Latest Findings of the Regenerative Materials Application in Periodontal and Peri-Implant Surgery: A Scoping Review
Abstract
1. Introduction
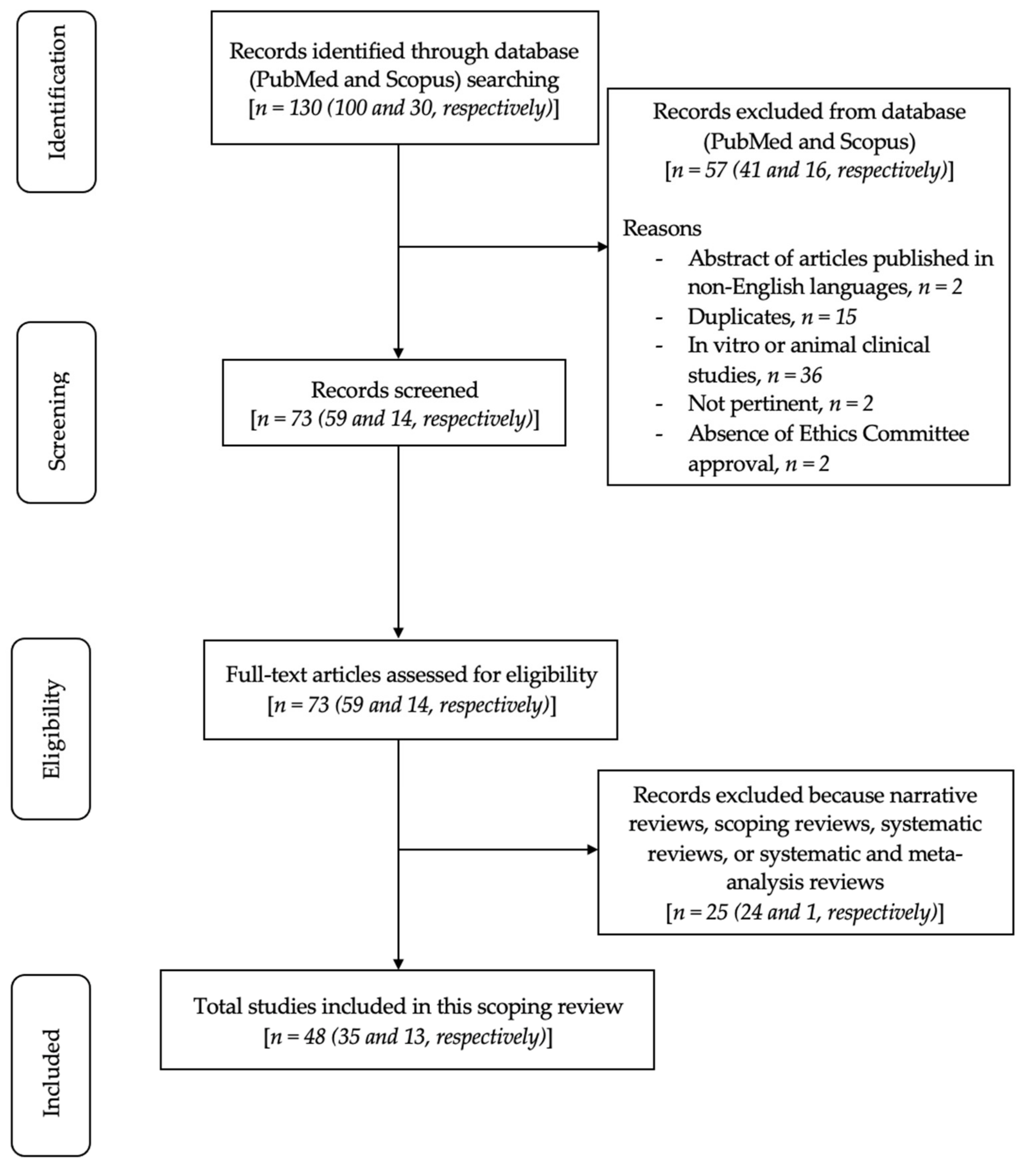
2. Materials and Methods
2.1. Focused Questions
2.2. Eligibility Criteria
2.3. Search Strategy
2.4. Quality Assessment of Included Studies
3. Results
Risk of Bias
4. Discussion
4.1. Periodontal Application: Intrabony Defects
4.2. Periodontal Application: Furcation Defects
4.3. Implant-Based Application: Treatment of Peri-Implantitis
4.4. Alveolar Ridge Preservation and Implant Site Development
5. Conclusions
Supplementary Materials
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Data Availability Statement
Protocol Registration
Conflicts of Interest
Abbreviations
| ABG | autogenous bone graft |
| APC | autogenous platelet concentrates |
| BMP-2 | bone morphogenetic protein-2 |
| BMP | bone morphogenetic proteins |
| BoP | bleeding on probing |
| CAL | clinical attachment level |
| CMs | collagen membranes |
| CSR | cumulative survival rate |
| DBBM | demineralized bovine bone mineral grafts |
| DFDBA | freeze-dried demineralized bone allograft |
| EMD | enamel matrix derivative |
| FGF-2 | fibroblast growth factor-2 |
| GBR | guided bone regeneration |
| GF | growth factors |
| GR | gingival recession |
| GTR | guided tissue regeneration |
| IBDs | intrabony defects |
| MeSH | medical subject heading |
| MPMs | modified perforated membranes |
| NHLBI | national heart, lung, and blood Institute |
| PBFP | pedicled buccal fat pad |
| PDGF | platelet-derived growth factor |
| PI | plaque index |
| PLGA | poly-lactic-glycolic acid |
| PPD | probing pocket depth |
| PRF | platelet-rich fibrin |
| PRP | platelet-rich plasma |
| β-TCP | β-tricalcium phosphate allograft |
References
- Larsson, L.; Decker, A.M.; Nibali, L.; Pilipchuk, S.P.; Berglundh, T.; Giannobile, W.V. Regenerative Medicine for Periodontal and Peri-implant Diseases. J. Dent. Res. 2016, 95, 255–266. [Google Scholar] [CrossRef] [PubMed]
- Chappuis, V.; Araújo, M.G.; Buser, D. Clinical relevance of dimensional bone and soft tissue alterations post-extraction in esthetic sites. Periodontology 2000 2017, 73, 73–83. [Google Scholar] [CrossRef] [PubMed]
- Singh, A.K. GTR membranes: The barriers for periodontal regeneration. DHR Int. J. Med. Sci. 2013, 4, 31–38. [Google Scholar]
- Susin, C.; Fiorini, T.; Lee, J.; De Stefano, J.A.; Dickinson, D.P.; Wikesjo, U.M.E. Wound healing following surgical and regenerative periodontal therapy. Periodontology 2000 2015, 68, 83–98. [Google Scholar] [CrossRef] [PubMed]
- Bosshardt, D.D.; Sculean, A. Does periodontal tissue regeneration really work? Periodontology 2000 2009, 51, 208–219. [Google Scholar] [CrossRef]
- Tatullo, M.; Codispoti, B.; Paduano, F.; Nuzzolese, M.; Makeeva, I. Strategic Tools in Regenerative and Translational Dentistry. Int. J. Mol. Sci. 2019, 20, 1879. [Google Scholar] [CrossRef]
- Aromataris, E.; Munn, Z. JBI Manual for Evidence Synthesis. 2020. Available online: https://synthesismanual.jbi.global (accessed on 4 September 2022).
- NHLBI; NIH. Study Quality Assessment Tool. Available online: https://www.nhlbi.nih.gov/health-topics/study-quality-assessment-tools (accessed on 4 September 2022).
- Aslan, S.; Buduneli, N.; Cortellini, P. Clinical outcomes of the entire papilla preservation technique with and without biomaterials in the treatment of isolated intrabony defects: A randomized controlled clinical trial. J. Clin. Periodontol. 2020, 47, 470–478. [Google Scholar] [CrossRef]
- Paolantonio, M.; Di Tullio, M.; Giraudi, M.; Romano, L.; Secondi, L.; Paolantonio, G.; Graziani, F.; Pilloni, A.; De Ninis, P.; Femminella, B. Periodontal regeneration by leukocyte and platelet-rich fibrin with autogenous bone graft versus enamel matrix derivative with autogenous bone graft in the treatment of periodontal intrabony defects: A randomized non-inferiority trial. J. Periodontol. 2020, 91, 1595–1608. [Google Scholar] [CrossRef]
- Gautam, K.; Kapoor, A.; Mathur, S.; Ali, A.R.; Choudhary, A.; Shekhawat, A. Comparative Evaluation of Autogenous Bone Graft and Autologous Platelet-Rich Fibrin with and Without 1.2 mg in situ Rosuvastatin Gel in the Surgical Treatment of Intrabony Defect in Chronic Periodontitis Patients. Contemp. Clin. Dent. 2022, 13, 69–77. [Google Scholar] [PubMed]
- Górski, B.; Jalowski, S.; Górska, R.; Zaremba, M. Treatment of intrabony defects with modified perforated membranes in aggressive periodontitis: A 4-year follow-up of a randomized controlled trial. Clin. Oral Investig. 2020, 24, 1183–1196. [Google Scholar] [CrossRef]
- Gamal, A.Y.; Aziz, M.; Salama, M.H.; Iacono, V.J. Gingival crevicular fluid bone morphogenetic protein-2 release profile following the use of modified perforated membrane barriers in localized intrabony defects: A randomized clinical trial. J. Int. Acad. Periodontol. 2014, 16, 55–63. [Google Scholar]
- Aggour, R.L.; Abd El-Hady, H.M.G. Platelet-Rich Fibrin for the Treatment of Intrabony Periodontal Defects in Patients with Generalized Aggressive Periodontitis: A Randomized Controlled Clinical Study. J. Int. Acad. Periodontol. 2017, 19, 28–34. [Google Scholar] [PubMed]
- Hazari, V.; Choudhary, A.; Mishra, R.; Chandrashekar, K.T.; Trivedi, A.; Pathak, P.K. Clinical and Radiographic Analysis of Novabone Putty with Platelet-Rich Fibrin in the Treatment of Periodontal Intrabony Defects: A Randomized Control Trial. Contemp. Clin. Dent. 2021, 12, 150–156. [Google Scholar]
- Cieplik, F.; Tabenski, L.; Hiller, K.-A.; Schmalz, G.; Buchalla, W.; Christgau, M. Influence of autogenous platelet concentrate on combined GTR/graft therapy in intra-bony defects: A 13-year follow-up of a randomized controlled clinical split-mouth study. J. Clin. Periodontol. 2018, 45, 382–391. [Google Scholar] [CrossRef] [PubMed]
- Temraz, A.; Ghallab, N.A.; Hamdy, R.; El-Dahab, O.A. Clinical and radiographic evaluation of amnion chorion membrane and demineralized bone matrix putty allograft for management of periodontal intrabony defects: A randomized clinical trial. Cell Tissue Bank. 2019, 20, 117–128. [Google Scholar] [CrossRef] [PubMed]
- Ferrarotti, F.; Romano, F.; Gamba, M.N.; Quirico, A.; Giraudi, M.; Audagna, M.; Aimetti, M. Human intrabony defect regeneration with micrografts containing dental pulp stem cells: A randomized controlled clinical trial. J. Clin. Periodontol. 2018, 45, 841–850. [Google Scholar] [CrossRef]
- Chen, F.-M.; Gao, L.-N.; Tian, B.-M.; Zhang, X.-Y.; Zhang, Y.-J.; Dong, G.-Y.; Lu, H.; Chu, Q.; Xu, J.; Wu, R.-X.; et al. Treatment of periodontal intrabony defects using autologous periodontal ligament stem cells: A randomized clinical trial. Stem Cell Res. Ther. 2016, 7, 33. [Google Scholar] [CrossRef] [PubMed]
- Gonçalves, P.F.; Lima, L.L.; Sallum, E.A.; Casati, M.Z.; Nociti, F.H., Jr. Root cementum may modulate gene expression during periodontal regeneration: A preliminary study in humans. J. Periodontol. 2008, 79, 323–331. [Google Scholar] [CrossRef]
- Rani, N.; Kaushal, S.; Singh, S.; Nandhal, L.; Khan, M.A.; Pathak, A.K. Evaluation of the relative efficacy of autologous platelet-rich fibrin membrane in combination with β-tricalcium phosphate (Septodont- resorbable tissue replacement) ™ alloplast versus β-TCP alloplast alone in the treatment of grade II furcation defects. Natl. J. Maxillofac. Surg. 2018, 9, 196–204. [Google Scholar]
- Bajaj, P.; Pradeep, A.R.; Agarwal, E.; Rao, N.S.; Naik, S.B.; Priyanka, N.; Kalra, N. Comparative evaluation of autologous platelet-rich fibrin and platelet-rich plasma in the treatment of mandibular degree II furcation defects: A randomized controlled clinical trial. J. Periodontal. Res. 2013, 48, 573–581. [Google Scholar] [CrossRef]
- Queiroz, L.A.; Santamaria, M.P.; Casati, M.Z.; Ruiz, K.S.; Nociti, F., Jr.; Sallum, A.W.; Sallum, E.A. Enamel matrix protein derivative and/or synthetic bone substitute for the treatment of mandibular class II buccal furcation defects. A 12-month randomized clinical trial. Clin. Oral Investig. 2016, 20, 1597–1606. [Google Scholar] [CrossRef] [PubMed]
- Pajnigara, N.G.; Kolte, A.P.; Kolte, R.A.; Pajnigara, N.G. Volumetric Assessment of Regenerative Efficacy of Demineralized Freeze-Dried Bone Allograft with or Without Amnion Membrane in Grade II Furcation Defects: A Cone Beam Computed Tomography Study. Int. J. Periodontics Restor. Dent. 2017, 37, 255–262. [Google Scholar] [CrossRef] [PubMed]
- Huidrom, E.; Srivastava, V.; Meenawat, A.; Srivastava, A.; Khan, Y.S.; Shahni, R. Evaluation of the efficacy of concentrated growth factor along with bovine-derived xenograft and collagen membrane in the treatment of Degree II mandibular molar furcation defect—A clinicoradiographic study. J. Indian Soc. Periodontol. 2022, 26, 130–136. [Google Scholar] [CrossRef] [PubMed]
- Sneha, K.; Sowjanya, K.; Vaishnavi, V.; Chandra, R.V. Comparative Evaluation of Efficacy between Recombinant Human Bone Morphogenetic Protein-2 Impregnated with Absorbable Sponge and Platelet-Rich Fibrin in the Treatment of Grade II Furcation Defects: A Randomized Controlled Trial. Contemp. Clin. Dent. 2021, 12, 419–425. [Google Scholar] [CrossRef] [PubMed]
- Isehed, C.; Svenson, B.; Lundberg, P.; Holmlund, A. Surgical treatment of peri-implantitis using enamel matrix derivative, an RCT: 3- and 5-year follow-up. J. Clin. Periodontol. 2018, 45, 744–753. [Google Scholar] [CrossRef]
- Lee, J.-H.; Jeong, S.-N. Effect of enamel matrix derivative on alveolar ridge preservation in the posterior maxilla: A randomized controlled clinical trial. Clin. Implant. Dent. Relat. Res. 2020, 22, 622–630. [Google Scholar] [CrossRef]
- Jo, D.-W.; Cho, Y.-D.; Seol, Y.-J.; Lee, Y.-M.; Lee, H.-J.; Kim, Y.-K. A randomized controlled clinical trial evaluating efficacy and adverse events of different types of recombinant human bone morphogenetic protein-2 delivery systems for alveolar ridge preservation. Clin. Oral Implant. Res. 2019, 30, 396–409. [Google Scholar] [CrossRef]
- Stumbras, A.; Galindo-Moreno, P.; Januzis, G.; Juodzbalys, G. Three-dimensional analysis of dimensional changes after alveolar ridge preservation with bone substitutes or plasma rich in growth factors: Randomized and controlled clinical trial. Clin. Implant. Dent. Relat. Res. 2021, 23, 96–106. [Google Scholar] [CrossRef]
- Saito, H.; Couso-Queiruga, E.; Shiau, H.J.; Stuhr, S.; Prasad, H.; Allareddy, T.V.; Reynolds, M.A.; Avila-Ortiz, G. Evaluation of poly lactic-co-glycolic acid-coated β-tricalcium phosphate for alveolar ridge preservation: A multicenter randomized controlled trial. J. Periodontol. 2021, 92, 524–535. [Google Scholar] [CrossRef]
- Gonshor, A.; McAllister, B.S.; Wallace, S.S.; Prasad, H. Histologic and histomorphometric evaluation of an allograft stem cell-based matrix sinus augmentation procedure. Int. J. Oral Maxillofac. Implant. 2011, 26, 123–131. [Google Scholar]
- Majzoub, J.; Barootchi, S.; Tavelli, L.; Wang, C.-W.; Travan, S.; Wang, H.-L. Treatment effect of guided tissue regeneration on the horizontal and vertical components of furcation defects: A retrospective study. J. Periodontol. 2020, 91, 1148–1158. [Google Scholar] [CrossRef] [PubMed]
- Canullo, L.; Signorini, L.; Pistilli, R.; Patini, R.; Pistilli, V.; Pesce, P. A prospective case series on surgical treatment of circumferential and semi-circumferential defects due to peri-implantitis. Braz. Oral Res. 2019, 33, e072. [Google Scholar] [CrossRef] [PubMed]
- Kadkhodazadeh, M.; Amid, R.; Moscowchi, A. Management of extensive peri-implant defects with titanium meshes. Oral Maxillofac. Surg. 2021, 25, 561–568. [Google Scholar] [CrossRef] [PubMed]
- Chiapasco, M.; Tommasato, G.; Palombo, D.; Del Fabbro, M. A retrospective 10-year mean follow-up of implants placed in ridges grafted using autogenous mandibular blocks covered with bovine bone mineral and collagen membrane. Clin. Oral Implant. Res. 2020, 31, 328–340. [Google Scholar] [CrossRef]
- Beretta, M.; Maiorana, C.; Manfredini, M.; Signorino, F.; Poli, P.P.; Vinci, R. Marginal Bone Resorption Around Dental Implants Placed in Alveolar Socket Preserved Sites: A 5 Years Follow-up Study. J. Maxillofac. Oral Surg. 2021, 20, 381–388. [Google Scholar] [CrossRef]
- Manavella, V.; Romano, F.; Corano, L.; Bignardi, C.; Aimetti, M. Three-Dimensional Volumetric Changes in Severely Resorbed Alveolar Sockets After Ridge Augmentation with Bovine-Derived Xenograft and Resorbable Barrier: A Preliminary Study on CBCT Imaging. Int. J. Oral Maxillofac. Implant. 2018, 33, 373–382. [Google Scholar] [CrossRef]
- Zafiropoulos, G.-G.; Kačarević, Z.P.; Qasim, S.S.B.; Trajkovski, B. Open-Healing Socket Preservation with a Novel Dense Polytetrafluoroethylene (dPTFE) Membrane: A Retrospective Clinical Study. Medicina 2020, 56, 216. [Google Scholar] [CrossRef]
- Beretta, M.; Cicciù, M.; Poli, P.P.; Rancitelli, D.; Bassi, G.; Grossi, G.B.; Maiorana, C. A Retrospective Evaluation of 192 Implants Placed in Augmented Bone: Long-Term Follow-Up Study. J. Oral Implant. 2015, 41, 669–674. [Google Scholar] [CrossRef]
- Yoshikawa, K.; Saito, A.; Tomita, S. Periodontal Regenerative Therapy with Enamel Matrix Derivative and Autogenous Bone Graft in Patient with Chronic Periodontitis: An 18-month Follow-up Report. Bull. Tokyo Dent. Coll. 2020, 61, 43–51. [Google Scholar] [CrossRef]
- Thakkalapati, P.; Chandran, C.R.; Ranganathan, A.T.; Jain, A.R.; Prabhakar, P.; Padmanaban, S. Management of a One-wall Intrabony Osseous Defect with Combination of Platelet Rich Plasma and Demineralized Bone Matrix- a Two-year Follow up Case Report. J. Dent. 2015, 16, 219–223. [Google Scholar]
- Pal, M.; Gupta, K.; Kumar, S.; Gopalkrishna, P. Use of a mandibular torus for autogenous grafting: A case report. Gen. Dent. 2018, 66, 73–76. [Google Scholar] [PubMed]
- Zhou, Z.; Qi, X.; Notice, T. Treatment of mandibular grade III furcation involvement using platelet-rich fibrin and allogenic graft with 12-month follow-up—A case report. J. Oral Biol. Craniofacial Res. 2020, 10, 542–546. [Google Scholar] [CrossRef] [PubMed]
- Panda, S.; Del Fabbro, M.; Satpathy, A.; Das, A.C. Pedicled buccal fat pad graft for root coverage in severe gingival recession defect. J. Indian Soc. Periodontol. 2016, 20, 216–219. [Google Scholar] [CrossRef] [PubMed]
- Bassi, F.; Poli, P.P.; Rancitelli, D.; Signorino, F.; Maiorana, C. Surgical Treatment of Peri-Implantitis: A 17-Year Follow-Up Clinical Case Report. Case Rep Dent. 2015, 2015, 574676. [Google Scholar] [CrossRef]
- Poli, P.P.; Souza, F.A.; Manfredini, M.; Maiorana, C.; Beretta, M. Regenerative Treatment of Peri-Implantitis Following Implant Surface Decontamination with Titanium Brush and Antimicrobial Photodynamic Therapy: A Case Series With Reentry. J. Oral Implant. 2020, 46, 619–626. [Google Scholar] [CrossRef]
- Bhide, V.M.; Goldberg, M.B.; Tenenbaum, H.C. Surgical Treatment of Peri-implantitis with Guided Bone Regeneration Using Dehydrated Amnion-Chorion Membranes: A Case Report with a 2-Year Follow-up. Int. J. Periodont. Restor. Dent. 2022, 42, e59–e66. [Google Scholar] [CrossRef]
- Park, J.-B. Application of enamel matrix derivative and deproteinized bovine bone for the treatment of peri-implantitis after decontamination with an ultrasonic scaler: A case report. Medicine 2018, 97, e13461. [Google Scholar] [CrossRef]
- Jensen, O.T.; Adams, M.; Cottam, J.R.; Ringeman, J. Occult peri-implant oroantral fistulae: Posterior maxillary peri-implantitis/sinusitis of zygomatic or dental implant origin. Treatment and prevention with bone morphogenetic protein-2/absorbable collagen sponge sinus grafting. Int J. Oral Maxillofac. Implant. 2013, 28, e512–e520. [Google Scholar] [CrossRef]
- Maeda, D.; Dos Reis, L.D.; Fermiano, D.; Giro, G.; Mauricio, J.M.; Marinho, K.O.; Faveri, M. Alveolar Ridge Preservation Using a Bovine derived Bone Graft in Association with Titanium Foil—A Prospective Case Series. J. Int. Acad. Periodontol. 2021, 23, 57–64. [Google Scholar]
- Urban, I.A.; Ravidà, A.; Saleh, M.H.A.; Galli, M.; Lozada, J.; Farkasdi, S.; Wang, H.-L. Long-term crestal bone changes in implants placed in augmented sinuses with minimal or moderate remaining alveolar bone: A 10-year retrospective case-series study. Clin. Oral Implant. Res. 2021, 32, 60–74. [Google Scholar] [CrossRef]
- Blume, O.; Donkiewicz, P.; Palkovics, D.; Götz, W.; Windisch, P. Volumetric Changes of a Customized Allogeneic Bone Block Measured by Two Image Matching Tools: Introduction of a Novel Assessment Technique for Graft Resorption. Acta Stomatol. Croat. 2021, 55, 406–417. [Google Scholar] [CrossRef] [PubMed]
- Urban, I.A.; Tattan, M.; Ravida, A.; Saleh, M.H.; Tavelli, L.; Avila-Ortiz, G. Simultaneous Alveolar Ridge Augmentation and Periodontal Regenerative Therapy Leveraging Recombinant Human Platelet-Derived Growth Factor-BB (rhPDGF-BB): A Case Report. Int. J. Periodontics Restor. Dent. 2022, in press. [Google Scholar] [CrossRef] [PubMed]
- Khojasteh, A.; Hosseinpour, S.; Rad, M.R.; Alikhasi, M. Buccal Fat Pad-Derived Stem Cells in Three-Dimensional Rehabilitation of Large Alveolar Defects: A Report of Two Cases. J. Oral Implant. 2019, 45, 45–54. [Google Scholar] [CrossRef] [PubMed]
- Windisch, P.; Orban, K.; Salvi, G.E.; Sculean, A.; Molnar, B. Vertical-guided bone regeneration with a titanium-reinforced d-PTFE membrane utilizing a novel split-thickness flap design: A prospective case series. Clin. Oral Investig. 2021, 25, 2969–2980. [Google Scholar] [CrossRef]
- Wu, D.T.; Munguia-Lopez, J.G.; Cho, Y.W.; Ma, X.; Song, V.; Zhu, Z.; Tran, S.D. Polymeric Scaffolds for Dental, Oral, and Craniofacial Regenerative Medicine. Molecules 2021, 26, 7043. [Google Scholar] [CrossRef]
- Correia, F.; Pozza, D.H.; Gouveia, S.; Felino, A.; Almeida, R.F.E. The applications of regenerative medicine in sinus lift procedures: A systematic review. Clin. Implant. Dent. Relat Res. 2018, 20, 229–242. [Google Scholar] [CrossRef]
- Avila-Ortiz, G.; Bartold, P.M.; Giannobile, W.; Katagiri, W.; Nares, S.; Rios, H.; Spagnoli, D.; Wikesjö, U.M. Biologics and Cell Therapy Tissue Engineering Approaches for the Management of the Edentulous Maxilla: A Systematic Review. Int J. Oral Maxillofac. Implants 2016, 31, s121–s164. [Google Scholar] [CrossRef]
- Deb, S.; Chana, S. Biomaterials in Relation to Dentistry. Front. Oral Biol. 2015, 17, 1–12. [Google Scholar]
- Egusa, H.; Sonoyama, W.; Nishimura, M.; Atsuta, I.; Akiyama, K. Stem cells in dentistry—Part II: Clinical applications. J. Prosthodont Res. 2012, 56, 229–248. [Google Scholar] [CrossRef]
- Sancho, F.M.; Leira, Y.; Orlandi, M.; Buti, J.; Giannobile, W.V.; D’Aiuto, F. Cell-Based Therapies for Alveolar Bone and Periodontal Regeneration: Concise Review. Stem Cells Transl. Med. 2019, 8, 1286–1295. [Google Scholar] [CrossRef]
- DeCarlo, A.A.; Whitelock, J.M. The role of heparan sulfate and perlecan in bone-regenerative procedures. J. Dent. Res. 2006, 85, 122–132. [Google Scholar] [CrossRef] [PubMed]
- Tavelli, L.; Ravidà, A.; Barootchi, S.; Chambrone, L.; Giannobile, W.V. Recombinant Human Platelet-Derived Growth Factor: A Systematic Review of Clinical Findings in Oral Regenerative Procedures. JDR Clin. Trans. Res. 2021, 6, 161–173. [Google Scholar] [CrossRef] [PubMed]
- Farimani, Z.; Shamshiri, A.R.; Roosta, H.A.; Akbari, S.; Bohlouli, M. Regenerative benefits of using growth factors in treatment of periodontal defects: A systematic review and meta-analysis with Trial Sequential Analysis on preclinical studies. J. Tissue Eng. Regen. Med. 2021, 15, 964–997. [Google Scholar] [CrossRef] [PubMed]
- Ripamonti, U.; Petit, J.-C. Bone morphogenetic proteins, cementogenesis, myoblastic stem cells and the induction of periodontal tissue regeneration. Cytokine Growth Factor Rev. 2009, 20, 489–499. [Google Scholar] [CrossRef]
- Goker, F.; Larsson, L.; Del Fabbro, M.; Asa’ad, F. Gene Delivery Therapeutics in the Treatment of Periodontitis and Peri-Implantitis: A State of the Art Review. Int. J. Mol. Sci. 2019, 20, 3551. [Google Scholar] [CrossRef]
- Marei, M.K.; El Backly, R.M. Dental Mesenchymal Stem Cell-Based Translational Regenerative Dentistry: From Artificial to Biological Replacement. Front. Bioeng. Biotechnol. 2018, 6, 49. [Google Scholar] [CrossRef]
- Fayzullin, A.; Bakulina, A.; Mikaelyan, K.; Shekhter, A.; Guller, A. Implantable Drug Delivery Systems and Foreign Body Reaction: Traversing the Current Clinical Landscape. Bioengineering 2021, 8, 205. [Google Scholar] [CrossRef]
- Liu, J.; Ruan, J.; Weir, M.D.; Ren, K.; Schneider, A.; Wang, P.; Oates, T.W.; Chang, X.; Xu, H.H.K. Periodontal Bone-Ligament-Cementum Regeneration via Scaffolds and Stem Cells. Cells 2019, 8, 537. [Google Scholar] [CrossRef]
- Xu, X.-Y.; Li, X.; Wang, J.; He, X.-T.; Sun, H.-H.; Chen, F.-M. Concise Review: Periodontal Tissue Regeneration Using Stem Cells: Strategies and Translational Considerations. Stem Cells Transl. Med. 2019, 8, 392–403. [Google Scholar] [CrossRef]
- Rios, H.F.; Lin, Z.; Oh, B.; Park, C.H.; Giannobile, W.V. Cell- and gene-based therapeutic strategies for periodontal regenerative medicine. J. Periodontol. 2011, 82, 1223–1237. [Google Scholar] [CrossRef]
- Iwata, T.; Yamato, M.; Ishikawa, I.; Ando, T.; Okano, T. Tissue engineering in periodontal tissue. Anat. Rec. 2014, 297, 16–25. [Google Scholar] [CrossRef] [PubMed]
- Chen, F.-M.; Zhang, J.; Zhang, M.; An, Y.; Chen, F.; Wu, Z.-F. A review on endogenous regenerative technology in periodontal regenerative medicine. Biomaterials 2010, 31, 7892–7927. [Google Scholar] [CrossRef] [PubMed]
- Kim, M.G.; Park, C.H. Tooth-Supporting Hard Tissue Regeneration Using Biopolymeric Material Fabrication Strategies. Molecules 2020, 25, 4802. [Google Scholar] [CrossRef] [PubMed]
- Iviglia, G.; Kargozar, S.; Baino, F. Biomaterials, Current Strategies, and Novel Nano-Technological Approaches for Periodontal Regeneration. J. Funct. Biomater. 2019, 10, 3. [Google Scholar] [CrossRef] [PubMed]
- Carter, S.-S.D.; Costa, P.F.; Vaquette, C.; Ivanovski, S.; Hutmacher, D.W.; Malda, J. Additive Biomanufacturing: An Advanced Approach for Periodontal Tissue Regeneration. Ann. Biomed. Eng. 2017, 45, 12–22. [Google Scholar] [CrossRef]
- Kim, J.H.; Park, C.H.; Perez, R.A.; Lee, H.Y.; Jang, J.H.; Lee, H.H.; Wall, I.B.; Shi, S.; Kim, H.W. Advanced biomatrix designs for regenerative therapy of periodontal tissues. J. Dent. Res. 2014, 93, 1203–1211. [Google Scholar] [CrossRef]
- Khoshkam, V.; Chan, H.-L.; Lin, G.-H.; Mailoa, J.; Giannobile, W.V.; Wang, H.-L.; Oh, T.-J. Outcomes of regenerative treatment with rhPDGF-BB and rhFGF-2 for periodontal intra-bony defects: A systematic review and meta-analysis. J. Clin. Periodontol. 2015, 42, 272–280. [Google Scholar] [CrossRef]
- Jepsen, S.; Gennai, S.; Hirschfeld, J.; Kalemaj, Z.; Buti, J.; Graziani, F. Regenerative surgical treatment of furcation defects: A systematic review and Bayesian network meta-analysis of randomized clinical trials. J. Clin. Periodontol. 2020, 47, 352–374. [Google Scholar] [CrossRef]
- Miron, R.; Zucchelli, G.; Pikos, M.A.; Salama, M.; Lee, S.; Guillemette, V.; Fujioka-Kobayashi, M.; Bishara, M.; Zhang, Y.; Wang, H.-L.; et al. Use of platelet-rich fibrin in regenerative dentistry: A systematic review. Clin. Oral Investig. 2017, 21, 1913–1927. [Google Scholar] [CrossRef]
- Del Amo, F.S.-L.; Monje, A.; Padial-Molina, M.; Tang, Z.; Wang, H.L. Biologic Agents for Periodontal Regeneration and Implant Site Development. BioMed Res. Int. 2015, 2015, 957518. [Google Scholar]
- Butera, A.; Pascadopoli, M.; Pellegrini, M.; Gallo, S.; Zampetti, P.; Scribante, A. Oral Microbiota in Patients with Peri-Implant Disease: A Narrative Review. Appl. Sci. 2022, 12, 3250. [Google Scholar] [CrossRef]
- Butera, A.; Pascadopoli, M.; Pellegrini, M.; Gallo, S.; Zampetti, P.; Cuggia, G.; Scribante, A. Domiciliary Use of Chlorhexidine vs Postbiotic Gels in Patients with Peri-Implant Mucositis: A Split-Mouth Randomized Clinical Trial. Appl. Sci. 2022, 12, 2800. [Google Scholar] [CrossRef]
| Random Sequence Generation | Allocation Concealment | Blinding | Incomplete Outcome Data | Selective Reporting | |
|---|---|---|---|---|---|
| Aslan et al., 2020 [9] |  |  |  |  |  |
| Paolantonio et al., 2020 [10] |  |  |  |  |  |
| Gautam et al., 2022 [11] |  |  |  |  |  |
| Górski et al., 2020 [12] |  |  |  |  |  |
| Gamal et al., 2014 [13] |  |  |  |  |  |
| Aggour et al., 2017 [14] |  |  |  |  |  |
| Hazari et al., 2021 [15] |  |  |  |  |  |
| Cieplik et al., 2018 [16] |  |  |  |  |  |
| Temraz et al., 2019 [17] |  |  |  |  |  |
| Ferrarotti et al., 2018 [18] |  |  |  |  |  |
| Chen et al., 2016 [19] |  |  |  |  |  |
| Gonçalves et al., 2008 [20] |  |  |  |  |  |
| Rani et al., 2018 [21] |  |  |  |  |  |
| Bajaj et al., 2013 [22] |  |  |  |  |  |
| Queiroz et al., 2016 [23] |  |  |  |  |  |
| Pajnigara et al., 2017 [24] |  |  |  |  |  |
| Huidrom et al., 2022 [25] |  |  |  |  |  |
| Sneha et al., 2021 [26] |  |  |  |  |  |
| Isehed et al., 2018 [27] |  |  |  |  |  |
| Lee et al., 2020 [28] |  |  |  |  |  |
| Jo et al., 2019 [29] |  |  |  |  |  |
| Stumbras et al., 2021 [30] |  |  |  |  |  |
| Saito et al., 2021 [31] |  |  |  |  |  |
| Gonshor et al., 2011 [32] |  |  |  |  |  |
| Majzoub et al., 2020 [33] |  |  |  |  |  |
| Canullo et al., 2019 [34] |  |  |  |  |  |
| Kadkhodazadeh et al., 2021 [35] |  |  |  |  |  |
| Chiapasco et al., 2020 [36] |  |  |  |  |  |
| Beretta et al., 2021 [37] |  |  |  |  |  |
| Manavella et al., 2018 [38] |  |  |  |  |  |
| Zafiropoulos et al., 2020 [39] |  |  |  |  |  |
| Beretta et al., 2015 [40] |  |  |  |  |  |
| Yoshikawa et al., 2020 [41] |  |  |  |  |  |
| Thakkalapati et al., 2015 [42] |  |  |  |  |  |
| Pal et al., 2018 [43] |  |  |  |  |  |
| Zhou et al., 2020 [44] |  |  |  |  |  |
| Panda et al., 2016 [45] |  |  |  |  |  |
| Bassi et al., 2015 [46] |  |  |  |  |  |
| Poli et al., 2020 [47] |  |  |  |  |  |
| Bhide et al., 2022 [48] |  |  |  |  |  |
| Park et al., 2018 [49] |  |  |  |  |  |
| Jensen et al., 2013 [50] |  |  |  |  |  |
| Maeda et al., 2021 [51] |  |  |  |  |  |
| Urban et al., 2021 [52] |  |  |  |  |  |
| Blume et al., 2021 [53] |  |  |  |  |  |
| Urban et al., 2022 [54] |  |  |  |  |  |
| Khojasteh et al., 2019 [55] |  |  |  |  |  |
| Windisch et al., 2021 [56] |  |  |  |  |  |
| References (Authors, Year of Publication and Study Details) | Study Methods | Results |
|---|---|---|
| Aslan et al., 2020 [9]: 1-year single-blinded randomized controlled clinical trial with 30 periodontal participants, each with one isolated intrabony defect. | Papilla preservation technique with EMD + plus bovine-derived bone substitutes (15 patients—test sites). Papilla preservation technique alone (15 patients—control sites). | Application of papilla preservation technique with and without regenerative biomaterials resulted in significant amounts of CAL gain and PPD reduction, with negligible increase in gingival recession. |
| Paolantonio et al., 2020 [10]: 1-year triple-blinded randomized controlled clinical trial with 44 periodontal patients exhibiting at least one unfavorable intraosseous defect. | Open flap debridement with PRF associated with ABG (22 patients—test sites). Open flap debridement with EMD + ABG (22 patients—control sites). | PRF-ABG combined treatment of non-contained IBDs produces non-inferior results in terms of CAL gain, PPD reduction, GR increase, and defect bone level gain in comparison with the EMD-ABG combination. |
| Gautam et al., 2022 [11]: 9-month randomized controlled clinical trial with 39 chronic periodontitis participants, each with one isolated intrabony defect. | Open flap debridement + placebo (13 participants—group A). Open flap debridement + BG + PRF (13 participants—group B). Open flap debridement + ABG + PRF + 1.2 mg Rosuvastatin (13 participants—group C). | Addition of 1.2 mg Rosuvastatin gel, PRF, and ABG has synergistic effects, explaining their role as a regenerative material in the treatment of intrabony defects. |
| Górski et al., 2020 [12]: 4-year double-blinded randomized controlled clinical split-mouth trial with 15 aggressive periodontitis patients, each with two deep intrabony defects (1 drop-out and 1 tooth extracted due to root fracture). | Papilla preservation technique with xenogenic graft plus MPMs (14 defects—test sites). Papilla preservation technique with xenogenic graft plus standard CMs (13 defects—control sites). | GTR of intrabony defects in aggressive periodontitis with either standard or MPMs yielded similarly successful and maintainable clinical benefits for compromised teeth 4 years following the surgery. The use of MPMs showed no additional benefit. |
| Gamal et al., 2014 [13]: 1-month single-blinded randomized controlled clinical split-mouth trial with 15 severe chronic periodontitis participants, each with two interproximal contralateral defects. | Open flap debridement with MPMs (15 defects—test sites). Open flap debridement with occlusive membrane (15 defects—control sites). | MPMs coverage of periodontal defects is associated with a significant initial increase in GCF levels of BMP-2, a factor that could improve the clinical outcomes of guided tissue regenerative surgery. |
| Aggour et al., 2017 [14]: 6-month single-blinded randomized controlled clinical split-mouth trial with 16 generalized aggressive periodontitis patients, each with paired contralateral intrabony defects. | Open flap debridement with ABG mixed with xenograft + PRF (16 defects—test sites). Open flap debridement with composite bone graft + CMs (16 defects—control sites). | PRF has shown favorable results that are comparable to CMs for treatment of intrabony periodontal defects in patients with generalized aggressive periodontitis. Better results concerning gingival margin have been reported when PRF was used. |
| Hazari et al., 2021 [15]: 6-month randomized controlled clinical trial with 20 chronic generalized periodontitis participants, each with intrabony defects. | Open flap debridement with premixed composite of bioactive calcium phosphosilicate particles and an absorbable synthetic binder (group A). Open flap debridement with premixed composite of bioactive calcium phosphosilicate particles and an absorbable synthetic binder along with PRF (group B). | Evaluation of efficacy of premixed composite of bioactive calcium phosphosilicate particles and an absorbable synthetic binder along with PRF produced more favorable results in relative attachment level gain and more reduction in probing pocket depth when compared to premixed composite alone. |
| Cieplik et al., 2018 [16]: 13-year double-blinded randomized controlled clinical split-mouth trial with 22 periodontal patients, each with two deep contralateral intrabony defect. | Open flap debridement with β-TCP and bio-resorbable membranes with the additional application of APC (11 defects). Open flap debridement with β-TCP and patient blood (11 defects). | CAL gain following GTR can be maintained over 13 years. The additional use of APC had no positive influence on the long-term stability. |
| Temraz et al., 2019 [17]: 6-month triple-blinded randomized controlled clinical trial with 22 severe chronic periodontitis participants, each with one intrabony defect. | Open flap debridement and amnion chorion membrane (11 defects—test sites). Open flap debridement and demineralized bone matrix putty (11 defects—control sites). | Amnion chorion membrane barrier and demineralized bone matrix putty allograft provided significant improvement in clinical and radiographic outcomes after 6 months, yet no significant differences were noticed between them. |
| Ferrarotti et al., 2018 [18]: 1-year double-blinded randomized controlled clinical trial with 29 chronic periodontitis patients presenting one deep intrabony defect. | Minimally invasive surgical technique using micrografts rich in autologous dental pulp stem cells seeded onto collagen sponge (15 defects—test sites). Minimally invasive surgical technique using collagen sponge alone (14 defects—control sites). | Application of dental pulp stem cells significantly improved clinical parameters of periodontal regeneration 1 year after treatment. |
| Chen et al., 2016 [19]: 1-year single-blinded randomized controlled clinical trial with 30 periodontal participants and a total of 48 intrabony defects. | GTR and autologous periodontal ligament stem cells sheets in combination with xenograft bone substitute (24 defects—test sites). GTR and xenograft bone substitute (24 defects—control sites). | Autologous periodontal ligament stem cells to treat periodontal intrabony defects is safe and does not produce significant adverse effects. |
| Gonçalves et al., 2008 [20]: 3-week randomized controlled clinical trial with 22 intrabony defects in 22 periodontal patients. | Scaling and root planing with the removal of granulation tissue and root cementum and soft microbial deposits by cleaning the root surface with a microbrush and saline solution (11 defects—test sites). Scaling and root planing with the removal of granulation tissue and root cementum (11 defects—control sites). | mRNA levels for platelet-derived growth factor-alpha, bone sialoprotein, and basic fibroblast growth factor were higher in the sites where root cementum was kept in place compared to the sites where root cementum was removed completely as part of the periodontal therapy. Root cementum may modulate the expression of growth and mineral-associated factors during periodontal regeneration. |
| Rani et al., 2018 [21]: 6-month randomized controlled clinical trial with 20 mandibular grade II furcation defects in 20 participants. | Open flap debridement with β-TCP with PRF membrane (10 defects—test sites). Open flap debridement with β-TCP alone (10 defects—control sites). | The combination of PRF with β-TCP allograft led to more favorable improvement in the management of grade II furcation defect except PPD. |
| Bajaj et al., 2013 [22]: 9-month double-blinded randomized controlled clinical trial with 72 mandibular grade II furcation defects in 72 patients. | Open flap debridement with PRF (24 defects—test sites). Open flap debridement with autologous PRP (25 defects—test sites). Open flap debridement alone (23 defects—control sites). | The use of autologous PRF or PRP were both effective in the treatment of furcation defects with uneventful healing of sites. |
| Queiroz et al., 2016 [23]: 1-year single-blinded randomized controlled clinical trial with 41 mandibular grade II furcation defects in 41 participants. | Open flap debridement with β-TCP + hydroxyapatite (14 defects—test sites). Open flap debridement with EMD + β-TCP + hydroxyapatite (14 defects—test sites). EMD (13 defects—control sites). | EMD + β-TCP + hydroxyapatite does not provide a significant advantage when compared to the isolated approaches. All three tested treatments promote significant improvements and partial closure of class II buccal furcation defects. EMD may be considered an attractive option for this type of defect |
| Pajnigara et al., 2017 [24]: 6-month double-blinded randomized controlled clinical trial with 20 grade II furcation defects in 20 participants. | Open flap debridement with DFDBA + amnion membrane (10 defects—test sites). Open flap debridement with DFDBA (10 defects—control sites). | DFDBA used with amnion membrane resulted in significant improvement in clinical and radiographic parameters when compared with DFDBA alone. |
| Huidrom et al., 2022 [25]: 6-month single-blinded randomized controlled clinical trial with 20 mandibular grade II furcation defects in 20 patients. | Open flap debridement with concentrated GF mixed with inorganic bovine bone along with GTR (10 defects—test sites). Open flap debridement with inorganic bovine bone along with GTR (10 defects—control sites). | The use of CGF showed a positive additive efficacy in enhancing the events of periodontal regeneration in the treatment of Degree II mandibular molar furcation defect. |
| Sneha et al., 2021 [26]: 6-month single-blinded randomized controlled clinical trial with 32 grade II furcation defects in 32 participants. | Open flap debridement with BMP-2 impregnated with absorbable collagen sponge (16 defects—test sites). Open flap debridement with PRF (16 defects—control sites). | The unique regenerative potential BMP-2 impregnated with absorbable collagen sponge makes it a potential agent to be used as a graft material for the treatment of grade II furcation defects. |
| Isehed et al., 2018 [27]: 5-year double-blinded randomized controlled clinical trial with 25 peri-implantitis patients. | Open flap debridement with EMD (13 patients—test group). Open flap debridement alone (12 patients—control group). | Adjunctive EMD is positively associated with implant survival up to 5 years. |
| Lee et al., 2020 [28]: 5-month single-blinded randomized controlled clinical trial with 28 post-extraction alveoli in 28 participants following posterior maxillary alveolar ridge preservation. | Extraction sockets filled with DBBM and CM with EMD (10 patients—test group). Extraction sockets filled with DBBM and CM without EMD (10 patients—test group). Spontaneous healing extraction sockets (8 patients—control group). | EMD failed to provide additional benefits in posterior maxillary alveolar ridge preservation in the posterior maxilla. |
| Jo et al., 2019 [29]: 12-week single-blinded randomized controlled clinical trial with 64 post-extraction alveoli in 64 patients following maxillary alveolar ridge preservation. | Extraction sockets filled with BMP-2-soaked absorbable collagen sponge (32 patients—test group). Extraction sockets filled with β-TCP and hydroxyapatite particles (32 patients—control group). | The delivery systems showed similar efficacy for alveolar ridge preservation without severe adverse events. |
| Stumbras et al., 2021 [30]: 3-month double-blinded randomized controlled clinical trial with 40 post-extraction alveoli in 40 participants following anterior maxillary alveolar ridge preservation. | Extraction sockets filled with DBBM covered with resorbable native CM (10 patients—test group). Extraction sockets filled with DFDBA covered with resorbable native CM (10 patients—test group). Extraction sockets filled with FGF-2 alone (10 patients—test group). Spontaneous healing extraction sockets (10 patients—control group). | Alveolar ridge preservation technique in the esthetic zone using natural DBBM covered with resorbable native CM or using FGF-2 beneficial to reduce horizontal and vertical bone changes. |
| Saito et al., 2021 [31]: 16-week single-blinded randomized controlled clinical trial with 45 post-extraction alveoli in 45 patients following alveolar ridge preservation. | Extraction sockets filled with alloplastic graft PLGA coated β-TCP (24 patients—test group). Extraction sockets filled with DFDBA particles covered with a rapidly absorbable collagen dressing (21 patients—control group). | Although a higher proportion of mineralized tissue was associated with the use of DFDBA particles covered with a rapidly absorbable collagen dressing compared to alloplastic graft PLGA-coated β-TCP, both approaches rendered comparable outcomes in terms of maintenance of alveolar bone dimensions. |
| Gonshor et al., 2011 [32]: 9-month randomized controlled clinical trial with 21 sinus augmentation in 18 participants. | Sinus-augmentation procedures using allograft cellular bone matrix (13 sinus augmentation procedures—test sites). Sinus-augmentation procedures using conventional allograft (8 sinus augmentation procedures—control sites). | The high percentage of vital bone content, after a relatively short healing phase, may encourage a more rapid initiation of implant placement or restoration when a cellular grafting approach is considered. |
| Majzoub et al., 2020 [33]: retrospective cohort study, with a 1–9.6-year follow-up on 83 patients with 98 treated grade III furcation defects. | GTR using an allogeneic cancellous bone graft and covered by an absorbable membrane. | GTR using allogeneic cancellous bone graft and absorbable collagen membrane to be a viable option for treating furcation-involved teeth if the defect morphology and the location of the defect are favorable. |
| Canullo et al., 2019 [34]: prospective clinical trial, with a 12-month follow-up on 6 participants with 6 circumferential and semi-circumferential defects due to peri-implantitis. | GBR technique using 50:50 mixture of ABG and allograft and CMs. | The proposed technique might represent a promising result for treatment of circumferential and semi-circumferential bone defects around implants affected by peri-implantitis. |
| Kadkhodazadeh et al., 2021 [35]: retrospective pilot study, with a 8-month follow-up on 7 patients with 11 peri-implant defects. | GBR using titanium mesh, a combination of ABG, allogenic graft material, and acellular dermal matrix. Soft tissue augmentation and vestibuloplasty were performed in the second-stage surgery, if required. | This technique may lead to promising outcomes in cautiously selected patients seeking to retain their failing implants. |
| Chiapasco et al., 2020 [36]: retrospective longitudinal cohort study, with a 3–16-year follow-up on 75 participants with 82 grafted bone defects and 182 implants placed post-regeneration bone. | Reconstructive bone procedure with autogenous mandibular bone blocks and rehabilitated with implant-supported prostheses. | Implants placed in areas reconstructed with mandibular bone blocks presented survival rates consistent with those obtained for implants placed in native bone. |
| Beretta et al., 2021 [37]: clinical and radiological prospective study, with a 5-year follow-up on 8 post-extraction alveoli in 8 patients. | Alveolar socket preservation with DBBM particles covered with a porcine-derived non-cross-linked collagen matrix. | Alveolar socket preservation using DBBM in combination with a porcine-derived non-cross-linked collagen matrix proved to be an effective technique to maintain stable dimensional volumes of both hard and soft tissues. |
| Manavella et al., 2018 [38]: retrospective cohort study, with a 12-month follow-up on 11 severely resorbed alveolar sockets in 11 participants. | Ridge augmentation procedure with DBBM with 10% collagen covered with a CMs. | A ridge preservation technique performed with DBBM, and a CMs was able to improve ridge shape and dimensions in compromised alveolar sockets. |
| Zafiropoulos et al., 2020 [39]: retrospective clinical study, with a 6-month follow-up on 44 post-extraction premolar alveoli in 44 patients. | Alveolar socket preservation with non-resorbable dense polytetrafluoroethylene membranes. | The use of the examined new non-resorbable dense polytetrafluoroethylene membranes consistently led to the preservation of hard tissue in the extraction sites. |
| Beretta et al., 2015 [40]: retrospective long-term follow-up study, with a 78-month follow-up on 61 participants with 192 implants placed after guided bone regeneration. | Assessing the CSR after GBR using DBBM (76 implants). Assessing the CSR after GBR using ABG (20 implants). Assessing the CSR after GBR using 1:1 ratio mixture of ABG and demineralized DBBM (96 implants). Assessing the CSR after GBR using resorbable membranes (101 grafted sites). Assessing the CSR after GBR using non-resorbable membranes (91 grafted sites). | All the procedures performed with different bone grafts and type of membranes guaranteed optimal results in CSR (> 90%). |
| Yoshikawa et al., 2020 [41]: case report, with an 18-month follow-up on a 57-year-old man participant with generalized chronic periodontitis. | Surgical periodontal therapy with using EMD and ABG. | Periodontal regenerative therapy using EMD with ABG resulted in improvement in vertical bone resorption. |
| Thakkalapati et al., 2015 [42]: case report, with a 2-year follow-up on a 30-year-old female patient with one-wall intrabony osseous defect. | Open flap debridement and placement of combination of autologous PRP and DBBM. | Radiographic evidence of bone formation was observed as early as 3 months with almost complete fill by 6 months post-operatively. The results were maintained over a period of 2 years. |
| Pal et al., 2018 [43]: case report, with a 1-year follow-up on a male participant with a mandibular torus and intrabony defect at the mandibular right central incisor. | ABG was obtained from a mandibular torus and utilized to fill the IBD. | The mandibular torus provided sufficient graft material and eliminated the need for a second surgical site. A follow-up at 1 year revealed reduction in clinical attachment loss and complete resolution of tooth mobility. |
| Zhou et al., 2020 [44]: case report, with a 12-month follow-up on a 56-year-old and 34-year-old female patients with mandibular grade III furcation involvement. | Open flap debridement with allogenic bone graft and PRF. | Successful periodontal regeneration of grade III furcation defects can be achieved by using PRF in combination with bone allograft. |
| Panda et al., 2016 [45]: case report, with a 6-month follow-up on a 36-year-old male participant with class III gingival recession defect along with furcation involvement. | Open flap debridement with PBFP. | PBFP may be considered as a reliable modality for root coverage of severe GR defects, which could not be repaired by other conventional procedures. |
| Bassi et al., 2015 [46]: case report, with a 17-year follow-up on a 48-year-old female patient with peri-implantitis affecting the mandibular right second molar. | Open flap debridement with DBBM and a CM. | The treatment seemed to show improved clinical results up to a relevant follow-up period. |
| Poli et al., 2020 [47]: case series, with a 9-month follow-up with reentry on 6 participants with peri-implantitis affecting maxillary or mandibular area. | Open flap debridement, antimicrobial photodynamic therapy with a solution of phenothiazine chloride dye consisting of methylenthioniniumchlorid photo-activated with 100-mW diode Laser (660 ± 10 nm) and regeneration with 70:30 mixture of ABG and DBBM particles, titanium mesh, and resorbable CM. | At the reentry surgery, no evidence of granulation tissue, purulence, or progressive bone resorption was found. Newly formed bonelike tissue hardly distinguishable from the existing bone was found in intimate contact with the implant surface at most of the implant sites. A modest amount of augmented bone was obtained at only one mandibular site, resulting in persistent dehiscence-type defects associated with a supracrestal component. |
| Bhide et al., 2022 [48]: case report, with a 2-year follow-up on a 68-year-old male participant with peri-implantitis affecting the endosseous implant that replaced the maxillary left first molar. | GBR performed using DBBM covered with a dehydrated human deepithelialized human amnion-chorion membrane. | Posttreatment clinical assessment suggested that the patient responded well to surgical regenerative therapy, with the reestablishment of healthy peri-implant soft tissues. It is possible to treat peri-implantitis successfully and obtain stable long-term results with a GBR approach utilizing a DBBM covered with a dehydrated human deepithelialized human amnion-chorion membrane. |
| Park et al., 2018 [49]: case report, with a 27-month follow-up on a 52-year-old male patient with peri-implantitis affecting mandibular right second premolar area. | Open flap debridement with bovine-derived hydroxyapatite and EMD. | The area showed maintenance of graft material with increased radiopacity around the dental implant. |
| Jensen et al., 2013 [50]: case series, with a 6-month follow-up on 4 participants with posterior maxillary peri-implantitis/sinusitis and occult peri-implant oroantral fistulae. | Sinus floor grafting with BMP-2 in an absorbable collagen sponge carrier. | Sinus floor grafting to salvage trans-sinus implants or to prevent peri-implantitis/sinusitis is suggested by the successful use of BMP-2 in an absorbable collagen sponge carrier. |
| Maeda et al., 2021 [51]: case series, with a 6-month follow-up on 16 patients with 16 post-extraction alveoli. | Alveolar ridge preservation a DBBM and anodized titanium foil. | The application of a DBBM covered with anodized titanium foil resulted in clinically important horizontal preservation of the alveolar ridge at 6 months after extraction. |
| Urban et al., 2021 [52]: case series, with a 10-year follow-up on 86 participants with 92 sinus lifts and 209 implants placed. | Sinus augmentation using particulate ABG and inorganic bovine bone-derived mineral (3:7 ratio). Implants were placed on baseline residual alveolar ridge height. | Implant placement after two-stage sinus grafting utilizing the sagittal sandwich technique is a relatively safe and predictable procedure with minimal complications and marginal bone loss after 10-year follow-up. |
| Blume et al., 2021 [53]: case report, with a 6-month follow-up on a 19-year-old male patient with a complex maxillary defect. | Reconstruction of a complex maxillary defect using allogeneic bone block. | The present case report demonstrates a limited resorption of the allogeneic bone block and further emphasizes the practicability of determining bone resorption by the here introduced method. |
| Urban et al., 2022 [54]: case report, with a 3-month follow-up on a 55-year-old male participant with peri-implantitis around an implant in the maxillary left central incisor position and with severe bone loss on the mesial aspect of the maxillary left lateral incisor. | Ridge augmentation using composite bone graft (ABG + xenograft particles), and a PDGF. Tissue augmentation with connective tissue grafts. Peri-implant keratinez mucosa augmentation using labial gingival graft strip and a xenogenic collagen matrix. | Simultaneous vertical ridge augmentation and periodontal regeneration can be achieved to manage a challenging clinical situation. |
| Khojasteh et al., 2019 [55]: case report, with a 10-month and 6-month follow-up, respectively, on a 19-year-old female and 22-year-old male participants with large alveolar defects. | GBR using adipose-derived stem cells, originated from buccal fat pad, loaded with natural bovine bone mineral. | The application of adipose-derived stem cells isolated from buccal fat pad in combination with natural bovine bone mineral can be considered an efficient treatment for bone regeneration in large alveolar bone defects. |
| Windisch et al., 2021 [56]: prospective case series, with a 9-month follow-up on 19 patients with 24 vertical alveolar ridge defects. | Vertical GBR consisting of a split-thickness flap and using titanium-reinforced high-density polytetrafluoroethylene membrane combined with particulated ABG and bovine-derived xenograft in 1:1 ratio. | Vertical GBR consisting of a split-thickness flap and using titanium-reinforced non-resorbable membrane in conjunction with a 1:1 mixture of particulated ABG and bovine-derived xenograft may lead to a predictable vertical and horizontal hard tissue reconstruction. |
| Agent | rhPDGF-BB | EMD | PRP/PRF | FGF-2 | BMP-2 |
|---|---|---|---|---|---|
| Origin | Blood platelets | Hertwig’s epithelial root sheath | Platelet alpha granules | Fibroblast growth factors family | Recombinant DNA biotechnology using mammalian cells |
| Composition | Protein | 90% Amelogenin | PDGF, I-LGF, VEGF, TGF-beta | Protein | Bone Morphogenic Protein-2 |
| Mechanism of action | Chemotaxis and mitogenesis | Precise mechanism of action still unknown | Combination of different mechanism of different growth factors contained with the platelet concentrates (e.g., revascularization, fibrogenesis, etc.) | Proliferation PDL cells Migration PDL cells Differentiation PDL cells ECM production | Increase of proliferation, mineralization, and expression of alkaline phosphatase and osteocalcin |
| Clinical indications | Intrabony defects; Furcations; Gingival recession defects | Intrabony defects; Optimize tissue height in esthetic zone | Recession coverage Barrier membrane | Peri-implant defects Intrabony defects | Use in case of systemic or anatomic or conditions where successful bone regeneration cannot be achieved with conventional grafts |
Publisher’s Note: MDPI stays neutral with regard to jurisdictional claims in published maps and institutional affiliations. |
© 2022 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Gallo, S.; Pascadopoli, M.; Pellegrini, M.; Pulicari, F.; Manfredini, M.; Zampetti, P.; Spadari, F.; Maiorana, C.; Scribante, A. Latest Findings of the Regenerative Materials Application in Periodontal and Peri-Implant Surgery: A Scoping Review. Bioengineering 2022, 9, 594. https://doi.org/10.3390/bioengineering9100594
Gallo S, Pascadopoli M, Pellegrini M, Pulicari F, Manfredini M, Zampetti P, Spadari F, Maiorana C, Scribante A. Latest Findings of the Regenerative Materials Application in Periodontal and Peri-Implant Surgery: A Scoping Review. Bioengineering. 2022; 9(10):594. https://doi.org/10.3390/bioengineering9100594
Chicago/Turabian StyleGallo, Simone, Maurizio Pascadopoli, Matteo Pellegrini, Federica Pulicari, Mattia Manfredini, Paolo Zampetti, Francesco Spadari, Carlo Maiorana, and Andrea Scribante. 2022. "Latest Findings of the Regenerative Materials Application in Periodontal and Peri-Implant Surgery: A Scoping Review" Bioengineering 9, no. 10: 594. https://doi.org/10.3390/bioengineering9100594
APA StyleGallo, S., Pascadopoli, M., Pellegrini, M., Pulicari, F., Manfredini, M., Zampetti, P., Spadari, F., Maiorana, C., & Scribante, A. (2022). Latest Findings of the Regenerative Materials Application in Periodontal and Peri-Implant Surgery: A Scoping Review. Bioengineering, 9(10), 594. https://doi.org/10.3390/bioengineering9100594











