FT-IR Analysis of Structural Changes in Ketoprofen Lysine Salt and KiOil Caused by a Pulsed Magnetic Field
Abstract
1. Introduction
2. Materials and Methods
2.1. Compounds
2.2. Magnetic Field
2.3. FTIR and Signal Processing
2.4. Study Design
2.5. Experimental Study
3. Results
3.1. Clinical Treatment with Ketoprofen
3.2. Clinical Treatment with KiOil
4. Discussion
5. Conclusions
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Data Availability Statement
Conflicts of Interest
References
- Caliceti, P.; Matricardi, P. Advances in drug delivery and biomaterials: Facts and vision. Pharmaceutics 2019, 11, 48. [Google Scholar] [CrossRef]
- Trucillo, P. Drug Carriers: Classification, Administration, Release Profiles, and Industrial Approach. Processes 2021, 9, 470. [Google Scholar] [CrossRef]
- Dobrzański, L.A.; Dobrzańska-Danikiewicz, A.D.; Dobrzański, L.B. Effect of biomedical materials in the implementation of a long and healthy life policy. Processes 2021, 9, 865. [Google Scholar] [CrossRef]
- Schneider, C.; Stratman, S.; Kirsner, R.S. Lower Extremity Ulcers. Med. Clin. North. Am. 2021, 105, 663–679. [Google Scholar] [CrossRef]
- Jogani, V.; Jinturkar, K.; Vyas, T.; Misra, A. Recent Patents Review on Intranasal Administration for CNS Drug Delivery. Recent Pat. Drug Deliv. Formul. 2008, 2, 25–40. [Google Scholar] [CrossRef] [PubMed]
- Hadgraft, J.; Lane, M.E. Passive transdermal drug delivery systems. Recent considerations and advances. Am. J. Drug Deliv. 2006, 4, 153–160. [Google Scholar] [CrossRef]
- Satoskar, R.S.; Rege, N.; Bhandarkar, S.D. Pharmacology and Pharmacotherapeutics, 24th ed.; Elsevier: New Delhi, India, 2015; ISBN 9783540732761. [Google Scholar]
- Benson, H.A.E.; Roberts, M.S. Challenges and Innovations of Controlled Drug Delivery. Fundam. Drug Deliv. 2021. [Google Scholar] [CrossRef]
- Critello, C.D.; Fiorillo, A.S.; Matula, T.J. Size of Sclerosing Foams Prepared by Ultrasound, Mechanical Agitation, and the Handmade Tessari Method for Treatment of Varicose Veins. J. Ultrasound Med. 2017, 36, 649–658. [Google Scholar] [CrossRef][Green Version]
- Critello, D.C.; Pullano, S.A.; Gallo, G.; Matula, T.J.; Fiorillo, A.S. Low frequency ultrasound as a potentially viable foaming option for pathological veins. Colloids Surf. A Physicochem. Eng. Asp. 2020, 599, 124919. [Google Scholar] [CrossRef]
- Kitaoka, K.; Kawata, S.; Yoshida, T.; Kadoriku, F.; Kitamura, M. Exposure to an extremely-low-frequency magnetic field stimulates adrenal steroidogenesis via inhibition of phosphodiesterase activity in a mouse adrenal cell line. PLoS ONE 2016, 11, 1–14. [Google Scholar] [CrossRef]
- Mohamad Darwish, S.; Alsamamra, H.R.; Abusharkh, S.E.; Khalid, I.M.; Alfaqeh, R.A.; Abuteir, M.M. Effects of Extremely Low Frequency Magnetic Field on the Secondary Structures of β-Amyloid and Human Serum Albumin. Eur. J. Biophys. 2017, 5, 89–103. [Google Scholar] [CrossRef][Green Version]
- Burlaka, A.; Selyuk, M.; Gafurov, M.; Lukin, S.; Potaskalova, V.; Sidorik, E. Changes in mitochondrial functioning with electromagnetic radiation of ultra high frequency as revealed by electron paramagnetic resonance methods. Int. J. Radiat. Biol. 2014, 90, 357–362. [Google Scholar] [CrossRef] [PubMed]
- Coşkun, Ö.; Naziroğlu, M.; Çömlekçi, S.; Özkorucuklu, S. Effects of 50 Hertz-1 mT magnetic field on action potential in isolated rat sciatic nerve. Toxicol. Ind. Health. 2011, 27, 127–132. [Google Scholar] [CrossRef] [PubMed]
- Lai, H.; Singh, N.P. Magnetic field-induced DNA strand breaks in brain cells of the rat. Environ. Health Perspect. 2004, 112, 687–694. [Google Scholar] [CrossRef] [PubMed]
- Xu, Y.; Wang, Y.; Yao, A.; Xu, Z.; Dou, H.; Shen, S.; Hou, Y.; Wang, T. Low Frequency Magnetic Fields Induce Autophagy-associated Cell Death in Lung Cancer through miR-486-mediated Inhibition of Akt/mTOR Signaling Pathway. Sci. Rep. 2017, 7, 1–14. [Google Scholar] [CrossRef]
- Pesqueira, T.; Costa-Almeida, R.; Gomes, M.E. Magnetotherapy: The quest for tendon regeneration. J. Cell. Physiol. 2018, 233, 6395–6405. [Google Scholar] [CrossRef]
- Zhang, X.; Yarema, K.; Xu, A. Biological Effects of Static Magnetic Fields; Springer: Singapore, 2017; ISBN 9789811035777. [Google Scholar]
- Zablotskii, V.; Polyakova, T.; Dejneka, A. Effects of high magnetic fields on the diffusion of biologically active molecules. Cells 2022, 11, 81. [Google Scholar] [CrossRef]
- Kuczynska, J.; Nieradko-Iwanicka, B. New uses of ketoprofen-a review of studies from 2015 to 2021. Curr. Issues Pharm. Med. Sci. 2022, 35, 1–5. [Google Scholar] [CrossRef]
- Harrou, F.; Sun, Y.; Madakyaru, M.; Bouyedou, B. An Improved Multivariate Chart Using Partial Least Squares with Continuous Ranked Probability Score. IEEE Sens. J. 2018, 18, 6715–6726. [Google Scholar] [CrossRef]
- Pullano, S.A.; Bianco, M.G.; Greco, M.; Mazzuca, D.; Nisticò, S.P.; Fiorillo, A.S. FT-IR saliva analysis for the diagnosis of psoriasis: A pilot study. Biomed. Signal. Process. Control. 2022, 74, 103525. [Google Scholar] [CrossRef]
- Pullano, S.A.; Greco, M.; Bianco, M.G.; Foti, D.P.; Brunetti, A.; Fiorillo, A.S. FT-IR Saliva Profiling in Patients with Obesity and Obesity-Related Insulin Resistance. In Proceedings of the 2019 IEEE International Symposium on Medical Measurements and Applications (MeMeA), Istanbul, Turkey, 26–28 June 2019; pp. 1–5. [Google Scholar] [CrossRef]
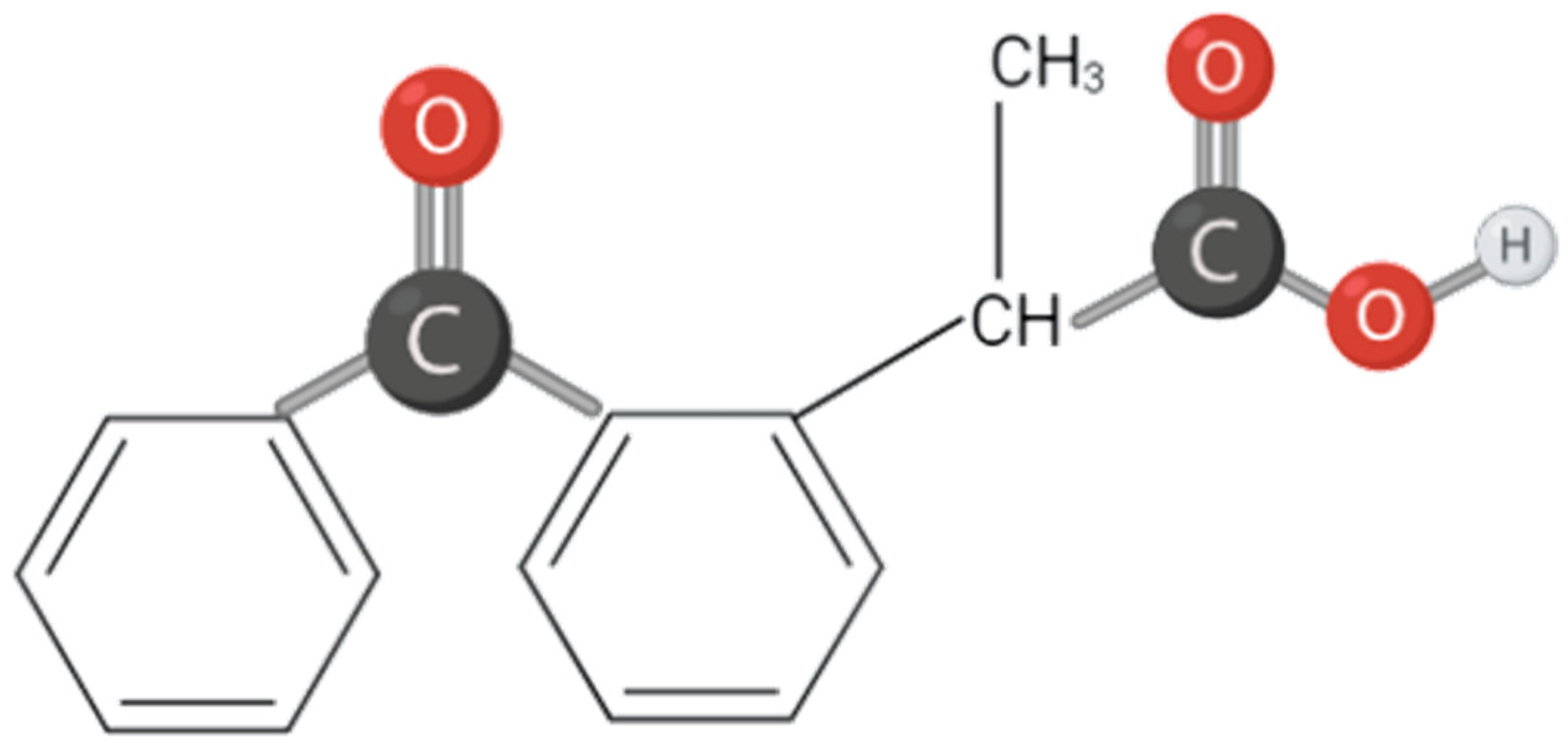
- PubChem National Institutes of Health Ketoprofen. Available online: https://pubchem.ncbi.nlm.nih.gov/compound/Ketoprofen (accessed on 27 August 2022).
- Kantor, T.G. Ketoprofen: A Review of Its Pharmacologic and Clinical Properties. Pharmacotherapy 1986, 6, 93–102. [Google Scholar] [CrossRef] [PubMed]
- AIFA Agenzia Italiana del Farmaco Riassunto delle Caratteristiche del Prodotto-Ketoprofene. Available online: https://farmaci.agenziafarmaco.gov.it/aifa/servlet/PdfDownloadServlet?pdfFileName=footer_000028_028511_RCP.pdf&sys=m0b1l3 (accessed on 7 June 2022).
- Gallelli, L.; Colosimo, M.; Pirritano, D.; Ferraro, M.; De Fazio, S.; Marigliano, N.M.; De Sarro, G. Retrospective evaluation of adverse drug reactions induced by nonsteroidal anti-inflammatory drugs. Clin. Drug Investig. 2007, 27, 115–122. [Google Scholar] [CrossRef] [PubMed]
- Smith, A.G.; Miles, V.N.; Holmes, D.T.; Chen, X.; Lei, W. Clinical Trials, Potential Mechanisms, and Adverse Effects of Arnica as an Adjunct Medication for Pain Management. Medicines 2021, 8, 58. [Google Scholar] [CrossRef]
- Widrig, R.; Suter, A.; Saller, R.; Melzer, J. Choosing between NSAID and arnica for topical treatment of hand osteoarthritis in a randomised, double-blind study. Rheumatol. Int. 2007, 27, 585–591. [Google Scholar] [CrossRef] [PubMed]
- Menghini, L.; Recinella, L.; Leone, S.; Chiavaroli, A.; Cicala, C.; Brunetti, L.; Vladimir-Knežević, S.; Orlando, G.; Ferrante, C. Devil’s claw (Harpagophytum procumbens) and chronic inflammatory diseases: A concise overview on preclinical and clinical data. Phyther. Res. 2019, 33, 2152–2162. [Google Scholar] [CrossRef]
- Oltean, H.; Robbins, C.; Mw, V.T.; Bm, B.; Bombardier, C.; Gagnier, J.J. Herbal medicine for low-back pain. Cochrane database Syst. Rev. 2014, 2014, 1–75. [Google Scholar] [CrossRef] [PubMed]
- Ouitas, N.A.; Heard, C. Estimation of the relative anti-inflammatory efficacies of six commercial preparations of Harpagophytum procumbens (Devil’s Claw). Phyther. Res. 2008, 24, 333–338. [Google Scholar] [CrossRef]
- Huang, T.H.W.; Tran, V.H.; Duke, R.K.; Tan, S.; Chrubasik, S.; Roufogalis, B.D.; Duke, C.C. Harpagoside suppresses lipopolysaccharide-induced iNOS and COX-2 expression through inhibition of NF-κB activation. J. Ethnopharmacol. 2006, 104, 149–155. [Google Scholar] [CrossRef] [PubMed]
- Negah, S.S.; Ghazavi, H.; Vafaee, F.; Rashidi, R.; Aminian, A.R.; Forouzanfar, F. The Potential Role of Green Tea and its Main Constituent (Epigallocatechin -3-Gallate) in Pain Relief: A Mechanistic Review. Curr. Drug Discov. Technol. 2021, 18. [Google Scholar] [CrossRef]
- Ohishi, T.; Goto, S.; Monira, P.; Isemura, M. Anti-inflammatory Action of Green Tea Anti-Inflammatory & Anti-Allergy Agentsin Medicinal Chemistry. Antiinf. Antiallergy Agents Med. Chem. 2016, 15, 74–90. [Google Scholar] [CrossRef] [PubMed]
- Eshghpour, M.; Mortazavi, H.; Mohammadzadeh Rezaei, N.; Nejat, A. Effectiveness of green tea mouthwash in postoperative pain control following surgical removal of impacted third molars: Double blind randomized clinical trial. Daru. J. Pharm. Sci. 2013, 21, 59. [Google Scholar] [CrossRef] [PubMed]
- Hashempur, M.H.; Sadrneshin, S.; Mosavat, S.H.; Ashraf, A. Green tea (Camellia sinensis) for patients with knee osteoarthritis: A randomized open-label active-controlled clinical trial. Clin. Nutr. 2018, 37, 85–90. [Google Scholar] [CrossRef] [PubMed]
- Banchero, M.; Manna, L. The use of lysine to enhance the supercritical complexation of ketoprofen and cyclodextrins. J. Supercrit. Fluids 2012, 67, 76–83. [Google Scholar] [CrossRef]
- Del Gaudio, P.; Russo, P.; Rosaria Lauro, M.; Colombo, P.; Aquino, R.P. Encapsulation of ketoprofen and ketoprofen lysinate by prilling for controlled drug release. AAPS Pharm. Sci. Tech. 2009, 10, 1178–1185. [Google Scholar] [CrossRef]
- Zhou, X.L.; Sun, P.N.; Bucheli, P.; Huang, T.H.; Wang, D. FT-IR methodology for quality control of arabinogalactan protein (AGP) Extracted from green tea (Camellia sinensis). J. Agric. Food Chem. 2009, 57, 5121–5128. [Google Scholar] [CrossRef] [PubMed]
- Agatonovic-Kustrin, S.; Ristivojevic, P.; Gegechkori, V.; Litvinova, T.M.; Morton, D.W. Essential oil quality and purity evaluation via ft-ir spectroscopy and pattern recognition techniques. Appl. Sci. 2020, 10, 7294. [Google Scholar] [CrossRef]
- Pawlaczyk, I.; Czerchawski, L.; Pilecki, W.; Lamer-Zarawska, E.; Gancarz, R. Polyphenolic-polysaccharide compounds from selected medicinal plants of Asteraceae and Rosaceae families: Chemical characterization and blood anticoagulant activity. Carbohydr. Polym. 2009, 77, 568–575. [Google Scholar] [CrossRef]
- Vicenzino, B.; Lawrenson, P.; Khan, A.; Stephenson, A.; Heales, L.; Benson, H.A.E.; Wright, A. A randomised pilot equivalence trial to evaluate diamagnetically enhanced transdermal delivery of key ground substance components in comparison to an established transdermal non-steroidal anti-inflammatory formulation in males with prior knee injury. PLoS ONE 2019, 14, 1–13. [Google Scholar] [CrossRef]
- Hochsprung, A.; Escudero-Uribe, S.; Ibáñez-Vera, A.J.; Izquierdo-Ayuso, G. Effectiveness of monopolar dielectric transmission of pulsed electromagnetic fields for multiple sclerosis–related pain: A pilot study. Neurologia (Engl. Ed.) 2021, 36, 433–439. [Google Scholar] [CrossRef] [PubMed]
- Roberti, R.; Marcianò, G.; Casarella, A.; Rania, V.; Palleria, C.; Muraca, L.; Citraro, R.; De Sarro, G.; Serra, R.; Romeo, P.; et al. High-Intensity, Low-Frequency Pulsed Electromagnetic Field as an Odd Treatment in a Patient with Mixed Foot Ulcer: A Case Report. Reports 2022, 5, 3. [Google Scholar] [CrossRef]
- Roberti, R.; Marcianò, G.; Casarella, A.; Rania, V.; Palleria, C.; Vocca, C.; Catarisano, L.; Muraca, L.; Citraro, R.; Romeo, P.; et al. Diamagnetic Therapy in a Patient with Complex Regional Pain Syndrome Type I and Multiple Drug Intolerance: A Case Report. Reports 2022, 5, 18. [Google Scholar] [CrossRef]
- Uzunca, K.; Birtane, M.; Taştekin, N. Effectiveness of pulsed electromagnetic field therapy in lateral epicondylitis. Clin. Rheumatol. 2007, 26, 69–74. [Google Scholar] [CrossRef]
- Cheing, G.L.Y.; Wan, J.W.H.; Kai Lo, S. Ice and pulsed electromagnetic field to reduce pain and swelling after distal radius fractures. J. Rehabil. Med. 2005, 37, 372–377. [Google Scholar] [CrossRef] [PubMed]
- Sun, X.; Huang, L.; Wang, L.; Fu, C.; Zhang, Q.; Cheng, H.; Pei, G.; Wang, Y.; He, C.; Wei, Q. Efficacy of pulsed electromagnetic field on pain and physical function in patients with low back pain: A systematic review and meta-analysis. Clin. Rehabil. 2022, 36, 636–649. [Google Scholar] [CrossRef] [PubMed]
- Osti, L.; Buono, A.D.; Maffuli, N. Pulsed electromagnetic fields after rotator cuff repair: A randomized, controlled study. Orthopedics 2015, 38, e223–e228. [Google Scholar] [CrossRef] [PubMed]
- Binder, A.; Parr, G.; Hazleman, B.; Fitton-Jackson, S. Pulsed electromagnetic field therapy of persistent rotator cuff tendinitis. A Double-blind Controlled Assessment. Lancet 1984, 323, 695–698. [Google Scholar] [CrossRef]
- Galace De Freitas, D.; Marcondes, F.B.; Monteiro, R.L.; Rosa, S.G.; Maria De Moraes Barros Fucs, P.; Fukuda, T.Y. Pulsed electromagnetic field and exercises in patients with shoulder impingement syndrome: A randomized, double-blind, placebo-controlled clinical trial. Arch. Phys. Med. Rehabil. 2014, 95, 345–352. [Google Scholar] [CrossRef] [PubMed]
- Aktas, I.; Akgun, K.; Cakmak, B. Therapeutic effect of pulsed electromagnetic field in conservative treatment of subacromial impingement syndrome. Clin. Rheumatol. 2007, 26, 1234–1239. [Google Scholar] [CrossRef] [PubMed]
- Markov, M.S. Magnetic field therapy: A review. Electromagn. Biol. Med. 2007, 26, 1–23. [Google Scholar] [CrossRef] [PubMed]
- Coskun, C.; Ocal, I.; Gunay, I. A Low-Frequency Pulsed Magnetic Field Reduces Neuropathic Pain by Regulating NaV1.8 and NaV1.9 Sodium Channels at the Transcriptional Level in Diabetic Rats. Bioelectromagnetics 2021, 42, 357–370. [Google Scholar] [CrossRef]
- Ahmed, Z.; Wieraszko, A. Pulsed magnetic stimulation modifies amplitude of action potentials in vitro via ionic channels-dependent mechanism. Bioelectromagnetics 2015, 36, 386–397. [Google Scholar] [CrossRef]
- Hu, H.; Yang, W.; Zeng, Q.; Chen, W.; Zhu, Y.B.; Liu, W.; Wang, S.; Wang, B.; Shao, Z.; Zhang, Y. Promising application of Pulsed Electromagnetic Fields (PEMFs) in musculoskeletal disorders. Biomed. Pharmacother. 2020, 131, 110767. [Google Scholar] [CrossRef] [PubMed]
- Peng, L.; Fu, C.; Wang, L.; Zhang, Q.; Liang, Z.; He, C.; Wei, Q. The Effect of Pulsed Electromagnetic Fields on Angiogenesis. Bioelectromagnetics 2021, 42, 250–258. [Google Scholar] [CrossRef] [PubMed]
- Premi, E.; Benussi, A.; La Gatta, A.; Visconti, S.; Costa, A.; Gilberti, N.; Cantoni, V.; Padovani, A.; Borroni, B.; Magoni, M. Modulation of long-term potentiation-like cortical plasticity in the healthy brain with low frequency-pulsed electromagnetic fields. BMC Neurosci. 2018, 19, 1–6. [Google Scholar] [CrossRef] [PubMed]
- PERISO CTU Mega 20. Available online: https://medecor.sk/wp-content/uploads/2017/02/CTU-MEGA-20-EN-1st-edition.pdf (accessed on 7 June 2022).
- Cohen, S.P.; Vase, L.; Hooten, W.M. Series Chronic Pain 1 Chronic pain: An update on burden, best practices, and new advances. Lancet 2021, 397, 2082–2097. [Google Scholar] [CrossRef]
- da Costa, B.R.; Pereira, T.V.; Saadat, P.; Rudnicki, M.; Iskander, S.M.; Bodmer, N.S.; Bobos, P.; Gao, L.; Kiyomoto, H.D.; Montezuma, T.; et al. Effectiveness and safety of non-steroidal anti-inflammatory drugs and opioid treatment for knee and hip osteoarthritis: Network meta-analysis. BMJ 2021, 375, n2321. [Google Scholar] [CrossRef]
- Barkin, R.L. The Pharmacology of Topical Analgesics. Postgrad. Med. 2013, 125, 7–18. [Google Scholar] [CrossRef]
- Smolen, J.S.; Aletaha, D. Rheumatoid Arthritis. Lancet 2016, 388, 2023–2038. [Google Scholar] [CrossRef]
- Chaturvedi, S.K. Prevalence of chronic pain in psychiatric patients. Pain 1987, 29, 231–237. [Google Scholar] [CrossRef]





| Protocol Name | Patients | Parameters |
|---|---|---|
| Chronic low back pain | n = 22 | Pain control (10 min): 5 Hz, 70 J Endogenous biostimulation (10 min): power 4, skeletal muscle Movement of liquids (5 min): Intracellular 80, extracellular 60 |
| Ketoprofen | KiOil | ||||||
|---|---|---|---|---|---|---|---|
| No MF treatment Wavenumber (cm−1) | No MF treatment Wavenumber (cm−1) | No MF treatment Wavenumber (cm−1) | No MF treatment Wavenumber (cm−1) | ||||
| 1480.62 1493.85 1499.32 1506.88 1512.65 1520.54 1525.72 1532.73 1538.24 1544.56 1550.67 1556.67 1564.00 1570.59 | 1578.08 1587.22 1596.15 1604.70 1613.88 1622.02 1629.21 1635.86 1643.09 1649.49 1655.99 1661.15 1671.53 1690.74 | 1478.05 1492.89 1501.57 1505.81 1511.88 1525.26 1532.20 1539.31 1545.11 1550.33 1556.24 1561.80 1566.75 1573.07 | 1590.51 1610.15 1615.21 1621.62 1628.74 1636.79 1643.72 1651.59 1659.18 1666.85 1674.64 1682.25 1691.26 | 813.95 831.84 850.43 866.69 894.65 913.48 930.98 947.37 962.93 975.85 988.77 1001.68 1013.41 1027.63 1038.15 1054.25 1060.68 1068.51 1089.40 1098.43 1119.60 1144.56 1172.38 1199.40 1221.32 1240.90 | 1259.83 1278.82 1303.73 1334.04 1357.59 1375.39 1387.70 1396.35 1415.01 1429.54 1457.17 1478.19 1505.45 1525.28 1547.70 1563.15 1581.13 1608.12 1635.53 1657.24 1678.04 1694.91 1710.84 1734.64 1753.47 | 755.69 770.81 782.00 797.49 815.80 835.68 844.22 859.77 872.34 889.91 906.33 927.07 946.95 967.22 995.53 1022.77 1036.04 1060.81 1075.40 1087.87 1100.83 1111.32 1121.79 | 1130.01 1141.47 1148.50 1161.34 1170.47 1184.63 1201.17 1226.97 1249.74 1275.81 1320.99 1356.49 1394.12 1429.87 1458.59 1487.75 1531.80 1550.50 1582.85 1627.62 1660.41 1703.38 1746.09 |
| Total (n = 11) | |
|---|---|
| Age, years | 64.09 ± 8.45 |
| Years of illness | 4.18 ± 4.62 |
| Females, n (%) | 8 (72.72) |
| Smokers or ever smokers, n (%) | 3 (27.27) |
| Diagnosis, n (%) | |
| Low back pain | 11 (100%) |
| Comorbidities; n (%) | |
| Cardiovascular illness | 5 (45.45) |
| Diabetes | 1 (9.09) |
| Dysthyroidism | 3 (27.27) |
| Gastroenterologic | 2 (18.18) |
| Hyperlypidaemia | 3 (27.27) |
| Hypertension | 7 (63.63) |
| Neurologic | 2 (18.18) |
| Obesity | 2 (18.18) |
| Osteoporosis/osteopenia | 3 (27.27) |
| Psychiatric | 2 (18.18) |
| Renal | 1 (9.09) |
| Rheumatologic | 1 (9.09) |
| Urologic | 1 (9.09) |
| Other | 2 (18.18) |
| Previous pain treatments, n (%) | 11 (100) |
| Corticosteroids | 2 (18.18) |
| Myorelaxants | 2 (18.18) |
| NSAIDs | 11 (100) |
| Opioids | 3 (27.27) |
| Paracetamol | 4 (36.36) |
| Pregabalin | 2 (18.18) |
| Other | 2 (18.18) |
| Concomitant pain treatments, n (%) | 9 (81.81) |
| Duloxetine | 1 (9.09) |
| L-acetylcarnitine | 3 (27.27) |
| Myorelaxants | 2 (18.18) |
| NSAIDs | 6 (54.54) |
| Opioids | 3 (27.27) |
| Paracetamol | 4 (36.36) |
| Pregabalin | 1 (9.09) |
| Medium number of sessions | 7.77 ± 3.09 |
| NRS before treatment | 7.77 ± 2.25 |
| NRS after treatment | 2.45 ± 2.38 |
| Pharmacologic treatment at last session, n (%) | 6 (54.54) |
| SF-36 Item | First Session | Last Session | Statistical Significancy |
|---|---|---|---|
| Physical functioning Limitations of physical health Limitations emotional problems | 44.09 ± 20.59 | 69.09 ± 17.86 | p < 0.0001 |
| 20.45 ± 31.26 | 56.82 ± 27.59 | p = 0.0039 | |
| 72.73 ± 38.92 | 93.93 ± 20.11 | p = 0.0456 | |
| Energy/fatigue Emotional well being | 47.27 ± 18.35 | 57.73 ± 16.49 | p = 0.0028 |
| 64.36 ± 15.95 | 72.00 ± 14.75 | p = 0.0032 | |
| Social functioning Pain General health Health change | 81.81 ± 17.99 | 90.90 ± 11.30 | p = 0.0119 |
| 32.04 ± 17.05 | 65.90 ± 19.04 | p < 0.0001 | |
| 38.18 ± 28.42 | 51.36 ± 18.04 | p = 0.0047 | |
| 29.55 ± 21.85 | 72.73 ± 23.60 | p < 0.0001 |
| Total (n = 11) | |
|---|---|
| Age, years | 58.63 ± 14.71 |
| Years of illness | 2.18 ± 1.72 |
| Females, n (%) | 8 (72.72) |
| Smokers or ever smokers, n (%) | 4 (36.36) |
| Diagnosis, n (%) | |
| Low back pain | 11 (100) |
| Comorbidities; n (%) | |
| Cardiovascular illness | 3 (27.27) |
| Diabetes | 2 (18.18) |
| Dysthyroidism | 2 (18.18) |
| Gastroenterological | 3 (27.27) |
| Hyperlipidaemia | 4 (36.36) |
| Hypertension | 5 (45.45) |
| Neurologic | 2 (18.18) |
| Obesity | 3 (27.27) |
| Osteoporosis/osteopenia | 2 (18.18) |
| Pneumological | 1 (9.09) |
| Renal | 1 (9.09) |
| Urologic | 2 (18.18) |
| Other | 5 (45.45) |
| Previous pain treatments, n (%) | 10 (90.90) |
| Corticosteroids | 2 (18.18) |
| L-acetylcarnitine | 3 (27.27) |
| Myorelaxants | 2 (18.18) |
| NSAIDs | 10 (90.90) |
| Opioids | 4 (36.36) |
| Paracetamol | 2 (18.18) |
| Pregabalin | 2 (18.18) |
| Other | 3 (27.27) |
| Concomitant pain treatments, n (%) | 10 (90.90) |
| Duloxetine | 3 (27.27) |
| L-acetylcarnitine | 3 (27.27) |
| Myorelaxants | 6 (54.54) |
| NSAIDs | 6 (54.54) |
| Opioids | 6 (54.54) |
| Paracetamol | 6 (54.54) |
| Pregabalin | 1 (9.09) |
| Steroids | 3 (27.27) |
| Other | 4 (36.36) |
| Medium number of sessions | 7.27 ± 1.48 |
| NRS before treatment | 7.59 ± 2.49 |
| NRS after treatment | 1.90 ± 2.26 |
| Pharmacologic treatment at last session, n (%) | 6 (54.54) |
| SF-36 Item | First Session | Last Session | Statistical Significancy |
|---|---|---|---|
| Physical functioning Limitations of physical health Limitations emotional problems | 55.45 ± 19.81 | 75.91 ± 18.41 | p = 0.0017 |
| 31.82 ± 31.80 | 68.18 ± 29.77 | p = 0.0039 | |
| 90.90 ± 21.56 | 96.97 ± 10.04 | p = 0.1669 | |
| Energy/fatigue Emotional well being | 58.18 ± 9.82 | 62.73 ± 9.32 | p = 0.0531 |
| 60.36 ± 8.66 | 65.82 ± 7.01 | p = 0.0024 | |
| Social functioning Pain General health Health change | 84.09 ± 17.75 | 94.31 ± 6.52 | p = 0.0552 |
| 37.04 ± 17.74 | 70.90 ± 10.91 | p < 0.0001 | |
| 28.18 ± 13.28 | 42.73 ± 14.72 | p = 0.0012 | |
| 18.18 ± 19.66 | 84.09 ± 12.61 | p < 0.0001 |
Publisher’s Note: MDPI stays neutral with regard to jurisdictional claims in published maps and institutional affiliations. |
© 2022 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Pullano, S.A.; Marcianò, G.; Bianco, M.G.; Oliva, G.; Rania, V.; Vocca, C.; Cione, E.; De Sarro, G.; Gallelli, L.; Romeo, P.; et al. FT-IR Analysis of Structural Changes in Ketoprofen Lysine Salt and KiOil Caused by a Pulsed Magnetic Field. Bioengineering 2022, 9, 503. https://doi.org/10.3390/bioengineering9100503
Pullano SA, Marcianò G, Bianco MG, Oliva G, Rania V, Vocca C, Cione E, De Sarro G, Gallelli L, Romeo P, et al. FT-IR Analysis of Structural Changes in Ketoprofen Lysine Salt and KiOil Caused by a Pulsed Magnetic Field. Bioengineering. 2022; 9(10):503. https://doi.org/10.3390/bioengineering9100503
Chicago/Turabian StylePullano, Salvatore Andrea, Gianmarco Marcianò, Maria Giovanna Bianco, Giuseppe Oliva, Vincenzo Rania, Cristina Vocca, Erika Cione, Giovambattista De Sarro, Luca Gallelli, Pietro Romeo, and et al. 2022. "FT-IR Analysis of Structural Changes in Ketoprofen Lysine Salt and KiOil Caused by a Pulsed Magnetic Field" Bioengineering 9, no. 10: 503. https://doi.org/10.3390/bioengineering9100503
APA StylePullano, S. A., Marcianò, G., Bianco, M. G., Oliva, G., Rania, V., Vocca, C., Cione, E., De Sarro, G., Gallelli, L., Romeo, P., La Gatta, A., & Fiorillo, A. S. (2022). FT-IR Analysis of Structural Changes in Ketoprofen Lysine Salt and KiOil Caused by a Pulsed Magnetic Field. Bioengineering, 9(10), 503. https://doi.org/10.3390/bioengineering9100503