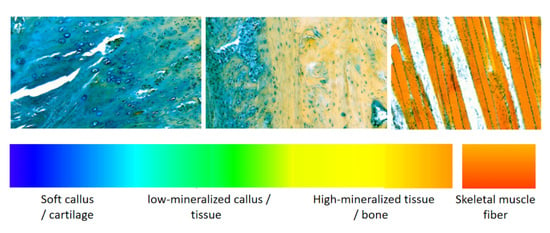
Multicolor Histochemical Staining for Identification of Mineralized and Non-Mineralized Musculoskeletal Tissue: Immunohistochemical and Radiological Validation in Decalcified Bone Samples
Abstract
1. Introduction
2. Materials and Methods
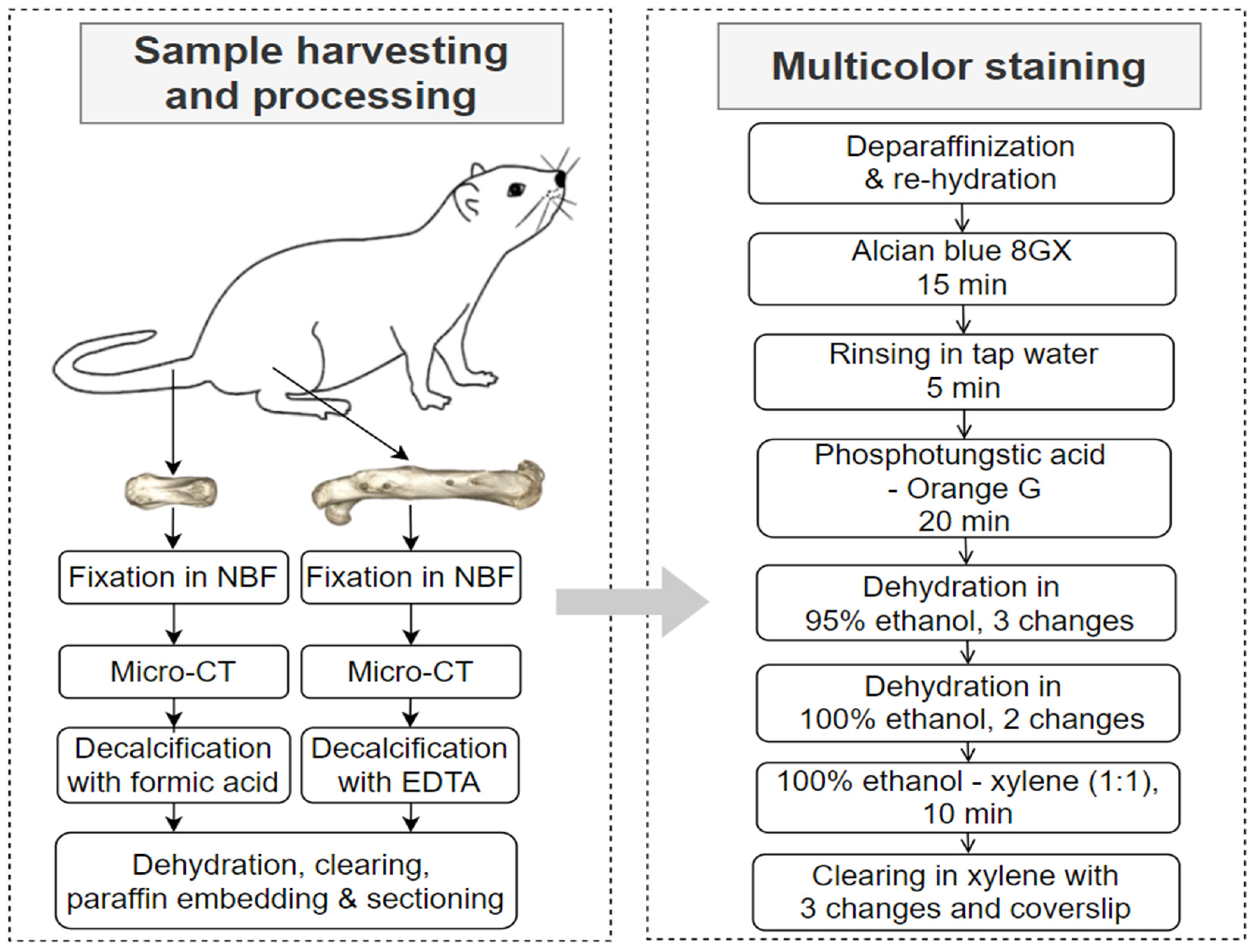
2.1. Sample Selection
2.2. Sample Processing
2.3. Histochemical and Immunohistochemical Staining
2.4. Micro-CT and Microscopy
3. Results
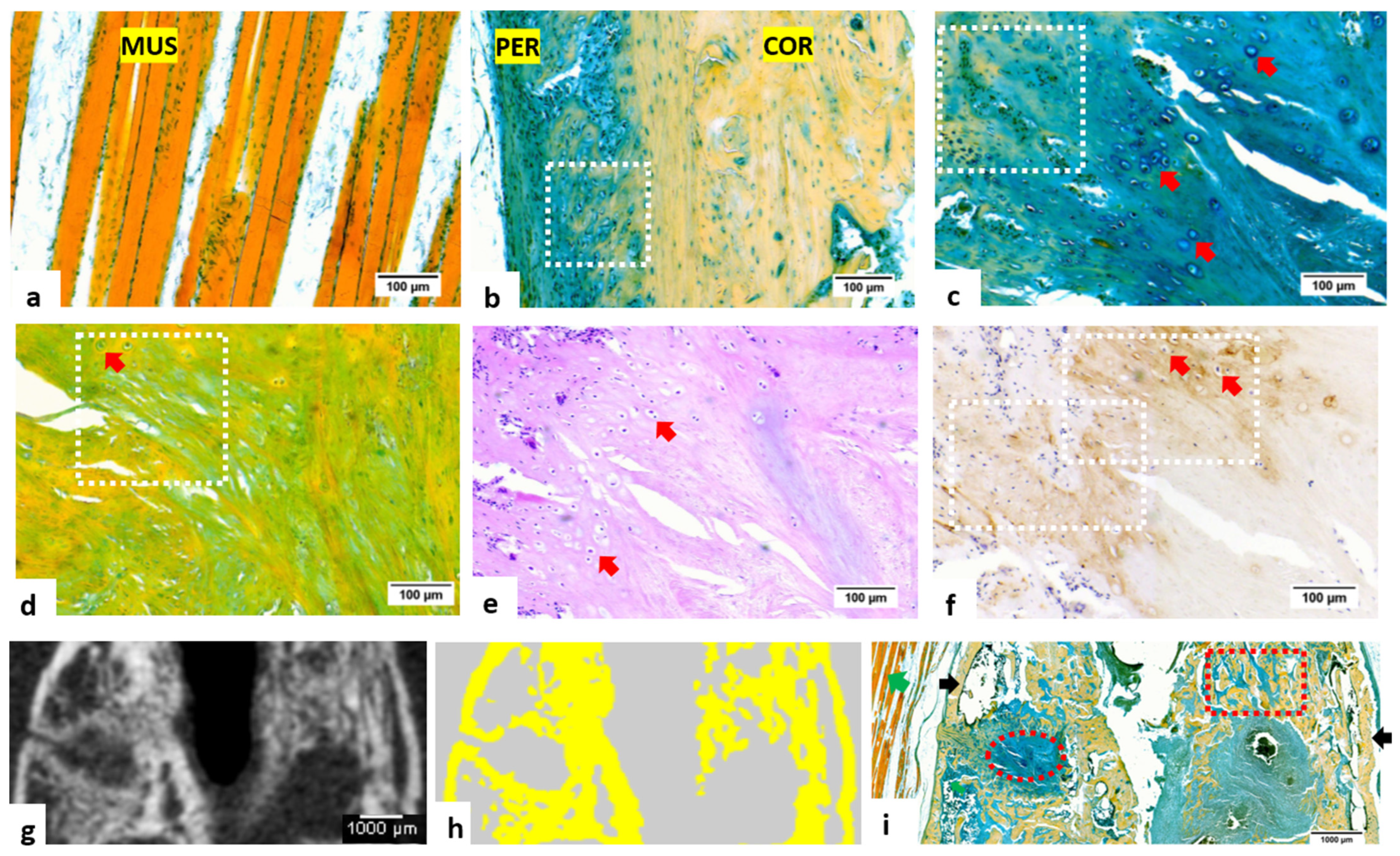
3.1. Staining and Imaging of Rat Femur Fracture Samples Decalcified with EDTA
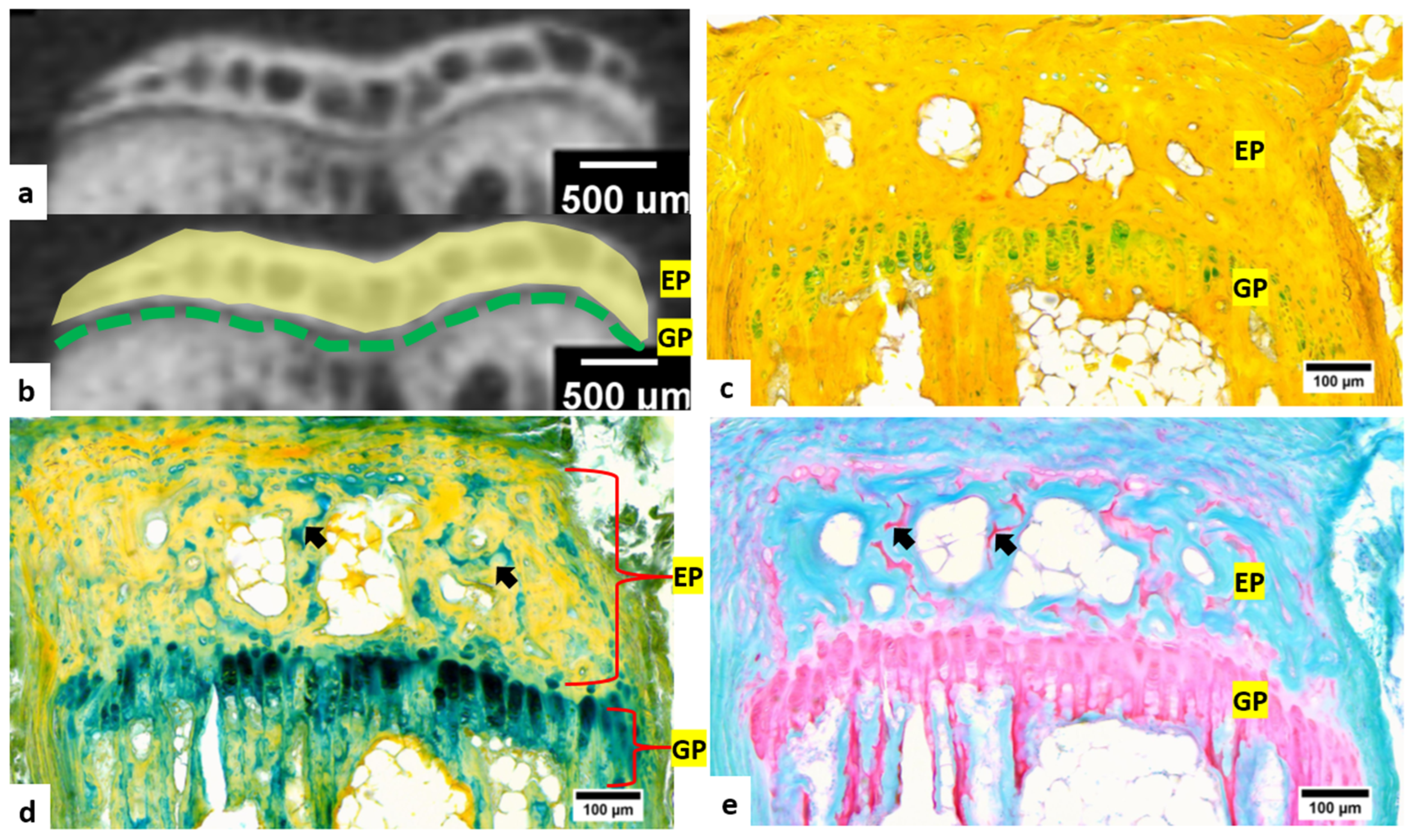
3.2. Micro-CT Imaging and Histochemical Staining of Tail Vertebra Decalcified with Formic Acid
4. Discussion
5. Conclusions
Supplementary Materials
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Data Availability Statement
Acknowledgments
Conflicts of Interest
References
- Sun, Y.; Helmholz, H.; Will, O.; Damm, T.; Wiese, B.; Luczak, M.; Peschke, E.; Luthringer-Feyerabend, B.; Ebel, T.; Hövener, J.-B.; et al. Dynamic in vivo monitoring of fracture healing process in response to magnesium implant with multimodal imaging: Pilot longitudinal study in a rat external fixation model. Biomater. Sci. 2022, 10, 1532–1543. [Google Scholar] [CrossRef] [PubMed]
- Angrisani, N.; Willumeit-Römer, R.; Windhagen, H.; Chathoth, B.M.; Scheper, V.; Wiese, B.; Helmholz, H.; Reifenrath, J. Small-sized magnesium cylinders influence subchondral bone quality in osteoarthritic rabbits—An in vivo pilot study. Eur. Cell Mater. 2021, 42, 179–195. [Google Scholar] [CrossRef] [PubMed]
- Panvini, F.M.; Pacini, S.; Mazzoni, S.; Méndez-Ferrer, S. Multicolor Immunofluorescence Staining of Paraffin-Embedded Human Bone Marrow Sections. Methods Mol. Biol. 2021, 2308, 119–126. [Google Scholar] [CrossRef]
- Bauer, M.; Vaxevanis, C.; Bethmann, D.; Massa, C.; Pazaitis, N.; Wickenhauser, C.; Seliger, B. Multiplex immunohistochemistry as a novel tool for the topographic assessment of the bone marrow stem cell niche. Methods Enzymol. 2020, 635, 67–79. [Google Scholar] [CrossRef]
- Sugita, S.; Hasegawa, T. Practical use and utility of fluorescence in situ hybridization in the pathological diagnosis of soft tissue and bone tumors. J. Orthop. Sci. 2017, 22, 601–612. [Google Scholar] [CrossRef]
- Mirando, A.J.; Hilton, M.J. Demineralized Murine Skeletal Histology. Methods Mol. Biol. 2021, 2230, 283–302. [Google Scholar] [CrossRef] [PubMed]
- Yakovleva, A.; Plieskatt, J.L.; Jensen, S.; Humeida, R.; Lang, J.; Li, G.; Bracci, P.; Silver, S.; Bethony, J.M. Fit for genomic and proteomic purposes: Sampling the fitness of nucleic acid and protein derivatives from formalin fixed paraffin embedded tissue. PLoS ONE 2017, 12, e0181756. [Google Scholar] [CrossRef]
- Groelz, D.; Viertler, C.; Pabst, D.; Dettmann, N.; Zatloukal, K. Impact of storage conditions on the quality of nucleic acids in paraffin embedded tissues. PLoS ONE 2018, 13, e0203608. [Google Scholar] [CrossRef]
- Rentsch, C.; Schneiders, W.; Manthey, S.; Rentsch, B.; Rammelt, S. Comprehensive histological evaluation of bone implants. Biomatter 2014, 4, e27993. [Google Scholar] [CrossRef]
- Schmitz, N.; Laverty, S.; Kraus, V.B.; Aigner, T. Basic methods in histopathology of joint tissues. Osteoarthr. Cartil. 2010, 18 (Suppl. S3), S113–S116. [Google Scholar] [CrossRef]
- Movat, H.Z. Demonstration of all connective tissue elements in a single section; pentachrome stains. AMA Arch. Pathol. 1955, 60, 289–295. [Google Scholar] [PubMed]
- Campo, R.D.; Betz, R.R. Loss of proteoglycans during decalcification of fresh metaphyses with disodium ethylenediaminetetraacetate (EDTA). Calcif. Tissue Int. 1987, 41, 52–55. [Google Scholar] [CrossRef] [PubMed]
- Bogoevski, K.; Woloszyk, A.; Blackwood, K.; Woodruff, M.; Glatt, V. Tissue Morphology and Antigenicity in Mouse and Rat Tibia: Comparing 12 Different Decalcification Conditions. J. Histochem. Cytochem. 2019, 67, 545–561. [Google Scholar] [CrossRef] [PubMed]
- Nakamura, T.; Sumi, K.; Tsuji, E.; Hosotani, M.; Namba, T.; Ichii, O.; Irie, T.; Nagasaki, K.-I.; Kon, Y.; Mishima, T.; et al. Novel polychrome staining distinguishing osteochondral tissue and bone cells in decalcified paraffin sections. Cell Tissue Res. 2021, 385, 727–737. [Google Scholar] [CrossRef] [PubMed]
- Schindelin, J.; Arganda-Carreras, I.; Frise, E.; Kaynig, V.; Longair, M.; Pietzsch, T.; Preibisch, S.; Rueden, C.; Saalfeld, S.; Schmid, B.; et al. Fiji: An open-source platform for biological-image analysis. Nat. Methods 2012, 9, 676–682. [Google Scholar] [CrossRef]
- Grant, W.T.; Wang, G.J.; Balian, G. Type X collagen synthesis during endochondral ossification in fracture repair. J. Biol. Chem. 1987, 262, 9844–9849. [Google Scholar] [CrossRef]
- Shen, G. The role of type X collagen in facilitating and regulating endochondral ossification of articular cartilage. Orthod. Craniofac Res. 2005, 8, 11–17. [Google Scholar] [CrossRef]
- Yang, L.; Tsang, K.Y.; Tang, H.C.; Chan, D.; Cheah, K.S.E. Hypertrophic chondrocytes can become osteoblasts and osteocytes in endochondral bone formation. Proc. Natl. Acad. Sci. USA 2014, 111, 12097–12102. [Google Scholar] [CrossRef]
- Frankel, A. Formalin fixation in the ’-omics’ era: A primer for the surgeon-scientist. ANZ J. Surg. 2012, 82, 395–402. [Google Scholar] [CrossRef]
- Magaki, S.; Hojat, S.A.; Wei, B.; So, A.; Yong, W.H. An Introduction to the Performance of Immunohistochemistry. Methods Mol. Biol. 2019, 1897, 289–298. [Google Scholar] [CrossRef]
- Scott, J.E.; Quintarelli, G.; Dellovo, M.C. The chemical and histochemical properties of Alcian Blue. I. The mechanism of Alcian Blue staining. Histochem. Cell Biol. 1964, 4, 73–85. [Google Scholar] [CrossRef] [PubMed]
- Sathawane, P.; Kamal, M.M.; Deotale, P.R.; Mankar, H. Nuances of the Papanicolaou stain. Cytojournal 2022, 19, 43. [Google Scholar] [CrossRef] [PubMed]
- Chantziantoniou, N.; Donnelly, A.D.; Mukherjee, M.; Boon, M.E.; Austin, R.M. Inception and Development of the Papanicolaou Stain Method. Acta Cytol. 2017, 61, 266–280. [Google Scholar] [CrossRef] [PubMed]
- Ballerini, L.; Franke-Stenport, V.; Borgefors, G.; Johansson, C.B. Comparison of histomorphometrical data obtained with two different image analysis methods. J. Mater Sci. Mater. Med. 2007, 18, 1471–1479. [Google Scholar] [CrossRef] [PubMed]
- Malhan, D.; Muelke, M.; Rosch, S.; Schaefer, A.B.; Merboth, F.; Weisweiler, D.; Heiss, C.; Arganda-Carreras, I.; Khassawna, T.E. An Optimized Approach to Perform Bone Histomorphometry. Front. Endocrinol. 2018, 9, 666. [Google Scholar] [CrossRef]
| Chemical Reagent | Catalogue Number | Manufacturer | Note |
|---|---|---|---|
| Alcian blue 8GX solution | 66011 | Sigma-Aldrich, Schneldorf, Germany | histochemical staining |
| Phosphotungstic acid-Orange G solution | 3470 | Carl Roth, Karlsruhe, Germany | histochemical staining |
| Safranin O | 84120 | Sigma-Aldrich, Schneldorf, Germany | histochemical staining |
| Fast green FCF | F7258 | Sigma-Aldrich, Schneldorf, Germany | histochemical staining |
| H&E fast staining kit | 9194.1 | Carl Roth, Karlsruhe, Germany | histochemical staining |
| Movat’s pentachrome staining kit | 12057 | MORPHISTO, Frankfurt am Main, Germany | histochemical staining |
| PBS Tablets | 1111.2 | Carl Roth, Karlsruhe, Germany | immunohistochemistry |
| PBS-TWEEN® Tablets | 524653-1EA | Merck, Darmstadt, Germany | immunohistochemistry |
| Triton™ X-100 | X100-100ML | Sigma-Aldrich, Schneldorf, Germany | immunohistochemistry |
| IHC Select Citrate Buffer pH 6.0, 10x | 21545 | Merck, Darmstadt, Germany | immunohistochemistry |
| ReadyProbes™ Endogenous HRP and AP Blocking Solution | R37629 | Thermo Fisher Scientific, Waltham, MA, USA | immunohistochemistry |
| Normal goat serum | 5425 | Cell Signaling Technology, Danvers, MA, USA | immunohistochemistry |
| Collagen X Monoclonal Antibody (X53) | 41-9771-82 | Thermo Fisher Scientific, Waltham, MA, USA | immunohistochemistry |
| Goat anti-Mouse IgG (H+L) Secondary Antibody, HRP | 31431 | Thermo Fisher Scientific, Waltham, MA, USA | immunohistochemistry |
| SignalStain® DAB Substrate Kit | 8059 | Cell Signaling Technology, Danvers, MA, USA | immunohistochemistry |
| Cytoseal 60 | 8310-4 | Thermo Fisher Scientific, Waltham, MA, USA | coverslipping |
Publisher’s Note: MDPI stays neutral with regard to jurisdictional claims in published maps and institutional affiliations. |
© 2022 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Sun, Y.; Helmholz, H.; Willumeit-Römer, R. Multicolor Histochemical Staining for Identification of Mineralized and Non-Mineralized Musculoskeletal Tissue: Immunohistochemical and Radiological Validation in Decalcified Bone Samples. Bioengineering 2022, 9, 488. https://doi.org/10.3390/bioengineering9100488
Sun Y, Helmholz H, Willumeit-Römer R. Multicolor Histochemical Staining for Identification of Mineralized and Non-Mineralized Musculoskeletal Tissue: Immunohistochemical and Radiological Validation in Decalcified Bone Samples. Bioengineering. 2022; 9(10):488. https://doi.org/10.3390/bioengineering9100488
Chicago/Turabian StyleSun, Yu, Heike Helmholz, and Regine Willumeit-Römer. 2022. "Multicolor Histochemical Staining for Identification of Mineralized and Non-Mineralized Musculoskeletal Tissue: Immunohistochemical and Radiological Validation in Decalcified Bone Samples" Bioengineering 9, no. 10: 488. https://doi.org/10.3390/bioengineering9100488
APA StyleSun, Y., Helmholz, H., & Willumeit-Römer, R. (2022). Multicolor Histochemical Staining for Identification of Mineralized and Non-Mineralized Musculoskeletal Tissue: Immunohistochemical and Radiological Validation in Decalcified Bone Samples. Bioengineering, 9(10), 488. https://doi.org/10.3390/bioengineering9100488