Single-Cell Protein Production as a Strategy to Reincorporate Food Waste and Agro By-Products Back into the Processing Chain
Abstract
1. Introduction
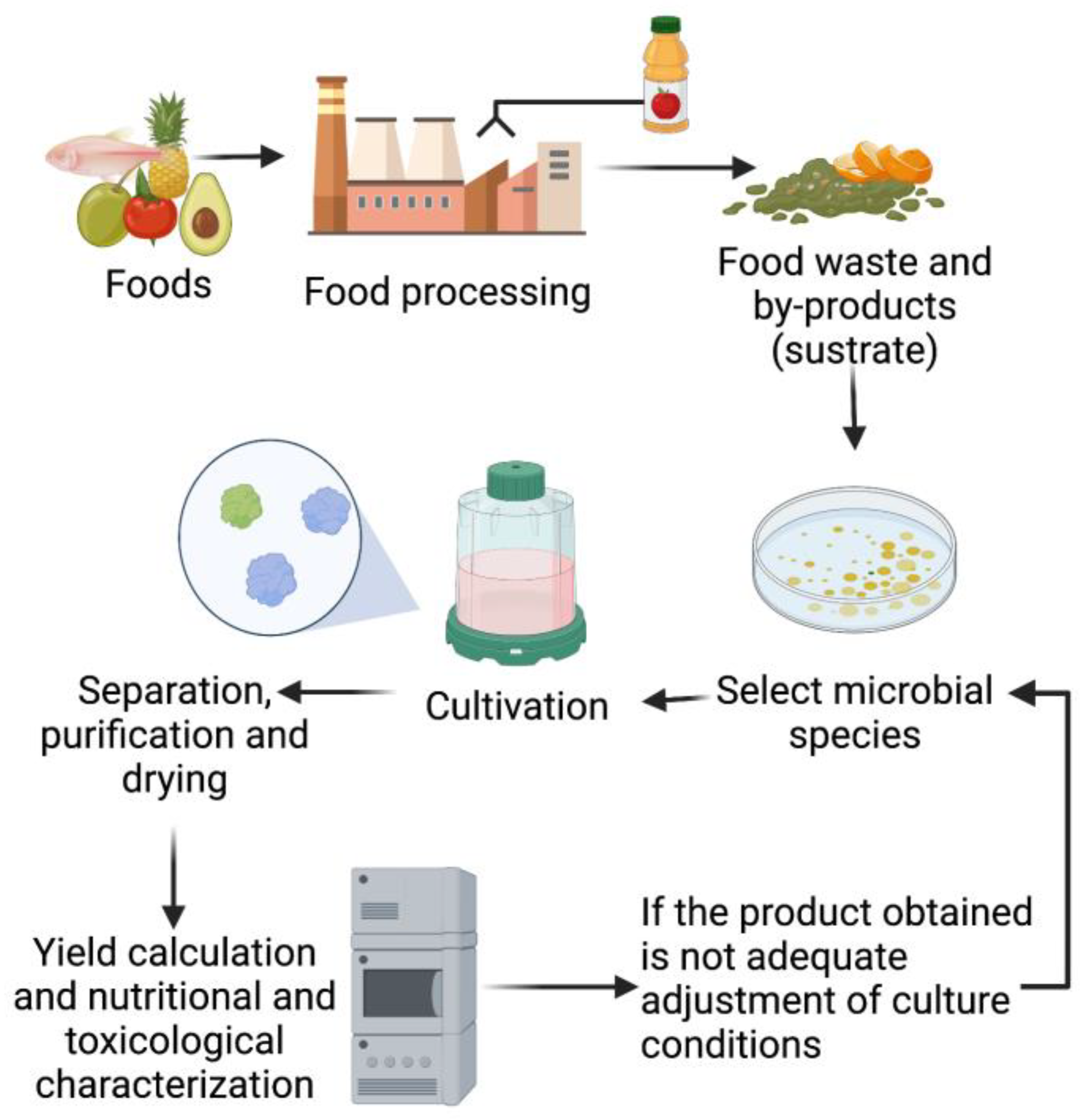
2. Production
| Microorganism | Substrate | Biomass Yield | Protein Content (%) | Reference |
|---|---|---|---|---|
| Yeasts | ||||
| Saccharomyces cerevisiae | Fish, pineapple, banana, apple, and citrus peels | NR | 40.2% | [27] |
| Saccharomyces cerevisiae | Food wastes (banana peel, citrus peel, carrot pomace and potato peel) | Up to 12 g/100 g of waste | 47.7% | [17] |
| Saccharomyces cerevisiae | Candy production effluent and digestate | 0.25 g/L per day | 28.0% | [11] |
| Saccharomyces cerevisiae | Vinasse | 73.2 g/L per day | 53.3% | [28] |
| Yarrowia lipolytica | Unspecified food waste | 8.4 ± 0.7 g/L | 38.8% | [29] |
| Candida utilis | Orange peel residues | 15.7 g/L | 6.2% | [30] |
| Candida tropicalis | Soy molasses | 8.4 g/L | 53.1% | [31] |
| Kluyveromyces marxianus | Food industry waste mixture | NR | 33.7% | [32] |
| Hanseniaspora guilliermondii and Issatchenkia orientalis | Wasted date molasses | Up to 70 g/100 g of waste | 54.3% | [33] |
| Fungi | ||||
| Aspergillus niger | Orange peels | NR | 29.7% | [34] |
| Geotrichum candidum (fungi) and Candida utilis (yeast) | Yellow wine lees and rice soaking waste fungi:yeast (1:1) | 4.91 g/g of waste | 68.5% | [35] |
| Fusarium moniliforme (fungi) and Saccharomyces cerevisiae (yeast) | Sweet potato residue | 13.9 g/L | 65.8% | [36] |
| Algae | ||||
| Haematococcus pluvialis | Synthetic brewery wastewater | 27 × 105 cells/mL | 64.9% | [37] |
| Chlorella sp. | Food processing wastes (tofu) | 42.5 × 106 cells/mL | 52.3% | [38] |
| Chlorella vulgaris (microalgae) and Yarrowia lipolytica (yeast) and mix culture | Liquid digestate of dairy wastewater | C. vulgaris 0.13 g/L per day; Y. lipolytica 0.1 g/L per day; Mix 0.2 g/L per day | C. vulgaris 21.8% Y. lipolytica 25.7% Mix 31.1% | [39] |
| Bacteria | ||||
| Brevibacterium lactofermentum and Candida utilis (yeast) | Beet pulp | 53 g/100 g of waste | 54.5% | [40] |
| Methylomonas and Methylophilus | Sewage sludge and discarded effluent | 11.54 g/g-NH4+ | 41% | [41] |
| Rhodopseudomonas faecalis | Sugar industry wastewater | 2.5 g/L per day | 51.5% | [42] |
3. Nutrient Content
4. Safety
5. Applications and Challenges of Single-Cell Protein
6. Conclusions
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Data Availability Statement
Acknowledgments
Conflicts of Interest
References
- United_Nations Population. Available online: https://www.un.org/en/global-issues/population (accessed on 9 August 2022).
- Shahbandeh, M. Per Capita Consumption of Meat Worldwide 2019–2031, by Region. Available online: https://www.statista.com/statistics/1037429/per-capita-consumption-of-meat-worldwide-by-region/#:~:text=The%20per%20capita%20consumption%20of,per%20year%20during%20that%20period (accessed on 19 July 2022).
- World Cancer Research Fund. Recommendations and Public Health and Policy Implications. Available online: https://www.wcrf.org/wp-content/uploads/2021/01/Recommendations.pdf (accessed on 19 July 2022).
- Wolk, A. Potential health hazards of eating red meat. J. Intern. Med. 2017, 281, 106–122. [Google Scholar] [CrossRef] [PubMed]
- WHO. El Centro Internacional de Investigaciones Sobre el Cáncer Evalúa el Consumo de la Carne Roja y de la Carne Procesada. Available online: https://apps.who.int/mediacentre/news/releases/2015/cancer-red-meat/es/index.html# (accessed on 19 July 2022).
- Hartmann, C.; Siegrist, M. Our daily meat: Justification, moral evaluation and willingness to substitute. Food Qual. Prefer. 2020, 80, 103799. [Google Scholar] [CrossRef]
- Onwezen, M.C.; Verain, M.C.D.; Dagevos, H. Positive emotions explain increased intention to consume five types of alternative proteins. Food Qual. Prefer. 2022, 96, 104446. [Google Scholar] [CrossRef]
- Lähteenmäki-Uutela, A.; Rahikainen, M.; Lonkila, A.; Yang, B. Alternative proteins and EU food law. Food Control 2021, 130, 108336. [Google Scholar] [CrossRef]
- van der Weele, C.; Feindt, P.; Jan van der Goot, A.; van Mierlo, B.; van Boekel, M. Meat alternatives: An integrative comparison. Trends Food Sci. Technol. 2019, 88, 505–512. [Google Scholar] [CrossRef]
- Sharif, M.; Zafar, M.H.; Aqib, A.I.; Saeed, M.; Farag, M.R.; Alagawany, M. Single cell protein: Sources, mechanism of production, nutritional value and its uses in aquaculture nutrition. Aquaculture 2021, 531, 735885. [Google Scholar] [CrossRef]
- Bertasini, D.; Binati, R.L.; Bolzonella, D.; Battista, F. Single Cell Proteins production from food processing effluents and digestate. Chemosphere 2022, 296, 134076. [Google Scholar] [CrossRef]
- Molnár, J.; Pal, M. Saccharomyces cerevisiae and Kluyveromyces marxianus: Possibilities and Role of the Most Popular Yeasts in Food Industry. Acta Sci. Microbiol. 2021, 4, 7–9. [Google Scholar] [CrossRef]
- Usmani, Z.; Sharma, M.; Awasthi, A.K.; Sharma, G.D.; Cysneiros, D.; Nayak, S.C.; Thakur, V.K.; Naidu, R.; Pandey, A.; Gupta, V.K. Minimizing hazardous impact of food waste in a circular economy—Advances in resource recovery through green strategies. J. Hazard. Mater. 2021, 416, 126154. [Google Scholar] [CrossRef]
- Thiviya, P.; Gamage, A.; Kapilan, R.; Merah, O.; Madhujith, T. Single Cell Protein Production Using Different Fruit Waste: A Review. Separations 2022, 9, 178. [Google Scholar] [CrossRef]
- Vilas-Boas, A.A.; Pintado, M.; Oliveira, A.L.S. Natural Bioactive Compounds from Food Waste: Toxicity and Safety Concerns. Foods 2021, 10, 1564. [Google Scholar] [CrossRef] [PubMed]
- Ritala, A.; Häkkinen, S.T.; Toivari, M.; Wiebe, M.G. Single Cell Protein—State-of-the-Art, Industrial Landscape and Patents 2001–2016. Front. Microbiol. 2017, 8, 2009. [Google Scholar] [CrossRef] [PubMed]
- Khan, M.K.I.; Asif, M.; Razzaq, Z.U.; Nazir, A.; Maan, A.A. Sustainable food industrial waste management through single cell protein production and characterization of protein enriched bread. Food Biosci. 2022, 46, 101406. [Google Scholar] [CrossRef]
- Zhu, Z.; Wu, Y.; Hu, W.; Zheng, X.; Chen, Y. Valorization of food waste fermentation liquid into single cell protein by photosynthetic bacteria via stimulating carbon metabolic pathway and environmental behaviour. Bioresour. Technol. 2022, 361, 127704. [Google Scholar] [CrossRef] [PubMed]
- Mantzouridou, F.T.; Paraskevopoulou, A.; Lalou, S. Yeast flavour production by solid state fermentation of orange peel waste. Biochem. Eng. J. 2015, 101, 1–8. [Google Scholar] [CrossRef]
- Karim, A.; Gerliani, N.; Aïder, M. Kluyveromyces marxianus: An emerging yeast cell factory for applications in food and biotechnology. Int. J. Food Microbiol. 2020, 333, 108818. [Google Scholar] [CrossRef] [PubMed]
- Lukjanenko, J.; Kovtuna, K.; Grube, M.; Vigants, A. Enhancement of protein content in “(‘Kluyveromyces marxianus’)” biomass produced on cheese whey lactose. J. Biotechnol. 2015, 208, S76. [Google Scholar] [CrossRef]
- Ai, Y.; Luo, T.; Yu, Y.; Zhou, J.; Lu, H. Downregulation of ammonium uptake improves the growth and tolerance of Kluyveromyces marxianus at high temperature. MicrobiologyOpen 2022, 11, e1290. [Google Scholar] [CrossRef]
- Amorim, M.L.; Soares, J.; Coimbra, J.S.D.R.; Leite, M.D.O.; Albino, L.F.T.; Martins, M.A. Microalgae proteins: Production, separation, isolation, quantification, and application in food and feed. Crit. Rev. Food Sci. Nutr. 2021, 61, 1976–2002. [Google Scholar] [CrossRef]
- Timira, V.; Meki, K.; Li, Z.; Lin, H.; Xu, M.; Pramod, S.N. A comprehensive review on the application of novel disruption techniques for proteins release from microalgae. Crit. Rev. Food Sci. Nutr. 2022, 62, 4309–4325. [Google Scholar] [CrossRef]
- Böcker, L.; Bertsch, P.; Wenner, D.; Teixeira, S.; Bergfreund, J.; Eder, S.; Fischer, P.; Mathys, A. Effect of Arthrospira platensis microalgae protein purification on emulsification mechanism and efficiency. J. Colloid Interface Sci. 2021, 584, 344–353. [Google Scholar] [CrossRef] [PubMed]
- Smetana, S.; Mathys, A.; Knoch, A.; Heinz, V. Meat alternatives: Life cycle assessment of most known meat substitutes. Int. J. Life Cycle Assess. 2015, 20, 1254–1267. [Google Scholar] [CrossRef]
- Tropea, A.; Ferracane, A.; Albergamo, A.; Potortì, A.G.; Lo Turco, V.; Di Bella, G. Single Cell Protein Production through Multi Food-Waste Substrate Fermentation. Fermentation 2022, 8, 91. [Google Scholar] [CrossRef]
- Pires, J.F.; Ferreira, G.M.R.; Reis, K.C.; Schwan, R.F.; Silva, C.F. Mixed yeasts inocula for simultaneous production of SCP and treatment of vinasse to reduce soil and fresh water pollution. J. Environ. Manag. 2016, 182, 455–463. [Google Scholar] [CrossRef]
- Yang, R.; Chen, Z.; Hu, P.; Zhang, S.; Luo, G. Two-stage fermentation enhanced single-cell protein production by Yarrowia lipolytica from food waste. Bioresour. Technol. 2022, 361, 127677. [Google Scholar] [CrossRef]
- Carranza-Méndez, R.C.; Chávez-González, M.L.; Sepúlveda-Torre, L.; Aguilar, C.N.; Govea-Salas, M.; Ramos-González, R. Production of single cell protein from orange peel residues by Candida utilis. Biocatal. Agric. Biotechnol. 2022, 40, 102298. [Google Scholar] [CrossRef]
- Gao, Y.; Li, D.; Liu, Y. Production of single cell protein from soy molasses using Candida tropicalis. Ann. Microbiol. 2012, 62, 1165–1172. [Google Scholar] [CrossRef]
- Aggelopoulos, T.; Katsieris, K.; Bekatorou, A.; Pandey, A.; Banat, I.M.; Koutinas, A.A. Solid state fermentation of food waste mixtures for single cell protein, aroma volatiles and fat production. Food Chem. 2014, 145, 710–716. [Google Scholar] [CrossRef]
- Hashem, M.; Al-Qahtani, M.S.; Alamri, S.A.; Moustafa, Y.S.; Lyberatos, G.; Ntaikou, I. Valorizing food wastes: Assessment of novel yeast strains for enhanced production of single-cell protein from wasted date molasses. Biomass Convers. Biorefin. 2022, 12, 4491–4502. [Google Scholar] [CrossRef]
- Azam, S.; Khan, Z.; Ahmad, B.; Khan, I.; Ali, J. Production of Single Cell Protein from Orange Peels Using Aspergillus niger and Saccharomyces cerevisiae. Glob. J. Biotechnol. Biochem. 2014, 9, 5. [Google Scholar] [CrossRef]
- Zhu, W.; He, Q.; Gao, H.; Nitayavardhana, S.; Khanal, S.K.; Xie, L. Bioconversion of yellow wine wastes into microbial protein via mixed yeast-fungus cultures. Bioresour. Technol. 2020, 299, 122565. [Google Scholar] [CrossRef] [PubMed]
- Aziz, N.H.; Mohsen, G.I. Bioconversion of acid- and gamma-ray-treated sweet potato residue to microbial protein by mixed cultures. J. Ind. Microbiol. Biotechnol. 2002, 29, 264–267. [Google Scholar] [CrossRef] [PubMed]
- Yap, S.M.; Lan, J.C.-W.; Kee, P.E.; Ng, H.S.; Yim, H.S. Enhancement of protein production using synthetic brewery wastewater by Haematococcus pluvialis. J. Biotechnol. 2022, 350, 1–10. [Google Scholar] [CrossRef] [PubMed]
- Putri, D.; Ulhidayati, A.; Musthofa, I.A.; Wardani, A.K. Single cell protein production of Chlorella sp. using food processing waste as a cultivation medium. IOP Conf. Ser. Earth Environ. Sci. 2018, 131, 012052. [Google Scholar] [CrossRef]
- Qin, L.; Liu, L.; Wang, Z.; Chen, W.; Wei, D. Efficient resource recycling from liquid digestate by microalgae-yeast mixed culture and the assessment of key gene transcription related to nitrogen assimilation in microalgae. Bioresour. Technol. 2018, 264, 90–97. [Google Scholar] [CrossRef]
- Rajoka, M.I.; Ahmed, S.; Hashmi, A.S.; Athar, M. Production of microbial biomass protein from mixed substrates by sequential culture fermentation of Candida utilis and Brevibacterium lactofermentum. Ann. Microbiol. 2012, 62, 1173–1179. [Google Scholar] [CrossRef]
- Zha, X.; Tsapekos, P.; Zhu, X.; Khoshnevisan, B.; Lu, X.; Angelidaki, I. Bioconversion of wastewater to single cell protein by methanotrophic bacteria. Bioresour. Technol. 2021, 320, 124351. [Google Scholar] [CrossRef]
- Saejung, C.; Salasook, P. Recycling of sugar industry wastewater for single-cell protein production with supplemental carotenoids. Environ. Technol. 2020, 41, 59–70. [Google Scholar] [CrossRef]
- Janssen, M.; Wijffels, R.H.; Barbosa, M.J. Microalgae based production of single-cell protein. Curr. Opin. Biotechnol. 2022, 75, 102705. [Google Scholar] [CrossRef]
- USDA. FoodData Central. Available online: https://fdc.nal.usda.gov/index.html (accessed on 1 September 2022).
- FAO. Amino-Acid Content of Foods and Biological Data on Proteins; FAO: Rome, Italy, 1981; ISBN 92-5-001102-4. [Google Scholar]
- Yu, H.; Liang, H.; Longshaw, M.; Wang, J.; Ge, X.; Ren, M.; Zhang, L. Methanotroph (Methylococcus capsulatus, Bath) bacteria meal (FeedKind®) could effectively improve the growth, apparent digestibility coefficient, blood biochemical parameters, antioxidant indices of juvenile Jian carp (Cyprinus carpio var. Jian). Anim. Feed. Sci. Technol. 2022, 288, 115293. [Google Scholar] [CrossRef]
- Dantas, E.M.; Valle, B.C.S.; Brito, C.M.S.; Calazans, N.K.F.; Peixoto, S.R.M.; Soares, R.B. Partial replacement of fishmeal with biofloc meal in the diet of postlarvae of the Pacific white shrimp Litopenaeus vannamei. Aquac. Nutr. 2016, 22, 335–342. [Google Scholar] [CrossRef]
- Muys, M.; Sui, Y.; Schwaiger, B.; Lesueur, C.; Vandenheuvel, D.; Vermeir, P.; Vlaeminck, S.E. High variability in nutritional value and safety of commercially available Chlorella and Spirulina biomass indicates the need for smart production strategies. Bioresour. Technol. 2019, 275, 247–257. [Google Scholar] [CrossRef] [PubMed]
- Alamgir, A.N.M. Therapeutic Use of Medicinal Plants and Their Extracts: Volume 2: Phytochemistry and Bioactive Compounds; Progress in Drug Research; Springer International Publishing: Cham, Switzerland, 2018; Volume 74, ISBN 978-3-319-92386-4. [Google Scholar]
- Petrus, M.; Culerrier, R.; Campistron, M.; Barre, A.; Rougé, P. First case report of anaphylaxis to spirulin: Identification of phycocyanin as responsible allergen: ALLERGYNet. Allergy 2009, 65, 924–925. [Google Scholar] [CrossRef]
- Yim, H.E.; Yoo, K.H.; Seo, W.H.; Won, N.H.; Hong, Y.S.; Lee, J.W. Acute tubulointerstitial nephritis following ingestion of Chlorella tablets. Pediatr. Nephrol. 2007, 22, 887–888. [Google Scholar] [CrossRef]
- Pillaca-Pullo, O.S.; Lopes, A.M.; Rodriguez-Portilla, L.M.I.; Estela-Escalante, W. Optimizing medium composition with wastewater from Coffea arabica processing to produce single-cell protein using Candida sorboxylosa. J. Chem. Technol. Biotechnol. 2022, jctb.7219. [Google Scholar] [CrossRef]
- Kurcz, A.; Błażejak, S.; Kot, A.M.; Bzducha-Wróbel, A.; Kieliszek, M. Application of Industrial Wastes for the Production of Microbial Single-Cell Protein by Fodder Yeast Candida utilis. Waste Biomass Valorization 2018, 9, 57–64. [Google Scholar] [CrossRef]
- Harguess, J.M.; Crespo, N.C.; Hong, M.Y. Strategies to reduce meat consumption: A systematic literature review of experimental studies. Appetite 2020, 144, 104478. [Google Scholar] [CrossRef]
- Özbal, B.; Çelekli, A.; Gün, D.; Bozkurt, H. Effect of Arthrospira platensis incorporation on nutritional and sensory attributes of white chocolate. Int. J. Gastron. Food Sci. 2022, 28, 100544. [Google Scholar] [CrossRef]
- Wang, K.; Luo, Q.; Hong, H.; Liu, H.; Luo, Y. Novel antioxidant and ACE inhibitory peptide identified from Arthrospira platensis protein and stability against thermal/pH treatments and simulated gastrointestinal digestion. Food Res. Int. 2021, 139, 109908. [Google Scholar] [CrossRef]
- Mirzaei, M.; Mirdamadi, S.; Safavi, M. Structural analysis of ACE-inhibitory peptide (VL-9) derived from Kluyveromyces marxianus protein hydrolysate. J. Mol. Struct. 2020, 1213, 128199. [Google Scholar] [CrossRef]
- Asmaz, E.D.; Seyidoglu, N. The prevention role of Spirulina platensis (Arthrospira platensis) on intestinal health. Food Sci. Hum. Wellness 2022, 11, 1342–1346. [Google Scholar] [CrossRef]
| Components | Meat (Beef) [44,45] | Milk (Cow, Whole) [44,45] | Fish (Carp, Raw) [44,45] | Rhodopseudomonas faecalis (Bacterium) [42] | Candida utilis (Yeast) and Brevibacterium lactofermentum (Corynebacterium) [40] | Saccharomyces cerevisiae (Yeast) [27] | Saccharomyces cerevisiae (Yeast) [17] | Saccharomyces cerevisiae (Yeast) [28] | Haematococcus pluvialis (Microalgae) [37] |
|---|---|---|---|---|---|---|---|---|---|
| Lipid % | 3.68 | 3.2 | 5.6 | NR | 0.10 | 14.4 | 2.3 | NR | NR |
| SFAs (%) | 1.65 | 1.86 | 1.08 | NR | NR | 24.68 | NR | NR | NR |
| MUFAs (%) | 1.22 | 0.69 | 2.33 | NR | NR | 47.02 | NR | NR | NR |
| PUFAs (%) | 0.22 | 0.11 | 1.43 | NR | NR | 26.39 | NR | NR | NR |
| Ash % | 1.02 | NR | 1.46 | NR | 11.5 | 1.08 | 7.85 | NR | NR |
| Fiber % | 0 | 0 | 0 | NR | 14.2 | NR | 3.38 | NR | NR |
| Carbohydrate (%) | 0.23 | 4.67 | 0 | NR | NR | NR | 34.88 | NR | NR |
| Protein % | 21.2 | 3.28 | 17.8 | 51.5 | 54.5 | NR | 47.7 | 53.31 | 64.93 |
| Essential amino acids (%) | |||||||||
| Isoleucine | 2.41 | 0.12045 | 2.71 | 3.7 | 3.45 | NR | 2.12 | 1.27 | 2.58 |
| Leucine | 4.06 | 0.2365 | 4.35 | 7.6 | 4.13 | NR | 4.35 | 2.28 | 10.87 |
| Lysine | 4.45 | 0.1364 | 5.16 | 5.6 | 25.00 | NR | 3.14 | 0.28 | 0.47–11.05 |
| Methionine | 1.35 | 0.0473 | 1.62 | 0.5 | 1.86 | NR | 1.12 | NR | 0.54 |
| Phenylalanine | 2.20 | 0.13145 | 2.22 | 4.1 | 1.65 | NR | 2.69 | 1.84 | 2.07–3.17 |
| Threonine | 2.29 | 0.08415 | 2.59 | 0.3 | 3.93 | NR | 2.49 | 3.03 | 1.59–7.41 |
| Tryptophan | - | - | - | 3.8 | NR | NR | NR | 14.22 | ND |
| Valine | 2.50 | 0.14025 | 3.46 | 5.5 | 2.81 | NR | 3.84 | 3.49 | ND |
| Histidine | 1.70 | 0.0649 | 2.00 | 1.9 | 3.12 | NR | 0.79 | 3.3 | 0.34–1.84 |
| Non-essential amino acids (%) | |||||||||
| Cysteine | 0.64 | NR | 0.66 | 1.0 | NR | NR | 0.19 | NR | 0.55–1.19 |
| Tyrosine | 1.80 | 0.12 | 2.07 | 2.5 | 2.49 | NR | 0.52 | 7.24 | 1.11–6.91 |
| Arginine | 3.16 | 0.05 | 3.21 | 1.1 | 3.35 | NR | 3.21 | 1.76 | 5.55–21.44 |
| Alanine | 2.92 | 0.08 | 3.39 | 6.6 | 2.82 | NR | 1.84 | 2.17 | 1.15–12.68 |
| Aspartic acid | 4.50 | 0.13 | 5.86 | 4.7 | 4.87 | NR | 3.9 | 6.27 | 7.24–18.71 |
| Glutamic acid | 7.65 | 0.35 | 7.99 | 3.7 | 12.00 | NR | NR | 7.83 | 0.85–5.62 |
| Glycine | 2.43 | 0.04 | 2.73 | 6.1 | 3.87 | NR | 1.52 | 5.6 | 9.38–28.12 |
| Proline | 1.89 | 0.15 | 2.08 | 5.4 | 2.74 | NR | 0.39 | 32.07 | 6.45–9.96 |
| Serine | 2.02 | 0.10 | 2.45 | 3.7 | 1.24 | NR | 1.44 | 9.31 | 2.72–7.7 |
| Asparagine | NR | NR | NR | NR | NR | NR | 1.39 | NR | 0.68–6.67 |
| Glutamine | NR | NR | NR | 4.3 | NR | NR | 2.04 | NR | 1.53–6.83 |
| Limiting amino acid | Tryptophan | Threonine | Isoleucine | ||||||
Publisher’s Note: MDPI stays neutral with regard to jurisdictional claims in published maps and institutional affiliations. |
© 2022 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Salazar-López, N.J.; Barco-Mendoza, G.A.; Zuñiga-Martínez, B.S.; Domínguez-Avila, J.A.; Robles-Sánchez, R.M.; Ochoa, M.A.V.; González-Aguilar, G.A. Single-Cell Protein Production as a Strategy to Reincorporate Food Waste and Agro By-Products Back into the Processing Chain. Bioengineering 2022, 9, 623. https://doi.org/10.3390/bioengineering9110623
Salazar-López NJ, Barco-Mendoza GA, Zuñiga-Martínez BS, Domínguez-Avila JA, Robles-Sánchez RM, Ochoa MAV, González-Aguilar GA. Single-Cell Protein Production as a Strategy to Reincorporate Food Waste and Agro By-Products Back into the Processing Chain. Bioengineering. 2022; 9(11):623. https://doi.org/10.3390/bioengineering9110623
Chicago/Turabian StyleSalazar-López, Norma Julieta, Gabriel A. Barco-Mendoza, B. Shain Zuñiga-Martínez, J. Abraham Domínguez-Avila, R. Maribel Robles-Sánchez, Monica A. Villegas Ochoa, and Gustavo A. González-Aguilar. 2022. "Single-Cell Protein Production as a Strategy to Reincorporate Food Waste and Agro By-Products Back into the Processing Chain" Bioengineering 9, no. 11: 623. https://doi.org/10.3390/bioengineering9110623
APA StyleSalazar-López, N. J., Barco-Mendoza, G. A., Zuñiga-Martínez, B. S., Domínguez-Avila, J. A., Robles-Sánchez, R. M., Ochoa, M. A. V., & González-Aguilar, G. A. (2022). Single-Cell Protein Production as a Strategy to Reincorporate Food Waste and Agro By-Products Back into the Processing Chain. Bioengineering, 9(11), 623. https://doi.org/10.3390/bioengineering9110623