Optimization of Spinal Reconstructions for Thoracolumbar Burst Fractures to Prevent Proximal Junctional Complications: A Finite Element Study
Abstract
1. Introduction
2. Materials and Methods
2.1. Generation of T10-L3 Finite Element Model
2.2. Simulation of TCSR Models
2.3. Loading and Boundary Conditions
2.4. Convergence Test
3. Results
3.1. Model Validation
3.2. Global Range of Motion in the TL Spine
3.3. Motion in the Fractured L1 Vertebral Body and Motion Distributions
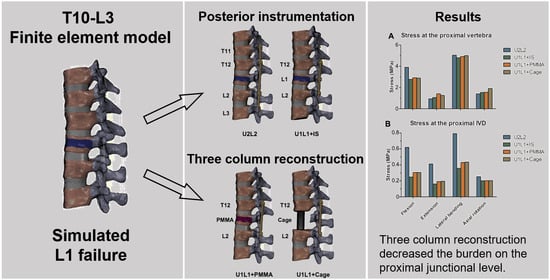
3.4. The Effect of PI and TCSR on the Proximal Junctional Level
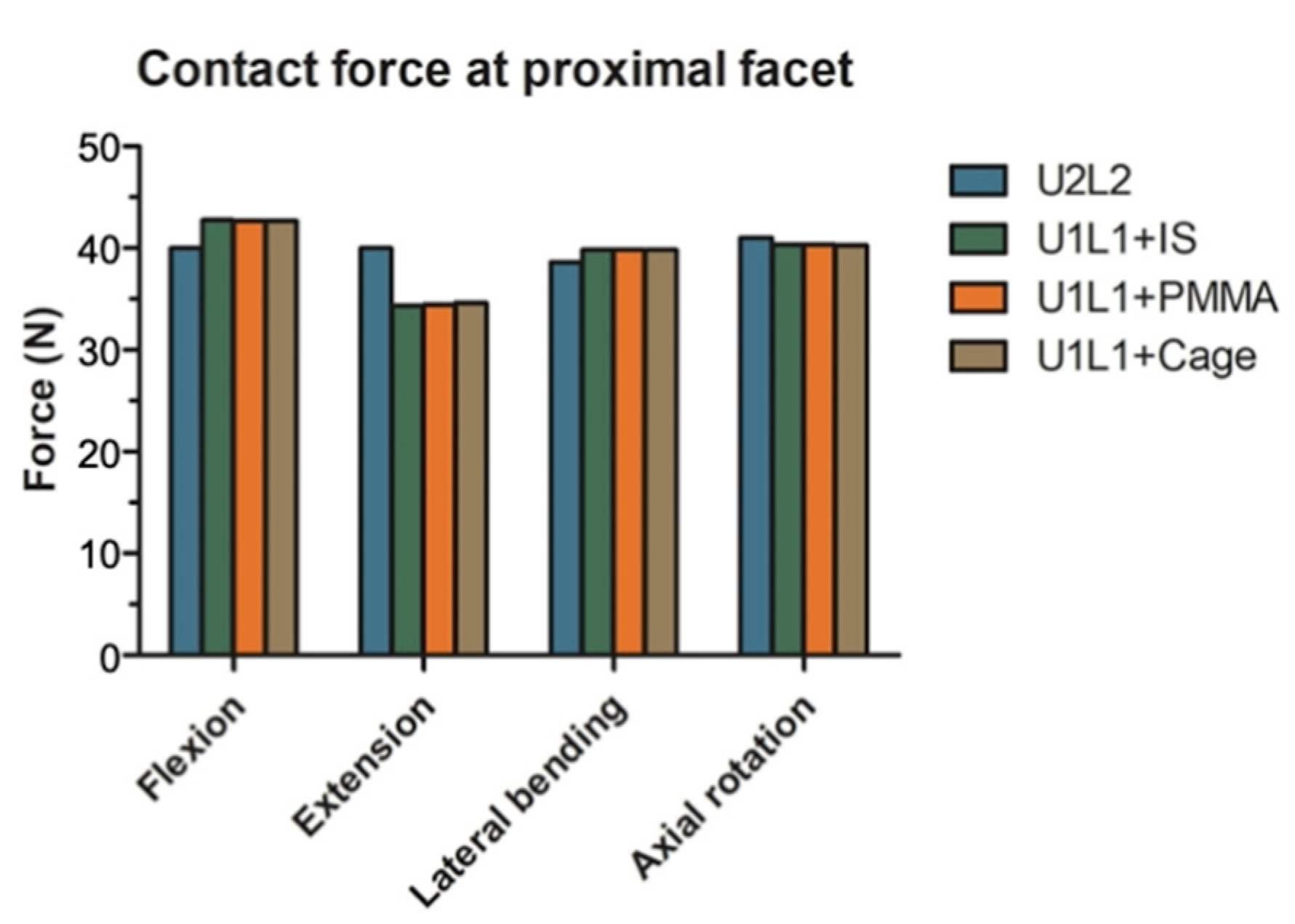
3.5. The Effect of PI and TCSR on the Proximal Articular Facets
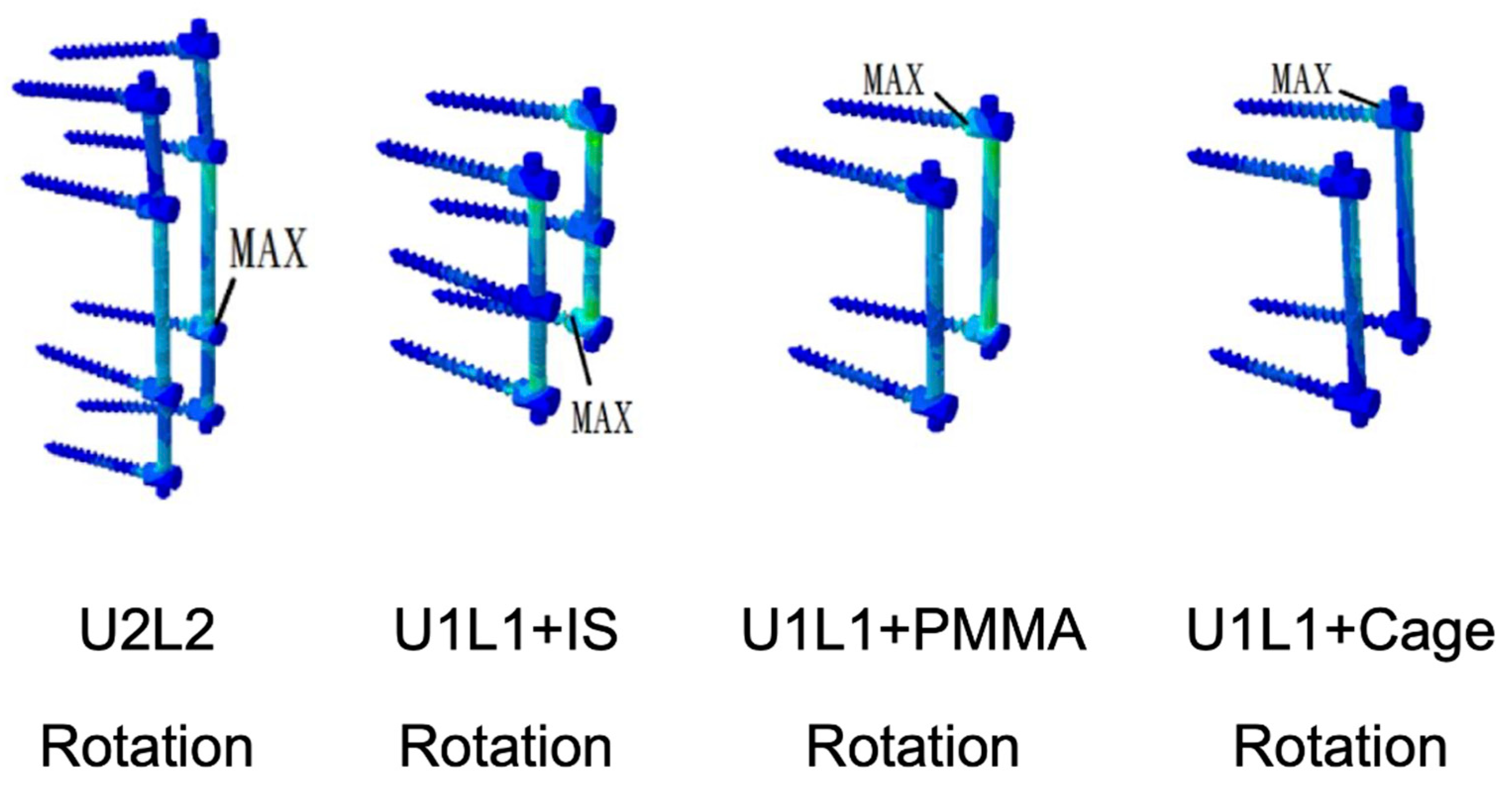
3.6. Von Mises Stress and Strain Energy Density on the Screw and Rod Construct
4. Discussion
5. Conclusions
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Data Availability Statement
Conflicts of Interest
References
- Denis, F. The Three Column Spine and Its Significance in the Classification of Acute Thoracolumbar Spinal Injuries. Spine (Phila Pa 1976) 1983, 8, 817–831. [Google Scholar] [CrossRef]
- Xu, R.; Garcés-Ambrossi, G.L.; McGirt, M.J.; Witham, T.F.; Wolinsky, J.P.; Bydon, A.; Gokaslan, Z.L.; Sciubba, D.M. Thoracic Vertebrectomy and Spinal Reconstruction via Anterior, Posterior, or Combined Approaches: Clinical Outcomes in 91 Consecutive Patients with Metastatic Spinal Tumors—Clinical Article. J. Neurosurg. Spine 2009, 11, 272–284. [Google Scholar] [CrossRef] [PubMed]
- Shannon, F.J.; DiResta, G.R.; Ottaviano, D.; Castro, A.; Healey, J.H.; Boland, P.J. Biomechanical Analysis of Anterior Poly-Methyl-Methacrylate Reconstruction Following Total Spondylectomy for Metastatic Disease. Spine (Phila Pa 1976) 2004, 29, 2096–3012. [Google Scholar] [CrossRef] [PubMed]
- Lai, O.; Hu, Y.; Yuan, Z.; Sun, X.; Dong, W.; Zhang, J.; Zhu, B. Modified One-Stage Posterior/Anterior Combined Surgery with Posterior Pedicle Instrumentation and Anterior Monosegmental Reconstruction for Unstable Denis Type B Thoracolumbar Burst Fracture. Eur. Spine J. 2017, 26, 1499–1505. [Google Scholar] [CrossRef] [PubMed]
- Nguyen, N.-L.M.; Kong, C.Y.; Hart, R.A. Proximal Junctional Kyphosis and Failure-Diagnosis, Prevention, and Treatment. Curr. Rev. Musculoskelet. Med. 2016, 9, 299–308. [Google Scholar] [CrossRef]
- Park, S.-J.; Lee, C.-S.; Chung, S.-S.; Lee, J.-Y.; Kang, S.-S.; Park, S.-H. Different Risk Factors of Proximal Junctional Kyphosis and Proximal Junctional Failure Following Long Instrumented Fusion to the Sacrum for Adult Spinal Deformity: Survivorship Analysis of 160 Patients. Neurosurgery 2017, 80, 279–286. [Google Scholar] [CrossRef]
- Kim, J.S.; Phan, K.; Cheung, Z.B.; Lee, N.; Vargas, L.; Arvind, V.; Merrill, R.K.; Gidumal, S.; di Capua, J.; Overley, S.; et al. Surgical, Radiographic, and Patient-Related Risk Factors for Proximal Junctional Kyphosis: A Meta-Analysis. Glob. Spine J. 2019, 9, 32–40. [Google Scholar] [CrossRef]
- Wang, W.; Pei, B.; Pei, Y.; Shi, Z.; Kong, C.; Wu, X.; Wu, N.; Fan, Y.; Lu, S. Biomechanical Effects of Posterior Pedicle Fixation Techniques on the Adjacent Segment for the Treatment of Thoracolumbar Burst Fractures: A Biomechanical Analysis. Comput. Methods Biomech. Biomed. Eng. 2019, 22, 1083–1092. [Google Scholar] [CrossRef]
- McDonnell, M.; Shah, K.N.; Paller, D.J.; Thakur, N.A.; Koruprolu, S.; Palumbo, M.A.; Daniels, A.H. Biomechanical Analysis of Pedicle Screw Fixation for Thoracolumbar Burst Fractures. Orthopedics 2016, 39, e514–e518. [Google Scholar] [CrossRef]
- Waqar, M.; Van-Popta, D.; Barone, D.G.; Bhojak, M.; Pillay, R.; Sarsam, Z. Short versus Long-Segment Posterior Fixation in the Treatment of Thoracolumbar Junction Fractures: A Comparison of Outcomes. Br. J. Neurosurg. 2017, 31, 54–57. [Google Scholar] [CrossRef]
- Thaker, R.A.; Gautam, V.K. Study of Vertebral Body Replacement with Reconstruction Spinal Cages in Dorsolumbar Traumatic and Koch’s Spine. Asian Spine J. 2014, 8, 786–792. [Google Scholar] [CrossRef][Green Version]
- Lindtner, R.A.; Mueller, M.; Schmid, R.; Spicher, A.; Zegg, M.; Kammerlander, C.; Krappinger, D. Monosegmental Anterior Column Reconstruction Using an Expandable Vertebral Body Replacement Device in Combined Posterior–Anterior Stabilization of Thoracolumbar Burst Fractures. Arch. Orthop. Trauma Surg. 2018, 138, 939–951. [Google Scholar] [CrossRef]
- Jordan, Y.; Buchowski, J.M.; Mokkarala, M.; Peters, C.; Bumpass, D.B. Outcomes and Cost-Minimization Analysis of Cement Spacers versus Expandable Cages for Posterior-Only Reconstruction of Metastatic Spine Corpectomies. Ann. Transl. Med. 2019, 7, 212. [Google Scholar] [CrossRef]
- Eleraky, M.; Papanastassiou, I.; Tran, N.D.; Dakwar, E.; Vrionis, F.D. Comparison of Polymethylmethacrylate versus Expandable Cage in Anterior Vertebral Column Reconstruction after Posterior Extracavitary Corpectomy in Lumbar and Thoraco-Lumbar Metastatic Spine Tumors. Eur. Spine J. 2011, 20, 1363–1370. [Google Scholar] [CrossRef][Green Version]
- Akamaru, T.; Kawahara, N.; Sakamoto, J.; Yoshida, A.; Murakami, H.; Hato, T.; Awamori, S.; Oda, J.; Tomita, K. The Transmission of Stress to Grafted Bone inside a Titanium Mesh Cage Used in Anterior Column Reconstruction after Total Spondylectomy: A Finite-Element Analysis. Spine (Phila Pa 1976) 2005, 30, 2783–2787. [Google Scholar] [CrossRef] [PubMed]
- Shin, G.; Mirka, G.A.; Loboa, E.G. Viscoelastic Responses of the Lumbar Spine during Prolonged Stooping. Proc. Hum. Factors Ergon. Soc. Annu. Meet. 2016, 49, 1269–1273. [Google Scholar] [CrossRef]
- Wilcox, R.K.; Allen, D.J.; Hall, R.M.; Limb, D.; Barton, D.C.; Dickson, R.A. A Dynamic Investigation of the Burst Fracture Process Using a Combined Experimental and Finite Element Approach. Eur. Spine J. 2004, 13, 481–488. [Google Scholar] [CrossRef] [PubMed]
- Imai, K. Computed Tomography-Based Finite Element Analysis to Assess Fracture Risk and Osteoporosis Treatment. World J. Exp. Med. 2015, 5, 182. [Google Scholar] [CrossRef]
- Sin, D.A.; Heo, D.H. Comparative Finite Element Analysis of Lumbar Cortical Screws and Pedicle Screws in Transforaminal and Posterior Lumbar Interbody Fusion. Neurospine 2019, 16, 298. [Google Scholar] [CrossRef] [PubMed]
- Rodriguez, A.G.; Rodriguez-Soto, A.E.; Burghardt, A.J.; Berven, S.; Majumdar, S.; Lotz, J.C. Morphology of the Human Vertebral Endplate. J. Orthop. Res. 2012, 30, 280. [Google Scholar] [CrossRef]
- Yeni, Y.N.; Dix, M.R.; Xiao, A.; Oravec, D.J.; Flynn, M.J. Measuring the Thickness of Vertebral Endplate and Shell Using Digital Tomosynthesis. Bone 2022, 157, 116341. [Google Scholar] [CrossRef]
- Palepu, V.; Rayaprolu, S.D.; Nagaraja, S. Differences in Trabecular Bone, Cortical Shell, and Endplate Microstructure across the Lumbar Spine. Int. J. Spine Surg. 2019, 13, 361–370. [Google Scholar] [CrossRef] [PubMed]
- Wong, C.-E.; Hu, H.-T.; Hsieh, M.-P.; Huang, K.-Y. Optimization of Three-Level Cervical Hybrid Surgery to Prevent Adjacent Segment Disease: A Finite Element Study. Front. Bioeng. Biotechnol. 2020, 8, 154. [Google Scholar] [CrossRef]
- Wong, C.-E.; Hu, H.-T.; Tsai, C.-H.; Li, J.-L.; Hsieh, C.-C.; Huang, K.-Y. Comparison of Posterior Fixation Strategies for Thoracolumbar Burst Fracture: A Finite Element Study. J. Biomech. Eng. 2021, 143, 071007. [Google Scholar] [CrossRef] [PubMed]
- Polikeit, A.; Ferguson, S.J.; Nolte, L.P.; Orr, T.E. Factors Influencing Stresses in the Lumbar Spine after the Insertion of Intervertebral Cages: Finite Element Analysis. Eur. Spine J. 2003, 12, 413–420. [Google Scholar] [CrossRef] [PubMed]
- Kumaresan, S.; Yoganandan, N.; Pintar, F.A. Finite Element Analysis of the Cervical Spine: A Material Property Sensitivity Study. Clin. Biomech. 1999, 14, 41–53. [Google Scholar] [CrossRef]
- Goel, V.K.; Park, H.; Kong, W. Investigation of Vibration Characteristics of the Ligamentous Lumbar Spine Using the Finite Element Approach. J. Biomech. Eng. 1994, 116, 377–383. [Google Scholar] [CrossRef]
- Hsieh, Y.Y.; Kuo, Y.J.; Chen, C.H.; Wu, L.C.; Chiang, C.J.; Lin, C.L. Biomechanical Assessment of Vertebroplasty Combined with Cement-Augmented Screw Fixation for Lumbar Burst Fractures: A Finite Element Analysis. Appl. Sci. 2020, 10, 2133. [Google Scholar] [CrossRef]
- Yamamoto, I.; Panjabi, M.M.; Crisco, T.; Oxland, T. Three-Dimensional Movements of the Whole Lumbar Spine and Lumbosacral Joint. Spine (Phila Pa 1976) 1989, 14, 1256–1260. [Google Scholar] [CrossRef]
- Lan, C.-C.; Kuo, C.-S.; Chen, C.-H.; Hu, H.-T. Finite Element Analysis of Biomechanical Behavior of Whole Thoraco-Lumbar Spine with Ligamentous Effect. Chang. J. Med. 2013, 11, 26–41. [Google Scholar]
- Chin, K.R.; Newcomb, A.G.U.; Reis, M.T.; Reyes, P.M.; Hickam, G.A.; Gabriel, J.; Pencle, F.J.R.; Sung, R.D.; Crawford, N.R. Biomechanics of Posterior Instrumentation in L1-L3 Lateral Interbody Fusion: Pedicle Screw Rod Construct vs. Transfacet Pedicle Screws. Clin. Biomech. 2016, 31, 59–64. [Google Scholar] [CrossRef]
- Rustenburg, C.M.E.; Faraj, S.S.A.; Holewijn, R.M.; Kingma, I.; van Royen, B.J.; Stadhouder, A.; Emanuel, K.S. The Biomechanical Effect of Single-Level Laminectomy and Posterior Instrumentation on Spinal Stability in Degenerative Lumbar Scoliosis: A Human Cadaveric Study. Neurosurg. Focus 2019, 46, E15. [Google Scholar] [CrossRef] [PubMed]
- Obid, P.; Danyali, R.; Kueny, R.; Huber, G.; Reichl, M.; Richter, A.; Niemeyer, T.; Morlock, M.; Püschel, K.; Übeyli, H. Hybrid Instrumentation in Lumbar Spinal Fusion. Glob. Spine J. 2017, 7, 47–53. [Google Scholar] [CrossRef] [PubMed]
- Cunningham, B.W.; Kotani, Y.; McNulty, P.S.; Cappuccino, A.; McAfee, P.C. The Effect of Spinal Destabilization and Instrumentation on Lumbar Intradiscal Pressure: An in Vitro Biomechanical Analysis. Spine (Phila Pa 1976) 1997, 22, 2655–2663. [Google Scholar] [CrossRef]
- Brinckmann, P.; Grootenboer, H. Change of Disc Height, Radial Disc Bulge, and Intradiscal Pressure from Discectomy. An in Vitro Investigation on Human Lumbar Discs. Spine (Phila Pa 1976) 1991, 16, 641–646. [Google Scholar] [CrossRef]
- Wilke, H.J.; Wolf, S.; Claes, L.E.; Arand, M.; Wiesend, A. Influence of Varying Muscle Forces on Lumbar Intradiscal Pressure: An in Vitro Study. J. Biomech. 1996, 29, 549–555. [Google Scholar] [CrossRef]
- Buttermann, G.R.; Beaubien, B.P. In Vitro Disc Pressure Profiles below Scoliosis Fusion Constructs. Spine (Phila Pa 1976) 2008, 33, 2134–2142. [Google Scholar] [CrossRef] [PubMed]
- Zhang, Q.; Chon, T.E.; Zhang, Y.; Baker, J.S.; Gu, Y. Finite Element Analysis of the Lumbar Spine in Adolescent Idiopathic Scoliosis Subjected to Different Loads. Comput. Biol. Med. 2021, 136, 104745. [Google Scholar] [CrossRef]
- Reid, J.J.; Johnson, J.S.; Wang, J.C. Challenges to Bone Formation in Spinal Fusion. J. Biomech. 2011, 44, 213–220. [Google Scholar] [CrossRef] [PubMed]
- Heggeness, M.H.; Esses, S.I. Classification of Pseudarthroses of the Lumbar Spine. Spine (Phila Pa 1976) 1991, 16, S449–S454. [Google Scholar] [CrossRef]
- Scholz, M.; Kandziora, F.; Tschauder, T.; Kremer, M.; Pingel, A. Prospective Randomized Controlled Comparison of Posterior vs. Posterior-Anterior Stabilization of Thoracolumbar Incomplete Cranial Burst Fractures in Neurological Intact Patients: The RASPUTHINE Pilot Study. Eur. Spine J. 2018, 27, 3016–3024. [Google Scholar] [CrossRef]
- Lau, D.; Funao, H.; Clark, A.J.; Nicholls, F.; Smith, J.; Bess, S.; Shaffrey, C.; Schwab, F.J.; Lafage, V.; Deviren, V.; et al. The Clinical Correlation of the Hart-ISSG Proximal Junctional Kyphosis Severity Scale With Health-Related Quality-of-Life Outcomes and Need for Revision Surgery. Spine (Phila Pa 1976) 2016, 41, 213–223. [Google Scholar] [CrossRef]
- Reames, D.L.; Kasliwal, M.K.; Smith, J.S.; Hamilton, D.K.; Arlet, V.; Shaffrey, C.I. Time to Development, Clinical and Radiographic Characteristics, and Management of Proximal Junctional Kyphosis Following Adult Thoracolumbar Instrumented Fusion for Spinal Deformity. J. Spinal Disord. Tech. 2015, 28, E106–E114. [Google Scholar] [CrossRef]
- Tezeren, G.; Kuru, I. Posterior Fixation of Thoracolumbar Burst Fracture: Short-Segment Pedicle Fixation versus Long-Segment Instrumentation. J. Spinal Disord. Tech. 2005, 18, 485–488. [Google Scholar] [CrossRef] [PubMed]
- Doblaré, M.; García, J.M.; Gómez, M.J. Modelling Bone Tissue Fracture and Healing: A Review. Eng. Fract. Mech. 2004, 71, 1809–1840. [Google Scholar] [CrossRef]
- Pattin, C.A.; Caler, W.E.; Carter, D.R. Cyclic Mechanical Property Degradation during Fatigue Loading of Cortical Bone. J. Biomech. 1996, 29, 69–79. [Google Scholar] [CrossRef]
- Ponnappan, R.K.; Serhan, H.; Zarda, B.; Patel, R.; Albert, T.; Vaccaro, A.R. Biomechanical Evaluation and Comparison of Polyetheretherketone Rod System to Traditional Titanium Rod Fixation. Spine J. 2009, 9, 263–267. [Google Scholar] [CrossRef]
- Machino, M.; Yukawa, Y.; Ito, K.; Nakashima, H.; Kato, F. Posterior/Anterior Combined Surgery for Thoracolumbar Burst Fractures—Posterior Instrumentation with Pedicle Screws and Laminar Hooks, Anterior Decompression and Strut Grafting. Spinal Cord. 2011, 49, 573–579. [Google Scholar] [CrossRef][Green Version]
- Bianchi, D.; Falcinelli, C.; Molinari, L.; Gizzi, A.; di Martino, A. Osteolytic vs. Osteoblastic Metastatic Lesion: Computational Modeling of the Mechanical Behavior in the Human Vertebra after Screws Fixation Procedure. J. Clin. Med. 2022, 11, 2850. [Google Scholar] [CrossRef]
- Rivera, A.F.; de Castro Magalhães, F.; Moreno, A.; Rubio, J.C. Assessment of the Highest Stress Concentration Area Generated on the Mandibular Structure Using Meshless Finite Elements Analysis. Bioengineering 2020, 7, 142. [Google Scholar] [CrossRef]
- Knapp, A.; Williams, L.N. Predicting the Effect of Localized ACL Damage on Neighbor Ligament Mechanics via Finite Element Modeling. Bioengineering 2022, 9, 54. [Google Scholar] [CrossRef]
- Molinari, L.; Falcinelli, C.; Gizzi, A.; di Martino, A. Effect of Pedicle Screw Angles on the Fracture Risk of the Human Vertebra: A Patient-Specific Computational Model. J. Mech. Behav. Biomed. Mater. 2021, 116, 104359. [Google Scholar] [CrossRef] [PubMed]





| Component | Young’s Modulus (MPa) | Poisson’s Ratio | Element Type |
|---|---|---|---|
| Annulus Fibers | Shell (STRI3) | ||
| Inner Laminate: Inner Layer | 360 | 0.30 | |
| Inner Laminate: Middle Layer | 385 | 0.30 | |
| Inner Laminate: Outer Layer | 420 | 0.30 | |
| Outer Laminate: Inner Layer | 440 | 0.30 | |
| Outer Laminate: Middle Layer | 495 | 0.30 | |
| Outer Laminate: Outer Layer | 550 | 0.30 | |
| Annulus Ground Substance | 4.2 | 0.30 | Tetrahedron (C3D4) |
| Cancellous Bone | 100 | 0.20 | Tetrahedron (C3D4) |
| Cancellous Bone (L1 failure) | 10 | ||
| Cortical Bone | 12,000 | 0.30 | Shell (S3R) |
| Cortical Bone (L1 failure) | 1200 | ||
| Posterior Bony Elements | 3500 | 0.25 | Tetrahedron (C3D4) |
| Endplate | 12,000 | 0.30 | Shell (S3R) |
| Nucleus Pulposus | 1 | 0.49 | Tetrahedron (C3D4) |
| ALL/PLL/LF/ISL/SSL | 20/20/20/10/15 | 0.25 | Truss (T3D2) |
| Titanium screw/rod/cage | 110,000 | 0.30 | Tetrahedron (C3D4) |
| PMMA | 2900 | 0.30 | Tetrahedron (C3D4) |
| Component | Element Type | No. of Elements | |||||
|---|---|---|---|---|---|---|---|
| T10 | T11 | T12 | L1 | L2 | L3 | ||
| Cortical bone | S3R | 2581 | 2401 | 2511 | 2789 | 2892 | 3098 |
| Cancellous bone | C3D4 | 15,144 | 17,500 | 18,509 | 21,312 | 23,452 | 19,079 |
| Endplate | S3R | 1905 | 1780 | 1796 | 2145 | 2010 | 2268 |
| Posterior elements | C3D4 | 17,472 | 16,613 | 16,820 | 19,951 | 20,628 | 21,503 |
| T10/11 | T11/12 | T12/L1 | L1/L2 | L2/L3 | |||
| Nucleus pulposus | C3D4 | 4513 | 3840 | 3076 | 5206 | 4565 | |
| Annulus fiber | STRI3 | 1025 | 812 | 732 | 1336 | 1436 | |
| Annulus ground substance | C3D4 | 5374 | 4937 | 3929 | 6479 | 5703 | |
| Ligaments | ALL | PLL | LF | ISL | SSL | ||
| No. of elements | T3D2 | 25 | 25 | 20 | 15 | 10 | |
| Maximum Stress in the Pedicle Screws | ||||
| construct | U2L2 | U1L1+IS | U1L1+PMMA | U1L1+Cage |
| Stress (MPa) | 27.98 | 24.01 | 27.31 | 16.78 |
| level | L2 | L2 | T12 | T12 |
| motion | rotation | rotation | rotation | rotation |
| Maximum Strain Energy Density in the Pedicle Screws | ||||
| construct | U2L2 | U1L1+IS | U1L1+PMMA | U1L1+Cage |
| Energy (mJ/mm3) | 12.41 | 8.05 | 5.72 | 4.55 |
| Motion | rotation | rotation | rotation | flexion |
Publisher’s Note: MDPI stays neutral with regard to jurisdictional claims in published maps and institutional affiliations. |
© 2022 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Wong, C.-E.; Hu, H.-T.; Huang, Y.-H.; Huang, K.-Y. Optimization of Spinal Reconstructions for Thoracolumbar Burst Fractures to Prevent Proximal Junctional Complications: A Finite Element Study. Bioengineering 2022, 9, 491. https://doi.org/10.3390/bioengineering9100491
Wong C-E, Hu H-T, Huang Y-H, Huang K-Y. Optimization of Spinal Reconstructions for Thoracolumbar Burst Fractures to Prevent Proximal Junctional Complications: A Finite Element Study. Bioengineering. 2022; 9(10):491. https://doi.org/10.3390/bioengineering9100491
Chicago/Turabian StyleWong, Chia-En, Hsuan-Teh Hu, Yu-Heng Huang, and Kuo-Yuan Huang. 2022. "Optimization of Spinal Reconstructions for Thoracolumbar Burst Fractures to Prevent Proximal Junctional Complications: A Finite Element Study" Bioengineering 9, no. 10: 491. https://doi.org/10.3390/bioengineering9100491
APA StyleWong, C.-E., Hu, H.-T., Huang, Y.-H., & Huang, K.-Y. (2022). Optimization of Spinal Reconstructions for Thoracolumbar Burst Fractures to Prevent Proximal Junctional Complications: A Finite Element Study. Bioengineering, 9(10), 491. https://doi.org/10.3390/bioengineering9100491