metrics | Retrieved Permittivity | Retrieved Conductivity | ||
|---|---|---|---|---|---|
| Mean | Standard Deviation | Mean | Standard Deviation | ||
| A (1422) | SSIM | 0.458 | 0.035 | 0.182 | 0.040 |
| NRMSE | 0.102 | 0.031 | 0.345 | 0.100 | |
| CORR | 0.795 | 0.066 | 0.779 | 0.067 | |
| B (2115) | SSIM | 0.453 | 0.037 | 0.179 | 0.043 |
| NRMSE | 0.102 | 0.030 | 0.253 | 0.066 | |
| CORR | 0.818 | 0.056 | 0.835 | 0.045 | |
| C (1837) | SSIM | 0.431 | 0.036 | 0.158 | 0.037 |
| NRMSE | 0.104 | 0.035 | 0.225 | 0.062 | |
| CORR | 0.818 | 0.060 | 0.845 | 0.046 | |
| D (626) | SSIM | 0.417 | 0.035 | 0.147 | 0.035 |
| NRMSE | 0.087 | 0.028 | 0.176 | 0.043 | |
| CORR | 0.849 | 0.048 | 0.871 | 0.037 | |
| SNR | 30 dB | 5 dB | |||||
|---|---|---|---|---|---|---|---|
| Quality Metric | SSIM | NRMSE | CORR | SSIM | NRMSE | CORR | |
| Breast 1 | 0.50 | 0.06 | 0.89 | 0.46 | 0.08 | 0.85 | |
| 0.21 | 0.23 | 0.86 | 0.21 | 0.31 | 0.81 | ||
| Breast 2 | 0.46 | 0.10 | 0.85 | 0.44 | 0.13 | 0.78 | |
| 0.21 | 0.21 | 0.86 | 0.20 | 0.29 | 0.80 | ||
| Breast 3 | 0.45 | 0.08 | 0.86 | 0.36 | 0.12 | 0.79 | |
| 0.27 | 0.17 | 0.89 | 0.18 | 0.26 | 0.82 | ||
| Breast 4 | 0.45 | 0.07 | 0.87 | 0.38 | 0.12 | 0.77 | |
| 0.19 | 0.17 | 0.88 | 0.09 | 0.28 | 0.80 | ||
Publisher’s Note: MDPI stays neutral with regard to jurisdictional claims in published maps and institutional affiliations. |
© 2022 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Ambrosanio, M.; Franceschini, S.; Pascazio, V.; Baselice, F. An End-to-End Deep Learning Approach for Quantitative Microwave Breast Imaging in Real-Time Applications. Bioengineering 2022, 9, 651. https://doi.org/10.3390/bioengineering9110651
Ambrosanio M, Franceschini S, Pascazio V, Baselice F. An End-to-End Deep Learning Approach for Quantitative Microwave Breast Imaging in Real-Time Applications. Bioengineering. 2022; 9(11):651. https://doi.org/10.3390/bioengineering9110651
Chicago/Turabian StyleAmbrosanio, Michele, Stefano Franceschini, Vito Pascazio, and Fabio Baselice. 2022. "An End-to-End Deep Learning Approach for Quantitative Microwave Breast Imaging in Real-Time Applications" Bioengineering 9, no. 11: 651. https://doi.org/10.3390/bioengineering9110651
APA StyleAmbrosanio, M., Franceschini, S., Pascazio, V., & Baselice, F. (2022). An End-to-End Deep Learning Approach for Quantitative Microwave Breast Imaging in Real-Time Applications. Bioengineering, 9(11), 651. https://doi.org/10.3390/bioengineering9110651








