Surgical Treatment of Pediatric Scoliosis: Historical Origins and Review of Current Techniques
Abstract
1. Introduction
2. Static Instrumentation
2.1. Early Rods
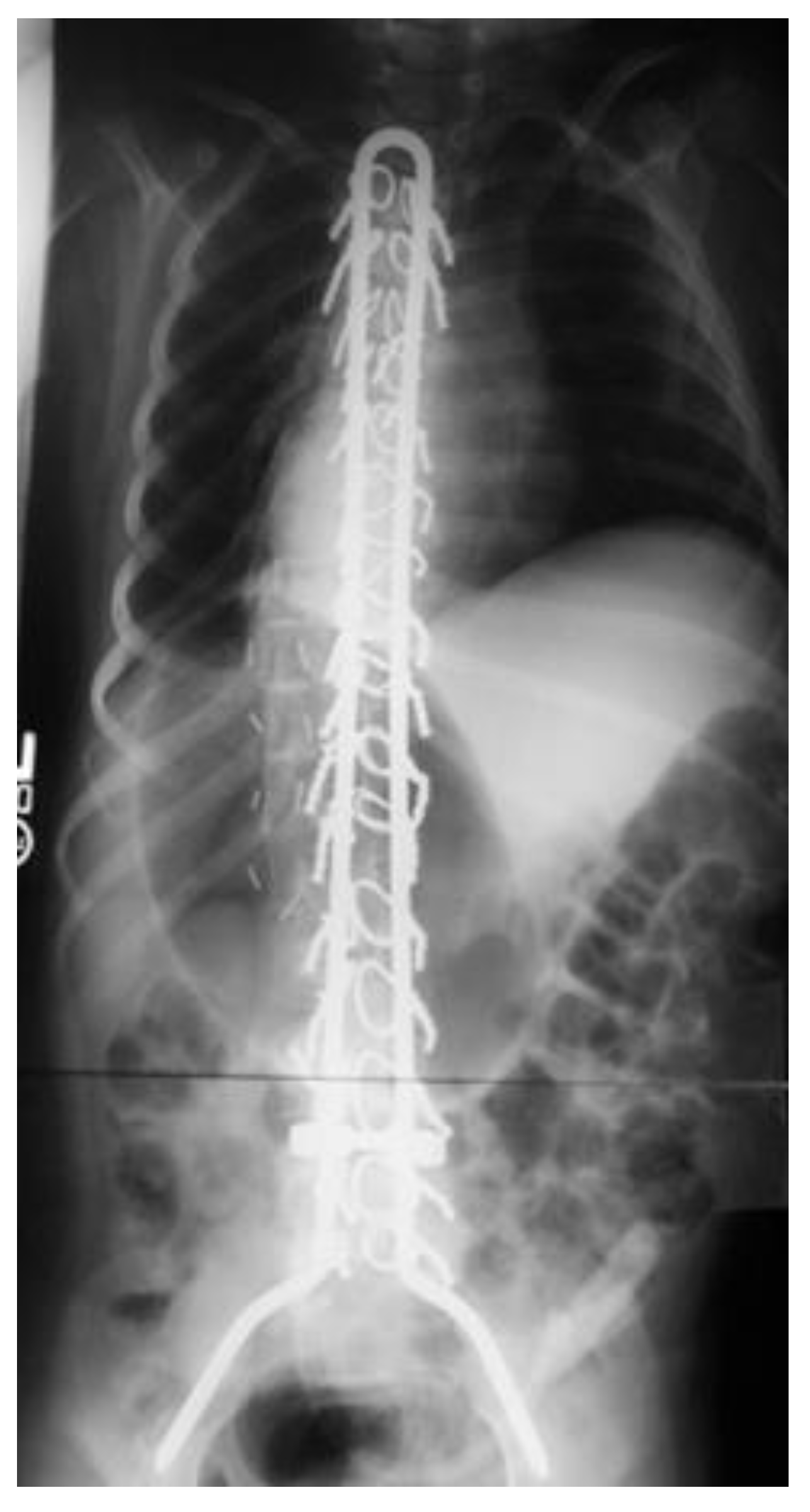
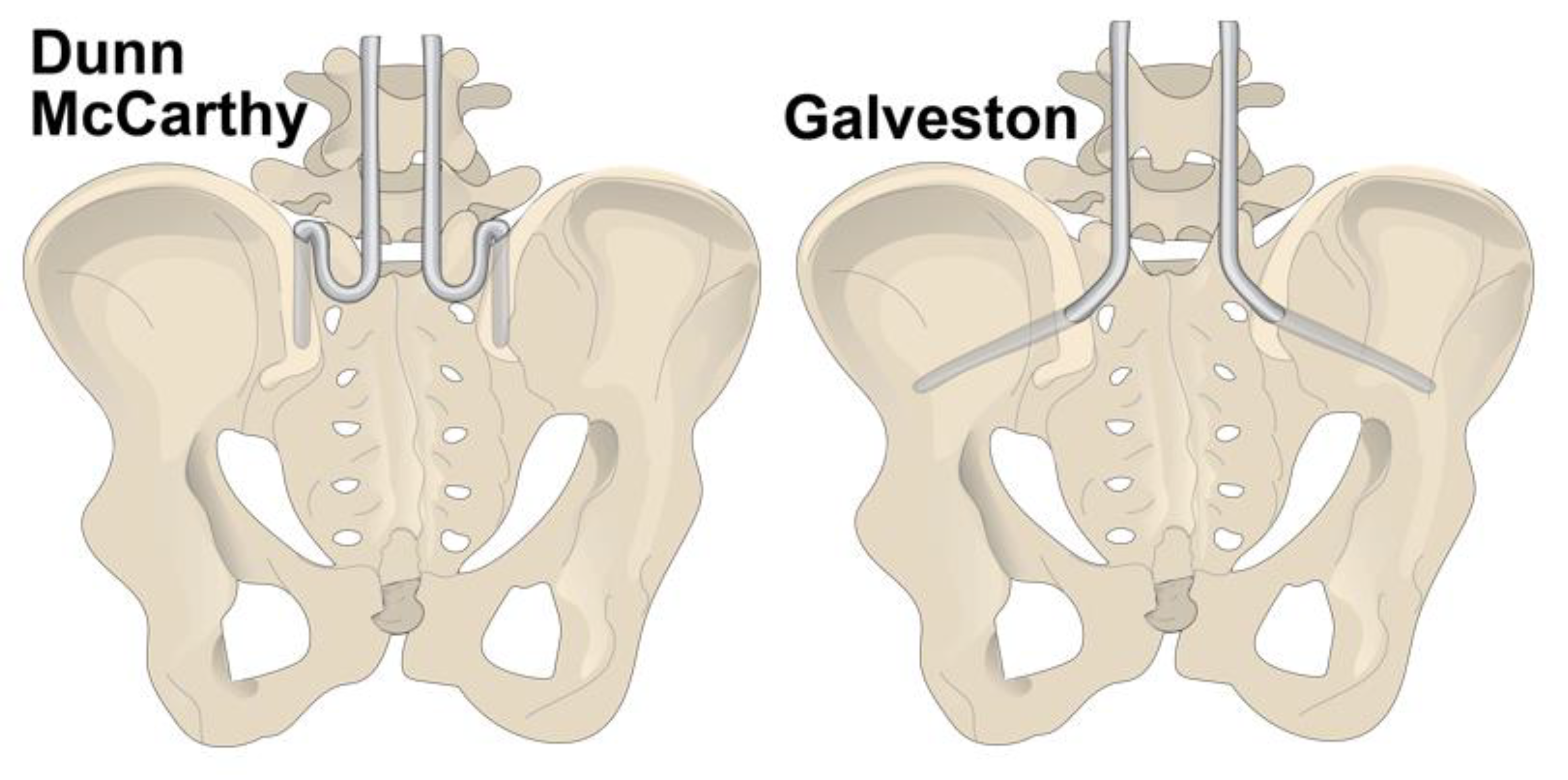
2.2. Pelvic Instrumentation
2.3. Fixation to the Spine
2.3.1. Screws
2.3.2. Hooks
2.3.3. Wires and Bands
3. Dynamic Instrumentation
3.1. The Luque Trolley System
3.2. The Shilla System
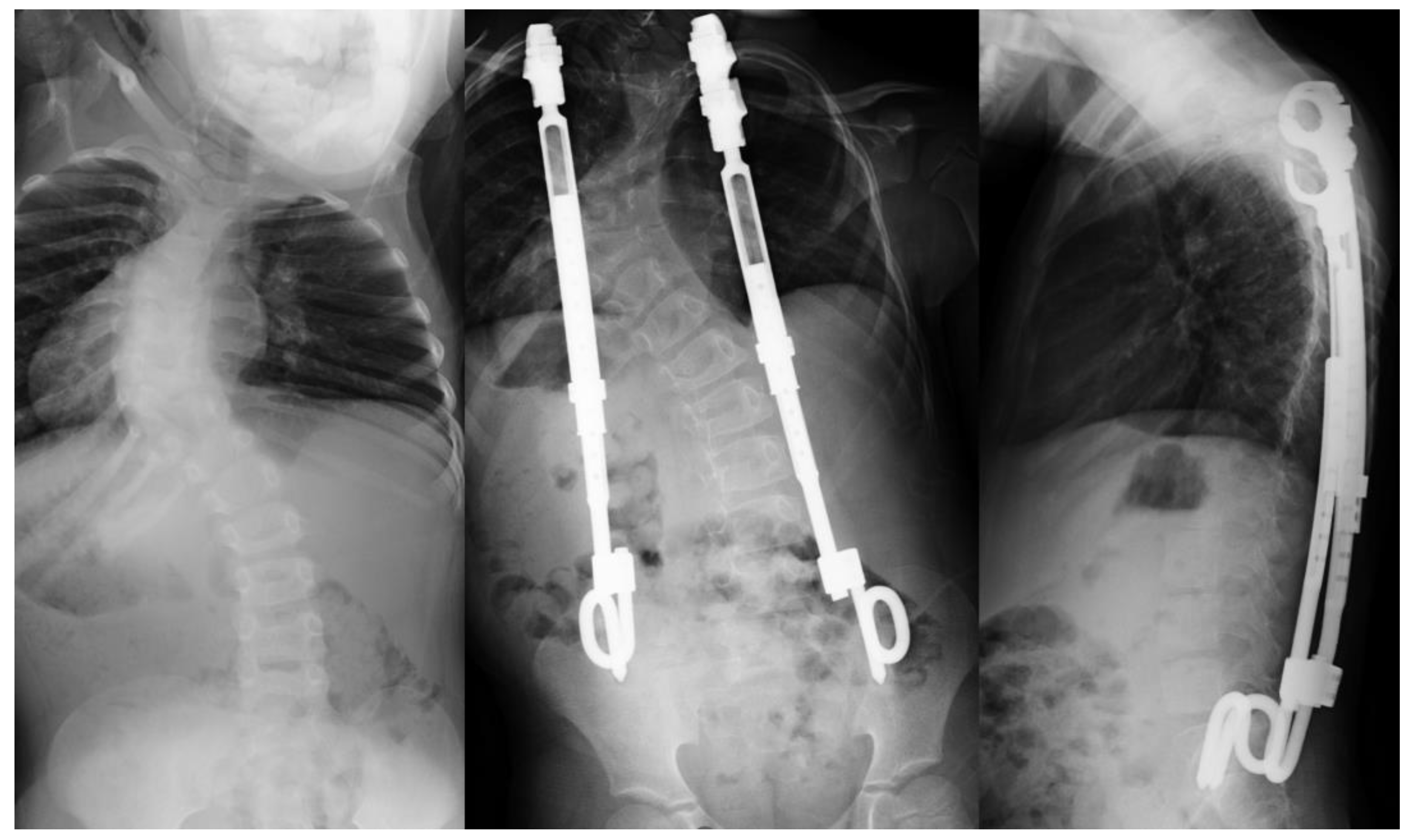
3.3. Traditional Growing Rods
3.4. Magnetically Controlled Growing Rods
3.5. Vertical Expandable Prosthetic Titanium Rib (VEPTR)
3.6. Staples
3.7. Vertebral Body Tethering
4. Conclusions
Author Contributions
Funding
Acknowledgments
Conflicts of Interest
References
- Kumar, K. Spinal deformity and axial traction. Spine 1996, 21, 653–655. [Google Scholar] [CrossRef] [PubMed]
- Heary, R.F.; Madhavan, K. The history of spinal deformity. Neurosurgery 2008, 63 (Suppl. 3), 5–15. [Google Scholar] [CrossRef]
- Hadra, B.E. Wiring the spinous processes in pott’s disease. JBJS 1891, s1–4, 206–210. [Google Scholar]
- Hibbs, R.A. An operation for progressive spinal deformities. A preliminary report of three cases from the service of the Orthopaedic hospital. NY Med. J. 1911, 93, 1013–1016. [Google Scholar]
- Moen, K.Y.; Nachemson, A.L. Treatment of scoliosis. An historical perspective. Spine 1999, 24, 2570–2575. [Google Scholar] [CrossRef]
- Harrington, P.R. The history and development of Harrington instrumentation. by Paul R. Harrington, 1973. Clin. Orthop. Relat. Res. 1988, 227, 3–5. [Google Scholar]
- Cochran, T.; Irstam, L.; Nachemson, A. Long-term anatomic and functional changes in patients with adolescent idiopathic scoliosis treated by Harrington rod fusion. Spine 1983, 8, 576–584. [Google Scholar] [CrossRef]
- Jain, A.; Hassanzadeh, H.; Strike, S.A.; Menga, E.N.; Sponseller, P.D.; Kebaish, K.M. Pelvic Fixation in Adult and Pediatric Spine Surgery: Historical Perspective, Indications, and Techniques: AAOS Exhibit Selection. J. Bone Jt. Surg. Am. 2015, 97, 1521–1528. [Google Scholar] [CrossRef]
- Luque, E.R. Segmental spinal instrumentation for correction of scoliosis. Clin. Orthop. Relat. Res. 1982, 163, 192–198. [Google Scholar] [CrossRef]
- McMaster, M. Luque rod instrumentation in the treatment of adolescent idiopathic scoliosis. A comparative study with Harrington instrumentation. J. Bone Jt. Surg. 1991, 73, 982–989. [Google Scholar] [CrossRef][Green Version]
- Herring, J.A.; Wenger, D.R. Segmental spinal instrumentation: A preliminary report of 40 consecutive cases. Spine 1982, 7, 285–298. [Google Scholar] [CrossRef] [PubMed]
- Roy-Camille, R.; Roy-Camille, M.; Demeulenaere, C. Osteosynthesis of dorsal, lumbar, and lumbosacral spine with metallic plates screwed into vertebral pedicles and articular apophyses. Presse Med. 1970, 78, 1447–1448. [Google Scholar]
- Cotrel, Y.; Dubousset, J. A new technic for segmental spinal osteosynthesis using the posterior approach. Rev. Chir. Orthop. Repar. Appar. Mot. 1984, 70, 489–494. [Google Scholar] [CrossRef]
- Camp, J.F.; Caudle, R.O.B.E.R.T.; Ashmun, R.D.; Roach, J.A.M.E.S. Immediate complications of Cotrel-Dubousset instrumentation to the sacro-pelvis. A clinical and biomechanical study. Spine 1990, 15, 932–941. [Google Scholar] [CrossRef] [PubMed]
- Liljenqvist, U.; Hackenberg, L.; Link, T.; Halm, H. Pullout strength of pedicle screws versus pedicle and laminar hooks in the thoracic spine. Acta Orthop. Belg. 2001, 67, 157–163. [Google Scholar]
- Liljenqvist, U.; Lepsien, U.; Hackenberg, L.; Niemeyer, T.; Halm, H. Comparative analysis of pedicle screw and hook instrumentation in posterior correction and fusion of idiopathic thoracic scoliosis. Eur. Spine J. 2002, 11, 336–343. [Google Scholar] [CrossRef]
- Suk, S.I.; Lee, C.K.; Kim, W.J.; Park, Y.B.; Chung, Y.J.; Song, K.Y. Segmental pedicle screw fixation in the treatment of thoracic idiopathic scoliosis. Spine 1995, 20, 1399–1405. [Google Scholar] [CrossRef]
- Allen, B.L., Jr.; Ferguson, R.L. The Galveston technique of pelvic fixation with L-rod instrumentation of the spine. Spine 1984, 9, 388–394. [Google Scholar] [CrossRef]
- Kornblatt, M.D.; Casey, M.P.; Jacobs, R.R. Internal fixation in lumbosacral spine fusion. A biomechanical and clinical study. Clin. Orthop. Relat. Res. 1986, 203, 141–150. [Google Scholar] [CrossRef]
- Dayer, R.; Ouellet, J.A.; Saran, N. Pelvic fixation for neuromuscular scoliosis deformity correction. Curr. Rev. Musculoskelet. Med. 2012, 5, 91–101. [Google Scholar] [CrossRef]
- Bell, D.F.; Moseley, C.F.; Koreska, J. Unit rod segmental spinal instrumentation in the management of patients with progressive neuromuscular spinal deformity. Spine 1989, 14, 1301–1307. [Google Scholar] [CrossRef]
- Canavese, F.; Rousset, M.; Le Gledic, B.; Samba, A.; Dimeglio, A. Surgical advances in the treatment of neuromuscular scoliosis. World J. Orthop. 2014, 5, 124–133. [Google Scholar] [CrossRef]
- Tsirikos, A.I.; Lipton, G.; Chang, W.N.; Dabney, K.W.; Miller, F. Surgical correction of scoliosis in pediatric patients with cerebral palsy using the unit rod instrumentation. Spine 2008, 33, 1133–1140. [Google Scholar] [CrossRef]
- Loughenbury, P.R.; Tsirikos, A.I. Current concepts in the treatment of neuromuscular scoliosis: Clinical assessment, treatment options, and surgical outcomes. Bone Jt. Open 2022, 3, 85–92. [Google Scholar] [CrossRef]
- McCarthy, R.E.; Dunn, H.; McCullough, F.L. Luque fixation to the sacral ala using the Dunn-McCarthy modification. Spine 1989, 14, 281–283. [Google Scholar] [CrossRef]
- Walick, K.S.; King, J.T.; Johnston, C.E.; Rathjen, K.E. Neuropathic lower extremity pain following Dunn-McCarthy instrumentation. Spine 2008, 33, E877–E880. [Google Scholar] [CrossRef]
- Schwend, R.M.; Sluyters, R.; Najdzionek, J. The pylon concept of pelvic anchorage for spinal instrumentation in the human cadaver. Spine 2003, 28, 542–547. [Google Scholar] [CrossRef]
- Gitelman, A.; Joseph, S.A., Jr.; Carrion, W.; Stephen, M. Results and morbidity in a consecutive series of patients undergoing spinal fusion with iliac screws for neuromuscular scoliosis. Orthopedics 2008, 31, 1–5. [Google Scholar]
- Phillips, J.H.; Gutheil, J.P.; Knapp, D.R., Jr. Iliac screw fixation in neuromuscular scoliosis. Spine 2007, 32, 1566–1570. [Google Scholar] [CrossRef] [PubMed]
- Chang, T.L.; Sponseller, P.D.; Kebaish, K.M.; Fishman, E.K. Low profile pelvic fixation: Anatomic parameters for sacral alar-iliac fixation versus traditional iliac fixation. Spine 2009, 34, 436–440. [Google Scholar] [CrossRef]
- Dalal, A.; Upasani, V.V.; Bastrom, T.P.; Yaszay, B.; Shah, S.A.; Shufflebarger, H.L.; Newton, P.O. Apical vertebral rotation in adolescent idiopathic scoliosis: Comparison of uniplanar and polyaxial pedicle screws. J. Spinal Disord. Tech. 2011, 24, 251–257. [Google Scholar] [CrossRef]
- Warburton, A.; Girdler, S.J.; Mikhail, C.M.; Ahn, A.; Cho, S.K. Biomaterials in Spinal Implants: A Review. Neurospine 2020, 17, 101–110. [Google Scholar] [CrossRef]
- Sielatycki, J.A.; Mitchell, K.; Leung, E.; Lehman, R.A. State of the art review of new technologies in spine deformity surgery-robotics and navigation. Spine Deform. 2022, 10, 5–17. [Google Scholar] [CrossRef]
- Cazzulino, A.; Gandhi, R.; Woodard, T.; Ackshota, N.; Janjua, M.B.; Arlet, V.; Saifi, C. Soft Landing technique as a possible prevention strategy for proximal junctional failure following adult spinal deformity surgery. J. Spine Surg. 2021, 7, 26–36. [Google Scholar] [CrossRef]
- Jaquith, B.P.; Chase, A.; Flinn, P.; Sawyer, J.R.; Warner, W.C.; Freeman, B.L.; Kelly, D.M. Screws versus hooks: Implant cost and deformity correction in adolescent idiopathic scoliosis. J. Children’s Orthop. 2012, 6, 137–143. [Google Scholar] [CrossRef]
- Fagerström, T.; Hedlund, R.; Bancel, P.; Robert, R.; Dupas, B. Laminar hook instrumentation in the cervical spine. An experimental study on the relation of hooks to the spinal cord. Eur. Spine J. 2001, 10, 340–344. [Google Scholar] [CrossRef]
- Wilber, R.G.; Thompson, G.H.; Shaffer, J.W.; Brown, R.H.; Nash, C.L., Jr. Postoperative neurological deficits in segmental spinal instrumentation. A study using spinal cord monitoring. J. Bone Jt. Surg. Am. 1984, 66, 1178–1187. [Google Scholar] [CrossRef]
- Mazda, K.; Ilharreborde, B.; Even, J.; Lefevre, Y.; Fitoussi, F.; Penneçot, G.F. Efficacy and safety of posteromedial translation for correction of thoracic curves in adolescent idiopathic scoliosis using a new connection to the spine: The Universal Clamp. Eur. Spine J. 2009, 18, 158–169. [Google Scholar] [CrossRef]
- Desai, S.K.; Sayama, C.; Vener, D.; Brayton, A.; Briceño, V.; Luerssen, T.G.; Jea, A. The feasibility and safety of using sublaminar polyester bands in hybrid spinal constructs in children and transitional adults for neuromuscular scoliosis. J. Neurosurg. Pediatr. 2015, 15, 328–337. [Google Scholar] [CrossRef]
- Albert, M.C.; LaFleur, B.C. Hybrid fixation with sublaminar polyester bands in the treatment of neuromuscular scoliosis: A comparative analysis. J. Pediatr. Orthop. 2015, 35, 172–177. [Google Scholar] [CrossRef]
- Strickland, B.A.; Sayama, C.; Briceño, V.; Lam, S.K.; Luerssen, T.G.; Jea, A. Use of subtransverse process polyester bands in pediatric spine surgery: A case series of 4 patients with a minimum of 12 months’ follow-up. J. Neurosurg. Pediatr. 2016, 17, 208–214. [Google Scholar] [CrossRef]
- Canavese, F.; Charles, Y.P.; Samba, A.; Dimeglio, A. Safety and efficacy of sublaminar bands and Ponte osteotomies in rigid deformity: Preliminary results in a prospective series of 20 neuromuscular scoliosis patients. J. Pediatr. Orthop. B 2017, 26, 233–239. [Google Scholar] [CrossRef] [PubMed]
- La Rosa, G.; Giglio, G.; Oggiano, L. Surgical treatment of neurological scoliosis using hybrid construct (lumbar transpedicular screws plus thoracic sublaminar acrylic loops). Eur. Spine J. 2011, 20 (Suppl. 1), 90–94. [Google Scholar] [CrossRef] [PubMed]
- Ferrero, E.; Pesenti, S.; Blondel, B.; Jouve, J.L.; Mazda, K.; Ilharreborde, B. Role of thoracoscopy for the sagittal correction of hypokyphotic adolescent idiopathic scoliosis patients. Eur. Spine J. 2014, 23, 2635–2642. [Google Scholar] [CrossRef]
- Hirsch, C.; Ilharreborde, B.; Fournier, J.; Mazda, K.; Bonnard, C. Adolescent idiopathic scoliosis correction achieved by posteromedial translation using polyester bands: A comparative study of subtransverse process versus sublaminar fixation. Orthop. Traumatol. Surg. Res. 2014, 100, 791–795. [Google Scholar] [CrossRef][Green Version]
- Ilharreborde, B.; Sebag, G.; Skalli, W.; Mazda, K. Adolescent idiopathic scoliosis treated with posteromedial translation: Radiologic evaluation with a 3D low-dose system. Eur. Spine J. 2013, 22, 2382–2391. [Google Scholar] [CrossRef]
- Sales de Gauzy, J.; Jouve, J.L.; Ilharreborde, B.; Blondel, B.; Accadbled, F.; Mazda, K. Use of the Universal Clamp in adolescent idiopathic scoliosis. Eur. Spine J. 2014, 23 (Suppl. 4), 446–451. [Google Scholar] [CrossRef]
- Eberle, C.F. Failure of fixation after segmental spinal instrumentation without arthrodesis in the management of paralytic scoliosis. J. Bone Jt. Surg. Am. 1988, 70, 696–703. [Google Scholar] [CrossRef]
- Mardjetko, S.M.; Hammerberg, K.W.; Lubicky, J.P.; Fister, J.S. The Luque trolley revisited. Review of nine cases requiring revision. Spine 1992, 17, 582–589. [Google Scholar] [CrossRef]
- McCarthy, R.E.; Luhmann, S.; Lenke, L.; McCullough, F.L. The Shilla growth guidance technique for early-onset spinal deformities at 2-year follow-up: A preliminary report. J. Pediatr. Orthop. 2014, 34, 1–7. [Google Scholar] [CrossRef]
- McCarthy, R.E.; McCullough, F.L. Shilla Growth Guidance for Early-Onset Scoliosis: Results After a Minimum of Five Years of Follow-up. J. Bone Jt. Surg. Am. 2015, 97, 1578–1584. [Google Scholar] [CrossRef] [PubMed]
- Nazareth, A.; Skaggs, D.L.; Illingworth, K.D.; Parent, S.; Shah, S.A.; Sanders, J.O.; Andras, L.M. Growth guidance constructs with apical fusion and sliding pedicle screws (SHILLA) results in approximately 1/3rd of normal T1-S1 growth. Spine Deform. 2020, 8, 531–535. [Google Scholar] [CrossRef] [PubMed]
- Andras, L.M.; Joiner, E.R.; McCarthy, R.E.; McCullough, L.; Luhmann, S.J.; Sponseller, P.D.; Emans, J.B.; Battett, K.K.; Skaggs, D.L.; Growing Spine Study Group. Growing Rods Versus Shilla Growth Guidance: Better Cobb Angle Correction and T1-S1 Length Increase But More Surgeries. Spine Deform. 2015, 3, 246–252. [Google Scholar] [CrossRef] [PubMed]
- Agarwal, A.; Aker, L.; Ahmad, A.A. Active apex correction with guided growth technique for controlling spinal deformity in growing children: A modified SHILLA technique. Glob. Spine J. 2020, 10, 438–442. [Google Scholar] [CrossRef]
- Akbarnia, B.A.; Marks, D.S.; Boachie-Adjei, O.; Thompson, A.G.; Asher, M.A. Dual growing rod technique for the treatment of progressive early-onset scoliosis: A multicenter study. Spine 2005, 30, S46–S57. [Google Scholar] [CrossRef]
- Thompson, G.H.; Akbarnia, B.A.; Campbell, R.M., Jr. Growing rod techniques in early-onset scoliosis. J. Pediatr. Orthop. 2007, 27, 354–361. [Google Scholar] [CrossRef]
- Blakemore, L.C.; Scoles, P.V.; Poe-Kochert, C.; Thompson, G.H. Submuscular Isola rod with or without limited apical fusion in the management of severe spinal deformities in young children: Preliminary report. Spine 2001, 26, 2044–2048. [Google Scholar] [CrossRef]
- Thompson, G.H.; Akbarnia, B.A.; Kostial, P.; Poe-Kochert, C.; Armstrong, D.G.; Roh, J.; Lowe, R.; Asher, M.A.; Marks, D.S. Comparison of single and dual growing rod techniques followed through definitive surgery: A preliminary study. Spine 2005, 30, 2039–2044. [Google Scholar] [CrossRef]
- Akbarnia, B.A.; Breakwell, L.M.; Marks, D.S.; McCarthy, R.E.; Thompson, A.G.; Canale, S.K.; Kostial, P.N.R.; Tambe, A.; Asher, M.A.; Growing Spine Study Group. Dual growing rod technique followed for three to eleven years until final fusion: The effect of frequency of lengthening. Spine 2008, 33, 984–990. [Google Scholar] [CrossRef]
- Neel, M.D.; Wilkins, R.M.; Rao, B.N.; Kelly, C.M. Early multicenter experience with a noninvasive expandable prosthesis. Clin. Orthop. Relat. Res. 2003, 415, 72–81. [Google Scholar] [CrossRef]
- Wick, J.M.; Konze, J. A magnetic approach to treating progressive early-onset scoliosis. Aorn J. 2012, 96, 163–173. [Google Scholar] [CrossRef] [PubMed]
- Rushton, P.R.P.; Smith, S.L.; Fender, D.; Bowey, A.J.; Gibson, M.J.; Joyce, T.J. Metallosis is commonly associated with magnetically controlled growing rods; results from an independent multicentre explant database. Eur. Spine J. 2021, 30, 1905–1911. [Google Scholar] [CrossRef] [PubMed]
- Tsirikos, A.I.; Roberts, S.B. Magnetic Controlled Growth Rods in the Treatment of Scoliosis: Safety, Efficacy and Patient Selection. Med. Devices 2020, 13, 75–85. [Google Scholar] [CrossRef]
- Klyce, W.; Mitchell, S.L.; Pawelek, J.; Skaggs, D.L.; Sanders, J.O.; Shah, S.A.; McCarthy, R.E.; Luhmann, S.J.; Sturm, P.F.; Flynn, J.M.; et al. Characterizing Use of Growth-friendly Implants for Early-onset Scoliosis: A 10-Year Update. J. Pediatr. Orthop. 2020, 40, e740–e746. [Google Scholar] [CrossRef]
- Rolton, D.; Richards, J.; Nnadi, C. Magnetic controlled growth rods versus conventional growing rod systems in the treatment of early onset scoliosis: A cost comparison. Eur. Spine J. 2015, 24, 1457–1461. [Google Scholar] [CrossRef]
- Su, A.W.; Milbrandt, T.A.; Larson, A.N. Magnetic Expansion Control System Achieves Cost Savings Compared to Traditional Growth Rods: An Economic Analysis Model. Spine 2015, 40, 1851–1856. [Google Scholar] [CrossRef]
- Inaparthy, P.; Queruz, J.C.; Bhagawati, D.; Thakar, C.; Subramanian, T.; Nnadi, C. Incidence of proximal junctional kyphosis with magnetic expansion control rods in early onset scoliosis. Eur. Spine J. 2016, 25, 3308–3315. [Google Scholar] [CrossRef]
- Teoh, K.H.; Winson, D.M.; James, S.H.; Jones, A.; Howes, J.; Davies, P.R.; Ahuja, S. Do magnetic growing rods have lower complication rates compared with conventional growing rods? Spine J. 2016, 16 (Suppl. 4), 40–44. [Google Scholar] [CrossRef]
- Teoh, K.H.; Winson, D.M.; James, S.H.; Jones, A.; Howes, J.; Davies, P.R.; Ahuja, S. Magnetic controlled growing rods for early-onset scoliosis: A 4-year follow-up. Spine J. 2016, 16 (Suppl. 4), S34–S39. [Google Scholar] [CrossRef]
- Gilday, S.E.; Schwartz, M.S.; Bylski-Austrow, D.I.; Glos, D.L.; Schultz, L.; O’Hara, S.; Jain, V.V.; Sturm, P.F. Observed Length Increases of Magnetically Controlled Growing Rods are Lower Than Programmed. J. Pediatr. Orthop. 2018, 38, e133–e137. [Google Scholar] [CrossRef]
- Campbell, R.M., Jr. VEPTR: Past experience and the future of VEPTR principles. Eur. Spine J. 2013, 22 (Suppl. 2), S106–S117. [Google Scholar] [CrossRef]
- Gavriliu, S.; Sora, E.M. The Use of VEPTR in Congenital Scoliosis. Int. J. Orthop. 2021, 8, 1452–1456. [Google Scholar]
- Konieczny, M.; Ehrlich, A.; Krauspe, R. Vertical expandable prosthetic titanium ribs (VEPTR) in early-onset scoliosis: Impact on thoracic compliance and sagittal balance. J. Child. Orthop. 2017, 11, 42–48. [Google Scholar] [CrossRef]
- Samdani, A.F.; Hilaire, T.S.; Emans, J.B.; Smith, J.T.; Song, K.; Campbell, R.J.; Betz, R.R. The usefulness of VEPTR in the older child with complex spine and chest deformity. Clin. Orthop. Relat. Res. 2010, 468, 700–704. [Google Scholar] [CrossRef]
- Ramirez, N.; Flynn, J.M.; Serrano, J.A.; Carlo, S.; Cornier, A.S. The Vertical Expandable Prosthetic Titanium Rib in the treatment of spinal deformity due to progressive early onset scoliosis. J. Pediatr. Orthop. B 2009, 18, 197–203. [Google Scholar] [CrossRef] [PubMed]
- Hasler, C.-C.; Mehrkens, A.; Hefti, F. Efficacy and safety of VEPTR instrumentation for progressive spine deformities in young children without rib fusions. Eur. Spine J. 2010, 19, 400–408. [Google Scholar] [CrossRef]
- Nachlas, I.W.; Borden, J.N. The cure of experimental scoliosis by directed growth control. J. Bone Jt. Surg. Am. 1951, 33, 24–34. [Google Scholar] [CrossRef]
- Smith, A.D.; Von Lackum, W.H.; Wylie, R. An operation for stapling vertebral bodies in congenital scoliosis. J. Bone Jt. Surg. Am. 1954, 36, 342–348. [Google Scholar] [CrossRef]
- Betz, R.R.; Kim, J.; D’Andrea, L.P.; Mulcahey, M.J.; Balsara, R.K.; Clements, D.H. An innovative technique of vertebral body stapling for the treatment of patients with adolescent idiopathic scoliosis: A feasibility, safety, and utility study. Spine 2003, 28, S255–S265. [Google Scholar] [CrossRef]
- Braun, J.T.; Ogilvie, J.W.; Akyuz, E.; Brodke, D.S.; Bachus, K.N. Fusionless scoliosis correction using a shape memory alloy staple in the anterior thoracic spine of the immature goat. Spine 2004, 29, 1980–1989. [Google Scholar] [CrossRef]
- Bylski-Austrow, D.I.; Wall, E.J.; Glos, D.L.; Ballard, E.T.; Montgomery, A.; Crawford, A.H. Spinal hemiepiphysiodesis decreases the size of vertebral growth plate hypertrophic zone and cells. J. Bone Jt. Surg. Am. 2009, 91, 584–593. [Google Scholar] [CrossRef] [PubMed]
- Cuddihy, L.; Danielsson, A.J.; Cahill, P.J.; Samdani, A.F.; Grewal, H.; Richmond, J.M.; Mulcahey, M.J.; Gaughan, J.P.; Antonacci, M.D.; Betz, R.R. Vertebral Body Stapling versus Bracing for Patients with High-Risk Moderate Idiopathic Scoliosis. Biomed. Res. Int. 2015, 2015, 438452. [Google Scholar] [CrossRef] [PubMed]
- O’Leary, P.T.; Sturm, P.F.; Hammerberg, K.W.; Lubicky, J.P.; Mardjetko, S.M. Convex hemiepiphysiodesis: The limits of vertebral stapling. Spine 2011, 36, 1579–1583. [Google Scholar] [CrossRef] [PubMed]
- Newton, P.O.; Farnsworth, C.L.; Faro, F.D.; Mahar, A.T.; Odell, T.R.; Mohamad, F.; Breisch, E.; Fricka, K.; Upasani, V.V.; Amiel, D. Spinal growth modulation with an anterolateral flexible tether in an immature bovine model: Disc health and motion preservation. Spine 2008, 33, 724–733. [Google Scholar] [CrossRef]
- Newton, P.O.; Fricka, K.B.; Lee, S.S.; Farnsworth, C.L.; Cox, T.G.; Mahar, A.T. Asymmetrical flexible tethering of spine growth in an immature bovine model. Spine 2002, 27, 689–693. [Google Scholar] [CrossRef]
- Samdani, A.F.; Ames, R.J.; Kimball, J.S.; Pahys, J.M.; Grewal, H.; Pelletier, G.J.; Betz, R.R. Anterior vertebral body tethering for idiopathic scoliosis: Two-year results. Spine 2014, 39, 1688–1693. [Google Scholar] [CrossRef]
- Crawford, C.H., 3rd; Lenke, L.G. Growth modulation by means of anterior tethering resulting in progressive correction of juvenile idiopathic scoliosis: A case report. J. Bone Jt. Surg. Am. 2010, 92, 202–209. [Google Scholar] [CrossRef]
- Lavelle, W.F.; Moldavsky, M.; Cai, Y.; Ordway, N.R.; Bucklen, B.S. An initial biomechanical investigation of fusionless anterior tether constructs for controlled scoliosis correction. Spine J. 2016, 16, 408–413. [Google Scholar] [CrossRef]
- Nicolini, L.F.; Kobbe, P.; Seggewiß, J.; Greven, J.; Ribeiro, M.; Beckmann, A.; Da Paz, S.; Eschweiler, J.; Prescher, A.; Markert, B.; et al. Motion preservation surgery for scoliosis with a vertebral body tethering system: A biomechanical study. Eur. Spine J. 2022, 31, 1013–1021. [Google Scholar] [CrossRef] [PubMed]
- Newton, P.O.; Kluck, D.G.; Saito, W.; Yaszay, B.; Bartley, C.E.; Bastrom, T.P. Anterior spinal growth tethering for skeletally immature patients with scoliosis: A retrospective look two to four years postoperatively. JBJS 2018, 100, 1691–1697. [Google Scholar] [CrossRef]
- Miyanji, F.; Pawelek, J.; Nasto, L.A.; Parent, S. A prospective, multicenter analysis of the efficacy of anterior vertebral body tethering (AVBT) in the treatment of idiopathic scoliosis. Spine Deform. 2018, 6, 820. [Google Scholar] [CrossRef]
- Newton, P.O.; Bartley, C.E.; Bastrom, T.P.; Kluck, D.G.; Saito, W.; Yaszay, B. Anterior Spinal Growth Modulation in Skeletally Immature Patients with Idiopathic Scoliosis: A Comparison with Posterior Spinal Fusion at 2 to 5 Years Postoperatively. J. Bone Jt. Surg. Am. 2020, 102, 769–777. [Google Scholar] [CrossRef] [PubMed]

Publisher’s Note: MDPI stays neutral with regard to jurisdictional claims in published maps and institutional affiliations. |
© 2022 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Block, A.M.; Tamburini, L.M.; Zeng, F.; Mancini, M.R.; Jackson, C.A.; Antonacci, C.L.; Karsmarski, O.P.; Stelzer, J.W.; Wellington, I.J.; Lee, M.C. Surgical Treatment of Pediatric Scoliosis: Historical Origins and Review of Current Techniques. Bioengineering 2022, 9, 600. https://doi.org/10.3390/bioengineering9100600
Block AM, Tamburini LM, Zeng F, Mancini MR, Jackson CA, Antonacci CL, Karsmarski OP, Stelzer JW, Wellington IJ, Lee MC. Surgical Treatment of Pediatric Scoliosis: Historical Origins and Review of Current Techniques. Bioengineering. 2022; 9(10):600. https://doi.org/10.3390/bioengineering9100600
Chicago/Turabian StyleBlock, Andrew M., Lisa M. Tamburini, Francine Zeng, Michael R. Mancini, Casey A. Jackson, Christopher L. Antonacci, Owen P. Karsmarski, John W. Stelzer, Ian J. Wellington, and Mark C. Lee. 2022. "Surgical Treatment of Pediatric Scoliosis: Historical Origins and Review of Current Techniques" Bioengineering 9, no. 10: 600. https://doi.org/10.3390/bioengineering9100600
APA StyleBlock, A. M., Tamburini, L. M., Zeng, F., Mancini, M. R., Jackson, C. A., Antonacci, C. L., Karsmarski, O. P., Stelzer, J. W., Wellington, I. J., & Lee, M. C. (2022). Surgical Treatment of Pediatric Scoliosis: Historical Origins and Review of Current Techniques. Bioengineering, 9(10), 600. https://doi.org/10.3390/bioengineering9100600






