Biocompatibility and Connectivity of Semiconductor Nanostructures for Cardiac Tissue Engineering Applications
Abstract
1. Introduction
2. Materials and Methods
2.1. ZnO Nanowire and Si Nanowire Synthesis
2.2. Cell Culture
2.3. AlamarBlue Metabolic Activity Assay
2.4. ZnO and Si NW Degradation Cytotoxicity Assay
2.5. Cell Morphology
2.6. Statistical Analysis
3. Results
3.1. ZnO and Si Nanowire Growth
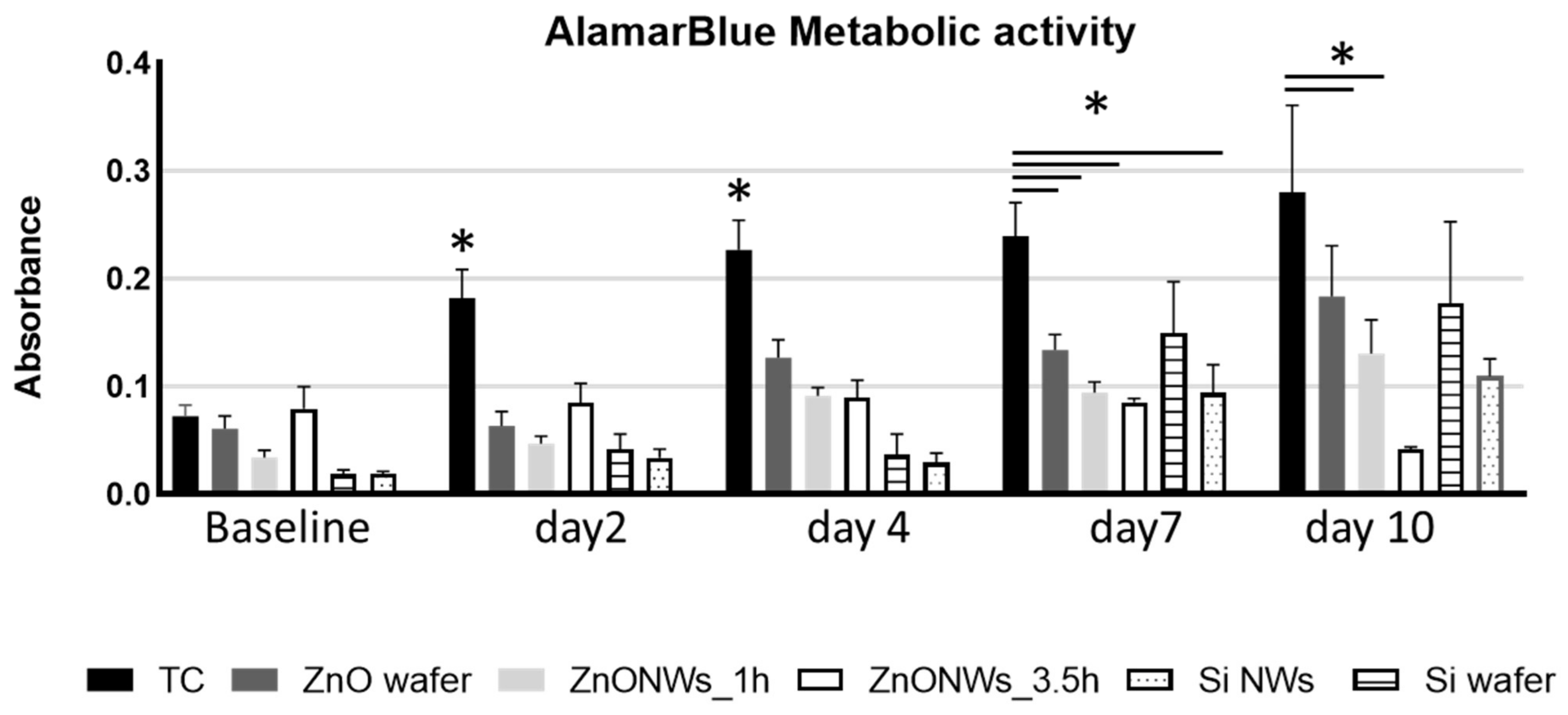
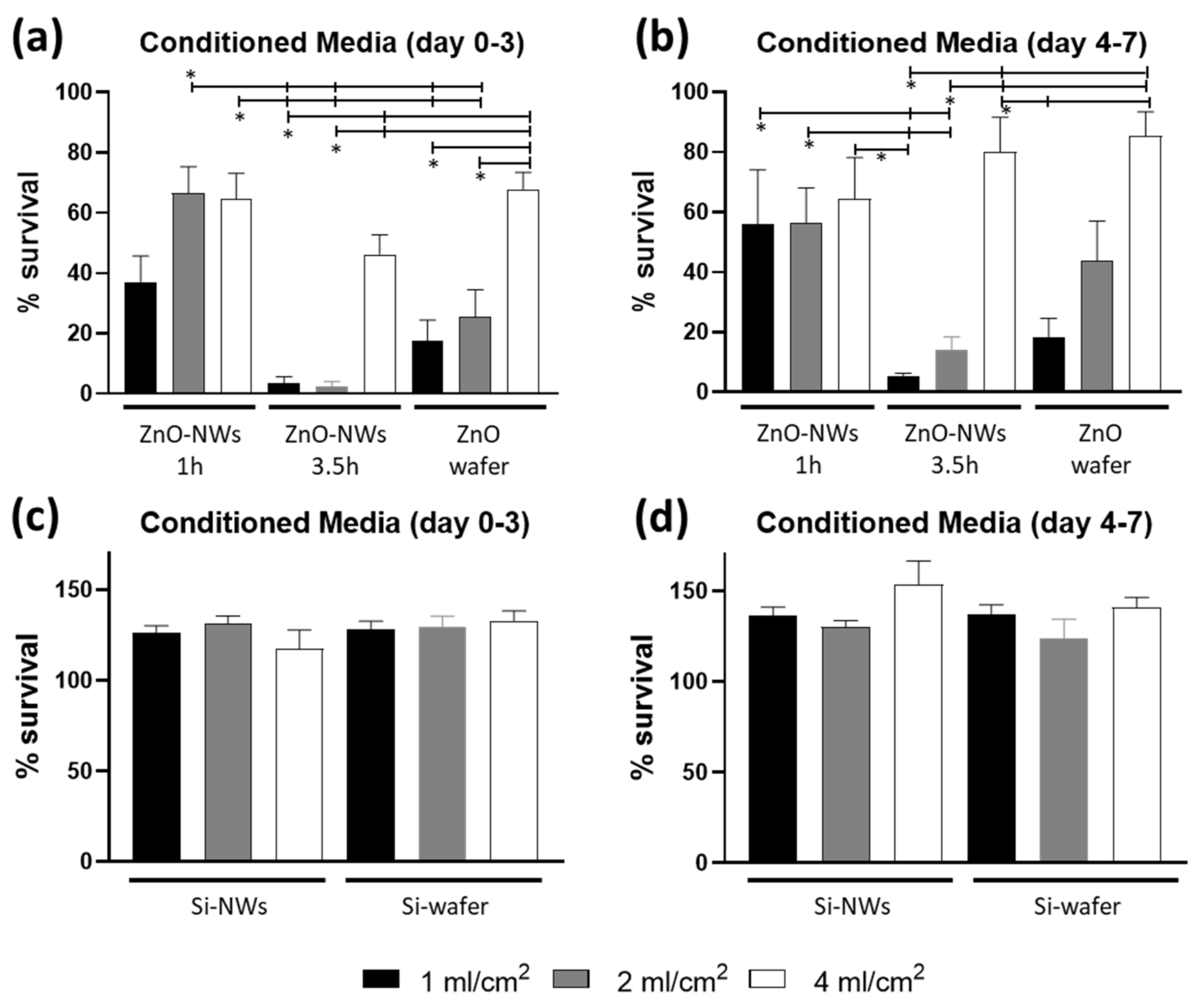
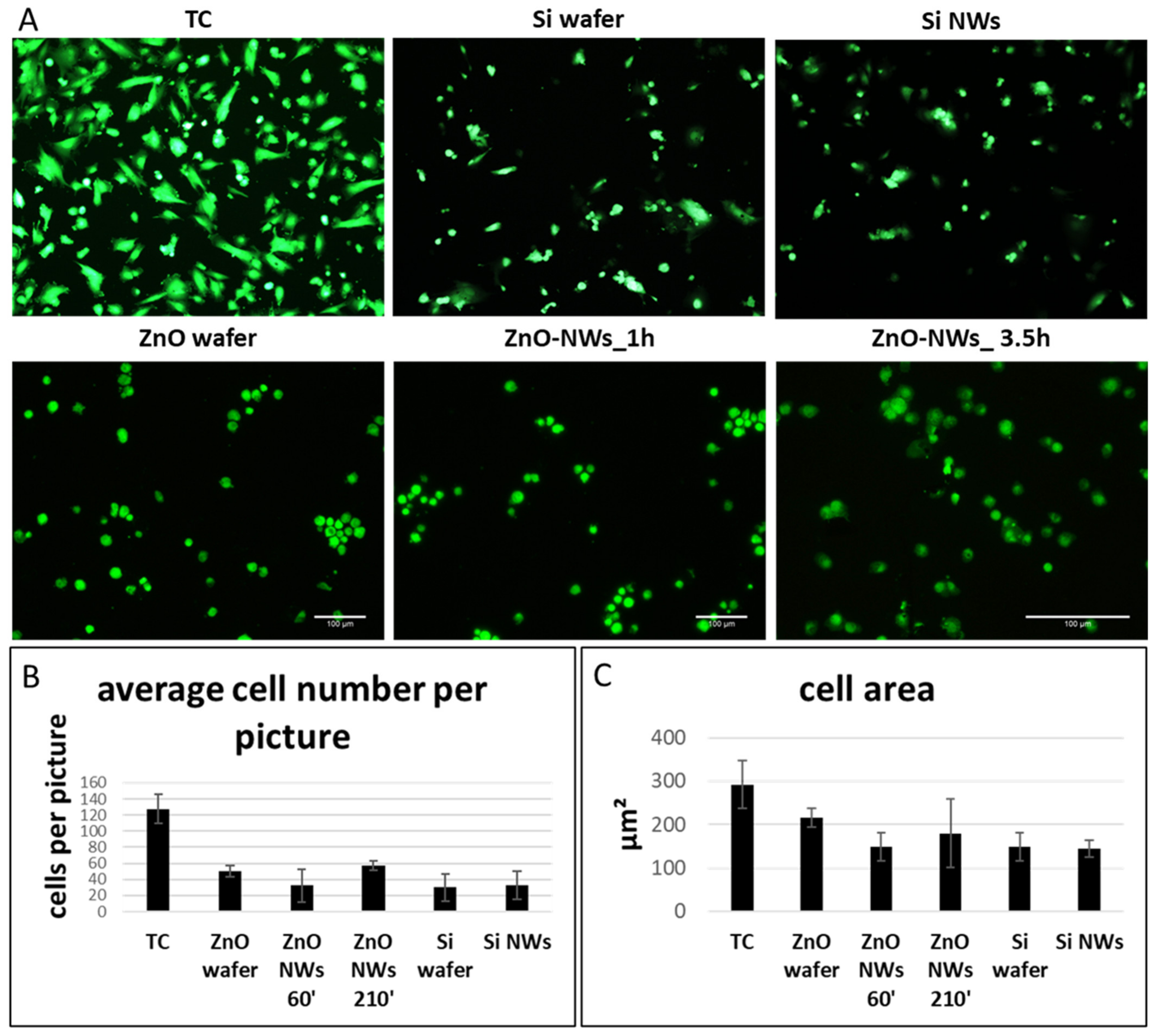
3.2. ZnO NW Biocompatibility
4. Discussion
Supplementary Materials
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Data Availability Statement
Conflicts of Interest
References
- De Lange, W.J.; Farrell, E.T.; Kreitzer, C.R.; Jacobs, D.R.; Lang, D.; Glukhov, A.V.; Carter Ralphe, J. Human iPSC-engineered cardiac tissue platform faithfully models important cardiac physiology. Am. J. Physiol.-Heart Circ. Physiol. 2021, 320, H1670–H1686. [Google Scholar] [CrossRef] [PubMed]
- Khedro, T.; Duran, J.M.; Adler, E.D. Modeling Nonischemic Genetic Cardiomyopathies Using Induced Pluripotent Stem Cells. Curr. Cardiol. Rep. 2022, 24, 631–644. [Google Scholar] [CrossRef] [PubMed]
- Salem, T.; Frankman, Z.; Churko, J.M. Tissue Engineering Techniques for Induced Pluripotent Stem Cell Derived Three-Dimensional Cardiac Constructs. Tissue Eng. Part B. Rev. 2022, 28, 891–911. [Google Scholar] [CrossRef] [PubMed]
- Stoppel, W.L.; Kaplan, D.L.; Black, L.D. Electrical and mechanical stimulation of cardiac cells and tissue constructs. Adv. Drug Deliv. Rev. 2016, 96, 135–155. [Google Scholar] [CrossRef] [PubMed]
- Ruan, J.L.; Tulloch, N.L.; Razumova, M.V.; Saiget, M.; Muskheli, V.; Pabon, L.; Reinecke, H.; Regnier, M.; Murry, C.E. Mechanical Stress Conditioning and Electrical Stimulation Promote Contractility and Force Maturation of Induced Pluripotent Stem Cell-Derived Human Cardiac Tissue. Circulation 2016, 134, 1557–1567. [Google Scholar] [PubMed]
- Nunes, S.S.; Miklas, J.W.; Liu, J.; Aschar-Sobbi, R.; Xiao, Y.; Zhang, B.; Jiang, J.; Massé, S.; Gagliardi, M.; Hsieh, A.; et al. Biowire: A platform for maturation of human pluripotent stem cell-derived cardiomyocytes. Nat. Methods 2013, 10, 781–787. [Google Scholar] [CrossRef]
- Ronaldson-Bouchard, K.; Yeager, K.; Teles, D.; Chen, T.; Ma, S.; Song, L.J.; Morikawa, K.; Wobma, H.M.; Vasciaveo, A.; Ruiz, E.C.; et al. Engineering of human cardiac muscle electromechanically matured to an adult-like phenotype. Nat. Protoc. 2019, 14, 2781–2817. [Google Scholar] [CrossRef]
- Guo, B.; Ma, P.X. Conducting Polymers for Tissue Engineering. Biomacromolecules 2018, 19, 1764–1782. [Google Scholar] [CrossRef]
- Ashtari, K.; Nazari, H.; Ko, H.; Tebon, P.; Akhshik, M.; Akbari, M.; Alhosseini, S.N.; Mozafari, M.; Mehravi, B.; Soleimani, M.; et al. Electrically conductive nanomaterials for cardiac tissue engineering. Adv. Drug Deliv. Rev. 2019, 144, 162–179. [Google Scholar] [CrossRef]
- Wang, L.; Wu, Y.; Hu, T.; Guo, B.; Ma, P.X. Electrospun conductive nanofibrous scaffolds for engineering cardiac tissue and 3D bioactuators. Acta Biomater. 2017, 59, 68–81. [Google Scholar] [CrossRef]
- Fromherz, P. Electrical interfacing of nerve cells and semiconductor chips. ChemPhysChem 2002, 3, 276–284. [Google Scholar] [CrossRef]
- Xie, C.; Lin, Z.; Hanson, L.; Cui, Y.; Cui, B. Intracellular recording of action potentials by nanopillar electroporation. Nat. Nanotechnol. 2012, 7, 185–190. [Google Scholar] [CrossRef]
- Eversmann, B.; Jenkner, M.; Hofmann, F.; Paulus, C.; Brederlow, R.; Holzapfl, B.; Fromherz, P.; Merz, M.; Brenner, M.; Schreiter, M.; et al. A 128 × 128 CMOS Biosensor Array for Extracellular Recording of Neural Activity. IEEE J. Solid-State Circuits 2003, 38, 2306–2317. [Google Scholar] [CrossRef]
- Spira, M.E.; Hai, A. Multi-electrode array technologies for neuroscience and cardiology. Nat. Nanotechnol. 2013, 8, 83–94. [Google Scholar] [CrossRef]
- Timko, B.P.; Cohen-Karni, T.; Yu, G.; Qing, Q.; Tian, B.; Lieber, C.M. Electrical Recording from Hearts with Flexible Nanowire Device Arrays. Nano Lett. 2009, 9, 914–918. [Google Scholar] [CrossRef]
- Duan, X.; Gao, R.; Xie, P.; Cohen-Karni, T.; Qing, Q.; Choe, H.S.; Tian, B.; Jiang, X.; Lieber, C.M. Intracellular recordings of action potentials by an extracellular nanoscale field-effect transistor. Nat. Nanotechnol. 2012, 7, 174–179. [Google Scholar] [CrossRef]
- Gao, J.; Liao, C.; Liu, S.; Xia, T.; Jiang, G. Nanotechnology: New opportunities for the development of patch-clamps. J. Nanobiotechnol. 2021, 19, 97. [Google Scholar] [CrossRef]
- Plikus, M.V.; Wang, X.; Sinha, S.; Forte, E.; Thompson, S.M.; Herzog, E.L.; Driskell, R.R.; Rosenthal, N.; Biernaskie, J.; Horsley, V. Fibroblasts: Origins, definitions, and functions in health and disease. Cell 2021, 184, 3852–3872. [Google Scholar] [CrossRef]
- Tallquist, M.D. Cardiac Fibroblast Diversity. Annu. Rev. Physiol. 2020, 82, 63–78. [Google Scholar] [CrossRef]
- Forte, E.; Furtado, M.B.; Rosenthal, N. The interstitium in cardiac repair: Role of the immune–stromal cell interplay. Nat. Rev. Cardiol. 2018, 15, 601–616. [Google Scholar] [CrossRef]
- Pea, M.; Maiolo, L.; Pilloton, R.; Rinaldi, A.; Araneo, R.; Giovine, E.; Orsini, A.; Notargiacomo, A. ZnO nanowires strips growth: Template reliability and morphology study. Microelectron. Eng. 2014, 121, 147–152. [Google Scholar] [CrossRef]
- Piedimonte, P.; Mazzetta, I.; Fucile, S.; Limatola, C.; Cattaruzza, E.; Riello, P.; Renzi, M.; Palma, F. Silicon nanowires to detect electric signals from living cells. Mater. Res. Express 2019, 6, 084005. [Google Scholar] [CrossRef]
- Messina, E.; De Angelis, L.; Frati, G.; Morrone, S.; Chimenti, S.; Fiordaliso, F.; Salio, M.; Battaglia, M.; Latronico, M.V.G.; Coletta, M.; et al. Isolation and expansion of adult cardiac stem cells from human and murine heart. Circ. Res. 2004, 95, 911–921. [Google Scholar] [CrossRef] [PubMed]
- De Souza, G.L.; Moura, C.C.G.; Silva, A.C.A.; Marinho, J.Z.; Silva, T.R.; Dantas, N.O.; Bonvicini, J.F.S.; Turrioni, A.P. Effects of zinc oxide and calcium–doped zinc oxide nanocrystals on cytotoxicity and reactive oxygen species production in different cell culture models. Restor. Dent. Endod. 2020, 45, e54. [Google Scholar] [CrossRef] [PubMed]
- Ferrone, E.; Araneo, R.; Notargiacomo, A.; Pea, M.; Rinaldi, A. ZnO nanostructures and electrospun ZnO–polymeric hybrid nanomaterials in biomedical, health, and sustainability applications. Nanomaterials 2019, 9, 1449. [Google Scholar]
- Liao, C.; Jin, Y.; Li, Y.; Tjong, S.C. Interactions of zinc oxide nanostructures with mammalian cells: Cytotoxicity and photocatalytic toxicity. Int. J. Mol. Sci. 2020, 21, 6305. [Google Scholar] [CrossRef]
- Ciofani, G.; Genchi, G.G.; Mattoli, V. ZnO nanowire arrays as substrates for cell proliferation and differentiation. Mater. Sci. Eng. C 2012, 32, 341–347. [Google Scholar]
- Wang, Y.; Wu, Y.; Quadri, F.; Prox, J.D.; Guo, L. Cytotoxicity of ZnO nanowire arrays on excitable cells. Nanomaterials 2017, 7, 80. [Google Scholar] [CrossRef]
- Lee, J.; Kang, B.S.; Hicks, B.; Chancellor, T.F.; Chu, B.H.; Wang, H.T.; Keselowsky, B.G.; Ren, F.; Lele, T.P. The control of cell adhesion and viability by zinc oxide nanorods. Biomaterials 2008, 29, 3743–3749. [Google Scholar] [CrossRef]
- Qin, X.; Zhang, J.; Wang, B.; Xu, G.; Yang, X.; Zou, Z.; Yu, C. Ferritinophagy is involved in the zinc oxide nanoparticles-induced ferroptosis of vascular endothelial cells. Autophagy 2021, 17, 4266–4285. [Google Scholar] [CrossRef]
- Zong, X.; Zhu, R. Zinc oxide nanorod field effect transistor for long-time cellular force measurement. Sci. Rep. 2017, 7, 43661. [Google Scholar] [CrossRef]
- Li, Z.; Yang, R.; Yu, M.; Bai, F.; Li, C.; Wang, Z.L. Cellular level biocompatibility and biosafety of ZnO nanowires. J. Phys. Chem. C 2008, 112, 20114–20117. [Google Scholar] [CrossRef]
- Ahmed, B.; Dwivedi, S.; Abdin, M.Z.; Azam, A.; Al-Shaeri, M.; Khan, M.S.; Saquib, Q.; Al-Khedhairy, A.A.; Musarrat, J. Mitochondrial and Chromosomal Damage Induced by Oxidative Stress in Zn2+ Ions, ZnO-Bulk and ZnO-NPs treated Allium cepa roots. Sci. Rep. 2017, 7, 40685. [Google Scholar] [CrossRef]
- Ahtzaz, S.; Nasir, M.; Shahzadi, L.; Iqbal, F.; Chaudhry, A.A.; Yar, M.; ur Rehman, I.; Amir, W.; Anjum, A.; Arshad, R. A study on the effect of zinc oxide and zinc peroxide nanoparticles to enhance angiogenesis-pro-angiogenic grafts for tissue regeneration applications. Mater. Des. 2017, 132, 409–418. [Google Scholar] [CrossRef]
- Zhang, R.; Huang, Q.; Liu, X.; Yang, X.; Yan, H.; Xiong, Z.; Xu, N.; Ma, J.; Feng, Q.; Shen, Z. ZnO nanostructures enhance the osteogenic capacity of SaOS-2 cells on acid-etched pure Ti. Mater. Lett. 2018, 215, 173–175. [Google Scholar] [CrossRef]
- Parnia, F.; Yazdani, J.; Javaherzadeh, V.; Dizaj, S.M. Overview of Nanoparticle Coating of Dental Implants for Enhanced Osseointegration and Antimicrobial Purposes. J. Pharm. Pharm. Sci. 2017, 20, 148–160. [Google Scholar] [CrossRef]
- Wingett, D.; Louka, P.; Anders, C.; Zhang, J.; Punnoose, A. A role of ZnO nanoparticle electrostatic properties in cancer cell cytotoxicity. Nanotechnol. Sci. Appl. 2016, 9, 29–45. [Google Scholar] [CrossRef]
- Pan, C.Y.; Lin, F.Y.; Kao, L.S.; Huang, C.C.; Liu, P.S. Zinc oxide nanoparticles modulate the gene expression of ZnT1 and ZIP8 to manipulate zinc homeostasis and stress-induced cytotoxicity in human neuroblastoma SH-SY5Y cells. PLoS ONE 2020, 15, e0232729. [Google Scholar] [CrossRef]
- Jung, E.B.; Yu, J.; Choi, S.J. Interaction between zno nanoparticles and albumin and its effect on cytotoxicity, cellular uptake, intestinal transport, toxicokinetics, and acute oral toxicity. Nanomaterials 2021, 11, 2922. [Google Scholar] [CrossRef]
- Gaetani, R.; Yin, C.; Srikumar, N.; Braden, R.; Doevendans, P.A.; Sluijter, J.P.G.; Christman, K.L. Cardiac-derived extracellular matrix enhances cardiogenic properties of human cardiac progenitor cells. Cell Transplant. 2016, 25, 1653–1663. [Google Scholar] [CrossRef]
- Gaetani, R.; Zizzi, E.A.; Deriu, M.A.; Morbiducci, U.; Pesce, M.; Messina, E. When Stiffness Matters: Mechanosensing in Heart Development and Disease. Front. Cell Dev. Biol. 2020, 8, 334. [Google Scholar] [CrossRef]
- Pagliarosi, O.; Picchio, V.; Chimenti, I.; Messina, E.; Gaetani, R. Building an Artificial Cardiac Microenvironment: A Focus on the Extracellular Matrix. Front. Cell Dev. Biol. 2020, 8, 559032. [Google Scholar] [CrossRef] [PubMed]
- Lee, J.; Chu, B.H.; Chen, K.H.; Ren, F.; Lele, T.P. Randomly oriented, upright SiO2 coated nanorods for reduced adhesion of mammalian cells. Biomaterials 2009, 30, 4488–4493. [Google Scholar] [CrossRef] [PubMed]
- Stewart, F.A.; Heeneman, S.; Te Poele, J.; Kruse, J.; Russell, N.S.; Gijbels, M.; Daemen, M. Ionizing radiation accelerates the development of atherosclerotic lesions in ApoE−/− mice and predisposes to an inflammatory plaque phenotype prone to hemorrhage. Am. J. Pathol. 2006, 168, 649–658. [Google Scholar] [CrossRef] [PubMed]
- Piedimonte, P.; Fucile, S.; Limatola, C.; Renzi, M.; Palma, F. Silicon Nanowires as Biocompatibile Electronics-Biology Interface. In Proceedings of the 2019 20th International Conference on Solid-State Sensors, Actuators and Microsystems and Eurosensors XXXIII, TRANSDUCERS 2019 and EUROSENSORS XXXIII, Berlin, Germany, 23–27 June 2019; Institute of Electrical and Electronics Engineers Inc.: Piscataway, NJ, USA, 2019; pp. 1052–1055. [Google Scholar]
- Piedimonte, P.; Fucile, S.; Limatola, C.; Renzi, M.; Palma, F. Biocompatibility of silicon nanowires: A step towards IC detectors. In AIP Conference Proceedings; American Institute of Physics Inc.: College Park, MD, USA, 2019; Volume 2145, p. 20011. [Google Scholar]
- Piedimonte, P.; Feyen, D.A.M.; Mercola, M.; Messina, E.; Renzi, M.; Palma, F. Silicon Nanowires as Contact Between the Cell Membrane and CMOS Circuits. In Lecture Notes in Electrical Engineering; Springer: Berlin/Heidelberg, Germany, 2020; Volume 627, pp. 243–250. [Google Scholar]
- Kim, I.Y.; Joachim, E.; Choi, H.; Kim, K. Toxicity of silica nanoparticles depends on size, dose, and cell type. Nanomed. Nanotechnol. Biol. Med. 2015, 11, 1407–1416. [Google Scholar] [CrossRef]
- Li, J.; Yang, H.; Sha, S.; Li, J.; Zhou, Z.; Cao, Y. Evaluation of in vitro toxicity of silica nanoparticles (NPs) to lung cells: Influence of cell types and pulmonary surfactant component DPPC. Ecotoxicol. Environ. Saf. 2019, 186, 109770. [Google Scholar] [CrossRef]
- Spataru, M.-C.; Cojocaru, F.D.; Sandu, A.V.; Solcan, C.; Duceac, I.A.; Baltatu, M.S.; Voiculescu, I.; Geanta, V.; Vizureanu, P. Assessment of the Effects of Si Addition to a New TiMoZrTa System. Materials 2021, 14, 7610. [Google Scholar] [CrossRef]
- Abbott, J.; Ye, T.; Qin, L.; Jorgolli, M.; Gertner, R.S.; Ham, D.; Park, H. CMOS nanoelectrode array for all-electrical intracellular electrophysiological imaging. Nat. Nanotechnol. 2017, 12, 460–466. [Google Scholar] [CrossRef]
- Abbott, J.; Ye, T.; Krenek, K.; Gertner, R.S.; Ban, S.; Kim, Y.; Qin, L.; Wu, W.; Park, H.; Ham, D. A nanoelectrode array for obtaining intracellular recordings from thousands of connected neurons. Nat. Biomed. Eng. 2020, 4, 232–241. [Google Scholar]
- Liu, R.; Lee, J.; Tchoe, Y.; Pre, D.; Bourhis, A.M.; D’Antonio-Chronowska, A.; Robin, G.; Lee, S.H.; Ro, Y.G.; Vatsyayan, R.; et al. Ultra-Sharp Nanowire Arrays Natively Permeate, Record, and Stimulate Intracellular Activity in Neuronal and Cardiac Networks. Adv. Funct. Mater. 2022, 32, 2108378. [Google Scholar] [CrossRef]
- Tan, Y.; Richards, D.; Coyle, R.C.; Yao, J.; Xu, R.; Gou, W.; Wang, H.; Menick, D.R.; Tian, B.; Mei, Y. Cell number per spheroid and electrical conductivity of nanowires influence the function of silicon nanowired human cardiac spheroids. Acta Biomater. 2017, 51, 495–504. [Google Scholar] [CrossRef]

Publisher’s Note: MDPI stays neutral with regard to jurisdictional claims in published maps and institutional affiliations. |
© 2022 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Gaetani, R.; Derevyanchuk, Y.; Notargiacomo, A.; Pea, M.; Renzi, M.; Messina, E.; Palma, F. Biocompatibility and Connectivity of Semiconductor Nanostructures for Cardiac Tissue Engineering Applications. Bioengineering 2022, 9, 621. https://doi.org/10.3390/bioengineering9110621
Gaetani R, Derevyanchuk Y, Notargiacomo A, Pea M, Renzi M, Messina E, Palma F. Biocompatibility and Connectivity of Semiconductor Nanostructures for Cardiac Tissue Engineering Applications. Bioengineering. 2022; 9(11):621. https://doi.org/10.3390/bioengineering9110621
Chicago/Turabian StyleGaetani, Roberto, Yuriy Derevyanchuk, Andrea Notargiacomo, Marialilia Pea, Massimiliano Renzi, Elisa Messina, and Fabrizio Palma. 2022. "Biocompatibility and Connectivity of Semiconductor Nanostructures for Cardiac Tissue Engineering Applications" Bioengineering 9, no. 11: 621. https://doi.org/10.3390/bioengineering9110621
APA StyleGaetani, R., Derevyanchuk, Y., Notargiacomo, A., Pea, M., Renzi, M., Messina, E., & Palma, F. (2022). Biocompatibility and Connectivity of Semiconductor Nanostructures for Cardiac Tissue Engineering Applications. Bioengineering, 9(11), 621. https://doi.org/10.3390/bioengineering9110621






