Antibiofilm Activity of Biocide Metal Ions Containing Bioactive Glasses (BGs): A Mini Review
Abstract
1. Introduction
2. Bioactive Glasses
- I.
- Rapid exchange of Ca2+ with a proton or hydrate proton;
- II.
- Generation of silanols (Si–OH) at the site of the breakdown of the silica network. Solution interface for BG. In this stage, soluble silica [Si(OH)4] is also produced and released to the bodily fluid;
- III.
- Condensation and repolymerization of the silica-rich layer take place on the BGs’surface. Consumption of Si–OH;
- IV.
- Ca2+ and PO43− migrate to the surface and form Ca–PO43− clusters on the top of the SiO2-rich layer, and the crystallization of the amorphous CaP takes place;
- V.
- Finally, the hydroxycarbonate apatite layer (HAC) is formed by the incorporation of OH– and CO32− anions from the solution.
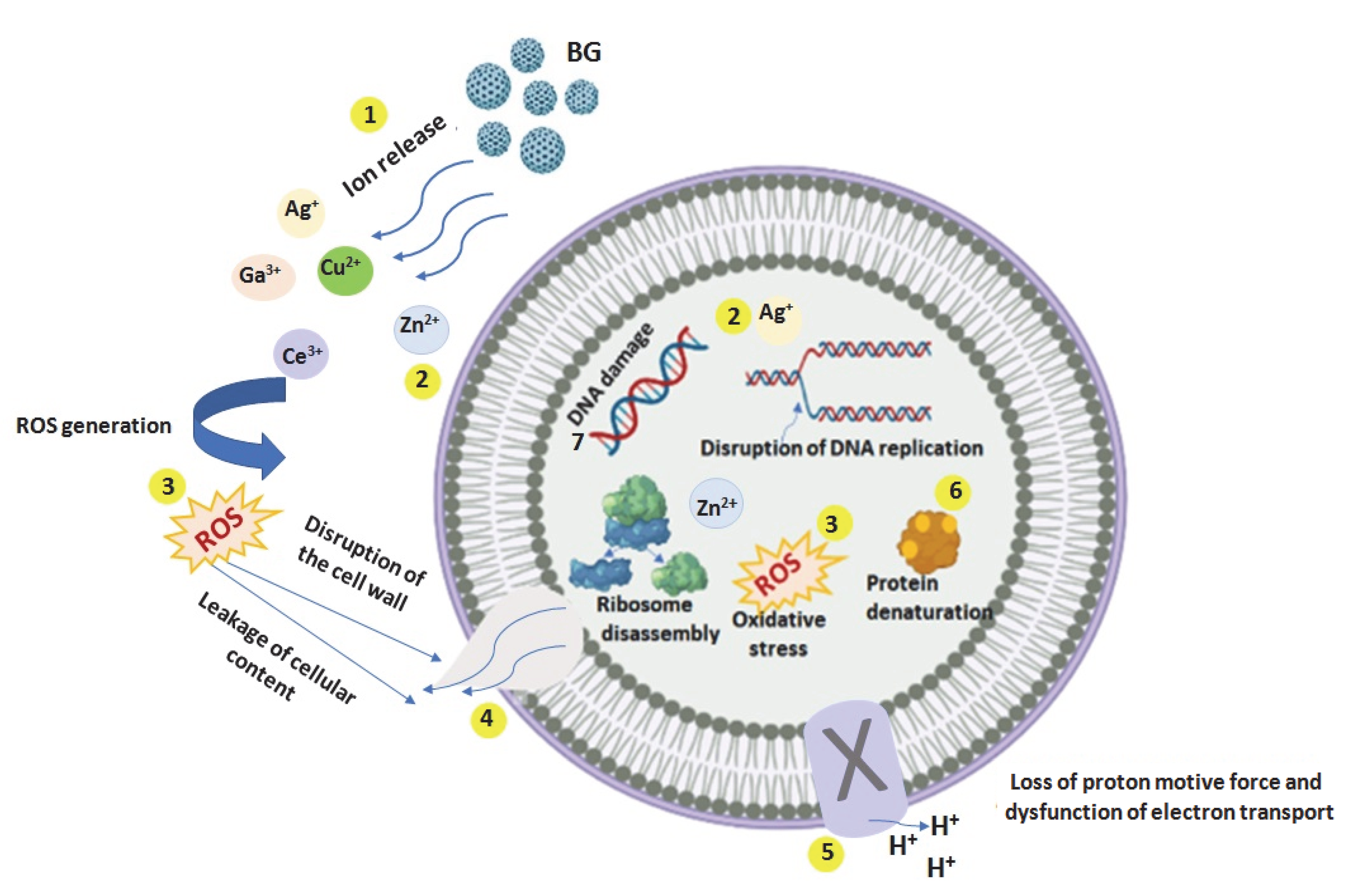
3. Mechanism of Antibacterial Action of Metal Ions
- (1)
- Release of the metal ions from the BGs;
- (2)
- Direct interaction of the metal ions with the cell wall through electrostatic interactions, compromising the membrane function and hindering nutrient assimilation;
- (3)
- Reactive oxygen species (ROS) generation, extracellular and intracellular, and oxidative stress cause damage to the proteins and DNA. Oxidative stress determined by ROS is crucial in the antibacterial effect of metal ions;
- (4)
- The high level of metal ions attached to the cell membranes and the high ROS levels can generate the disruption of the cellular wall, and hence the leaking of the cellular content;
- (5)
- A high level of ROS induces loss of the proton motive force and dysfunction of electron transport;
- (6)
- Depending on metal ions uptake, these can interfere with both proteins and DNA, destruction their function, and interrupt cellular metabolism, besides the metal ions mediated ROS production [70]. The production of ROS, due to the incomplete reduction of oxygen molecules, is often reported in bacterial cells treated with metal ions. ROS are oxygen-containing derivatives composed of highly unstable oxygen radicals, such as superoxide (O2−), hydroxyl (OH−), hydrogen peroxide (H2O2), and singlet oxygen (O2) [70]. When the ratio of the generated ROS to antioxidant defenses is perturbed, the ROS concentration continuously increases and causes damage to bacterial proteins and DNA, accumulating oxidative stress and leading to a change in their functionality and the death of the bacteria [70].
4. Metal Ions Incorporated Bioactive Glasses with Antibiofilm Efficiency
5. Conclusions and Future Perspectives
Funding
Conflicts of Interest
References
- Sahli, C.; Moya, S.E.; Lomas, J.S.; Gravier-Pelletier, C.; Briandet, R.; Hémadi, M. Recent advances in nanotechnology for eradicating bacterial biofilm. Theranostics 2022, 12, 2383–2405. [Google Scholar] [CrossRef] [PubMed]
- WHO. Résistance aux Antimicrobiens: Les Enjeux de la Réuniondes Nations Unies. Available online: http://www.who.int/bulletin/volumes/94/9/16-020916/fr/ (accessed on 2 August 2022).
- Ministère des Solidarités et de la Santé. L’antibiorésistance: Pourquoi est-ce si grave? Available online: https://solidaritessante.gouv.fr/prevention-en-sante/les-antibiotiques-des-medicaments-essent (accessed on 2 August 2022).
- Muhammad, M.H.; Idris, A.L.; Fan, X.; Guo, Y.; Yu, Y.; Jin, X.; Qiu, J.; Guan, X.; Huang, T. Beyond Risk: Bacterial Biofilms and Their Regulating Approaches. Front. Microbiol. 2020, 11, 928. [Google Scholar] [CrossRef] [PubMed]
- Drago, L.; Vassena, C.; Fenu, S.; De Vecchi, E.; Signori, V.; De Francesco, R.; Romanò, C.L. In vitro antibiofilm activity of bioactive glass S53P4. Future Microbiol. 2014, 9, 593–601. [Google Scholar] [CrossRef] [PubMed]
- Drago, L.; Toscano, M.; Bottagisio, M. Recent Evidence on Bioactive Glass Antimicrobial and Antibiofilm Activity: A Mini-Review. Materials 2018, 11, 326. [Google Scholar] [CrossRef] [PubMed]
- Del Pozo, J.L.; Patel, R. The Challenge of Treating Biofilm-associated Bacterial Infections. Trans. Med. 2007, 82, 204–209. [Google Scholar] [CrossRef] [PubMed]
- Staneva, A.D.; Dimitrov, D.K.; Gospodinova, D.N.; Vladkova, T.G. Antibiofouling Activity of Graphene Materials and Graphene-Based Antimicrobial Coatings. Microorganisms 2021, 9, 1839. [Google Scholar] [CrossRef] [PubMed]
- Vladkova, T.; Akuzov, D.; Klöppel, A.; Brümmer, F. Current Approaches to Reduction Marine Biofilm Formation. J. Chem. Technol. Metall. 2014, 49, 345–355. [Google Scholar]
- Khatoon, Z.; McTiernan, C.D.; Suuronen, E.J.; Mah, T.-F.; Alarcon, E.I. Bacterial biofilm formation on implantable devices and approaches to its treatment and prevention. Heliyon 2018, 4, e01067. [Google Scholar] [CrossRef]
- Hall-Stoodley, L.; Stoodley, P. Evolving concepts in biofilm infections. Cell. Microbiol. 2009, 11, 1034–1043. [Google Scholar] [CrossRef]
- De Oliveira, D.M.P.; Forde, B.M.; Kidd, T.J.; Harris, P.N.A.; Schembri, M.A.; Beatson, S.A.; Paterson, D.L.; Walker, M.J. Antimicrobial resistance in ESKAPE pathogens. Clin. Microbiol. Rev. 2020, 33, e00181-19. [Google Scholar] [CrossRef]
- Chen, M.; Yu, Q.; Sun, H. Novel strategies for the prevention and treatment of biofilm related infections. Int. J. Mol. Sci. 2013, 14, 18488–18501. [Google Scholar] [CrossRef] [PubMed]
- Otto, M. Staphylococcal Biofilms. Curr. Top Microbiol. Immunol. 2008, 322, 207–228. [Google Scholar] [PubMed]
- Gottenbos, B.; Grijpma, D.W.; van der Mei, H.C.; Feijen, J.; Busscher, H.J. Antimicrobial effects of positively charged surfaces on adhering Gram-positive and Gram-negative bacteria. J. Antimicrob. Chemother. 2001, 48, 7–13. [Google Scholar] [CrossRef] [PubMed]
- Chang, C.Y. Surface sensing for biofilm formation in Pseudomonas aeruginosa. Front. Microbiol. 2017, 8, 2671. [Google Scholar] [CrossRef] [PubMed]
- Erriu, M.; Blus, C.; Szmukler-Moncler, S.; Buogo, S.; Levi, R.; Barbato, G.; Madonnaripa, D.; Denotti, G.; Piras, V.; Orru, G. Microbial biofilm modulation by ultrasound: Current concepts and controversies. Ultrason. Sonochem. 2014, 21, 15–22. [Google Scholar] [CrossRef]
- Li, J.; Nickel, R.; Wu, J.; Lin, F.; van Lierop, J.; Liu, S. A new tool to attack biofilms: Driving magnetic iron-oxide nanoparticles to disrupt the matrix. Nanoscale 2019, 11, 6905–6915. [Google Scholar] [CrossRef]
- Yin, W.; Xu, S.; Wang, Y.; Zhang, Y.; Chou, S.H.; Galperin, M.Y.; He, J. Ways to control harmful biofilms: Prevention, inhibition, and eradication. Crit. Rev. Microbiol. 2021, 47, 57–78. [Google Scholar] [CrossRef]
- Llama-Palacios, A.; Sánchez, M.C.; Díaz, L.A.; Cabal, B.; Suárez, M.; Moya, J.S.; Torrecillas, R.; Figuero, E.; Sanz, M.; Herrera, D. In vitro biofilm formation on different ceramic biomaterial surfaces: Coating with two bactericidal glasses. Dent. Mater. 2019, 35, 883–892. [Google Scholar] [CrossRef]
- Dominguez, E.G.; Zarnowski, R.; Choy, H.L.; Zhao, M.; Sanchez, H.; Nett, J.E.; Andes, D.R. Conserved role for biofilm matrix polysaccharides in Candida auris drug resistance. mSphere 2019, 4, e00680. [Google Scholar] [CrossRef]
- Nandi, S.K.; Bandyopadhyay, S.; Das, P.; Samanta, I.; Mukherjee, P.; Roy, S.; Kundu, B. Understanding osteomyelitis and its treatment through local drug delivery system. Biotechnol. Adv. 2016, 34, 1305–1317. [Google Scholar] [CrossRef]
- Kim, J.Y.; Choi, W.; Mangal, U.; Seo, J.Y.; Kang, T.Y.; Lee, J.; Kim, T.; Cha, J.Y.; Kim, K.-M.; Kim, D.; et al. Multivalent network modifier upregulates bioactivity of multispecies biofilm-resistant polyalkenoate cement. Bioact. Mater. 2022, 14, 219–233. [Google Scholar] [CrossRef] [PubMed]
- Passos, T.F.; Souza, M.T.; Zanotto, E.D.; de Souza, C.W.O. Bactericidal activity and biofilm inhibition of F18 bioactive glass against Staphylococcus aureus. Mater. Sci. Eng. C 2021, 118, 111475. [Google Scholar] [CrossRef] [PubMed]
- Cunha, M.T.; Murça, M.A.; Nigro, S.; Klautau, G.B.; Salles, M.J.C. In vitro antibacterial activity of bioactive glass S53P4 on multiresistant pathogens causing osteomyelitis and prosthetic joint infection. BMC Infect. Dis. 2018, 18, 157. [Google Scholar] [CrossRef] [PubMed]
- Singh, B.N.; Pandey, G.P.; Jadaun, V.; Singh, S.; Bajpai, R.; Nayaka, S.; Naqvi, A.H.; Rawat, A.K.S.; Upreti, D.K.; Singh, B.R. Development and characterization of a novel swarna-based herbo-metallic colloidal nano-formulation– inhibitor of streptococcus mutans quorum sensing. RSC Adv. 2015, 5, 5809–5822. [Google Scholar] [CrossRef]
- Chen, Q.Z.; Li, Y.; Jin, L.Y.; Quinn, J.M.W.; Komesaroff, P.A. A new sol-gel process for producing Na2O-containing bioactive glass ceramics. Acta Biomater. 2010, 6, 4143–4153. [Google Scholar] [CrossRef]
- Hench, L.L.; Splinter, R.J.; Allen, W.C.; Greenlee, T.K. Bonding mechanisms at the interface of ceramic prosthetic materials. J. Biomed. Mater. Res. 1971, 5, 117–141. [Google Scholar] [CrossRef]
- Hench, L.L. Bioceramics. J. Am. Ceram. Soc. 1998, 81, 1705–1728. [Google Scholar] [CrossRef]
- Rainer, A.; Giannitelli, S.M.; Abbruzzese, F.; Traversa, E.; Licoccia, S.; Trombetta, M. Fabrication of bioactive glass–ceramic foams mimicking human bone portions for regenerative medicine. Acta Biomater. 2008, 4, 362–369. [Google Scholar] [CrossRef]
- Ma, J.; Chen, C.Z.; Wang, D.G.; Meng, X.G.; Shi, J.Z. Influence of the sintering temperature on the structural feature and bioactivity of sol–gel derived SiO2–CaO–P2O5 bioglass. Ceram. Int. 2010, 36, 1911–1916. [Google Scholar] [CrossRef]
- Xynos, I.D.; Edgar, A.J.; Buttery, L.D.K.; Hench, L.L.; Polak, J.M. Ionic products of bioactive glass dissolution increase proliferation of human osteoblasts and induce insulin-like growth factor II mRNA expression and protein synthesis. Biochem. Biophys. Res. Commun. 2000, 276, 461–465. [Google Scholar] [CrossRef]
- Boccaccini, A.R.; Erol, M.; Stark, W.J.; Mohn, D.; Hong, Z.; Mano, J.F. Polymer/bioactive glass nanocomposites for biomedical applications. A Review. Compos. Sci. Technol. 2010, 70, 1764–1776. [Google Scholar] [CrossRef]
- Li, R.; Clark, A.E.; Hench, L.L. An investigation of bioactive glass powders by sol-gel processing. J. Appl. Biomater. 1991, 2, 231–239. [Google Scholar] [CrossRef]
- Baino, F.; Fiorilli, S.; Vitale-Brovarone, C. Bioactive glass-based materials with hierarchical porosity for medical applications: Review of recent advances. Acta Biomater. 2016, 42, 16–32. [Google Scholar] [CrossRef]
- Zheng, K.; Boccaccini, A.R. Sol-gel processing of bioactive glass nanoparticles: A review. Adv. Colloid Interface Sci. 2017, 249, 363–373. [Google Scholar] [CrossRef] [PubMed]
- Yan, X.; Yu, C.; Zhou, X.; Tang, J.; Zhao, D. Highly ordered mesoporous bioactive glasses with superior in vitro bone-forming bioactivities. Angew. Chem. Int. Ed. 2004, 43, 5980–5984. [Google Scholar] [CrossRef] [PubMed]
- Ben-Nissan, B.; Choi, A.H.; Macha, I.J.; Cazalbou, S. Sol-Gel Nanocoatings of Bioceramics. In Handbook of Bioceramics and Biocomposites; Antoniac, I.V., Ed.; Springer: Cham, Switzerland, 2015. [Google Scholar]
- Wu, C.J.; Chang, J. Mesoporous bioactive glasses: Structure characteristics, drug/growth factor delivery and bone regeneration application. Interface Focus 2012, 2, 292–306. [Google Scholar] [CrossRef] [PubMed]
- Wu, C.; Chang, J. Multifunctional mesoporous bioactive glasses for effective delivery of therapeutic ions and drug/growth factors. J. Control. Release 2014, 193, 282–295. [Google Scholar] [CrossRef]
- Wu, C.; Chang, J.; Xiao, Y. Mesoporous bioactive glasses as drug delivery and bone tissue regeneration platforms. Ther. Deliv. 2011, 2, 1189–1198. [Google Scholar] [CrossRef]
- Lucas-Girot, A.; Mezahi, F.Z.; Mami, M.; Oudadesse, H.; Harabi, A.; Le Floch, M. Sol–gel synthesis of a new composition of bioactive glass in the quaternary system SiO2–CaO–Na2O–P2O5. Comparison with melting method. J. Non-Cryst. Solids 2011, 357, 3322–3327. [Google Scholar] [CrossRef]
- Zhou, P.; Garcia, B.L.; Kotsakis, G.A. Comparison of antibacterial and antibiofilm activity of bioactive glass compounds S53P4 and 45S5. BMC Microbiol. 2022, 22, 212. [Google Scholar] [CrossRef]
- Bortolin, M.; De Vecchi, E.; Romano, C.L.; Toscano, M.; Mattina, R.; Drago, L. Antibiofilm agents against MDR bacterial strains: Is bioactive glass BAG-S53P4 also effective? J. Antimicrob. Chemother. 2016, 71, 123–127. [Google Scholar] [CrossRef]
- Coraca-Huber, D.C.; Fille, M.; Hausdorfer, J.; Putzer, D.; Nogler, M. Efficacy of antibacterial bioactive glass S53P4 against S. aureus biofilms grown on titanium discs in vitro. J. Orthop. Res. 2014, 32, 175–177. [Google Scholar] [CrossRef] [PubMed]
- Oliver, J.N.; Akande, O.; Ecker, M. Incorporation of Novel Elements in Bioactive Glass Compositions to Enhance Implant Performance. Book chapter in Current Concepts in Dental Implantology—From Science to Clinical Research; IntechOpen: London, UK, 2022; ISBN 978-1-83969-864-4. [Google Scholar]
- Valappil, S.P.; Higham, S.M. Antibacterial effect of gallium and silver on Pseudomonas aeruginosa treated with gallium–silver–phosphate-based glasses. Bio-Med. Mater. Eng. 2014, 24, 1589–1594. [Google Scholar] [CrossRef]
- Esfahanizadeh, N.; Nourani, M.R.; Bahador, A.; Akhondi, N.; Montazeri, M. The Anti-biofilm Activity of Nanometric Zinc doped Bioactive Glass against Putative Periodontal Pathogens: An in vitro Study. Biomed. Glasses. 2018, 4, 95–107. [Google Scholar] [CrossRef]
- Paramita, P.; Ramachandran, M.; Narashiman, S.; Nagarajan, S.; Sukumar, D.K.; Chung, T.W.; Ambigapathi, M. Sol–gel based synthesis and biological properties of zinc integrated nanobioglass ceramics for bone tissue regeneration. J. Mater. Sci. Mater. Med. 2021, 32, 5. [Google Scholar] [CrossRef] [PubMed]
- Neel, E.A.A.; Hossain, K.M.Z.; Abuelenain, D.; Abuhaimed, T.; Ahmed, I.; Valappil, S.P.; Knowles, J.C. Antibacterial effect of titanium dioxide-doped phosphate glass microspheres filled total-etch dental adhesive on S. mutans biofilm. Int. J. Adhes. Adhes. 2021, 108, 102886. [Google Scholar]
- Wilkinson, H.N.; Iveson, S.; Catherall, P.; Hardman, M.J. A Novel Silver Bioactive Glass Elicits Antimicrobial Efficacy Against Pseudomonas aeruginosa and Staphylococcus aureus in an ex Vivo Skin Wound Biofilm Model. Front. Microbiol. 2018, 9, 1450. [Google Scholar] [CrossRef]
- Naseri, S.; Griffanti, G.; Lepry, W.C.; Maisuria, V.B.; Tufenkji, N.; Nazhat, S.N. Silver-doped sol-gel borate glasses: Dose-dependenteffect on Pseudomonas aeruginosa biofilms and keratinocyte function. J. Am. Ceram. Soc. 2021, 105, 1711–1722. [Google Scholar] [CrossRef]
- Bari, A.; Bloise, N.; Fiorilli, S.; Novajra, G.; Vallet-Regí, M.; Bruni, G.; Torres-Pardo, A.; González-Calbet, J.M.; Visai, L.; Vitale-Brovarone, C. Copper-containing mesoporous bioactive glass nanoparticles as multifunctional agent for bone regeneration. Acta Biomater. 2017, 55, 493–504. [Google Scholar] [CrossRef]
- Miola, M.; Massera, J.; Cochis, A.; Kumar, A.; Rimondini, L.; Verne, E. Tellurium: A new active element for innovative multifunctional bioactive glasses. Mater. Sci. Eng. C 2021, 123, 111957. [Google Scholar] [CrossRef]
- Rivadeneira, J.; Gorustovich, A. Bioactive glasses as delivery systems for antimicrobial agents. J. Appl. Microbiol. 2017, 122, 1424–1437. [Google Scholar] [CrossRef] [PubMed]
- Jacobs, A.; Renaudin, G.; Forestier, C.; Nedelec, J.M.; Descamps, S. Biological properties of copper-doped biomaterials for orthopedic applications: A review of antibacterial, angiogenic and osteogenic aspects. Acta Biomater. 2020, 117, 21–39. [Google Scholar] [CrossRef]
- Baino, F. Copper-Doped Ordered Mesoporous Bioactive Glass: A Promising Multifunctional Platform for Bone Tissue Engineering. Bioengineering 2020, 7, 45. [Google Scholar] [CrossRef] [PubMed]
- Atkinson, I.; Anghel, E.M.; Predoana, L.; Mocioiu, O.C.; Jecu, L.; Raut, I.; Munteanu, C.; Culita, D.; Zaharescu, M. Influence of ZnO addition on the structural, in vitro behavior and antimicrobial activity of sol–gel derived CaO–P2O5–SiO2 bioactive glasses. Ceram. Int. 2016, 42, 3033–3045. [Google Scholar] [CrossRef]
- Sánchez-Salcedo, S.; Shruti, S.; Salinas, A.J.; Malavasi, G.; Menabue, L.; Vallet Regí, M. In vitro antibacterial capacity and cytocompatibility of SiO2–CaO–P2O5 meso-macroporous glass scaffolds enriched with ZnO. J. Mater. Chem. B 2014, 2, 4836–4847. [Google Scholar] [CrossRef] [PubMed]
- Kurtuldu, F.; Mutlu, N.; Michalek, M.; Zheng, K.; Masar, M.; Liverani, L.; Chen, S.; Galusek, D.; Boccaccini, A.R. Cerium and gallium containing mesoporous bioactive glass nanoparticles for bone regeneration: Bioactivity, biocompatibility and antibacterial activity. Mater. Sci. Eng. C 2021, 124, 112050. [Google Scholar] [CrossRef]
- Kaya, S.; Cresswell, M.; Boccaccini, A.R. Mesoporous silica-based bioactive glasses for antibiotic-free antibacterial applications. Mater. Sci. Eng. C 2018, 83, 99–107. [Google Scholar] [CrossRef]
- Atkinson, I.; Anghel, E.M.; Petrescu, S.; Seciu, A.M.; Stefan, L.M.; Mocioiu, O.C.; Predoana, L.; Voicescu, M.; Somacescu, S.; Culita, D.; et al. Cerium-containing mesoporous bioactive glasses: Material characterization, in vitro bioactivity, biocompatibility and cytotoxicity evaluation. Microporous Mesoporous Mater. 2019, 276, 76–88. [Google Scholar] [CrossRef]
- Atkinson, I.; Seciu-Grama, A.M.; Petrescu, S.; Culita, D.; Mocioiu, O.C.; Voicescu, M.; Mitran, R.A.; Lincu, D.; Prelipceanu, A.M.; Craciunescu, O. Cerium-Containing Mesoporous Bioactive Glasses (MBGs)-Derived Scaffolds with Drug Delivery Capability for Potential Tissue Engineering Applications. Pharmaceutics 2022, 14, 1169. [Google Scholar] [CrossRef]
- Akhtach, S.; Tabia, Z.; Bricha, M.; Mabrouk, K.E. Structural characterization, in vitro bioactivity, and antibacterial evaluation of low silver-doped bioactive glasses. Ceram. Int. 2021, 47, 29036–29046. [Google Scholar] [CrossRef]
- Bouhazma, S.; Herradi, S.; Adouar, I.; Sadiki, M.; Elabed, S.; Koraichi, S.I.; El Bali, B.; Lachkar, M. A new composition silver doped-bioglass: Synthesis, in vitro bioactivity and antibacterial study and comparison with the binary SiO2-CaO and ternary SiO2-CaO-P2O5 systems. Mater. Today Proc. 2022, 53, 345–350. [Google Scholar] [CrossRef]
- Vale, A.C.; Pereira, P.R.; Barbosa, A.M.; Torrado, E.; Alves, N.M. Optimization of silver-containing bioglassnanoparticles envisaging biomedical applications. Mater. Sci. Eng. C 2019, 94, 161–168. [Google Scholar] [CrossRef] [PubMed]
- Mariappan, C.R.; Ranga, N. Influence of silver on the structure, dielectric and antibacterial effect of silver doped bioglass-ceramic nanoparticles. Ceram. Int. 2017, 43, 2196–2201. [Google Scholar] [CrossRef]
- Moghaniana, A.; Sedghic, A.; Ghorbanoghlid, A.; Salarie, E. The effect of magnesium content on in vitro bioactivity, biological behavior and antibacterial activity of sol–gel derived 58S bioactive glass. Ceram. Int. 2018, 44, 9422–9432. [Google Scholar] [CrossRef]
- Flemming, H.C.; Baveye, P.; Neu, T.R.; Stoodley, P.; Szewzyk, U.; Wingender, J.; Wuertz, S. Who put the film in biofilm? The migration of a term from wastewater engineering to medicine and beyond. Npj Biofilms Microbiomes 2021, 7, 10. [Google Scholar] [PubMed]
- Nakai, K.; Tsuruta, D. What Are Reactive Oxygen Species, Free Radicals, and Oxidative Stress in Skin Diseases? Int. J. Mol. Sci. 2021, 22, 10799. [Google Scholar] [CrossRef]
- Mustila, H.; Allahverdiyeva, Y.; Isojarvi, J.; Aro, E.M.; Eisenhut, M. The bacterial-type [4Fe-4S] ferredoxin 7 has a regulatory function under photooxidative stress conditions in the Cyanobacterium Synechocystis sp. PCC 6803. Biochim. Biophys. Acta Bioenerg. 2014, 1837, 1293–1304. [Google Scholar] [CrossRef]
- Harrison, J.J.; Tremaroli, V.; Stan, M.A.; Chan, C.S.; Vacchi-Suzzi, C.; Heyne, B.J.; Parsek, M.R.; Ceri, H.; Turner, R.J. Chromosomal antioxidant genes have metal ion-specific roles as determinants of bacterial metal tolerance. Environ. Microbiol. 2009, 11, 2491–2509. [Google Scholar] [CrossRef]
- Shaligram, S.; Campbell, A. Toxicity of copper salts is dependent on solubility profile and cell type tested. Toxicol. In Vitro 2013, 27, 844–851. [Google Scholar] [CrossRef]
- Wu, C.; Labrie, J.; Tremblay, Y.; Haine, D.; Mourez, M.; Jacques, M. Zinc as an agent for the prevention of biofilm formation by pathogenic bacteria. J. Appl. Microbiol. 2013, 115, 30–40. [Google Scholar] [CrossRef]
- Hutchings, C.; Rajasekharan, S.K.; Reifen, R.; Shemesh, M. Mitigating Milk-Associated Bacteria through Inducing Zinc Ions Antibiofilm Activity. Foods 2020, 9, 1094. [Google Scholar] [CrossRef]
- Shruti, S.; Salinas, A.J.; Ferrari, E.; Malavasi, G.; Lusvardi, G.; Doadrio, A.L.; Menabue, L.; Vallet-Regi, M. Curcumin release from cerium, gallium and zinc containing mesoporous bioactive glasses. Microporous Mesoporous Mater. 2013, 180, 92–101. [Google Scholar] [CrossRef]
- Heras, C.; Holguín, J.J.; Doadrio, A.L.; Vallet-Regí, M.; Sánchez-Salcedo, S.; Salinas, A.J. Multifunctional antibiotic and zinc containing mesoporous bioactive glass scaffolds to fight against bone infection. Acta Biomater. 2020, 114, 395–406. [Google Scholar] [CrossRef] [PubMed]
- Ruparelia, J.P.; Chatterjee, A.K.; Duttagupta, S.P.; Mukherji, S. Strain specificity in antimicrobial activity of silver and copper nanoparticles. Acta Biomater. 2008, 4, 707–716. [Google Scholar] [CrossRef] [PubMed]
- Kazemian, Z.; Varzandeh, M.; Labbaf, S. A facile synthesis of mono dispersed spherical silver doped bioactive glass nanoparticle. J. Mater. Sci. Mater. Med. 2021, 32, 29. [Google Scholar] [CrossRef] [PubMed]
- Palza, H.; Escobar, B.; Bejarano, J.; Bravo, D.; Diaz-Dosque, M.; Perez, J. Designing antimicrobial bioactive glass materials with embedded metal ions synthesized by the sol-gel method. J. Mater. Sci. Eng. C 2013, 33, 3795–3801. [Google Scholar] [CrossRef]
- Mekkawy, A.I.; El-Mokhtar, M.A.; Nafady, N.A.; Yousef, N.; Hamad, M.A.; El-Shanawany, S.M. In vitro and in vivo evaluation of biologically synthesized silver nanoparticles for topical applications: Effect of surface coating and loading into hydrogels. Int. J. Nanomed. 2017, 12, 759–777. [Google Scholar] [CrossRef]
- Da Silva Buriti, J.; Barreto, M.E.V.; Barbosa, F.C.; de Brito Buriti, B.M.A.; de Lima Souza, J.W.; de Vasconcelos Pina, H.; de Luna Rodrigues, P.; Fook, M.V.L. Synthesis and characterization of Ag-doped 45S5 bioglass and chitosan/45S5-Ag biocomposites for biomedical applications. J. Therm. Anal. Calorim. 2020, 145, 39–50. [Google Scholar] [CrossRef]
- Bellantone, M.; Coleman, N.J.; Hench, L.L. Bacteriostatic action of a novel four-component bioactive glass. J. Biomed. Mater. Res. Part A 2000, 51, 484–490. [Google Scholar] [CrossRef]
- Baino, F.; Hamzehlou, S.; Kargozar, S. Bioactive Glasses: Where Are We and Where Are We Going? J. Funct. Biomater. 2018, 9, 25. [Google Scholar] [CrossRef]
- Hoppe, A.; Mourino, V.; Boccaccini, A.R. Therapeutic inorganic ions in bioactive glasses to enhance bone formation and beyond. Biomater. Sci. 2013, 1, 254–256. [Google Scholar] [CrossRef] [PubMed]
- El-Kady, A.M.; Ali, A.F.; Rizk, R.A.; Ahmed, M.M. Synthesis, characterization and microbiological response of silver doped bioactive glass nanoparticles. Ceram. Int. 2012, 38, 177–188. [Google Scholar] [CrossRef]
- Fan, W.; Wu, D.; Ma, T.; Fan, B. Ag-loaded mesoporous bioactive glasses against Enterococcus faecalis biofilm in root canal of human teeth. Dent. Mater. J. 2015, 34, 54–60. [Google Scholar] [CrossRef] [PubMed]
- Gomes, S.; Vichery, C.; Descamps, S.; Martinez, H.; Kaur, A.; Jacobs, A.; Nedelec, J.M.; Renaudin, G. Cu-doping of calcium phosphate bioceramics: From mechanism to the control of cytotoxicity. Acta Biomater. 2018, 65, 462–474. [Google Scholar] [CrossRef] [PubMed]
- Wu, C.; Zhou, Y.; Xu, M.; Han, P.; Chen, L.; Chang, J.; Xiao, Y. Copper-containing mesoporous bioactive glass scaffolds with multifunctional properties of angiogenesis capacity, osteostimulation and antibacterial activity. Biomaterials 2013, 34, 422–433. [Google Scholar] [CrossRef]
- Zambanini, T.; Borges, R.; Kai, K.C.; Marchi, J. Bioactive Glasses for Treatment of Bone Infections. Book chapter in Biomedical, Therapeutic and Clinical Applications of Bioactive Glasses; Woodhead Publishing Series in Biomaterials; Woodhead Publishing: Cambridge, UK, 2019; pp. 383–415. [Google Scholar]
- Copper Development Associtaion Inc. U.S. EPA Approves Registration of Antimicrobial Copper Alloys. Available online: https://www.copper.org/about/pressreleases/2008 (accessed on 16 August 2022).
- Jiménez-Holguín, J.; Sánchez-Salcedo, S.; Cicuéndez, M.; Vallet-Regí, M.; Salinas, A.J. Cu-Doped Hollow Bioactive Glass Nanoparticles for Bone Infection Treatment. Pharmaceutics 2022, 14, 845. [Google Scholar] [CrossRef]
- Sbarra, M.S.; Arciola, C.R.; Di Poto, A.; Saino, E.; Rohde, H.; Speziale, P.; Visai, L. The photodynamic effect of tetra-substituted N-methyl-pyridyl-porphine combined with the action of vancomycin or host defense mechanisms disrupts Staphylococcus epidermidis biofilms. Int. J. Artif. Organs 2009, 32, 574–583. [Google Scholar] [CrossRef]
- Villanueva, M.E.; Diez, A.M.; Gonzàlez, J.A.; Pérez, C.J.; Orrego, M.; Piehl, L.; Teves, S.; Copello, G.J. Antimicrobial activity of starch hydrogel incorporated with copper nanoparticles. ACS Appl. Mater. Interfaces 2016, 8, 16280–16288. [Google Scholar] [CrossRef]
- Raffi, M.; Mehrwan, S.; Bhatti, T.M.; Akhter, J.I.; Hameed, A.; Yawar, W.; Masood Hasan, M. Investigations into the antibacterial behavior of copper nanoparticles against Escherichia coli. Ann. Microbiol. 2010, 60, 75–80. [Google Scholar] [CrossRef]
- Chitambar, C.R.; Narasimhan, J. Targeting iron-dependent DNA synthesis with gallium and transferrin-gallium. Pathobiology 1991, 59, 3–10. [Google Scholar] [CrossRef]
- Hedley, D.W.; Tripp, E.H.; Slowiaczek, P.; Mann, G.J. Effect of gallium on DNA synthesis by human T-cell lymphoblasts. Cancer Res. 1988, 48, 3014–3018. [Google Scholar] [PubMed]
- Kaneko, Y.; Thoendel, M.; Olakanmi, O.; Britigan, B.E.; Singh, P.K. The transition metal gallium disrupts Pseudomonas aeruginosa iron metabolism and has antimicrobial and antibiofilm activity. J. Clin. Investig. 2007, 117, 877–888. [Google Scholar] [CrossRef] [PubMed]
- Mutlu, N.; Kurtuldu, F.; Unalan, I.; Neščáková, Z.; Kaňková, H.; Galusková, D.; Michálek, M.; Liverani, L.; Galusek, D.; Boccaccini, A.R. Effect of Zn and Ga doping on bioactivity, degradation, and antibacterial properties of borate 1393-B3 bioactive glass. Ceram. Int. 2022, 48, 16404–16417. [Google Scholar] [CrossRef]
- Rahimnejad Yazdi, A.; Torkan, L.; Waldman, S.D.D.; Towler, M.R.R. Development of a novel bioactive glass suitable for osteosarcoma-related bone grafts. J. Biomed. Mater. Res. B Appl. Biomater. 2018, 106, 1186–1193. [Google Scholar] [CrossRef]
- Sahdev, R.; Ansari, T.I.; Higham, S.M.; Valappil, S.P. Potential use of gallium-doped phosphate-based glass material for periodontitis treatment. J. Biomater. Appl. 2015, 30, 85–92. [Google Scholar] [CrossRef]
- Yazdi, A.R.; Torkan, L.; Stone, W.; Towler, M.R.R. The Impact of Gallium Content on Degradation, Bioactivity, and Antibacterial Potency of Zinc Borate Bioactive Glass. J. Biomed. Mater. Res. 2018, 106, 367–376. [Google Scholar] [CrossRef]
- Valappil, S.P.; Coombes, M.; Wright, L.; Owens, G.J.; Lynch, R.J.M.; Hope, C.K.; Higham, S.M. Role of gallium and silver from phosphate-based glasses on in vitro dual species oral biofilm models of Porphyromonasgingivalis and Streptococcus gordonii. Acta Biomater. 2012, 8, 1957–1965. [Google Scholar] [CrossRef]
- Matharu, R.K.; Charani, Z.; Ciric, L.; Illangakoon, U.E.; Edirisinghe, M. Antimicrobial activity of tellurium-loaded polymeric fibre meshes. J. Appl. Polym. Sci. 2018, 135, 46368. [Google Scholar]
- Morena, A.G.; Bassegoda, A.; Hoyo, J.; Tzanov, T. Hybrid Tellurium–Lignin Nanoparticles with Enhanced Antibacterial Properties. ACS Appl. Mater. Interfaces 2021, 13, 14885–14893. [Google Scholar] [CrossRef]
- Zonaro, E.; Lampis, S.; Turner, R.J.; Qazi, S.J.S.; Vallini, G. Biogenic selenium and tellurium nanoparticles synthesized by environmental microbial isolates efficaciously inhibit bacterial planktonic cultures and biofilms. Front. Microb. 2015, 6, 584. [Google Scholar] [CrossRef]
- Taylor, D.E. Bacterial tellurite resistance. Trends Microbiol. 1999, 7, 111–115. [Google Scholar] [CrossRef]
- Sorrentino, R.; Cochis, A.; Azzimonti, B.; Caravaca, C.; Chevalier, J.; Kuntz, M.; Porporati, A.A.; Streicher, R.M.; Rimondini, L. Reduced bacterial adhesion on ceramics used for arthroplasty applications. J. Eur. Ceram. Soc. 2018, 38, 963–970. [Google Scholar] [CrossRef]
- Turner, R.J.; Borghese, R.; Zannoni, D. Microbial processing of tellurium as a tool in biotechnology. Biotechnol. Adv. 2012, 30, 954–963. [Google Scholar] [CrossRef]
- Borsetti, F.; Francia, F.; Turner, R.J.; Zannoni, D. The disulfide binding protein DsbB allows the transfer of oxidizing equivalents from the toxic metalloid tellurite (TeO32−) to the plasma membrane electron transport system of Rhodobactercapsulatus. J. Bacteriol. 2007, 189, 851–859. [Google Scholar] [CrossRef] [PubMed]

| Ions | BG Composition | Synthesis | Microbial Biofilm | Ref. |
|---|---|---|---|---|
| Ga3++Ag+ | 10CaO-37Na2O-45P2O5-3Ga2O3-5Ag2O (mol %) | Melting | Inhibition of P. aeruginosa biofilm | [47] |
| Zn2+ | Zn doped BG (5 mol %) | Sol–gel | Reduced biofilm formation of A. actinomycetemcomitans, P. gingivalis, and P. intermedia | [48,49] |
| 55SiO2-40CaO-5P2O5 (mol %) Zn (2.39 wt. %) | Sol–gel | Inhibition of S. aureus, P. aeruginosa, and A. aceti biofilms | ||
| Ti4+ | 40P2O5⋅16CaO⋅24MgO⋅17.5NaO⋅2.5TiO2 (mol %) | Melting | Inhibitory effect on S. mutans biofilm | [50] |
| Ag+ | 70SiO2-28CaO-2AgO (mol %) | Sol–gel | S. aureus and P. aeruginosa biofilms formation was entirely inhibited | [51,52] |
| 60B2O3–36CaO–(4–X)P2O5–(X)Ag2O x = 0.3, 0.5, 1 (mol %) | Sol–gel | Eradicated P. aeruginosa biofilm by up to 99.7%. | ||
| Cu2+ | Cu (2 mol %)-doped MBGs | Sol–gel | Disrupted the biofilm matrix of S. epidermidis | [53] |
| Te4+ | 48.6-xSiO2-16.7Na2O-34.2CaO-0.5P2O5-xTeO2 x = 1, 5 (mol %) | Melting | Ability to inhibit S. aureus and S. epidermidis biofilms formation | [54] |
Publisher’s Note: MDPI stays neutral with regard to jurisdictional claims in published maps and institutional affiliations. |
© 2022 by the author. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Atkinson, I. Antibiofilm Activity of Biocide Metal Ions Containing Bioactive Glasses (BGs): A Mini Review. Bioengineering 2022, 9, 489. https://doi.org/10.3390/bioengineering9100489
Atkinson I. Antibiofilm Activity of Biocide Metal Ions Containing Bioactive Glasses (BGs): A Mini Review. Bioengineering. 2022; 9(10):489. https://doi.org/10.3390/bioengineering9100489
Chicago/Turabian StyleAtkinson, Irina. 2022. "Antibiofilm Activity of Biocide Metal Ions Containing Bioactive Glasses (BGs): A Mini Review" Bioengineering 9, no. 10: 489. https://doi.org/10.3390/bioengineering9100489
APA StyleAtkinson, I. (2022). Antibiofilm Activity of Biocide Metal Ions Containing Bioactive Glasses (BGs): A Mini Review. Bioengineering, 9(10), 489. https://doi.org/10.3390/bioengineering9100489







