Comparative Evaluation of Various Extraction Techniques for Secondary Metabolites from Bombax ceiba L. Flowering Plants along with In Vitro Anti-Diabetic Performance
Abstract
1. Introduction
2. Materials and Methods
2.1. Collection and Identification of Plant Material
2.2. Chemicals
2.3. Hot Extraction Method
2.4. Cold Extraction Method
2.5. Organoleptic Characterization
| Sr. No. | Sample | Physical Characteristics | Sign of Instability |
| 1. | Powder | Light brown and amorphous | No Apparent Change |
| 2. | PBC | Yellowish green semi-solid with petroleum smell | No Apparent Change |
| 3. | CBC | Greenish semi-solid with characteristic smell | No Apparent Change |
| 4. | MBC | Dark brown semi-solid with no smell | No Apparent Change |
| 5. | EBC | Light brown semi-solid with no smell | No Apparent Change |
| 6. | WBC | Dark brown semi-solid with no smell | Semisolid to Solid |
2.6. The General Procedure of Determination of Total Polyphenolic Contents
2.7. The General Procedure of Determination of Total Flavonoid Contents
2.8. The General Procedure of Determination of Total Glycosaponin Contents
2.9. The General Procedure of Determination of Total Polysaccharides
2.10. The Procedure of In Vitro Anti-Diabetic Activity
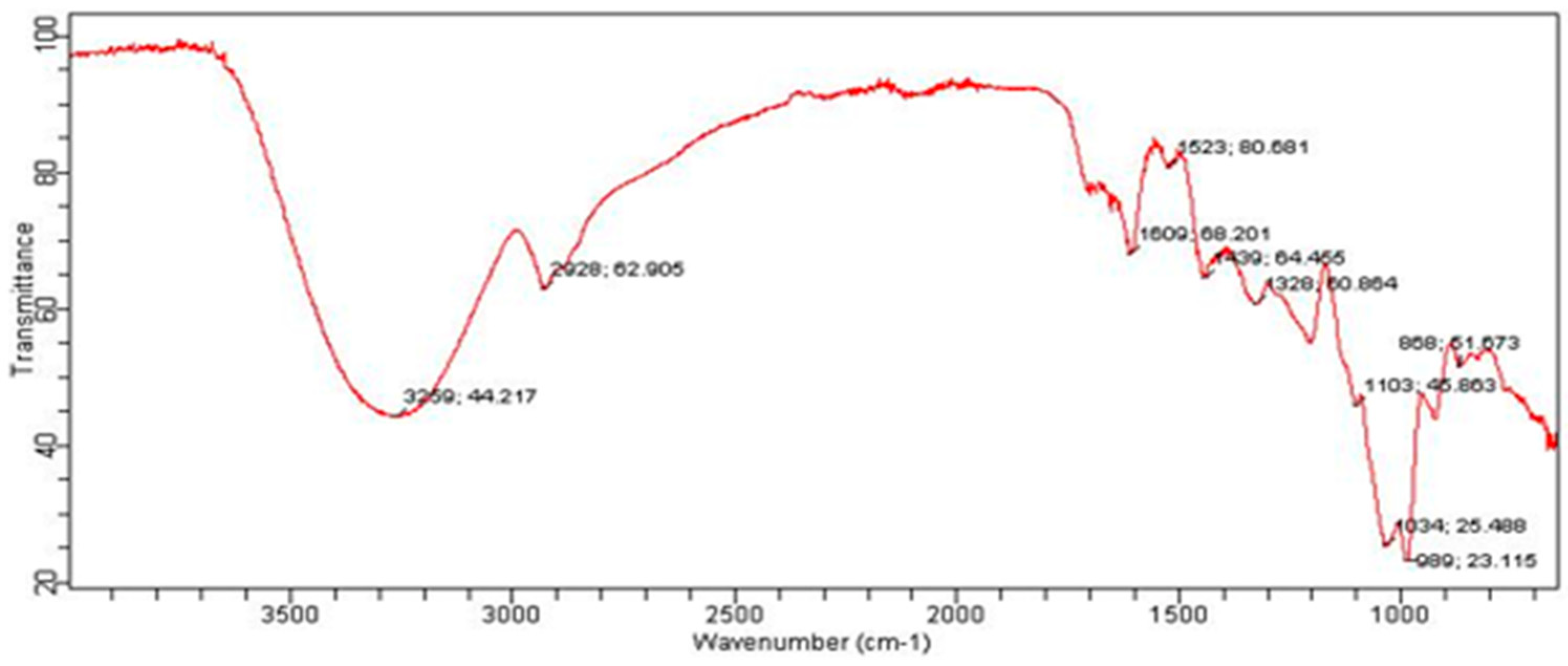
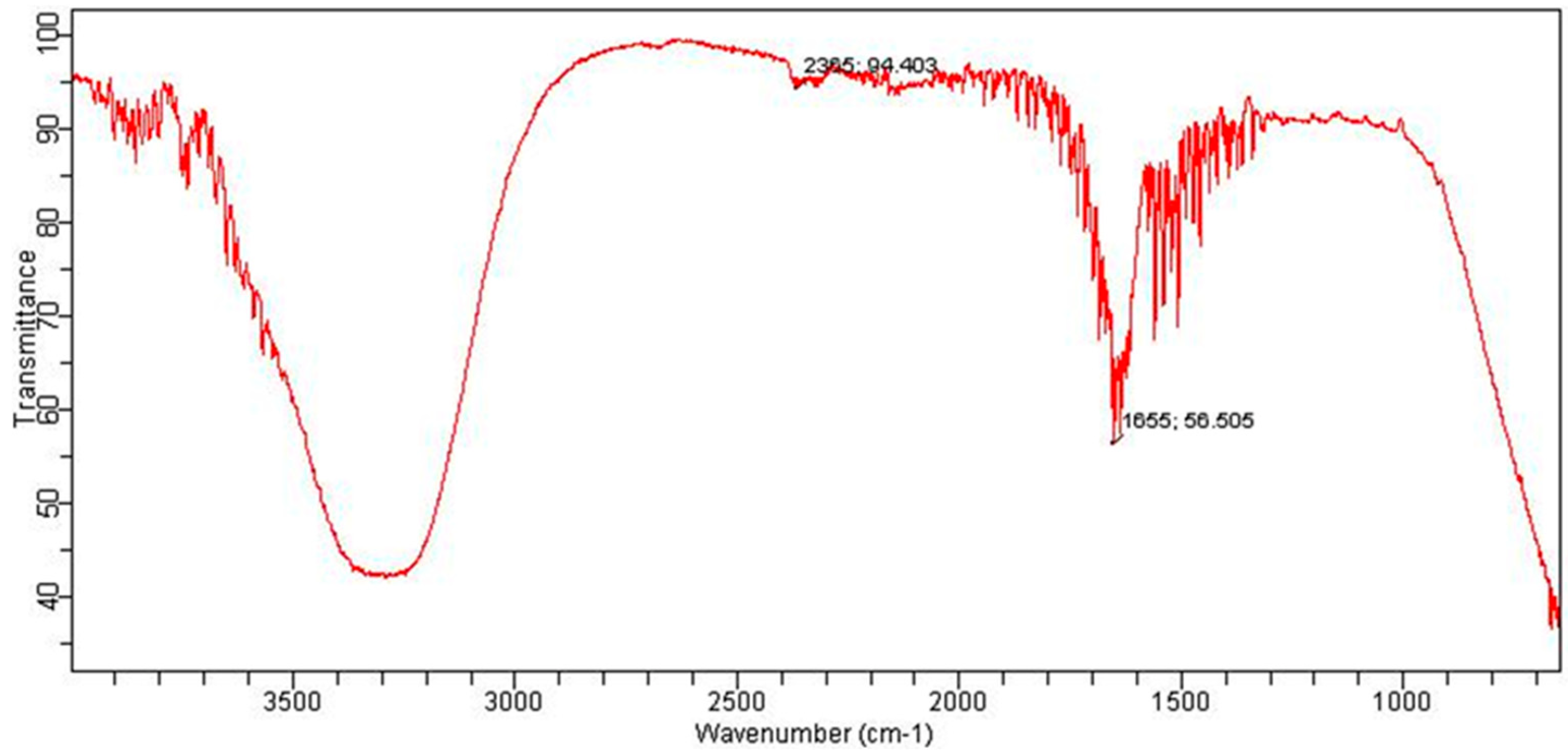
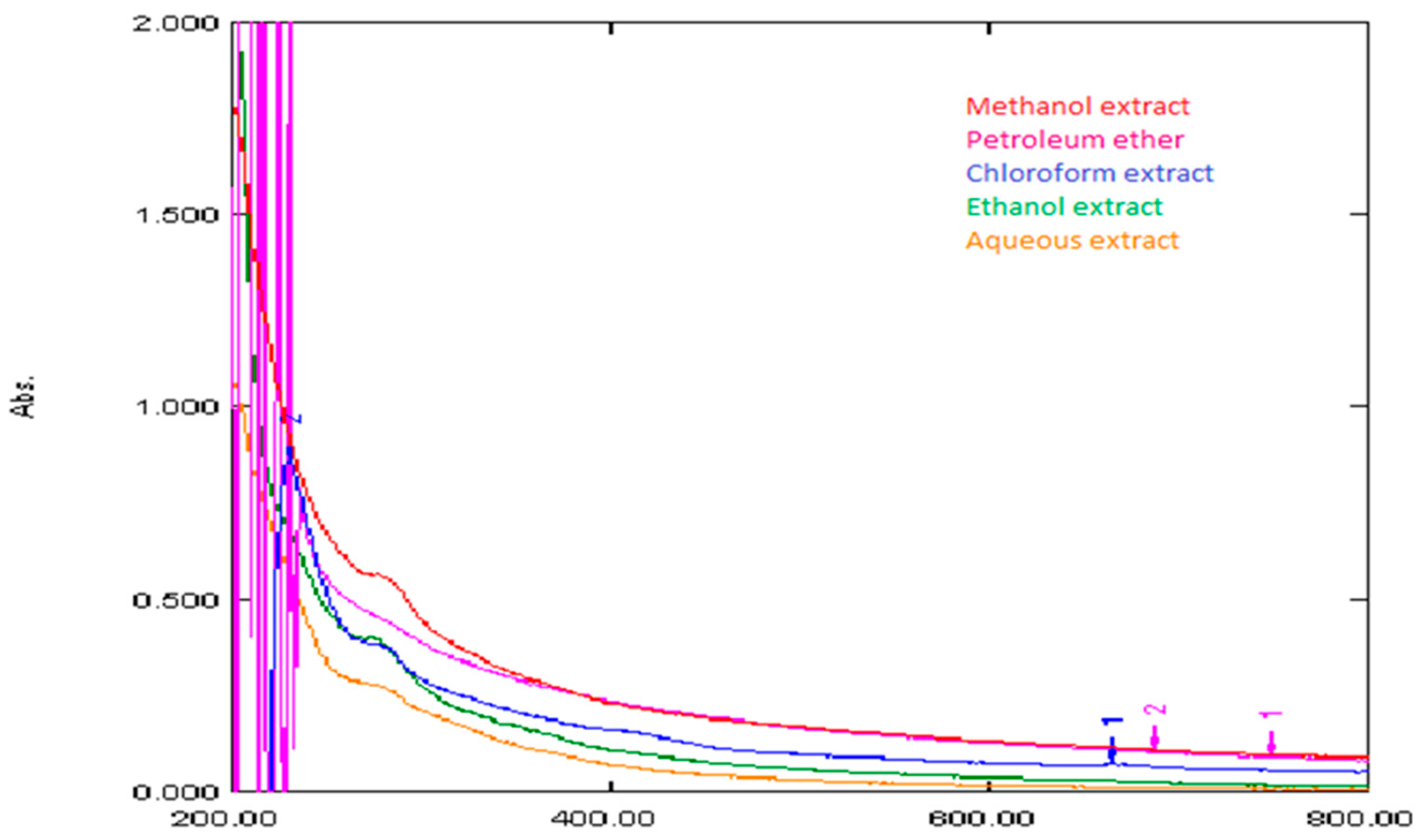
2.11. Instrumentation
3. Results and Discussion
4. Conclusions
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Data Availability Statement
Conflicts of Interest
References
- Anand, U.; Tudu, C.K.; Nandy, S.; Sunita, K.; Tripathi, V.; Loake, G.J.; Dey, A.; Proćków, J. Ethnodermatological use of medicinal plants in India: From ayurvedic formulations to clinical perspectives—A review. J. Ethnopharmacol. 2022, 284, 114744. [Google Scholar] [CrossRef]
- Arafa, A.F.; Foda, D.; Mahmoud, A.; Metwally, N.; Farrag, A. Bombax ceiba flowers extract ameliorates hepatosteatosis induced by ethanol and relatively moderate fat diet in rats. Toxicol. Rep. 2019, 6, 401–408. [Google Scholar] [CrossRef] [PubMed]
- Arunachalam, K.; Yang, X.; San, T.T. Tinospora cordifolia (Willd.) Miers: Protection mechanisms and strategies against oxidative stress-related diseases. J. Ethnopharmacol. 2022, 283, 114540. [Google Scholar] [CrossRef] [PubMed]
- Balde, E.S.; Traoré, M.; Balde, M.; Baldé, A.; Bah, F.; Camara, A.; Kéita, S. Traditional Guinean management of breast diseases in low and Middle Guinea. J. Herb. Med. 2022, 31, 100520. [Google Scholar] [CrossRef]
- Bhavsar, C.; Talele, G.S. Potential anti-diabetic activity of Bombax ceiba. Bangladesh J. Pharmacol. 2013, 8, 102–106. [Google Scholar] [CrossRef]
- Bi, T.E.Z.; Monnet, T.Y.; Ya, K.C.; Ekissi, E.S.G.; Bedel, J.; Fagbohoun, P.L.K. Phytochemicals compound and antioxidant activity study using different solvents and drying mode of flower Bombax costatum Pellgr and Vuillet (Bombacaceae) from Côte d’Ivoire. Eur. J. Biotechnol. Biosci. 2019, 7, 38–48. [Google Scholar]
- Chand, S.; Singh, A. In vitro propagation of Bombax ceiba L. (Silkcotton). Silvae Genet. 1999, 48, 313–317. [Google Scholar]
- Charoux, C.M.; Patange, A.; Lamba, S.; O’Donnell, C.P.; Tiwari, B.K.; Scannell, A.G. Applications of nonthermal plasma technology on safety and quality of dried food ingredients. J. Appl. Microbiol. 2021, 130, 325–340. [Google Scholar] [CrossRef]
- Dbeibia, A.; Ben Taheur, F.; Altammar, K.A.; Haddaji, N.; Mahdhi, A.; Amri, Z.; Mzoughi, R.; Jabeur, C. Control of Staphylococcus aureus methicillin resistant isolated from auricular infections using aqueous and methanolic extracts of Ephedra alata. Saudi J. Biol. Sci. 2021, 29, 1021–1028. [Google Scholar] [CrossRef]
- de Brum, T.F.; Camponogara, C.; da Silva Jesus, R.; Belke, B.V.; Piana, M.; Boligon, A.A.; Pires, F.B.; Oliveira, S.M.; da Rosa, M.B.; de Freitas Bauermann, L. Ethnopharmacological study and topical anti-inflammatory activity of crude extract from Poikilacanthus glandulosus (Nees) Ariza leaves. J. Ethnopharmacol. 2016, 193, 60–67. [Google Scholar] [CrossRef]
- De, D.; Ali, K.M.; Ghosh, D.; Chatterjee, K.; Bera, T.K. Antihyperglycemic and antihyperlipidemic effects of n-hexane fraction from the hydro-methanolic extract of sepals of Salmalia malabarica in streptozotocin-induced diabetic rats. J. Complementary Integr. Med. 2012, 9. [Google Scholar] [CrossRef]
- El-Toumy, S.A.; Hawas, U.W.; Taie, H.A.A. Xanthones and antitumor activity of Bombax ceiba against ehrlich ascites carcinoma cells in mice. Chem. Nat. Compd. 2013, 49, 945–950. [Google Scholar] [CrossRef]
- Estévez, L.; Queizán, M.; Mosquera, R.A.; Guidi, L.; Piccolo, E.L.; Landi, M. First Characterization of the Formation of Anthocyanin–Ge and Anthocyanin–B Complexes through UV–Vis Spectroscopy and Density Functional Theory Quantum Chemical Calculations. J. Agric. Food Chem. 2021, 69, 1272–1282. [Google Scholar] [CrossRef] [PubMed]
- Freitas, T.S.; Campina, F.F.; Costa, M.S.; Rocha, J.E.; Cruz, R.P.; Pinheiro, J.C.A.; Pereira-Júnior, F.N.; Lima, M.A.; de Sá, M.F.C.P.; Teixeira, A.M.; et al. UPLC-QTOF-MS/MS analysis and antibacterial activity of the Manilkara zapota (L.) P. Royen against Escherichia coli and other MDR bacteria. Cell. Mol. Biol. 2021, 67, 116–124. [Google Scholar] [CrossRef]
- Hassan, M.O. Leaf litter of Bombax ceiba L. threatens plant cover and floristic diversity in a new urban ecosystem. Flora 2018, 242, 22–30. [Google Scholar] [CrossRef]
- Jain, V.; Verma, S.K. Phytochemical Studies. In Pharmacology of Bombax Ceiba Linn; Springer: Berlin/Heidelberg, Germany, 2012; pp. 25–50. [Google Scholar]
- Khalivulla, S.I.; Mohammed, A.; Mallikarjuna, K. Novel Phytochemical Constituents and their Potential to Manage Diabetes. Curr. Pharm. Des. 2021, 27, 775–788. [Google Scholar] [CrossRef] [PubMed]
- Khan, A.; Ali, S.; Murad, W.; Hayat, K.; Siraj, S.; Jawad, M.; Khan, R.A.; Uddin, J.; Al-Harrasi, A.; Khan, A. Phytochemical and pharmacological uses of medicinal plants to treat cancer: A case study from Khyber Pakhtunkhwa, North Pakistan. J. Ethnopharmacol. 2021, 281, 114437. [Google Scholar] [CrossRef] [PubMed]
- Kikowska, M.; Thiem, B. In vitro systems of selected Eryngium species (E. planum, E. campestre, E. maritimum, and E. alpinum) for studying production of desired secondary metabolites (phenolic acids, flavonoids, triterpenoid saponins, and essential oil). J. Ref. Ser. Phytochem. Plant Cell Tissue Differ. Second. Metab. 2021, 869–901. [Google Scholar] [CrossRef]
- Kongthitilerd, P.; Thilavech, T.; Marnpae, M.; Rong, W.; Yao, S.; Adisakwattana, S.; Cheng, H.; Suantawee, T. Cyanidin-3-rutinoside stimulated insulin secretion through activation of L-type voltage-dependent Ca2+ channels and the PLC-IP3 pathway in pancreatic β-cells. Biomed. Pharmacother. 2022, 146, 112494. [Google Scholar] [CrossRef] [PubMed]
- Lall, N.; Kishore, N. Are plants used for skin care in South Africa fully explored? J. Ethnopharmacol. 2014, 153, 61–84. [Google Scholar] [CrossRef] [PubMed]
- Madzinga, M.; Kritzinger, Q.; Lall, N. Chapter 8—Medicinal Plants Used in the Treatment of Superficial Skin Infections: From Traditional Medicine to Herbal Soap Formulations. In Medicinal Plants for Holistic Health and Well-Being; Lall, N., Ed.; Academic Press: Cambridge, MA, USA, 2018; pp. 255–275. [Google Scholar]
- Mahmoud, A.H.; Metwally, N.S.; Youness, E.R.; El-Toukhy, S.E.; Elmalt, H.A.; Al-Mokaddem, A.K. Antiproliferative Activity of Bombax ceiba Flower Extract against Mammary Gland Carcinoma in Rats. Syst. Rev. Pharm. 2020, 11, 1406–1415. [Google Scholar]
- Mansoor, S.; Shahid, S.; Javed, M.; Saad, M.; Iqbal, S.; Alsaab, H.O.; Awwad, N.S.; Ibrahium, H.A.; Zaman, S.; Sarwar, M.N. Green synthesis of a MnO-GO-Ag nanocomposite using leaf extract of Fagonia arabica and its antioxidant and anti-inflammatory performance. Nano Struct. Nano Objects 2022, 29, 100835. [Google Scholar] [CrossRef]
- Mehta, C.; Kushwaha, K.; Gupta, J. Luteolin: Protective effects against diabetes and diabetes associated complications. Rom. J. Diabetes Nutr. Metab. Dis. 2021, 28, 303–310. [Google Scholar]
- Mohyuddin, A.; Kurniawan, T.A.; Khan, Z.-U.; Nadeem, S.; Javed, M.; Dera, A.A.; Iqbal, S.; Awwad, N.S.; Ibrahium, H.A.; Abourehab, M.A.S.; et al. Comparative Insights into the Antimicrobial, Antioxidant, and Nutritional Potential of the Solanum nigrum Complex. Processes 2022, 10, 1455. [Google Scholar] [CrossRef]
- Mosena, A.; Steinlein, T.; Beyschlag, W. Reconstructing the historical spread of non-native plants in the North American West from herbarium specimens. Flora 2018, 242, 45–52. [Google Scholar] [CrossRef]
- Mungole, A.; Chaturvedi, A. Hibiscus sabdariffa L. a rich source of secondary metabolites. Int. J. Pharm. Sci. Rev. Res. 2011, 6, 83–87. [Google Scholar]
- Niranjan, G.; Gupta, P.C. Anthocyanins from the flowers of Bombax malabaricum. Planta Med. 1973, 24, 196–199. [Google Scholar] [CrossRef]
- Noguchi, M.; Aizawa, R.; Nakazawa, D.; Hakumura, Y.; Furuhashi, Y.; Yang, S.; Ninomiya, K.; Takahashi, K.; Honda, R. Application of real treated wastewater to starch production by microalgae: Potential effect of nutrients and microbial contamination. Biochem. Eng. J. 2021, 169, 107973. [Google Scholar] [CrossRef]
- Othman, M.S.; Khaled, A.M.; Al-Bagawi, A.H.; Fareid, M.A.; Ghany, R.A.; Habotta, O.A.; Moneim, A.E.A. Hepatorenal protective efficacy of flavonoids from Ocimum basilicum extract in diabetic albino rats: A focus on hypoglycemic, antioxidant, anti-inflammatory and anti-apoptotic activities. Biomed. Pharmacother. 2021, 144, 112287. [Google Scholar] [CrossRef]
- Paramanya, A.; Sharma, S.; Bagdat, R.B.; Ali, A. Chapter 18—Recent practices of medicinal and aromatic plants in nanotechnology. In Nanomaterials for Agriculture and Forestry Applications; Husen, A., Jawaid, M., Eds.; Elsevier: Amsterdam, The Netherlands, 2020; pp. 435–467. [Google Scholar]
- Qasim, M.; Bashir, M.S.; Iqbal, S.; Mahmood, Q. Recent advancements in α-diimine-nickel and-palladium catalysts for ethylene polymerization. Eur. Polym. J. 2021, 160, 110783. [Google Scholar] [CrossRef]
- Riaz, T.; Munnwar, A.; Shahzadi, T.; Zaib, M.; Shahid, S.; Javed, M.; Iqbal, S.; Rizwan, K.; Waqas, M.; Khalid, B.; et al. Phyto-mediated synthesis of nickel oxide (NiO) nanoparticles using leaves’ extract of Syzygium cumini for antioxidant and dyes removal studies from wastewater. Inorg. Chem. Commun. 2022, 142, 109656. [Google Scholar] [CrossRef]
- Rufa’i, A.; Haruna, S. EFFECT of METHANOL and ACETONIC EXTRACTS of Bombax ceiba LEAVES on ALLOXAN INDUCED DIABETIC RATS. FUW Trends Sci. Technol. J. 2021, 6, 196–199. [Google Scholar]
- Safaei, M.; Tarkesh, M.; Bashari, H.; Bassiri, M. Modeling potential habitat of Astragalus verus Olivier for conservation decisions: A comparison of three correlative models. Flora 2018, 242, 61–69. [Google Scholar] [CrossRef]
- Sampathkumar, N.; Guruswamy, M. Quercetagetin Glycoside from the Flowers of Bombax ceiba 1. Asian J. Res. Chem. 2010, 3, 78–80. [Google Scholar]
- Shanmugam, K.R.; Shanmugam, B.; Subbaiah, G.V.; Ravi, S.; Reddy, K.S. Medicinal Plants and Bioactive Compounds for Diabetes Management: Important Advances in Drug Discovery. Curr. Pharm. Des. 2021, 27, 763–774. [Google Scholar] [CrossRef]
- Sharma, J.; Gairola, S.; Sharma, Y.; Gaur, R. Ethnomedicinal plants used to treat skin diseases by Tharu community of district Udham Singh Nagar, Uttarakhand, India. J. Ethnopharmacol. 2014, 158, 140–206. [Google Scholar] [CrossRef]
- Shoaib, M.; Bahadur, A.; Rahman, M.S.U.; Iqbal, S.; Arshad, M.I.; Tahir, M.A.; Mahmood, T. Sustained drug delivery of doxorubicin as a function of pH, releasing media, and NCO contents in polyurethane urea elastomers. J. Drug Deliv. Sci. Technol. 2017, 39, 277–282. [Google Scholar] [CrossRef]
- Sinha, S.; Kumar, B.; Luqman, S.; Singh, D. Neuroprotective potential of Cucurbita maxima Duchesne ex Poir, Caeselpenia bunduc (L.) Roxb and Bombax ceiba Linn extracts. S. Afr. J. Bot. 2019, 120, 319–325. [Google Scholar] [CrossRef]
- Sok Yen, F.; Qin, C.S.; Xuan, S.T.S.; Ying, P.J.; Le, H.Y.; Darmarajan, T.; Gunasekaran, B.; Salvamani, S. Hypoglycemic Effects of Plant Flavonoids: A Review. Evid. Based Complement. Altern. Med. 2021, 2021, 2057333. [Google Scholar] [CrossRef]
- Srivastava, R.; Mishra, N.; Mishra, N. Evaluation of the extraction hours and solvent concentrations on secondary metabolites and antioxidant activity of Feronia limonia fruit. Indian J. Nat. Prod. Resour. 2021, 12, 463–471. [Google Scholar]
- Ullah, F.; Zafar, M.; Amhad, M.; Sultana, S.; Ullah, A.; Shah, S.N.; Butt, M.A.; Mir, S. Taxonomic implications of foliar epidermal characteristics in subfamily Alsinoideae (Caryophyllaceae). Flora 2018, 242, 31–44. [Google Scholar] [CrossRef]
- Ullah, N.; Khan, A.; Mehwish, S.; Haq, I.-U. Comprehensive investigations of the mechanism of action of Bombax ceiba extracts against Leishmania tropica and its phytochemical investigation. Int. J. Infect. Dis. 2020, 101, 309. [Google Scholar] [CrossRef]
- Wahab, S.; Hussain, A.; Ahmad, P.; Usmani, S. Ethanobotanical, pharmacognostical and physico-chemical studies of stem bark of Bombax ceiba L.; commonly growing in eastern Uttar Pradesh region of India. Pharmacogn. J. 2012, 4, 55–60. [Google Scholar] [CrossRef]
- Xu, G.-K.; Qin, X.-Y.; Wang, G.-K.; Xie, G.-Y.; Li, X.-S.; Sun, C.-Y.; Liu, B.-L.; Qin, M.-J. Antihyperglycemic, antihyperlipidemic and antioxidant effects of standard ethanol extract of Bombax ceiba leaves in high-fat-diet- and streptozotocin-induced Type 2 diabetic rats. Chin. J. Nat. Med. 2017, 15, 168–177. [Google Scholar] [CrossRef]
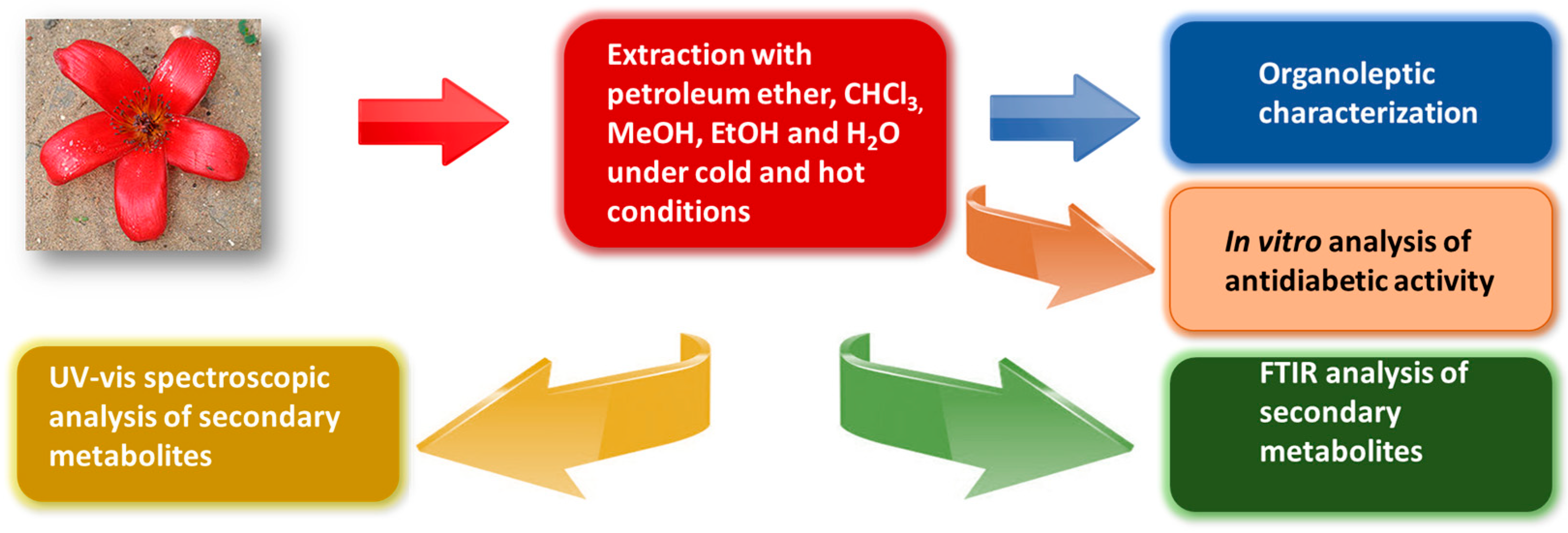
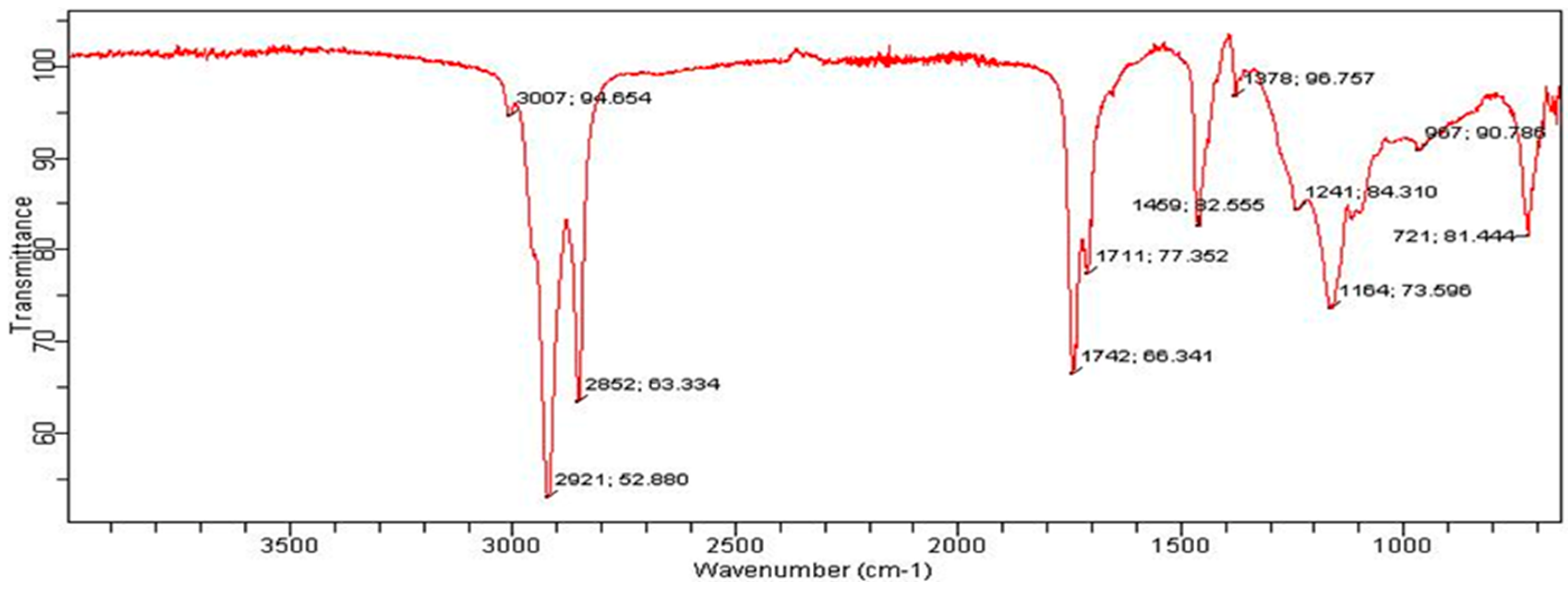
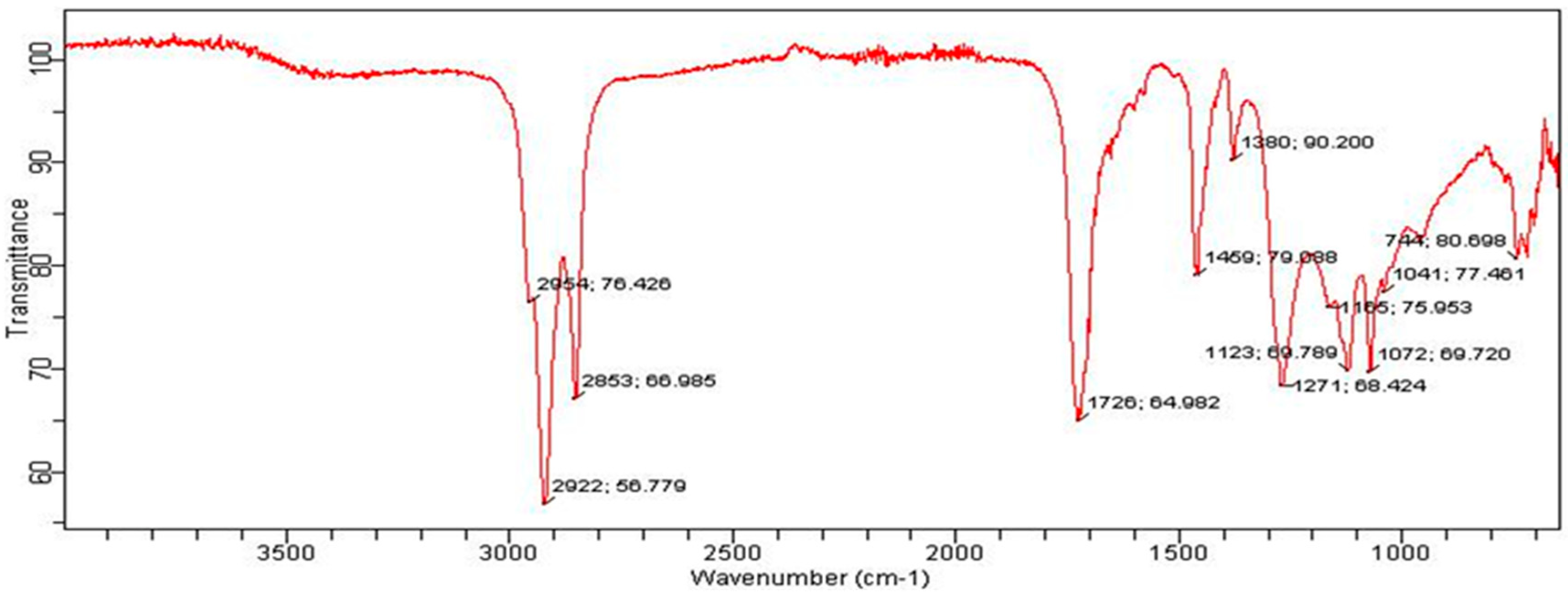
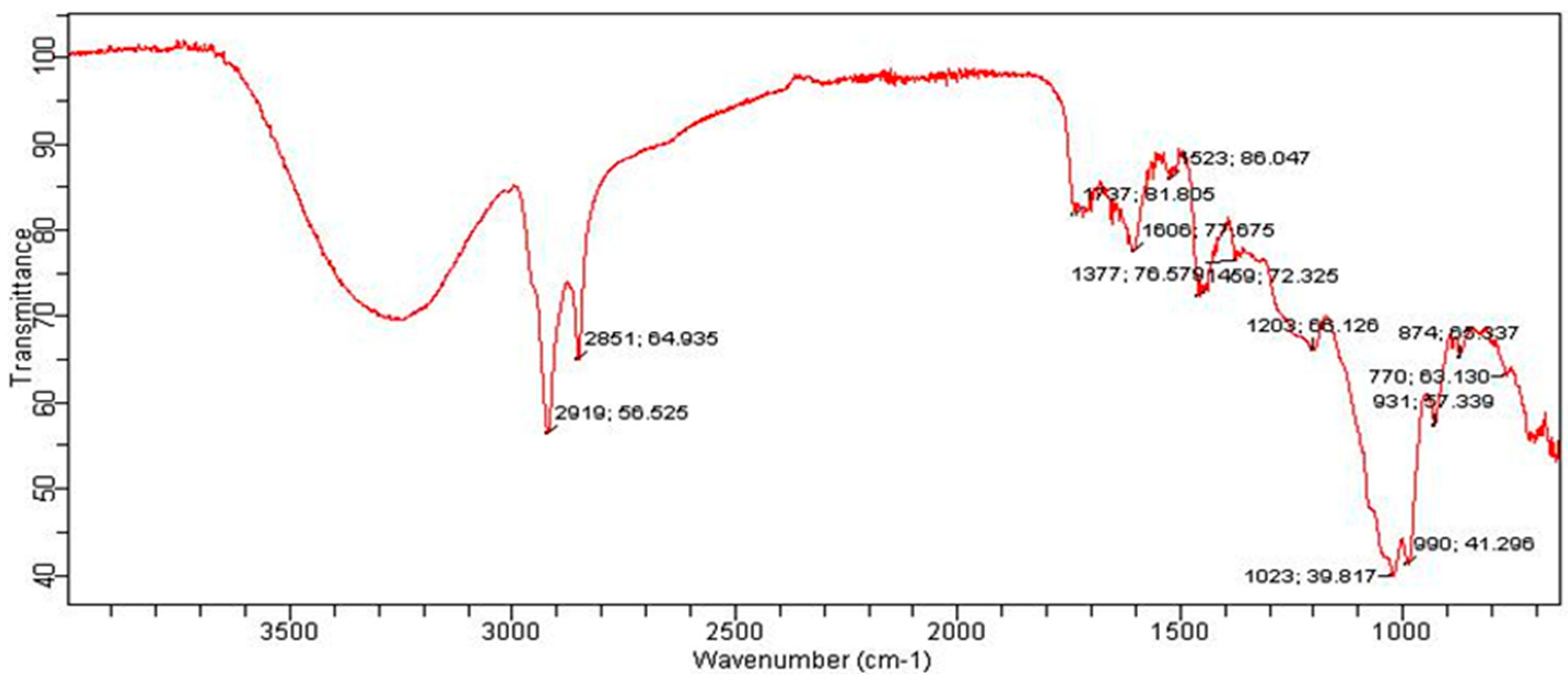
| Entry | Sample | Extract Weight (g) | Yield (%) |
|---|---|---|---|
| 1 | PBC | 06.02 | 1.40 |
| 2 | CBC | 02.74 | 0.64 |
| 3 | MBC | 23.42 | 5.44 |
| 4 | EBC | 02.10 | 0.48 |
| 5 | WBC | 21.64 | 5.23 |
| Entry | Sample | Physical Characteristics | Sign of Instability |
|---|---|---|---|
| 1 | Flower of Bombax ceiba L. | Light brown and amorphous powder | No Apparent Change |
| 2 | PBC | Yellowish green semi-solid petroleum smell | No Apparent Change |
| 3 | CBC | Greenish semi-solid with characteristic smell | No Apparent Change |
| 4 | MBC | Dark brown semi-solid with No smell | No Apparent Change |
| 5 | EBC | Light brown semi-solid with no smell | No Apparent Change |
| 6 | WBC | Dark brown semi-solid with no smell | Semisolid to Solid |
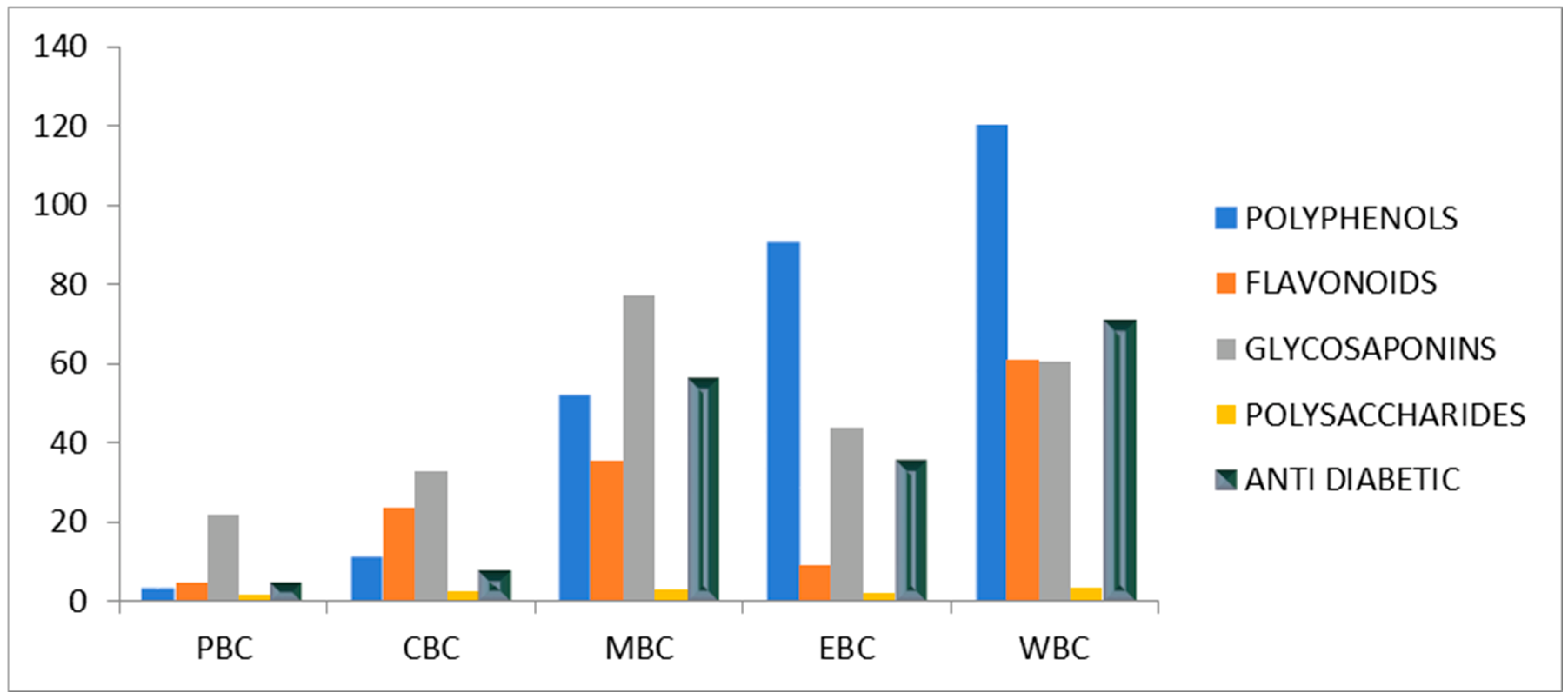
| Entry | Extract | Polyphenols (mg/g) | Flavonoids (mg/g) | Glycosaponins (mg/g) | Polysaccharides (mg/g) |
|---|---|---|---|---|---|
| 1 | PBC | 3.33 ± 1.53 | 1.89 ± 1.39 | 21.67 ± 1.24 | 0.005 ± 0.01 |
| 2 | CBC | 11.33 ± 0.58 | 23.66 ± 1.76 | 32.8 ± 0.75 | 0.013 ± 0.02 |
| 3 | MBC | 52.00 ± 2.64 | 35.22 ± 0.38 | 72.26 ± 1.05 | 0.147 ± 0.01 |
| 4 | EBC | 91.00 ± 1.00 | 9.22 ± 1.02 | 43.90 ± 0.30 | 0.090 ± 0.03 |
| 5 | WBC | 120.33 ± 2.31 | 60.77 ± 1.02 | 60.26 ± 1.20 | 0.167 ± 0.02 |
Publisher’s Note: MDPI stays neutral with regard to jurisdictional claims in published maps and institutional affiliations. |
© 2022 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Yasien, S.; Iqbal, M.M.; Javed, M.; Alnuwaiser, M.A.; Iqbal, S.; Mahmood, Q.; Elkaeed, E.B.; Dera, A.A.; Alrbyawi, H.; Pashameah, R.A.; et al. Comparative Evaluation of Various Extraction Techniques for Secondary Metabolites from Bombax ceiba L. Flowering Plants along with In Vitro Anti-Diabetic Performance. Bioengineering 2022, 9, 486. https://doi.org/10.3390/bioengineering9100486
Yasien S, Iqbal MM, Javed M, Alnuwaiser MA, Iqbal S, Mahmood Q, Elkaeed EB, Dera AA, Alrbyawi H, Pashameah RA, et al. Comparative Evaluation of Various Extraction Techniques for Secondary Metabolites from Bombax ceiba L. Flowering Plants along with In Vitro Anti-Diabetic Performance. Bioengineering. 2022; 9(10):486. https://doi.org/10.3390/bioengineering9100486
Chicago/Turabian StyleYasien, Sara, Muhammad Muntazir Iqbal, Mohsin Javed, Maha Abdallah Alnuwaiser, Shahid Iqbal, Qaiser Mahmood, Eslam B. Elkaeed, Ayed A. Dera, Hamad Alrbyawi, Rami Adel Pashameah, and et al. 2022. "Comparative Evaluation of Various Extraction Techniques for Secondary Metabolites from Bombax ceiba L. Flowering Plants along with In Vitro Anti-Diabetic Performance" Bioengineering 9, no. 10: 486. https://doi.org/10.3390/bioengineering9100486
APA StyleYasien, S., Iqbal, M. M., Javed, M., Alnuwaiser, M. A., Iqbal, S., Mahmood, Q., Elkaeed, E. B., Dera, A. A., Alrbyawi, H., Pashameah, R. A., Alzahrani, E., & Farouk, A.-E. (2022). Comparative Evaluation of Various Extraction Techniques for Secondary Metabolites from Bombax ceiba L. Flowering Plants along with In Vitro Anti-Diabetic Performance. Bioengineering, 9(10), 486. https://doi.org/10.3390/bioengineering9100486









