Membrane Technological Pathways and Inherent Structure of Bacterial Cellulose Composites for Drug Delivery
Abstract
:1. Introduction
2. Bacterial Cellulose
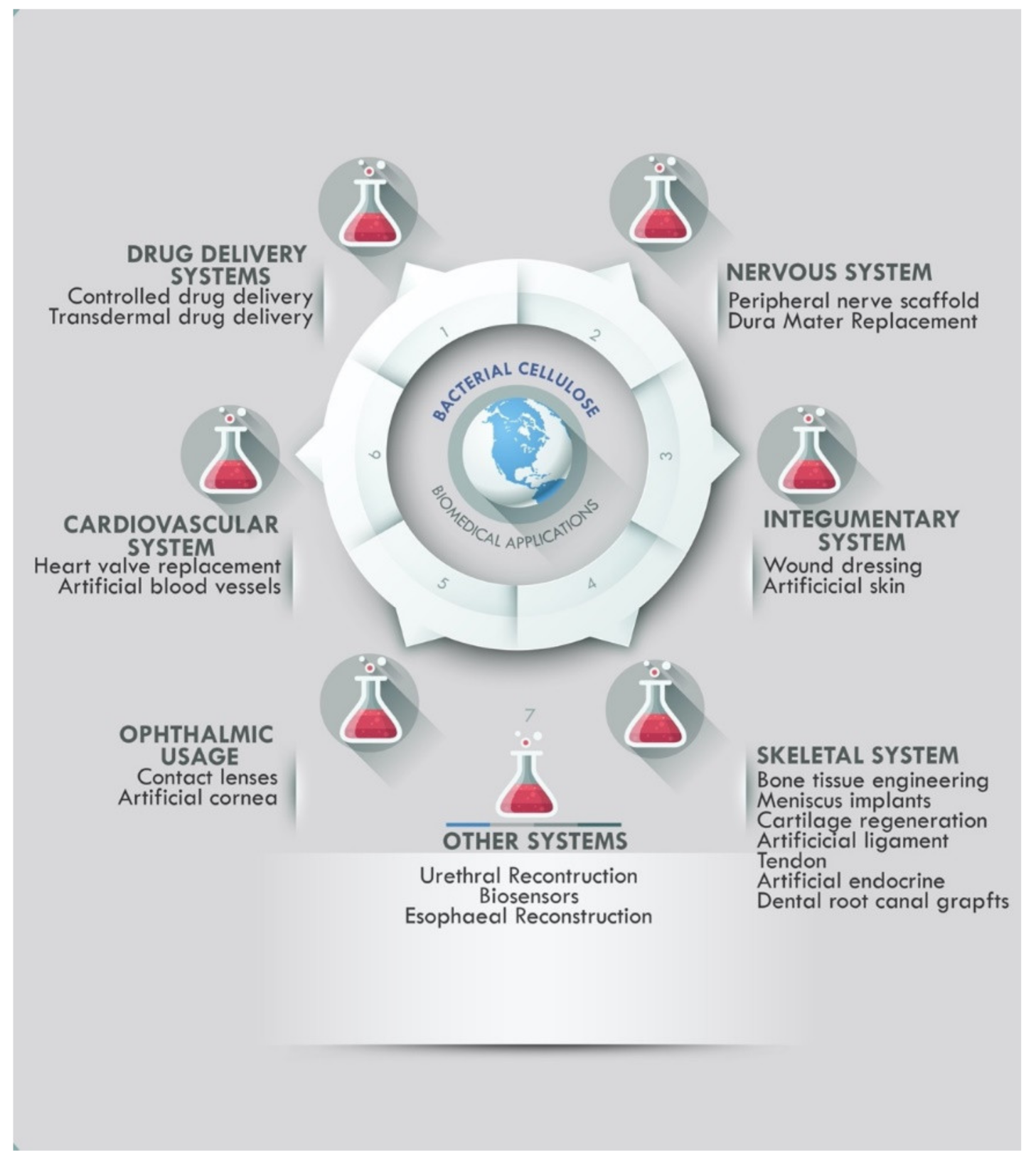
2.1. Bacterial Cellulose Medical Applications and Commercial Usage
2.2. Bacterial Cellulose for Drug Delivery
2.3. Critical Aspects Vital for BC-DDS and Biomedical Applications
2.3.1. Bacteria
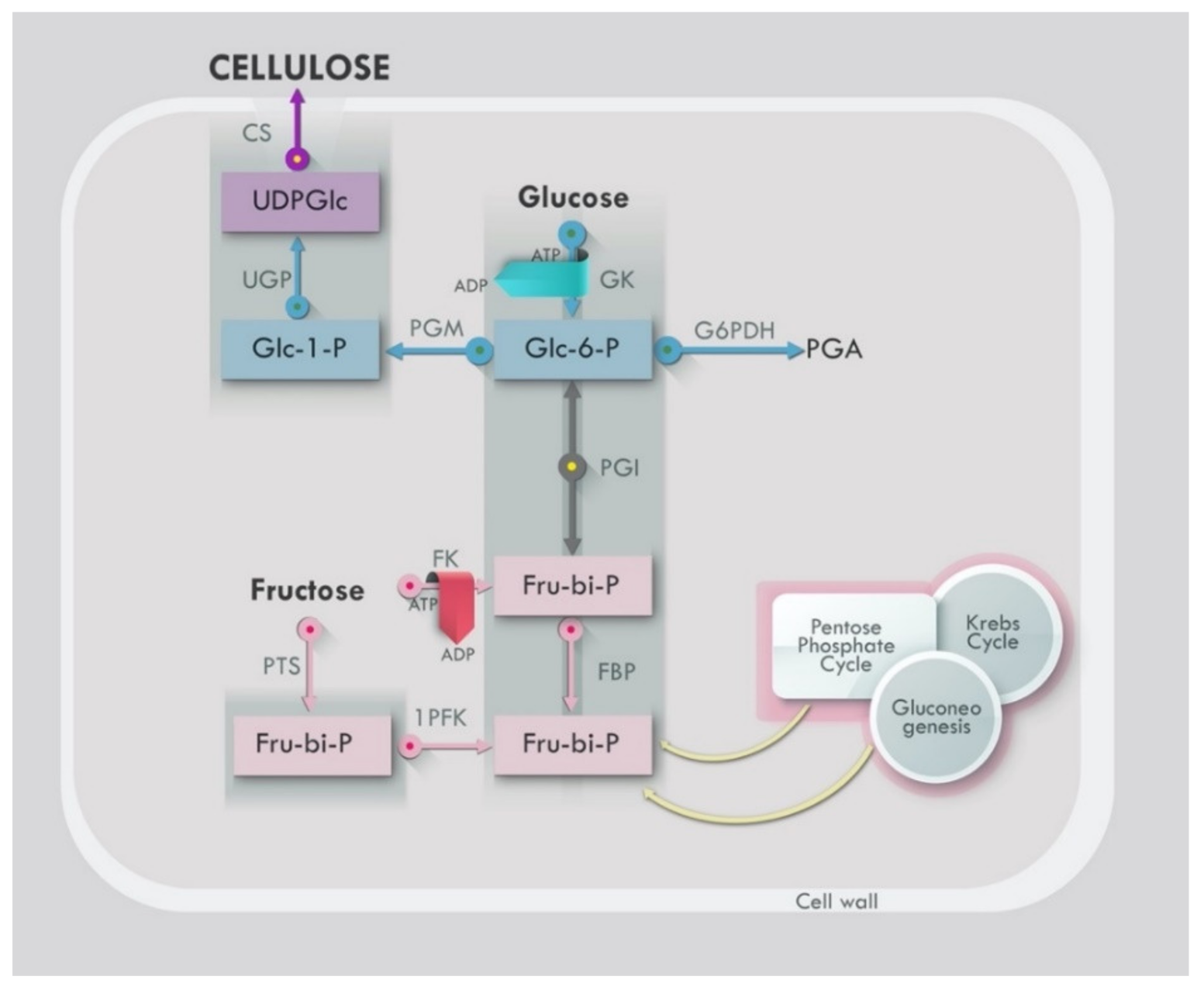
Bacteria Strains and Growth Factors (Biosynthetic Pathways)
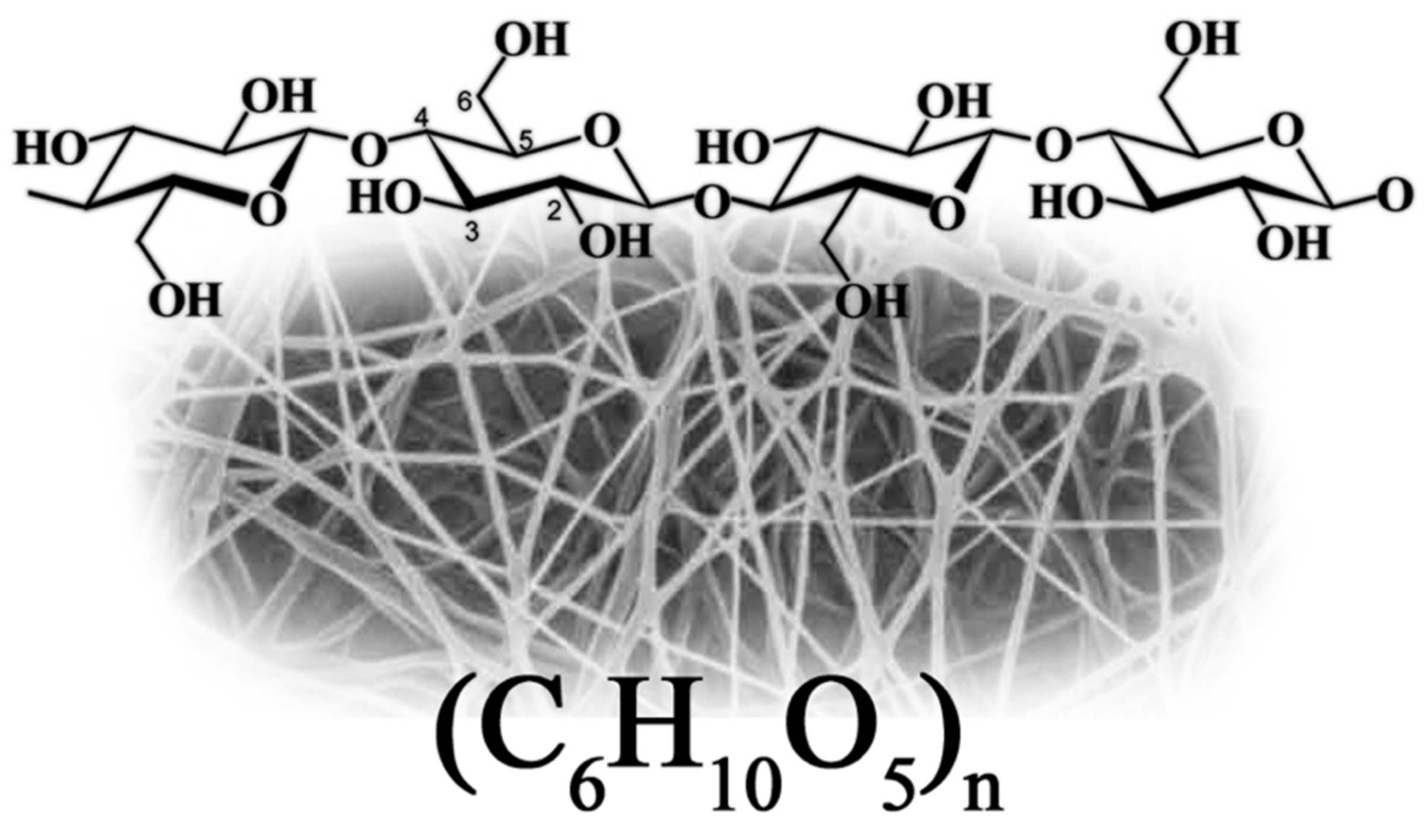
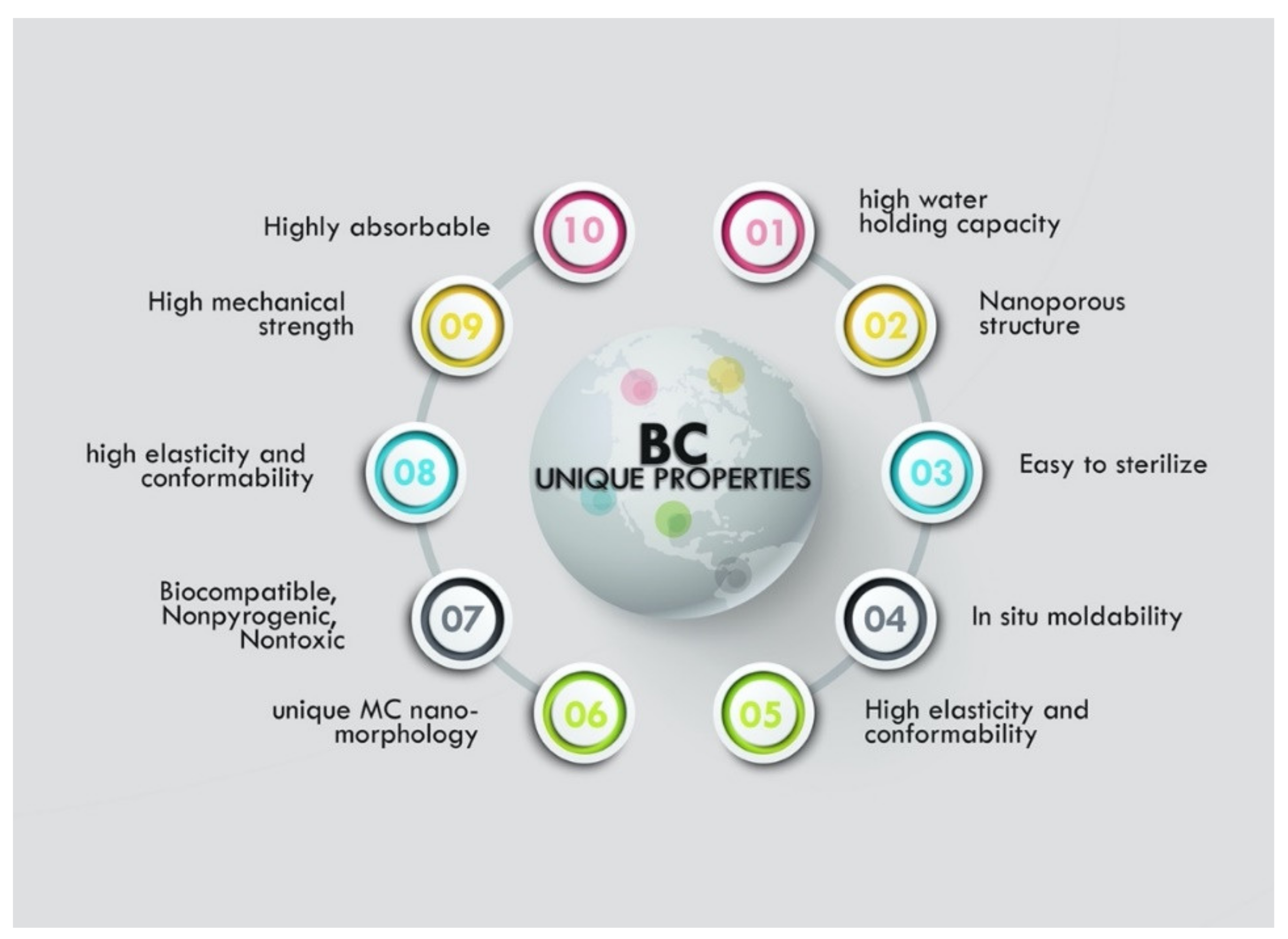
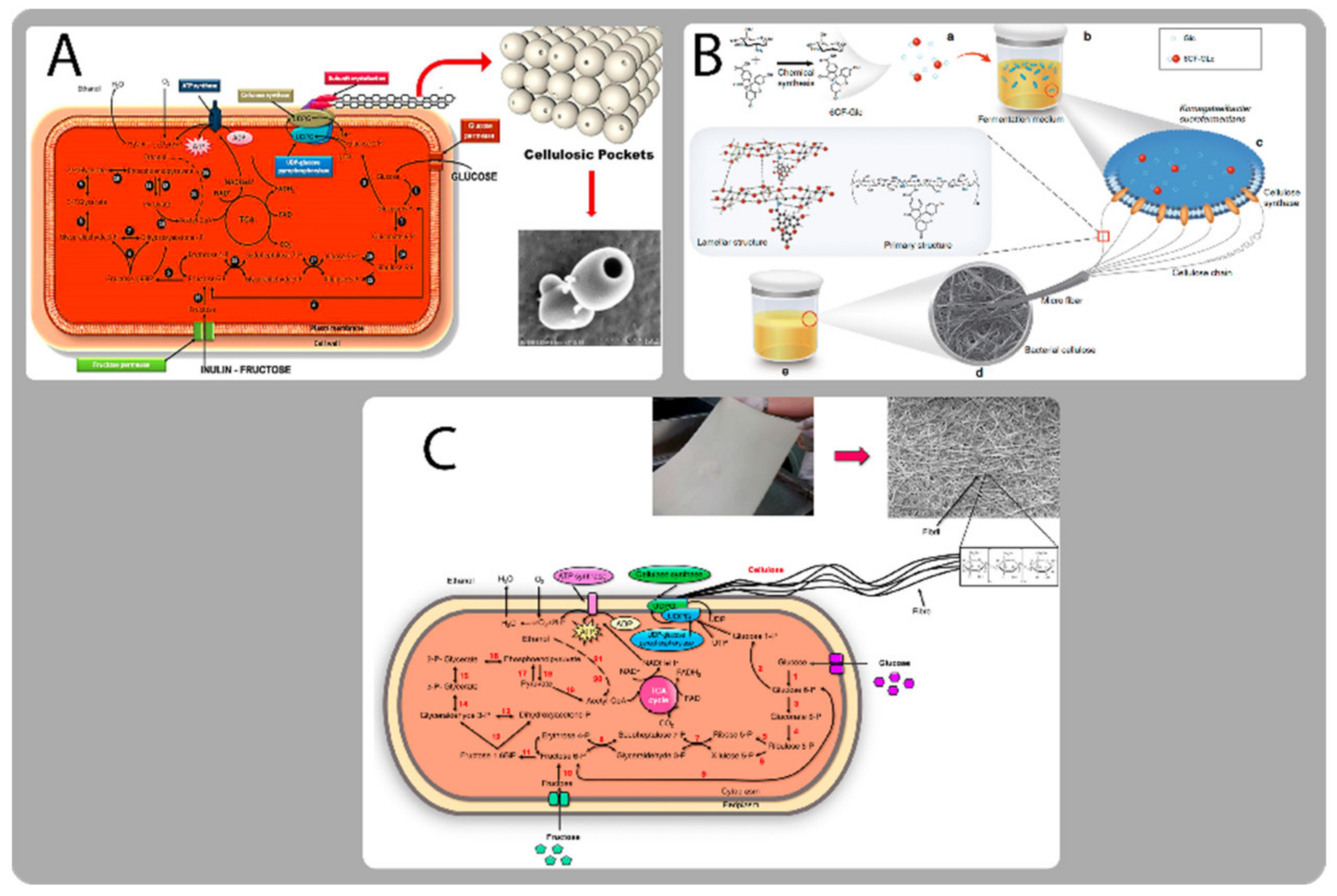
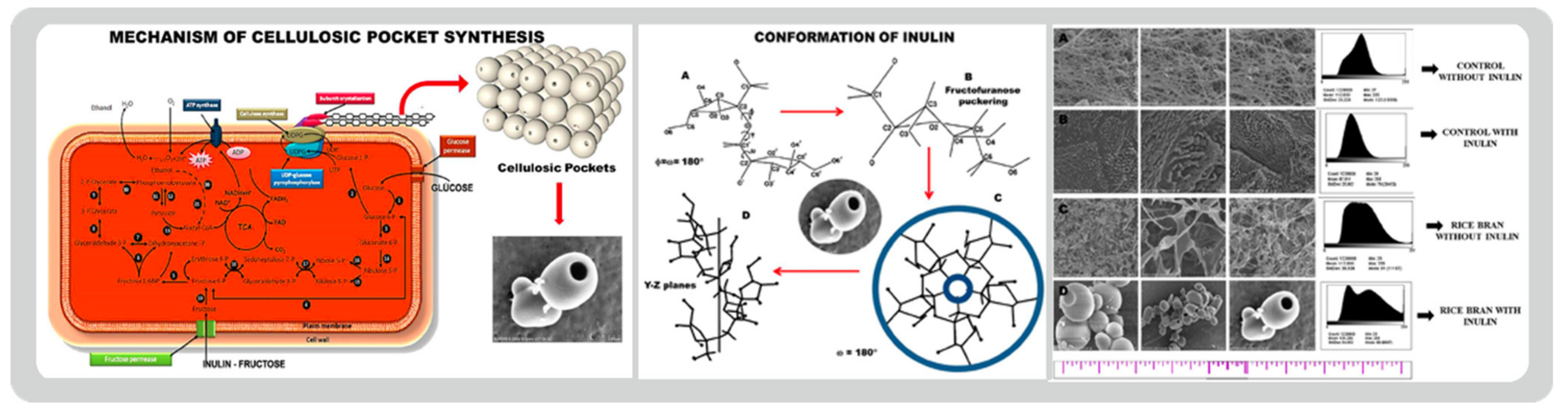
Bacterial Cellulose Structure and Unique Properties
2.3.2. Production Technology
Preparation Methods and Strategies for BC DDS Membranes
- (a)
- In situ pathway
- (b)
- Ex situ ‘unprocessed pellicle’ pathway
- (c)
- Ex situ “suspension/solution” (ExSSuSol) pathway
- (d)
- Hybrid pathway
2.3.3. Some Modes of BC Modifications for Drug Delivery
Modification via Cross-Linking Reactions
Modification via Grafting
Modification via Mineralization on and across the Fiber
Reactivity via Hydroxyl Sites
Modification via Etherification and Esterification
3. Perspectives, Challenges and Future Prospects for BC
4. Conclusions
Author Contributions
Funding
Acknowledgments
Conflicts of Interest
References
- Orasugh, J.T.; Saha, N.R.; Rana, D.; Sarkar, G.; Mollick, M.M.R.; Chattoapadhyay, A.; Mitra, B.C.; Mondal, D.; Ghosh, S.K.; Chattopadhyay, D. Jute cellulose nano-fibrils/hydroxypropylmethylcellulose nanocomposite: A novel material with potential for application in packaging and transdermal drug delivery system. Ind. Crops Prod. 2018, 112, 633–643. [Google Scholar] [CrossRef]
- Azarniya, A.; Tamjid, E.; Eslahi, N.; Simchi, A. Modification of bacterial cellulose/keratin nanofibrous mats by a tragacanth gum-conjugated hydrogel for wound healing. Int. J. Biol. Macromol. 2019, 134, 280–289. [Google Scholar] [CrossRef] [PubMed]
- Leitner, S.; Grijalvo, S.; Solans, C.; Eritja, R.; García-Celma, M.J.; Calderó, G. Ethylcellulose nanoparticles as a new “in vitro” transfection tool for antisense oligonucleotide delivery. Carbohydr. Polym. 2020, 229, 115451. [Google Scholar] [CrossRef] [PubMed]
- Maleki, R.; Afrouzi, H.H.; Hosseini, M.; Toghraie, D.; Rostami, S. Molecular dynamics simulation of Doxorubicin loading with N-isopropyl acrylamide carbon nanotube in a drug delivery system. Comput. Methods Programs Biomed. 2020, 184, 105303. [Google Scholar] [CrossRef] [PubMed]
- Zhang, Z.Y.; Sun, Y.; Zheng, Y.D.; He, W.; Yang, Y.Y.; Xie, Y.J.; Feng, Z.X.; Qiao, K. A biocompatible bacterial cellulose/tannic acid composite with antibacterial and anti-biofilm activities for biomedical applications. Mater. Sci. Eng. C 2020, 106, 110249. [Google Scholar] [CrossRef] [PubMed]
- Charreau, H.; Foresti, M.L.; Vazquez, A. Nanocellulose Patents Trends: A Comprehensive Review on Patents on Cellulose Nanocrystals, Microfibrillated and Bacterial Cellulose. Recent Pat. Nanotechnol. 2012, 7, 56–80. [Google Scholar] [CrossRef]
- Hokkanen, S.; Bhatnagar, A.; Sillanpää, M. A review on modification methods to cellulose-based adsorbents to improve adsorption capacity. Water Res. 2016, 91, 156–173. [Google Scholar] [CrossRef]
- Sharma, A.; Thakur, M.; Bhattacharya, M.; Mandal, T.; Goswami, S. Commercial application of cellulose nano-composites—A review. Biotechnol. Rep. 2019, 21, e00316. [Google Scholar] [CrossRef]
- Barros, S.C.; da Silva, A.A.; Costa, D.B.; Costa, C.M.; Lanceros-Mendez, S.; Maciavello, M.N.T.; Ribelles, J.L.G.; Sentanin, F.; Pawlicka, A.; Silva, M.M. Thermal–mechanical behaviour of chitosan–cellulose derivative thermoreversible hydrogel films. Cellulose 2015, 22, 1911–1929. [Google Scholar] [CrossRef] [Green Version]
- Norrrahim, M.N.F.; Mohd Kasim, N.A.; Knight, V.F.; Ujang, F.A.; Janudin, N.; Abdul Razak, M.A.I.; Shah, N.A.A.; Noor, S.A.M.; Jamal, S.H.; Ong, K.K.; et al. Nanocellulose: The next super versatile material for the military. Mater. Adv. 2021, 2, 1485–1506. [Google Scholar] [CrossRef]
- Picheth, G.F.; Pirich, C.L.; Sierakowski, M.R.; Woehl, M.A.; Sakakibara, C.N.; de Souza, C.F.; Martin, A.A.; da Silva, R.; de Freitas, R.A. Bacterial cellulose in biomedical applications: A review. Int. J. Biol. Macromol. 2017, 104, 97–106. [Google Scholar] [CrossRef]
- Boateng, J.S.; Matthews, K.H.; Stevens, H.N.E.; Eccleston, G.M. Wound healing dressings and drug delivery systems: A review. J. Pharm. Sci. 2008, 97, 2892–2923. [Google Scholar] [CrossRef]
- Żur, J.; Piński, A.; Michalska, J.; Hupert-Kocurek, K.; Nowak, A.; Wojcieszyńska, D.; Guzik, U. A whole-cell immobilization system on bacterial cellulose for the paracetamol-degrading Pseudomonas moorei KB4 strain. Int. Biodeterior. Biodegrad. 2020, 149, 104919. [Google Scholar] [CrossRef]
- Cacicedo, M.L.; Islan, G.A.; León, I.E.; Álvarez, V.A.; Chourpa, I.; Allard-Vannier, E.; García-Aranda, N.; Díaz-Riascos, Z.V.; Fernández, Y.; Schwartz, S.; et al. Bacterial cellulose hydrogel loaded with lipid nanoparticles for localized cancer treatment. Colloids Surf. B Biointerfaces 2018, 170, 596–608. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Coelho, F.; do Vale Braido, G.V.; Cavicchioli, M.; Mendes, L.S.; Specian, S.S.; Franchi, L.P.; Ribeiro, S.J.L.; Messaddeq, Y.; Scarel-Caminaga, R.M.; Capotea, T.S.O. Toxicity of therapeutic contact lenses based on bacterial cellulose with coatings to provide transparency. Contact Lens Anterior Eye 2019, 42, 512–519. [Google Scholar] [CrossRef]
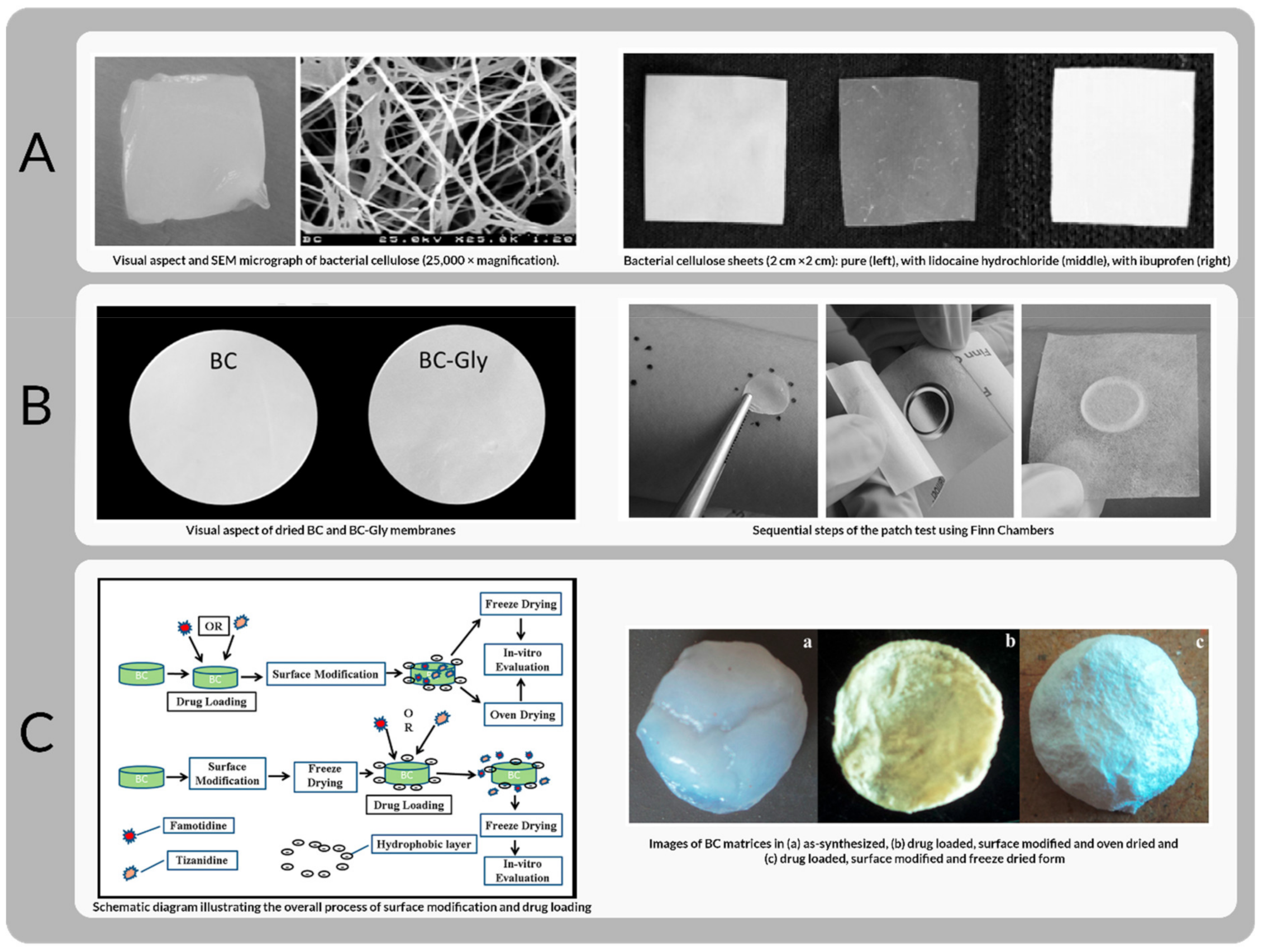
- Trovatti, E.; Freire, C.S.R.; Pinto, P.C.; Almeida, I.F.; Costa, P.; Silvestre, A.J.D.; Neto, C.P.; Rosado, C. Bacterial cellulose membranes applied in topical and transdermal delivery of lidocaine hydrochloride and ibuprofen: In vitro diffusion studies. Int. J. Pharm. 2012, 435, 83–87. [Google Scholar] [CrossRef]
- Ho, J.; Walsh, C.; Yue, D.; Dardik, A.; Cheema, U. Current Advancements and Strategies in Tissue Engineering for Wound Healing: A Comprehensive Review. Adv. Wound Care 2017, 6, 191–209. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Dutta, S.D.; Patel, D.K.; Lim, K.T. Functional cellulose-based hydrogels as extracellular matrices for tissue engineering. J. Biol. Eng. 2019, 13, 55. [Google Scholar] [CrossRef] [PubMed]
- Van Vlierberghe, S.; Dubruel, P.; Schacht, E. Biopolymer-based hydrogels as scaffolds for tissue engineering applications: A review. Biomacromolecules 2011, 12, 1387–1408. [Google Scholar] [CrossRef] [PubMed]
- Abeer, M.M.; Mohd Amin, M.C.I.; Martin, C. A review of bacterial cellulose-based drug delivery systems: Their biochemistry, current approaches and future prospects. J. Pharm. Pharmacol. 2014, 66, 1047–1061. [Google Scholar] [CrossRef]
- Committee on Drugs. American Academy of Alternative Routes of Drug Administration—Advantages and Disadvantages (Subject Review). Pediatrics 1997, 100, 143–152. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Coelho, J.F.; Ferreira, P.C.; Alves, P.; Cordeiro, R.; Fonseca, A.C.; Góis, J.R.; Gil, M.H. Drug delivery systems: Advanced technologies potentially applicable in personalized treatments. EPMA J. 2010, 1, 164–209. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Jeffreys, D. Aspirin: The Story of a Wonder Drug. BMJ 2004, 329, 1408. [Google Scholar] [CrossRef] [Green Version]
- Jain, K.K. An Overview of Drug Delivery Systems. Methods Mol. Biol. 2020, 2059, 1–54. [Google Scholar] [CrossRef] [PubMed]
- Koo, O.M.; Rubinstein, I.; Onyuksel, H. Role of nanotechnology in targeted drug delivery and imaging: A concise review. Nanomed. Nanotechnol. Biol. Med. 2005, 1, 193–212. [Google Scholar] [CrossRef] [PubMed]
- Sahraei, R.; Ghaemy, M.; Ma, H.-L.; Zhang, Y.; Hu, Q.-H.; Yan, D.; Yu, Z.-Z.; Zhai, M.; Liu, G.; He, F.; et al. Ultra-light nanocomposite aerogels of bacterial cellulose and reduced graphene oxide for specific absorption and separation of organic liquids. J. Ind. Eng. Chem. 2017, 4, 21553–21558. [Google Scholar] [CrossRef]
- Kamoun, E.A.; Kenawy, E.R.S.; Chen, X. A review on polymeric hydrogel membranes for wound dressing applications: PVA-based hydrogel dressings. J. Adv. Res. 2017, 8, 217–233. [Google Scholar] [CrossRef] [PubMed]
- Barakat, M.A. New trends in removing heavy metals from industrial wastewater. Arab. J. Chem. 2011, 4, 361–377. [Google Scholar] [CrossRef] [Green Version]
- Portela, R.; Leal, C.R.; Almeida, P.L.; Sobral, R.G. Bacterial cellulose: A versatile biopolymer for wound dressing applications. Microb. Biotechnol. 2019, 12, 586–610. [Google Scholar] [CrossRef]
- Almeida, I.F.; Pereira, T.; Silva, N.H.C.S.; Gomes, F.P.; Silvestre, A.J.D.; Freire, C.S.R.; Lobo, J.M.S.; Costa, P.C. Bacterial cellulose membranes as drug delivery systems: An in vivo skin compatibility study. Eur. J. Pharm. Biopharm. 2014, 86, 332–336. [Google Scholar] [CrossRef]
- Agarwal, P.; Hefner, R.E.; Ge, S.; Tomlinson, I.; Rao, Y.Q.; Dikic, T. Nanofiltration membranes from crosslinked Troger’s base Polymers of Intrinsic Microporosity (PIMs). J. Memb. Sci. 2020, 595, 117501. [Google Scholar] [CrossRef]
- Tapia-Rodriguez, M.R.; Hernandez-Mendoza, A.; Gonzalez-Aguilar, G.A.; Martinez-Tellez, M.A.; Martins, C.M.; Ayala-Zavala, J.F. Carvacrol as potential quorum sensing inhibitor of Pseudomonas aeruginosa and biofilm production on stainless steel surfaces. Food Control 2017, 75, 255–261. [Google Scholar] [CrossRef]
- Chantereau, G.; Brown, N.; Dourges, M.A.; Freire, C.S.R.; Silvestre, A.J.D.; Sebe, G.; Coma, V. Silylation of bacterial cellulose to design membranes with intrinsic anti-bacterial properties. Carbohydr. Polym. 2019, 220, 71–78. [Google Scholar] [CrossRef]
- Morone, J.; Alfeus, A.; Vasconcelos, V.; Martins, R. Revealing the potential of cyanobacteria in cosmetics and cosmeceuticals—A new bioactive approach. Algal Res. 2019, 41, 101541. [Google Scholar] [CrossRef]
- Gopinath, V.; Saravanan, S.; Al-Maleki, A.R.; Ramesh, M.; Vadivelu, J. A review of natural polysaccharides for drug delivery applications: Special focus on cellulose, starch and glycogen. Biomed. Pharmacother. 2018, 107, 96–108. [Google Scholar] [CrossRef] [PubMed]
- Infographic: Visualizing the Future of the Pharma Market. Available online: https://www.visualcapitalist.com/future-pharma-market/ (accessed on 4 December 2021).
- Yan, H.; Chen, X.; Feng, M.; Shi, Z.; Zhang, W.; Wang, Y.; Ke, C.; Lin, Q. Entrapment of bacterial cellulose nanocrystals stabilized Pickering emulsions droplets in alginate beads for hydrophobic drug delivery. Colloids Surf. B Biointerfaces 2019, 177, 112–120. [Google Scholar] [CrossRef] [PubMed]
- Wang, P.; Jiang, F.; Chen, B.; Tang, H.; Zeng, X.; Cai, D.; Zhu, M.; Long, R.; Yang, D.; Kankala, R.K.; et al. Bioinspired red blood cell membrane-encapsulated biomimetic nanoconstructs for synergistic and efficacious chemo-photothermal therapy. Colloids Surf. B Biointerfaces 2020, 189, 110842. [Google Scholar] [CrossRef]
- Liu, J.; Li, S.; Zhang, S.; Shen, C.; Zhu, J.; Yang, G.; Zhang, X. Piperazine multi-substituted triarylboron compound as an aqueous soluble fluorescent probe for imaging nucleoli, nuclear matrix and nuclear membrane. Sens. Actuators B Chem. 2018, 261, 531–536. [Google Scholar] [CrossRef]
- Liu, C.; Fan, L. A hybrid evolutionary algorithm based on tissue membrane systems and CMA-ES for solving numerical optimization problems. Knowl. Based Syst. 2016, 105, 38–47. [Google Scholar] [CrossRef]
- Okuyama, H.; Umeda, S.; Takama, Y.; Terasawa, T.; Nakayama, Y. Patch esophagoplasty using an in-body-tissue-engineered collagenous connective tissue membrane. J. Pediatr. Surg. 2018, 53, 223–226. [Google Scholar] [CrossRef]
- Larentis, G.R.; Camozzato, G.C.; Bastos, H.B.A.; Gregory, R.M.; Mattos, R.C. Equine Sperm Selection by Synthetic Membrane Filter. J. Equine Vet. Sci. 2018, 63, 69–73. [Google Scholar] [CrossRef]
- Giri, A.; Bhunia, T.; Pal, A.; Goswami, L.; Bandyopadhyay, A. In-situ synthesis of polyacrylate grafted carboxymethyl guargum-carbon nanotube membranes for potential application in controlled drug delivery. Eur. Polym. J. 2016, 74, 13–25. [Google Scholar] [CrossRef]
- Yoosefian, M.; Sabaei, S.; Etminan, N. Encapsulation efficiency of single-walled carbon nanotube for Ifosfamide anti-cancer drug. Comput. Biol. Med. 2019, 114, 103433. [Google Scholar] [CrossRef]
- Harikrishnan, S.; Sedev, R.; Beh, C.C.; Priest, C.; Foster, N.R. Loading of 5-fluorouracil onto Halloysite nanotubes for targeted drug delivery using a subcritical gas antisolvent process (GAS). J. Supercrit. Fluids 2020, 159, 104756. [Google Scholar] [CrossRef]
- Majumder, M.; Stinchcomb, A.; Hinds, B.J. Towards mimicking natural protein channels with aligned carbon nanotube membranes for active drug delivery. Life Sci. 2010, 86, 563–568. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Dong, X.; Wei, C.; Liang, J.; Liu, T.; Kong, D.; Lv, F. Thermosensitive hydrogel loaded with chitosan-carbon nanotubes for near infrared light triggered drug delivery. Colloids Surf. B Biointerfaces 2017, 154, 253–262. [Google Scholar] [CrossRef]
- Pippa, N.; Chronopoulos, D.D.; Stellas, D.; Fernández-Pacheco, R.; Arenal, R.; Demetzos, C.; Tagmatarchis, N. Design and development of multi-walled carbon nanotube-liposome drug delivery platforms. Int. J. Pharm. 2017, 528, 429–439. [Google Scholar] [CrossRef]
- Risoluti, R.; Gullifa, G.; Carcassi, E.; Masotti, A.; Materazzi, S. TGA/Chemometrics addressing innovative preparation strategies for functionalized carbon nanotubes. J. Pharm. Anal. 2020, 10, 351–355. [Google Scholar] [CrossRef]
- Tangboriboon, N. Carbon and Carbon Nanotube Drug Delivery and Its Characterization, Properties, and Applications. Nanocarr. Drug Deliv. 2019, 451–467. [Google Scholar] [CrossRef]
- Kaur, J.; Gill, G.S.; Jeet, K. Applications of Carbon Nanotubes in Drug Delivery: A Comprehensive Review. Charact. Biol. Nanomater. Drug. Deliv. 2018, 113–135. [Google Scholar] [CrossRef]
- Mahajan, S.; Patharkar, A.; Kuche, K.; Maheshwari, R.; Deb, P.K.; Kalia, K.; Tekade, R.K. Functionalized carbon nanotubes as emerging delivery system for the treatment of cancer. Int. J. Pharm. 2018, 548, 540–558. [Google Scholar] [CrossRef] [PubMed]
- Löbmann, K.; Svagan, A.J. Cellulose nanofibers as excipient for the delivery of poorly soluble drugs. Int. J. Pharm. 2017, 533, 285–297. [Google Scholar] [CrossRef] [PubMed]
- Wang, Y.; Yan, J.; Wen, N.; Xiong, H.; Cai, S.; He, Q.; Hu, Y.; Peng, D.; Liu, Z.; Liu, Y. Metal-organic frameworks for stimuli-responsive drug delivery. Biomaterials 2020, 230, 119619. [Google Scholar] [CrossRef]
- Kumar, G.; Kant, A.; Kumar, M.; Masram, D.T. Synthesis, characterizations and kinetic study of metal organic framework nanocomposite excipient used as extended release delivery vehicle for an antibiotic drug. Inorg. Chim. Acta 2019, 496, 119036. [Google Scholar] [CrossRef]
- Javanbakht, S.; Pooresmaeil, M.; Hashemi, H.; Namazi, H. Carboxymethylcellulose capsulated Cu-based metal-organic framework-drug nanohybrid as a pH-sensitive nanocomposite for ibuprofen oral delivery. Int. J. Biol. Macromol. 2018, 119, 588–596. [Google Scholar] [CrossRef] [PubMed]
- Cabrera-García, A.; Checa-Chavarria, E.; Rivero-Buceta, E.; Moreno, V.; Fernández, E.; Botella, P. Amino modified metal-organic frameworks as pH-responsive nanoplatforms for safe delivery of camptothecin. J. Colloid Interface Sci. 2019, 541, 163–174. [Google Scholar] [CrossRef] [PubMed]
- Sun, Q.; Bi, H.; Wang, Z.; Li, C.; Wang, X.; Xu, J.; Zhu, H.; Zhao, R.; He, F.; Gai, S.; et al. Hyaluronic acid-targeted and pH-responsive drug delivery system based on metal-organic frameworks for efficient antitumor therapy. Biomaterials 2019, 223, 119473. [Google Scholar] [CrossRef]
- Hashemipour, S.; Ahmad Panahi, H. Fabrication of magnetite nanoparticles modified with copper based metal organic framework for drug delivery system of letrozole. J. Mol. Liq. 2017, 243, 102–107. [Google Scholar] [CrossRef]
- Li, Z.; Zhao, S.; Wang, H.; Peng, Y.; Tan, Z.; Tang, B. Functional groups influence and mechanism research of UiO-66-type metal-organic frameworks for ketoprofen delivery. Colloids Surf. B Biointerfaces 2019, 178, 1–7. [Google Scholar] [CrossRef] [PubMed]
- Kim, S.N.; Park, C.G.; Huh, B.K.; Lee, S.H.; Min, C.H.; Lee, Y.Y.; Kim, Y.K.; Park, K.H.; Choy, Y.B. Metal-organic frameworks, NH2-MIL-88(Fe), as carriers for ophthalmic delivery of brimonidine. Acta Biomater. 2018, 79, 344–353. [Google Scholar] [CrossRef] [PubMed]
- Abuçafy, M.P.; Caetano, B.L.; Chiari-Andréo, B.G.; Fonseca-Santos, B.; do Santos, A.M.; Chorilli, M.; Chiavacci, L. Supramolecular cyclodextrin-based metal-organic frameworks as efficient carrier for anti-inflammatory drugs. Eur. J. Pharm. Biopharm. 2018, 127, 112–119. [Google Scholar] [CrossRef] [Green Version]
- Kritskiy, I.; Volkova, T.; Surov, A.; Terekhova, I. γ-Cyclodextrin-metal organic frameworks as efficient microcontainers for encapsulation of leflunomide and acceleration of its transformation into teriflunomide. Carbohydr. Polym. 2019, 216, 224–230. [Google Scholar] [CrossRef] [PubMed]
- Banerjee, S.; Lollar, C.T.; Xiao, Z.; Fang, Y.; Zhou, H.C. Biomedical Integration of Metal–Organic Frameworks. Trends Chem. 2020, 2, 467–479. [Google Scholar] [CrossRef]
- Singco, B.; Liu, L.H.; Chen, Y.T.; Shih, Y.H.; Huang, H.Y.; Lin, C.H. Approaches to drug delivery: Confinement of aspirin in MIL-100(Fe) and aspirin in the de novo synthesis of metal-organic frameworks. Microporous Mesoporous Mater. 2016, 223, 254–260. [Google Scholar] [CrossRef]
- Gulcay, E.; Erucar, I. Metal-Organic Frameworks for Biomedical Applications; Elsevier B.V.: Amsterdam, The Netherlands, 2020. [Google Scholar] [CrossRef]
- Rose, I.; Bezzu, C.G.; Carta, M.; Comesanã-Gándara, B.; Lasseuguette, E.; Ferrari, M.C.; Bernardo, P.; Clarizia, G.; Fuoco, A.; Jansen, J.C.; et al. Polymer ultrapermeability from the inefficient packing of 2D chains. Nat. Mater. 2017, 16, 932–937. [Google Scholar] [CrossRef]
- Carta, M.; Croad, M.; Malpass-Evans, R.; Jansen, J.C.; Bernardo, P.; Clarizia, G.; Friess, K.; Lanč, M.; McKeown, N.B. Triptycene induced enhancement of membrane gas selectivity for microporous Tröger’s base polymers. Adv. Mater. 2014, 26, 3526–3531. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Alameddine, B.; Shetty, S.; Baig, N.; Al-Mousawi, S.; Al-Sagheer, F. Synthesis and characterization of metalorganic polymers of intrinsic microporosity based on iron(II) clathrochelate. Polymer 2017, 122, 200–207. [Google Scholar] [CrossRef]
- Inoue, B.S.; Streit, S.; dos Santos Schneider, A.L.; Meier, M.M. Bioactive bacterial cellulose membrane with prolonged release of chlorhexidine for dental medical application. Int. J. Biol. Macromol. 2020, 148, 1098–1108. [Google Scholar] [CrossRef] [PubMed]
- Prabudiansyah, I.; Kusters, I.; Caforio, A.; Driessen, A.J.M. Characterization of the annular lipid shell of the Sec translocon. Biochim. Biophys. Acta Biomembr. 2015, 1848, 2050–2056. [Google Scholar] [CrossRef] [Green Version]
- LeVine, M.V.; Khelashvili, G.; Shi, L.; Quick, M.; Javitch, J.A.; Weinstein, H. Role of Annular Lipids in the Functional Properties of Leucine Transporter LeuT Proteomicelles. Biochemistry 2016, 55, 850–859. [Google Scholar] [CrossRef] [Green Version]
- Krebs, M.P.; Noorwez, S.M.; Malhotra, R.; Kaushal, S. Quality control of integral membrane proteins. Trends Biochem. Sci. 2004, 29, 648–655. [Google Scholar] [CrossRef]
- Radaic, A.; de Jesus, M.B.; Kapila, Y.L. Bacterial anti-microbial peptides and nano-sized drug delivery systems: The state of the art toward improved bacteriocins. J. Control. Release 2020, 321, 100–118. [Google Scholar] [CrossRef]
- Schramm, M.; Hestrin, S. Synthesis of Cellulose by Acetobacter xylinum. J. Bacteriol. 1954, 56, 163–166. [Google Scholar] [CrossRef] [Green Version]
- Park, J.K.; Jung, J.Y.; Park, Y.H. Cellulose production by Gluconacetobacter hansenii in a medium containing ethanol. Biotechnol. Lett. 2003, 25, 2055–2059. [Google Scholar] [CrossRef]
- Lee, K.Y.; Buldum, G.; Mantalaris, A.; Bismarck, A. More than meets the eye in bacterial cellulose: Biosynthesis, bioprocessing, and applications in advanced fiber composites. Macromol. Biosci. 2014, 14, 10–32. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Mensah, A.; Lv, P.; Narh, C.; Huang, J.; Wang, D.; Wei, Q. Sequestration of Pb(II) ions from aqueous systems with novel green bacterial cellulose graphene oxide composite. Materials 2019, 12, 218. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- De Oliveira Barud, H.G.; da Silva, R.R.; da Silva Barud, H.; Tercjak, A.; Gutierrez, J.; Lustri, W.R.; de Oliveira, O.B.; Ribeiro, S.J.L. A multipurpose natural and renewable polymer in medical applications: Bacterial cellulose. Carbohydr. Polym. 2016, 153, 406–420. [Google Scholar] [CrossRef] [Green Version]
- Gorgieva, S.; Trček, J. Bacterial cellulose: Production, modification and perspectives in biomedical applications. Nanomaterials 2019, 9, 1352. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Keshk, S.M. Bacterial Cellulose Production and its Industrial Applications. J. Bioprocess. Biotech. 2014, 4, 1–10. [Google Scholar] [CrossRef]
- Ausmees, N.; Jonsson, H.; Höglund, S.; Ljunggren, H.; Lindberg, M. Structural and putative regulatory genes involved in cellulose synthesis in Rhizobium leguminosarum bv. trifolii. Microbiology 1999, 145, 1253–1262. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Bielecki, S.; Krystynowicz, A. Marianna Bacterial. Cellulose 1989, 3, 37–46. [Google Scholar] [CrossRef] [Green Version]
- Lustri, W.R.; de Oliveira Barud, H.G.; da Silva Barud, H.; Peres, M.F.S.; Gutierrez, J.; Tercjak, A.; de Oliveira Junior, O.B.; Ribeiro, S.J.L. Microbial Cellulose. Biosynth. Mech. Med Appl. 2015, 1, 133–157. [Google Scholar]
- Czaja, W.K.; Young, D.J.; Kawecki, M.; Brown, R.M. The future prospects of microbial cellulose in biomedical applications. Biomacromolecules 2007, 8, 1–12. [Google Scholar] [CrossRef]
- Zhang, L.K.; Du, S.; Wang, X.; Jiao, Y.; Yin, L.; Zhang, Y.; Guan, Y.Q. Bacterial cellulose based composites enhanced transdermal drug targeting for breast cancer treatment. Chem. Eng. J. 2019, 370, 749–759. [Google Scholar] [CrossRef]
- Chawla, P.R.; Bajaj, I.B.; Survase, S.A.; Singhal, R.S. Microbial cellulose: Fermentative production and applications. Food Technol. Biotechnol. 2009, 47, 107–124. [Google Scholar]
- Shi, J.; Votruba, A.R.; Farokhzad, O.C.; Langer, R. Nanotechnology in Drug Delivery and Tissue Engineering: From Discovery to Applications. Nano Lett. 2010, 10, 3223–3230. [Google Scholar] [CrossRef] [Green Version]
- Mohammadi, H.; Boughner, D.; Millon, L.E.; Wan, W.K. Design and simulation of a poly(vinyl alcohol)-bacterial cellulose nanocomposite mechanical aortic heart valve prosthesis. Proc. Inst. Mech. Eng. Part H J. Eng. Med. 2009, 223, 697–711. [Google Scholar] [CrossRef]
- Lee, S.E.; Park, Y.S. The role of bacterial cellulose in artificial blood vessels. Mol. Cell. Toxicol. 2017, 13, 257–261. [Google Scholar] [CrossRef]
- Zang, S.; Zhang, R.; Chen, H.; Lu, Y.; Zhou, J.; Chang, X.; Qiu, G.; Wu, Z.; Yang, G. Investigation on artificial blood vessels prepared from bacterial cellulose. Mater. Sci. Eng. C 2015, 46, 111–117. [Google Scholar] [CrossRef]
- Klemm, D.; Schumann, D.; Udhardt, U.; Marsch, S. Bacterial synthesized cellulose—Artificial blood vessels for microsurgery. Prog. Polym. Sci. 2001, 26, 1561–1603. [Google Scholar] [CrossRef]
- Scherner, M.; Reutter, S.; Klemm, D.; Sterner-Kock, A.; Guschlbauer, M.; Richter, T.; Langebartels, G.; Madershahian, N.; Wahlers, T.; Wippermann, J. In vivo application of tissue-engineered blood vessels of bacterial cellulose as small arterial substitutes: Proof of concept? J. Surg. Res. 2014, 189, 340–347. [Google Scholar] [CrossRef]
- Levinson, D.; Glonek, T. (12) United States Patent (10) Patent No.: (45) Date of Patent: I The city, Ritact lens from Glucon. Microb. Cellul. Contact Lens 2010, 2, 1–6. [Google Scholar]
- Han, Y.; Li, C.; Cai, Q.; Bao, X.; Tang, L.; Ao, H.; Liu, J.; Jin, M.; Zhou, Y.; Wan, Y.; et al. Studies on bacterial cellulose/poly vinyl alcohol (BC/PVA) hydrogel composites as tissue-engineered corneal stroma. Biomed. Mater. 2019, 1–31, in press. [Google Scholar]
- Jia, H.; Jia, Y.; Wang, J.; Hu, Y.; Zhang, Y.; Jia, S. Potentiality of Bacterial Cellulose as the Scaffold of Tissue Engineering of Cornea. In Proceedings of the 2009 2nd International Conference on Biomedical Engineering and Informatics, Tianjin, China, 17–19 October 2009; Volume 79–82, pp. 147–150. [Google Scholar] [CrossRef]
- Sepúlveda, R.V.; Valente, F.L.; Reis, E.C.C.; Araújo, F.R.; Eleotério, R.B.; Queiroz, P.V.S.; Borges, A.P.B. Bacterial cellulose and bacterial cellulose/polycaprolactone composite as tissue substitutes in rabbits’ cornea. Pesqui. Vet. Bras. 2016, 6, 986–992. [Google Scholar] [CrossRef] [Green Version]
- Fu, L.; Zhang, Y.; Li, C.; Wu, Z.; Zhuo, Q.; Huang, X.; Qiu, G.; Zhou, P.; Yang, G. Skin tissue repair materials from bacterial cellulose by a multilayer fermentation method. J. Mater. Chem. 2012, 22, 12349–12357. [Google Scholar] [CrossRef]
- Hou, Y.; Wang, X.; Yang, J.; Zhu, R.; Zhang, Z.; Li, Y. Development and biocompatibility evaluation of biodegradable bacterial cellulose as a novel peripheral nerve scaffold. J. Biomed. Mater. Res. Part A 2018, 106, 1288–1298. [Google Scholar] [CrossRef]
- Hickey, R.J.; Pelling, A.E. Cellulose biomaterials for tissue engineering. Front. Bioeng. Biotechnol. 2019, 7, 45. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Bačáková, L.; Novotná, K.; Pařzek, M. Polysaccharides as cell carriers for tissue engineering: The use of cellulose in vascular wall reconstruction. Physiol. Res. 2014, 63, S29–S47. [Google Scholar] [CrossRef] [PubMed]
- Kowalska-Ludwicka, K.; Cala, J.; Grobelski, B.; Sygut, D.; Jesionek-Kupnicka, D.; Kolodziejczyk, M.; Bielecki, S.; Pasieka, Z. Modified bacterial cellulose tubes for regeneration of damaged peripheral nerves. Arch. Med. Sci. 2013, 9, 527–534. [Google Scholar] [CrossRef] [PubMed]
- Binnetoglu, A.; Demir, B.; Akakin, D.; Kervancioglu Demirci, E.; Batman, C. Bacterial cellulose tubes as a nerve conduit for repairing complete facial nerve transection in a rat model. Eur. Arch. Oto-Rhino-Laryngol. 2020, 277, 277–283. [Google Scholar] [CrossRef]
- Xu, C.; Ma, X.; Chen, S.; Tao, M.; Yuan, L.; Jing, Y. Bacterial cellulose membranes used as artificial substitutes for dural defection in rabbits. Int. J. Mol. Sci. 2014, 15, 10855–10867. [Google Scholar] [CrossRef] [Green Version]
- De Lima, F.d.M.T.; Pinto, F.C.M.; da Silveira Andrade-da-Costa, B.L.; da Silva, J.G.M.; Campos Júnior, O.; de Andrade Aguiar, J.L. Biocompatible bacterial cellulose membrane in dural defect repair of rat. J. Mater. Sci. Mater. Med. 2017, 28, 37. [Google Scholar] [CrossRef] [PubMed]
- Wei, B.; Yang, G.; Hong, F. Preparation and evaluation of a kind of bacterial cellulose dry films with antibacterial properties. Carbohydr. Polym. 2011, 84, 533–538. [Google Scholar] [CrossRef]
- Hutchens, S.A.; Benson, R.S.; Evans, B.R.; O’Neill, H.M.; Rawn, C.J. Biomimetic synthesis of calcium-deficient hydroxyapatite in a natural hydrogel. Biomaterials 2006, 27, 4661–4670. [Google Scholar] [CrossRef]
- Liu, H.; Hu, Y.; Zhu, Y.; Wu, X.; Zhou, X.; Pan, H.; Chen, S.; Tian, P. A simultaneous grafting/vinyl polymerization process generates a polycationic surface for enhanced antibacterial activity of bacterial cellulose. Int. J. Biol. Macromol. 2020, 143, 224–234. [Google Scholar] [CrossRef] [PubMed]
- Ye, S.; Jiang, L.; Wu, J.; Su, C.; Huang, C.; Liu, X.; Shao, W. Flexible Amoxicillin-Grafted Bacterial Cellulose Sponges for Wound Dressing: In Vitro and In Vivo Evaluation. ACS Appl. Mater. Interfaces 2018, 10, 5862–5870. [Google Scholar] [CrossRef]
- Schramm, M.; Gromet, Z.; Hestrin, S. Synthesis of cellulose by Acetobacter xylinum. Biochem. J. 1957, 67, 669–679. [Google Scholar] [CrossRef] [Green Version]
- Colvin, J.R.; Beer, M. The formation of cellulose microfibrils in suspensions of Acetobacter xylinum. Can. J. Microbiol. 1960, 6, 631–637. [Google Scholar] [CrossRef]
- Terawaki, S.S.; Taguchi, A.; Kawamata, J. Synthesis of Cellulose in Ethanol Extracts of Acetobacter xylinum. Nature 1959, 183, 1135–1136. [Google Scholar]
- Narh, C.; Frimpong, C.; Mensah, A.; Wei, Q. Rice Bran, an Alternative Nitrogen Source for Acetobacter xylinum Bacterial Cellulose Synthesis. Bioresources 2018, 13, 4346–4363. [Google Scholar] [CrossRef]
- Park, J.K.; Park, Y.H.; Jung, J.Y. Production of bacterial cellulose by Gluconacetobacter hansenif PJK isolated from rotten apple. Biotechnol. Bioprocess. Eng. 2003, 8, 83–88. [Google Scholar] [CrossRef]
- Dellaglio, F.; Cleenwerck, I.; Felis, G.E.; Engelbeen, K.; Janssens, D.; Marzotto, M. Description of Gluconacetobacter swingsii sp. nov. and Gluconacetobacter rhaeticus sp. nov., isolated from Italian apple fruit. Int. J. Syst. Evol. Microbiol. 2005, 55, 2365–2370. [Google Scholar] [CrossRef] [Green Version]
- Matthysse, A.G. Role of bacterial cellulose fibrils in Agrobacterium tumefaciens infection. J. Bacteriol. 1983, 154, 906–915. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Cannon, R.E.; Anderson, S.M. Biogenesis of Bacterial Cellulose. Crit. Rev. Microbiol. 1991, 17, 435–447. [Google Scholar] [CrossRef] [PubMed]
- Farag, S.; Asker, M.M.S.; Mahmoud, M.G.; Ibrahim, H.; Amr, A. Comparative study for bacterial cellulose production Using Egyptian Achromobacter sp. Res. J. Pharm. Biol. Chem. Sci. 2016, 7, 954–969. [Google Scholar]
- Robledo, M.; Rivera, L.; Jiménez-Zurdo, J.I.; Rivas, R.; Dazzo, F.; Velázquez, E.; Martínez-Molina, E.; Hirsch, A.M.; Mateos, P.F. Role of Rhizobium endoglucanase CelC2 in cellulose biosynthesis and biofilm formation on plant roots and abiotic surfaces. Microb. Cell Factories 2012, 11, 125. [Google Scholar] [CrossRef] [Green Version]
- Tanaka, M.; Murakami, S.; Shinke, R.; Aoki, K. Genetic characteristics of cellulose-forming acetic acid bacteria identified phenotypically as Gluconacetobacter xylinus. Biosci. Biotechnol. Biochem. 2014, 64, 757–760. [Google Scholar] [CrossRef]
- Ross, P.; Mayer, R.; Benziman, M. Cellulose biosynthesis and function in bacteria. Microbiol. Rev. 1991, 55, 35–58. [Google Scholar] [CrossRef]
- Sharma, C.; Bhardwaj, N.K. Bacterial nanocellulose: Present status, biomedical applications and future perspectives. Mater. Sci. Eng. C 2019, 104, 109963. [Google Scholar] [CrossRef]
- Bae, S.; Sugano, Y.; Shoda, M. Improvement of Bacterial Cellulose Production by Addition of Agar in a Jar Fermentor. J. Biosci. Bioeng. 2004, 97, 33–38. [Google Scholar] [CrossRef]
- Hwang, J.W.; Yang, Y.K.; Hwang, J.K.; Pyun, Y.R.; Kim, Y.S. Effects of pH and dissolved oxygen on cellulose production by Acetobacter xylinum BRC5 in agitated culture. J. Biosci. Bioeng. 1999, 88, 183–188. [Google Scholar] [CrossRef]
- Wu, J.; Liu, R. Thin stillage supplementation greatly enhances bacterial cellulose production by Gluconacetobacter xylinus. Carbohydr. Polym. 2012, 90, 116–121. [Google Scholar] [CrossRef]
- Cheng, K.; Catchmark, M.; Demirci, A. Effects of CMC Addition on Bacterial Cellulose Production in a Biofilm Reactor and Its Paper Sheets Analysis. Biomacromolecules 2011, 12, 730–736. [Google Scholar] [CrossRef] [PubMed]
- Cavka, A.; Guo, X.; Tang, S.J.; Winestrand, S.; Jönsson, L.J.; Hong, F. Production of bacterial cellulose and enzyme from waste fiber sludge. Biotechnol. Biofuels 2013, 6, 25. [Google Scholar] [CrossRef] [Green Version]
- Moosavi-Nasab, M.; Yousefi, A. Biotechnological production of cellulose by Gluconacetobacter xylinus from agricultural waste. Iran. J. Biotechnol. 2011, 9, 94–101. [Google Scholar]
- Chao, Y.; Ishida, T.; Sugano, Y.; Shoda, M. Bacterial cellulose production by Acetobacter xylinum in a 50-L internal-loop airlift reactor. Biotechnol. Bioeng. 2000, 68, 345–352. [Google Scholar] [CrossRef]
- Keshk, S.; Sameshima, K. Influence of lignosulfonate on crystal structure and productivity of bacterial cellulose in a static culture. Enzym. Microb. Technol. 2006, 40, 4–8. [Google Scholar] [CrossRef]
- Son, H.; Kim, H.; Kim, K.; Kim, H.; Kim, Y.; Lee, S. Increased production of bacterial cellulose by Acetobacter sp. V6 in synthetic media under shaking culture conditions. Bioresour. Technol. 2003, 86, 215–219. [Google Scholar] [CrossRef]
- Son, H.-J.; Heo, M.-S.; Kim, Y.-G.; Lee, S.-J. Optimization of fermentation conditions for the production of bacterial cellulose by a newly isolated Acetobacter sp. A9 in shaking cultures. Biotechnol. Appl. Biochem. 2001, 33, 1–5. [Google Scholar] [CrossRef] [PubMed]
- Keshk, S.; Sameshima, K. The utilization of sugar cane molasses with/without the presence of lignosulfonate for the production of bacterial cellulose. Appl. Microbiol. Biotechnol. 2006, 72, 291–296. [Google Scholar] [CrossRef]
- Seto, A.; Saito, Y.; Matsushige, M.; Kobayashi, H.; Sasaki, Y.; Tonouchi, N.; Tsuchida, T.; Yoshinaga, F.; Ueda, K.; Beppu, T. Effective cellulose production by a coculture of Gluconacetobacter xylinus and Lactobacillus mali. Appl. Microbiol. Biotechnol. 2006, 73, 915–921. [Google Scholar] [CrossRef]
- Zahan, K.A.; Pa’e, N.; Muhamad, I.I. An evaluation of fermentation period and discs rotation speed of rotary discs reactor for bacterial cellulose production. Sains Malays. 2016, 45, 393–400. [Google Scholar]
- Hungund, B. Production of Bacterial Cellulose from Gluconacetobacter persimmonis GH-2 using Dual and Cheaper Carbon Sources. J. Microb. Biochem. Technol. 2013, 5, 31–33. [Google Scholar] [CrossRef] [Green Version]
- Ross, P.; Benziman, M.; de Vroom, E.; Fidder, A.; Van Boom, J.H. The Cyclic Diguanylic Acid Regulatory in Acetobacter xylinum System of Cellulose Synthesis. Biochemistry 1990, 265, 18933–18943. [Google Scholar]
- Narh, C.; Charles, F.; Mensah, A.; Qufu, W. Synthesis of highly stable bacterial cellulosic pocket for drug storage. Carbohydr. Polym. 2019, 206, 625–632. [Google Scholar] [CrossRef] [PubMed]
- Gao, M.; Li, J.; Bao, Z.; Hu, M.; Nian, R.; Feng, D.; An, D.; Li, X.; Xian, M.; Zhang, H. A natural in situ fabrication method of functional bacterial cellulose using a microorganism. Nat. Commun. 2019, 10, 437. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Jacek, P.; Dourado, F.; Gama, M.; Bielecki, S. Molecular aspects of bacterial nanocellulose biosynthesis. Microb. Biotechnol. 2019, 12, 633–649. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Valla, S.; Coucheron, D.H.; Fjærvik, E.; Kjosbakken, J.; Weinhouse, H.; Ross, P.; Amikam, D.; Benziman, M. Cloning of a gene involved in cellulose biosynthesis in Acetobacter xylinum: Complementation of cellulose-negative mutants by the UDPG pyrophosphorylase structural gene. MGG Mol. Gen. Genet. 1989, 217, 26–30. [Google Scholar] [CrossRef]
- van Zyl, E.M.; Coburn, J.M. Hierarchical structure of bacterial-derived cellulose and its impact on biomedical applications. Curr. Opin. Chem. Eng. 2019, 24, 122–130. [Google Scholar] [CrossRef]
- Brown, R.M., Jr.; Willison, J.H.; Richardson, C.L. Cellulose biosynthesis in Acetobacter xylinum: Visualization of the site of synthesis and direct measurement of the in vivo process. Proc. Natl. Acad. Sci. USA 1976, 73, 4565–4569. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Tokoh, C.; Takabe, K.; Sugiyama, J.; Fujita, M. Cellulose synthesized by Acetobacter xylinum in the presence of plant cell wall polysaccharides. Cellulose 2002, 9, 65–74. [Google Scholar] [CrossRef]
- Watanabe, K.; Tabuchi, M.; Morinaga, Y.; Yoshinaga, F. Structural features and properties of bacterial cellulose produced in agitated culture. Cellulose 1998, 5, 187–200. [Google Scholar] [CrossRef]
- Fontana, J.D.; Koop, H.S.; Tiboni, M.; Grzybowski, A.; Pereira, A.; Kruger, C.D.; da Silva, M.G.R.; Wielewski, L.P. New Insights on Bacterial Cellulose; Elsevier Inc.: Amsterdam, The Netherlands, 2017. [Google Scholar] [CrossRef]
- Mirtalebi, S.S.; Almasi, H.; Alizadeh Khaledabad, M. Physical, morphological, antimicrobial and release properties of novel MgO-bacterial cellulose nanohybrids prepared by in-situ and ex-situ methods. Int. J. Biol. Macromol. 2019, 128, 848–857. [Google Scholar] [CrossRef] [PubMed]
- Kadivar, N.; Tavanai, H.; Allafchian, A. Fabrication of cellulose nanoparticles through electrospraying. IET Nanobiotechnol. 2018, 12, 807–813. [Google Scholar] [CrossRef]
- Amin, M.C.I.M.; Abadi, A.G.; Katas, H. Purification, characterization and comparative studies of spray-dried bacterial cellulose microparticles. Carbohydr. Polym. 2014, 99, 180–189. [Google Scholar] [CrossRef] [PubMed]
- Luo, H.; Dong, J.; Yao, F.; Yang, Z.; Li, W.; Wang, J.; Xu, X.; Hu, J.; Wan, Y. Layer-by-Layer Assembled Bacterial Cellulose/Graphene Oxide Hydrogels with Extremely Enhanced Mechanical Properties. Nano-Micro Lett. 2018, 10, 42. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Luo, H.; Xie, J.; Wang, J.; Yao, F.; Yang, Z.; Wan, Y. Step-by-step self-assembly of 2D few-layer reduced graphene oxide into 3D architecture of bacterial cellulose for a robust, ultralight, and recyclable all-carbon absorbent. Carbon N. Y. 2018, 139, 824–832. [Google Scholar] [CrossRef]
- Pircher, N.; Veigel, S.; Aigner, N.; Nedelec, J.M.; Rosenau, T.; Liebner, F. Reinforcement of bacterial cellulose aerogels with biocompatible polymers. Carbohydr. Polym. 2014, 111, 505–513. [Google Scholar] [CrossRef] [PubMed]
- Zakaria, M.N.; Hishammuddin, N.; Fairos, N.N.; Syafiq, M.N. Effect of Silane Concentration on the Physical and Mechanical Properties of Bacterial Cellulose Silylated Aerogels. Int. J. Eng. Technol. 2018, 7, 242–246. [Google Scholar]
- Ramli, S.; Ja’afar, S.M.; Sisak, M.A.A.; Zainuddin, N.; Rahman, I.A. Formulation and physical characterization of microemulsions based carboxymethyl cellulose as vitamin c carrier. Malays. J. Anal. Sci. 2015, 19, 275–283. [Google Scholar]
- Shezad, O.; Khan, S.; Khan, T.; Park, J.K. Physicochemical and mechanical characterization of bacterial cellulose produced with an excellent productivity in static conditions using a simple fed-batch cultivation strategy. Carbohydr. Polym. 2010, 82, 173–180. [Google Scholar] [CrossRef]
- Kralisch, D.; Hessler, N.; Klemm, D.; Erdmann, R.; Schmidt, W. White biotechnology for cellulose manufacturing—The HoLiR concept. Biotechnol. Bioeng. 2010, 105, 740–747. [Google Scholar] [CrossRef] [PubMed]
- Song, H.J.; Li, H.; Seo, J.H.; Kim, M.J.; Kim, S.J. Pilot-scale production of bacterial cellulose by a spherical type bubble column bioreactor using saccharified food wastes. Korean J. Chem. Eng. 2009, 26, 141–146. [Google Scholar] [CrossRef]
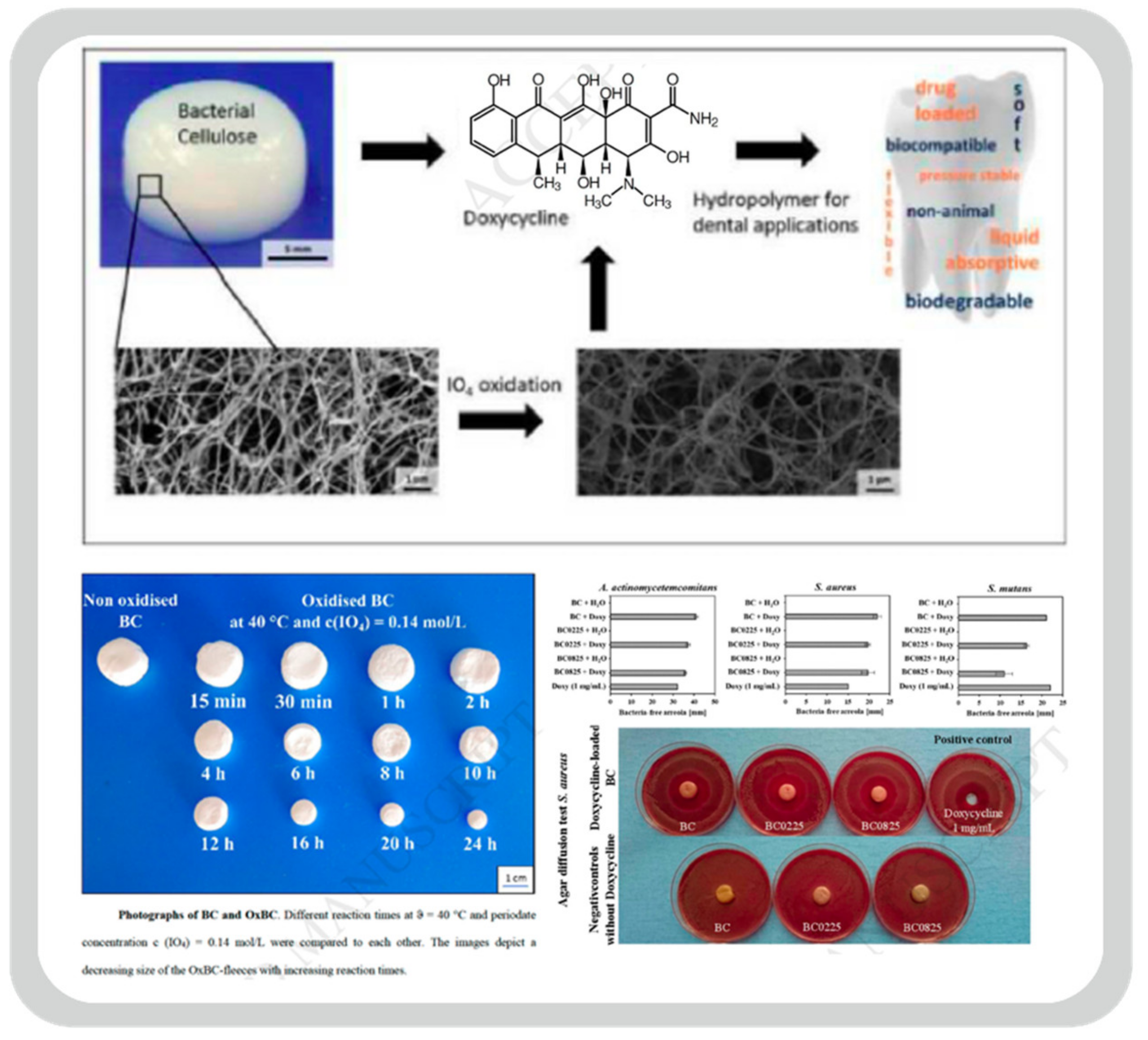
- Weyell, P.; Beekmann, U.; K¨upper, C.; Dederichs, M.; Thamm, J.; Fischer, D.; Kralisch, D. Tailor-made material characteristics of bacterial cellulose for drug delivery applications in dentistry. Carbohydr. Polym. 2019, 207, 1–10. [Google Scholar] [CrossRef]
- Luo, H.; Ao, H.; Li, G.; Li, W.; Xiong, G.; Zhu, Y.; Wan, Y. Bacterial cellulose/graphene oxide nanocomposite as a novel drug delivery system. Curr. Appl. Phys. 2017, 17, 249–254. [Google Scholar] [CrossRef]
- Faria, M.; Vilela, C.; Mohammadkazemi, F.; Silvestre, A.J.D.; Freire, C.S.R.; Cordeiro, N. Poly(glycidyl methacrylate)/bacterial cellulose nanocomposites: Preparation, characterization and post-modification. Int. J. Biol. Macromol. 2019, 127, 618–627. [Google Scholar] [CrossRef]
- Ciechańska, D. Multifunctional bacterial cellulose/chitosan composite materials for medical applications. Fibres Text. East. Eur. 2004, 12, 69–72. [Google Scholar]
- Romanov, D.P.; Khripunov, A.K.; Baklagina, Y.G.; Severin, A.V.; Lukasheva, N.V.; Tolmachev, D.A.; Lavrent’Ev, V.K.; Tkachenko, A.A.; Arkharova, N.A.; Klechkovskaya, V.V. Nanotextures of composites based on the interaction between hydroxyapatite and cellulose Gluconacetobacter xylinus. Glass Phys. Chem. 2014, 40, 367–374. [Google Scholar] [CrossRef]
- Arias, S.L.; Shetty, A.R.; Senpan, A.; Echeverry-Rendón, M.; Reece, L.M.; Allain, J.P. Fabrication of a functionalized magnetic bacterial nanocellulose with iron oxide nanoparticles. J. Vis. Exp. 2016, 2016, 52951. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Torgbo, S.; Sukyai, P. Fabrication of microporous bacterial cellulose embedded with magnetite and hydroxyapatite nanocomposite scaffold for bone tissue engineering. Mater. Chem. Phys. 2019, 237, 121868. [Google Scholar] [CrossRef]
- De Lima Fontes, M.; Meneguin, A.B.; Tercjak, A.; Gutierrez, J.; Cury, B.S.F.; dos Santos, A.M.; Ribeiro, S.J.L.; Barud, H.S. Effect of in situ modification of bacterial cellulose with carboxymethylcellulose on its nano/microstructure and methotrexate release properties. Carbohydr. Polym. 2018, 179, 126–134. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Zhu, W.; Li, W.; He, Y.; Duan, T. In-situ biopreparation of biocompatible bacterial cellulose/graphene oxide composites pellets. Appl. Surf. Sci. 2015, 338, 22–26. [Google Scholar] [CrossRef]
- Stoica-Guzun, A.; Stroescu, M.; Tache, F.; Zaharescu, T.; Grosu, E. Effect of electron beam irradiation on bacterial cellulose membranes used as transdermal drug delivery systems. Nucl. Instrum. Methods Phys. Res. Sect. B Beam Interact. Mater. At. 2007, 265, 434–438. [Google Scholar] [CrossRef]
- Badshah, M.; Ullah, H.; Khan, A.R.; Khan, S.; Park, J.K.; Khan, T. Surface modification and evaluation of bacterial cellulose for drug delivery. Int. J. Biol. Macromol. 2018, 113, 526–533. [Google Scholar] [CrossRef]
- Faisul Aris, F.A.; Mohd Fauzi, F.N.A.; Tong, W.Y.; Syed Abdullah, S.S. Interaction of silver sulfadiazine wıth bacterial cellulose via ex-situ modification method as an alternative diabetic wound healing. Biocatal. Agric. Biotechnol. 2019, 21, 101332. [Google Scholar] [CrossRef]
- Malmir, S.; Karbalaei, A.; Pourmadadi, M.; Hamedi, J.; Yazdian, F.; Navaee, M. Antibacterial properties of a bacterial cellulose CQD-TiO2 nanocomposite. Carbohydr. Polym. 2020, 234, 115835. [Google Scholar] [CrossRef] [PubMed]
- Cacicedo, M.L.; Pacheco, G.; Islan, G.A.; Alvarez, V.A.; Barud, H.S.; Castro, G.R. Chitosan-bacterial cellulose patch of ciprofloxacin for wound dressing: Preparation and characterization studies. Int. J. Biol. Macromol. 2020, 147, 1136–1145. [Google Scholar] [CrossRef]
- Sulaeva, I.; Hettegger, H.; Bergen, A.; Rohrer, C.; Kostic, M.; Konnerth, J.; Rosenau, T.; Potthast, A. Fabrication of bacterial cellulose-based wound dressings with improved performance by impregnation with alginate. Mater. Sci. Eng. C 2020, 110, 110619. [Google Scholar] [CrossRef]
- Volova, T.G.; Shumilova, A.A.; Nikolaeva, E.D.; Kirichenko, A.K.; Shishatskaya, E.I. Biotechnological wound dressings based on bacterial cellulose and degradable copolymer P(3HB/4HB). Int. J. Biol. Macromol. 2019, 131, 230–240. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Zahan, K.A.; Azizul, N.M.; Mustapha, M.; Tong, W.Y.; Abdul Rahman, M.S.; Sahuri, I.S. Application of bacterial cellulose film as a biodegradable and antimicrobial packaging material. Mater. Today Proc. 2020, 31, 83–88. [Google Scholar] [CrossRef]
- Zhijiang, C.; Guang, Y. Bacterial Cellulose/Collagen Composite: Characterization and First Evaluation of Cytocompatibility. J. Appl. Polym. Sci. 2011, 120, 2938–2944. [Google Scholar] [CrossRef]
- Treesuppharat, W.; Rojanapanthu, P.; Siangsanoh, C.; Manuspiya, H.; Ummartyotin, S. Synthesis and characterization of bacterial cellulose and gelatin-based hydrogel composites for drug-delivery systems. Biotechnol. Rep. 2017, 15, 84–91. [Google Scholar] [CrossRef] [PubMed]
- Khamrai, M.; Banerjee, S.L.; Paul, S.; Samanta, S.; Kundu, P.P. Curcumin entrapped gelatin/ionically modified bacterial cellulose based self-healable hydrogel film: An eco-friendly sustainable synthesis method of wound healing patch. Biol. Macromol. 2019, 122, 940–953. [Google Scholar] [CrossRef] [PubMed]
- Amin, M.C.I.M.; Ahmad, N.; Halib, N.; Ahmad, I. Synthesis and characterization of thermo- and pH-responsive bacterial cellulose/acrylic acid hydrogels for drug delivery. Carbohydr. Polym. 2012, 88, 465–473. [Google Scholar] [CrossRef]
- López De Dicastillo, C.; Rodríguez, F.; Guarda, A.; Galotto, M.J. Antioxidant films based on cross-linked methyl cellulose and native Chilean berry for food packaging applications. Carbohydr. Polym. 2016, 136, 1052–1060. [Google Scholar] [CrossRef] [PubMed]
- Sun, S.; Liu, P.; Ji, N.; Hou, H.; Dong, H. Effects of various cross-linking agents on the physicochemical properties of starch/PHA composite films produced by extrusion blowing. Food Hydrocoll. 2018, 77, 964–975. [Google Scholar] [CrossRef]
- Liang, J.; Wang, R.; Chen, R. The impact of cross-linking mode on the physical and antimicrobial properties of a chitosan/bacterial cellulose composite. Polymers 2019, 11, 491. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Quero, F.; Nogi, M.; Lee, K.Y.; Poel, G.V.; Bismarck, A.; Mantalaris, A.; Yano, H.; Eichhorn, S.J. Cross-linked bacterial cellulose networks using glyoxalization. ACS Appl. Mater. Interfaces 2011, 3, 490–499. [Google Scholar] [CrossRef] [PubMed]
- Liu, Y.; Zhou, J.; Zhu, E.; Tang, J.; Liu, X.; Tang, W. Facile synthesis of bacterial cellulose fibers covalently intercalated graphene oxide by one-step cross-linking for robust supercapacitors. J. Mater. Chem. C 2014, 3, 1011–1017. [Google Scholar] [CrossRef]
- Kirdponpattara, S.; Phisalaphong, M.; Kongruang, S. Gelatin-bacterial cellulose composite sponges thermally cross-linked with glucose for tissue engineering applications. Carbohydr. Polym. 2017, 177, 361–368. [Google Scholar] [CrossRef]
- Brown, E.E.; Laborie, M.P.G.; Zhang, J. Glutaraldehyde treatment of bacterial cellulose/fibrin composites: Impact on morphology, tensile and viscoelastic properties. Cellulose 2012, 19, 127–137. [Google Scholar] [CrossRef]
- Pandey, M.; Amin, M.C.I.M. Accelerated preparation of novel bacterial cellulose/acrylamide-based hydrogel by microwave irradiation. Int. J. Polym. Mater. Polym. Biomater. 2013, 62, 402–405. [Google Scholar] [CrossRef]
- Coelho, F.; Cavicchioli, M.; Specian, S.S.; Scarel-Caminaga, R.M.; de Aquino Penteado, L.; de Medeiros, A.I.; de Lima Ribeiro, S.J.; de Oliveira Capote, T.S. Bacterial cellulose membrane functionalized with hydroxiapatite and anti-bone morphogenetic protein 2: A promising material for bone regeneration. PLoS ONE 2019, 14, e0221286. [Google Scholar] [CrossRef]
- Petrauskaite, O.; Gomes, P.D.S.; Fernandes, M.H.; Juodzbalys, G.; Stumbras, A.; Maminskas, J.; Liesiene, J.; Cicciù, M. Biomimetic mineralization on a macroporous cellulose-based matrix for bone regeneration. BioMed Res. Int. 2013, 2013, 452750. [Google Scholar] [CrossRef]
- Yin, N.; Chen, S.Y.; Ouyang, Y.; Tang, L.; Yang, J.X.; Wang, H.P. Biomimetic mineralization synthesis of hydroxyapatite bacterial cellulose nanocomposites. Prog. Nat. Sci. Mater. Int. 2011, 21, 472–477. [Google Scholar] [CrossRef] [Green Version]
- Tolmachev, D.A.; Lukasheva, N.V. Study of the process of mineralization of nanofibrils of native bacterial cellulose in solutions of mineral ions: Modeling via the method of molecular dynamics. Polym. Sci. Ser. A 2014, 56, 545–557. [Google Scholar] [CrossRef]
- Nge, T.T.; Sugiyama, J. Surface functional group dependent apatite formation on bacterial cellulose microfibrils network in a simulated body fluid. J. Biomed. Mater. Res. Part A 2006, 81, 124–134. [Google Scholar] [CrossRef] [PubMed]
- Schlufter, K.; Schmauder, H.P.; Dorn, S.; Heinze, T. Efficient homogeneous chemical modification of bacterial cellulose in the ionic liquid 1-N-butyl-3-methylimidazolium chloride. Macromol. Rapid Commun. 2006, 27, 1670–1676. [Google Scholar] [CrossRef]
- Lee, K.Y.; Blaker, J.J.; Bismarck, A. Surface functionalisation of bacterial cellulose as the route to produce green polylactide nanocomposites with improved properties. Compos. Sci. Technol. 2009, 69, 2724–2733. [Google Scholar] [CrossRef] [Green Version]
- Lee, K.Y.; Bismarck, A. Susceptibility of never-dried and freeze-dried bacterial cellulose towards esterification with organic acid. Cellulose 2012, 19, 891–900. [Google Scholar] [CrossRef] [Green Version]
- Lin, Y.T.; Yu, C.J.; Zhu, L.; Yin, X.Q.; Lao, B.S.; Lin, Q. Synthesis and characterization of alkyl bacterial cellulose through etherification with alkyl bromide in DMAc/LiCL. Appl. Mech. Mater. 2013, 320, 478–482. [Google Scholar] [CrossRef]
- Stenstad, P.; Andresen, M.; Tanem, B.S.; Stenius, P. Chemical surface modifications of microfibrillated cellulose. Cellulose 2008, 15, 35–45. [Google Scholar] [CrossRef]
- Tomé, L.C.; Pinto, R.J.B.; Trovatti, E.; Freire, C.S.R.; Silvestre, A.J.D.; Neto, C.P.; Gandini, A. Transparent bionanocomposites with improved properties prepared from acetylated bacterial cellulose and poly(lactic acid) through a simple approach. Green Chem. 2011, 13, 419–427. [Google Scholar] [CrossRef] [Green Version]
- Zhu, L.Y.; Lin, D.Q.; Yao, S.J. Biodegradation of polyelectrolyte complex films composed of chitosan and sodium cellulose sulfate as the controllable release carrier. Carbohydr. Polym. 2010, 82, 323–328. [Google Scholar] [CrossRef]


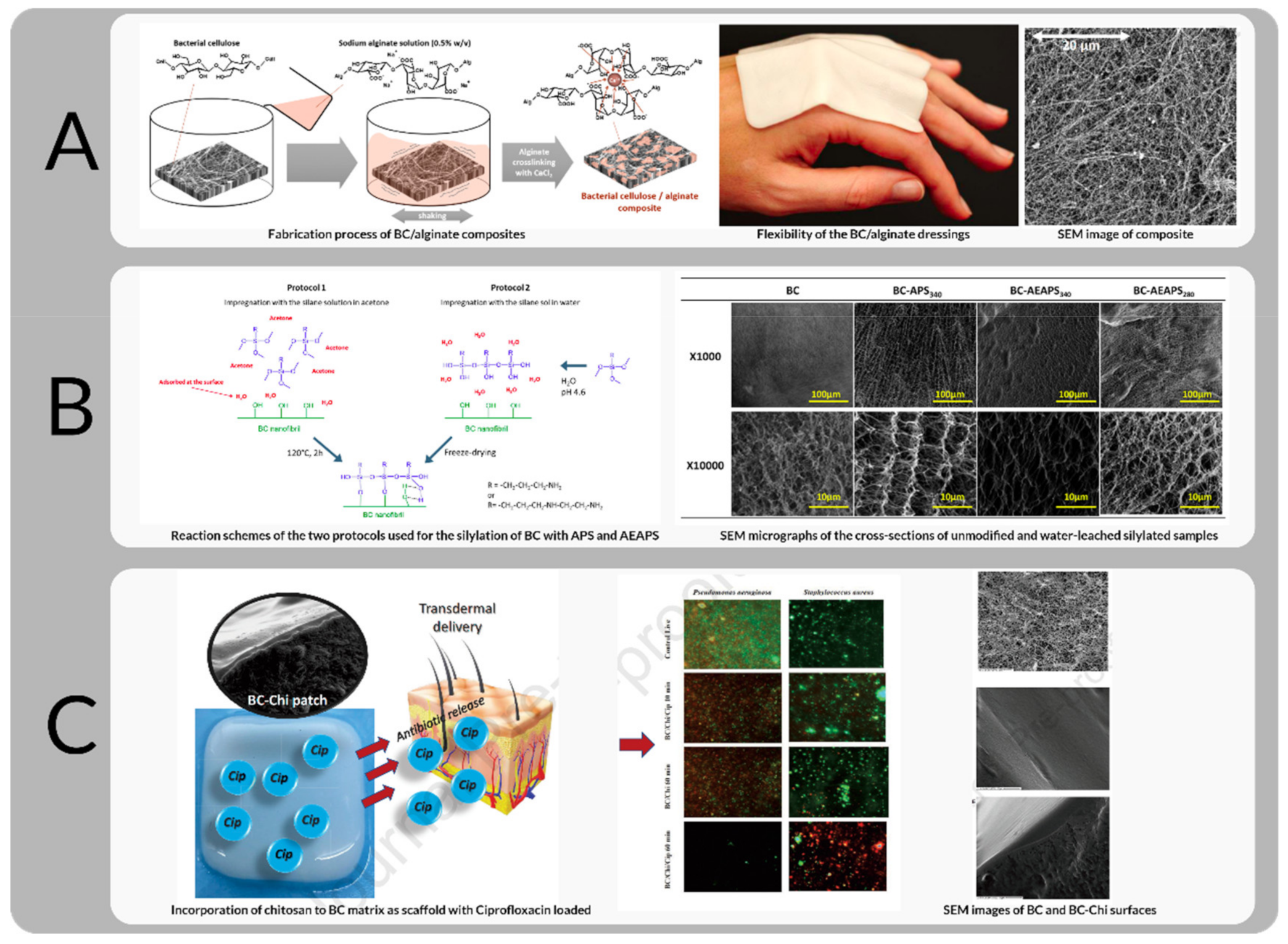
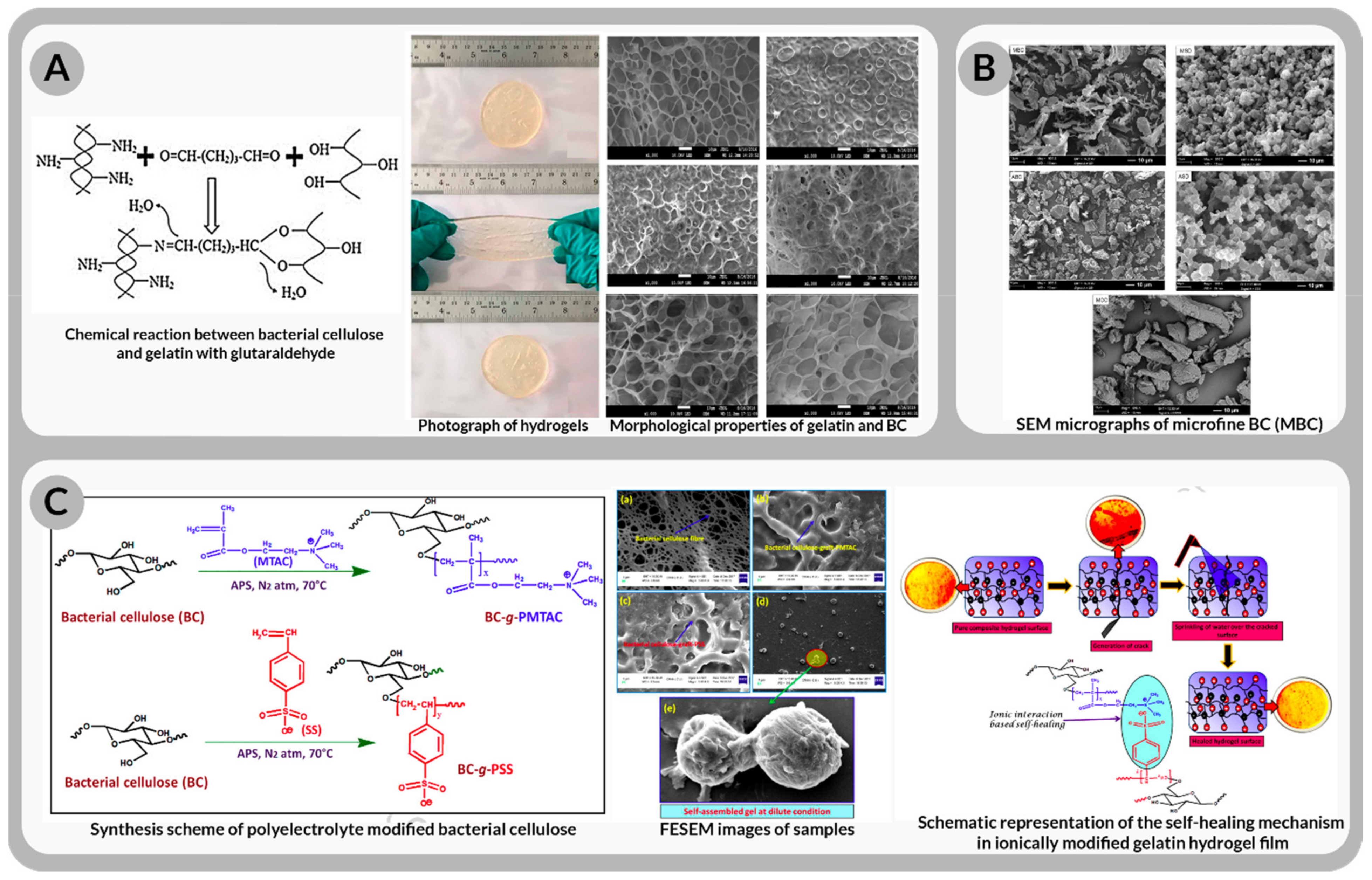
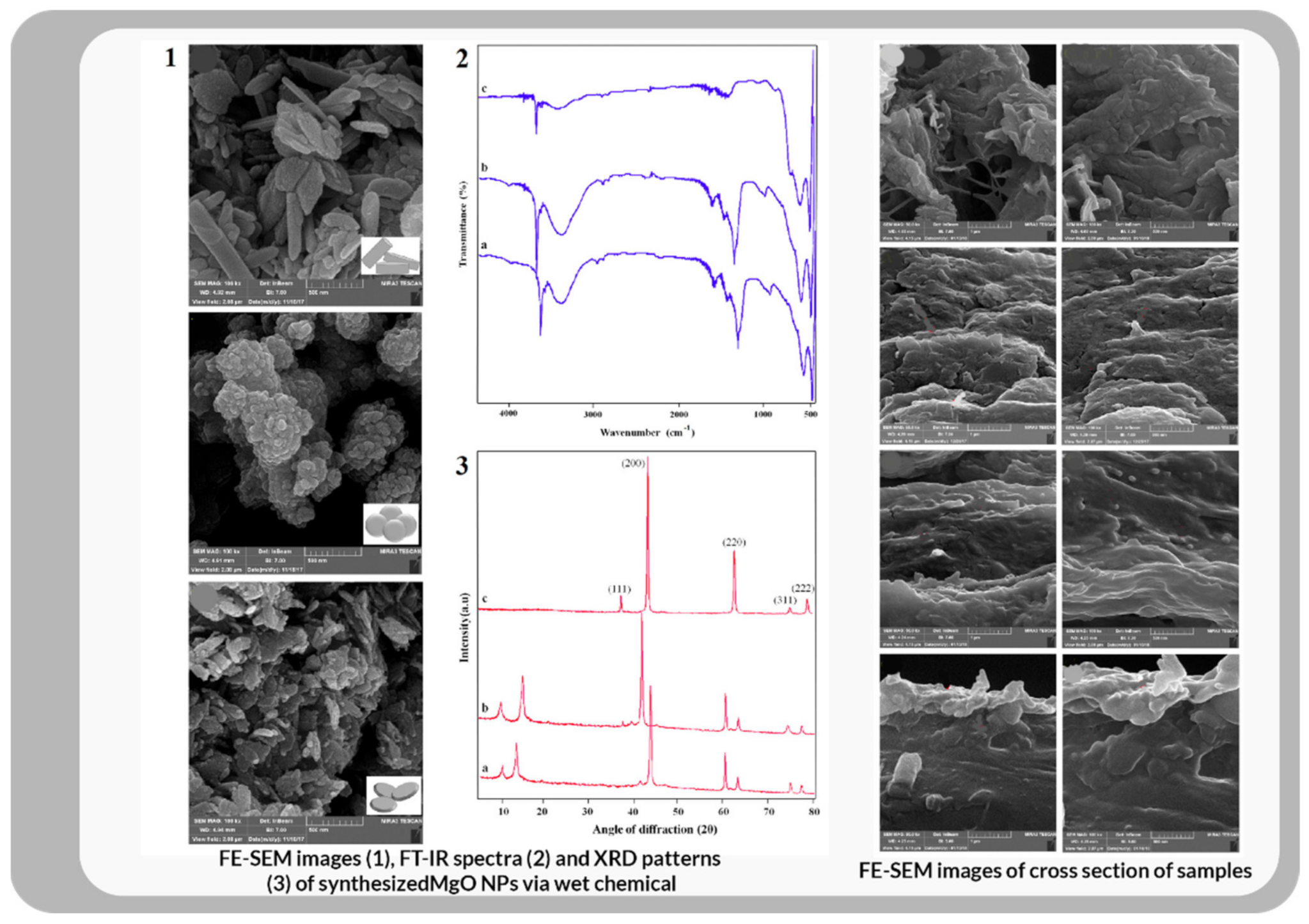
| Commercial Product Name | Clinical Utilization | Form for Usage | Company/Agency |
|---|---|---|---|
| Bio Fill® | Burns | Wound care systems | Robin goad, Milwaukee, WI, USA |
| Cellulon® | Medical applications including non-woven structures | Binder | CP Kelco, Atlanta, GA, USA |
| Basyc® | CABG (Coronary artery bypass surgery) | Vessel implants (tubes) | Jenpolymer materials Ltd. & co., Jena, Germany |
| Bioprocess® | Burns | Artificial skin | Biofill Produtos Biotechnologicos, São Paulo, Brazil |
| Dermafill® | Burns | Wound care dressing | Fibrocel Produtos Biotechnologicos Ltd.a, Ibipora, PR, Brazil |
| Cellulon PX microfibrous cellulose® | Suspensions of particles, encapsulated enzymes | Suspending agent | CP Kelco, Atlanta, GA, USA |
| Gengiflex® | Periodentitis | Non-resorbable cellulose membrane | Biofill Produtos Biotechnologicos, São Paulo, Brazil |
| CelMat ® MG & CelM®(R) MG | Protection for miners from potential burns | Protective dressings/jackets | Government of Poland, Warsaw, Poland |
| Securian® | Tendon repair | Tissue reinforcement matrix | Xylos corporation, Langhorne, PA, USA |
| MTA protective tissue | Injury and wound care | Biocompatible implant | Xylos corporation, Langhorne, PA, USA |
| Membracell® | Ulcers, burns, lacerations | Temporary skin substitute | Vuelo Pharma, Curitiba, PR, Brazil |
| Xcell® | Venous ulcer wounds | Wound care | XCELL BIOLOGIX, Kennesaw, GA, USA |
| Bionext® | Ulcers, burns, lacerations | Wound dressing | Bionext Produtos Biotechnologicos, Pacaembu, São Paulo, Brazil |
| Invention Field | Patent Title | Patent Number | Registration |
|---|---|---|---|
| Calcium alginate capsules embedded and prepared in situ; containing drugs and probiotics | Bacterial cellulose composite with capsules embedded therein and preparation thereof | US 2012308649A1 | United states patent and trade mark office (USPTO) |
| Implantable device; soft tissue repair-drug delivery carriers | A method for producing implantable microbial cellulose materials for various medical applications | EP1795213 B1 (Heather Beam et al.) | European patent office |
| Network meshed hydrogel, drug delivery carrier, skin substitute | Novel network meshed hydrogel structure | TW M428771U1 (Yung Kai Lin, Che Yung Kuan) | Intellectual Property Office Taiwan (TIPO) |
| Implantable bacterial cellulose; in-vivo application | Thermally modified microbial-derived cellulose for in-vivo implantation | EP1662976 A2 US20050042250 US8198261, (Ann Hethearbeam et al.) | USPTO, 2006 & EPO, 2005 |
| Use of microbial (bacterial) cellulose in transdermal drug delivery | Microbial cellulose materials for use in transdermal drug delivery systems, method of manufacture and use | US 20060240084 (Serafica et al.) | USPTO, 2006 |
| Cellulose hydrogels, making and applications; implant and ocular devices; sustained release drug delivery systems | Cellulose-based hydrogels and methods of making thereof | US20130032059 A1 (Morgana M Trexeler et al.) | USPTO 2013 |
| Medical implant; orthopeaedic | Medical device including bacterial cellulose reinforced by resorbable or non-resorbable materials | US 20110262521A1 (Bayon et al.) | USPTO, 2011 |
| Wide range of applications, dependent on density gradient dictated by thickness; number of drugs can be delivered | Bacterial cellulose films and uses thereof | EP 2390344 A1 US20110286948 (Mei-Ling Lee et al.) | EPO, 2011 USPTO, 2011 |
| Strain | Carbon Source | Production Quantity (g/L) | Incubation Mode | Duration of Incubation | Reference |
|---|---|---|---|---|---|
| G. xylinus (BPR 2001) | Fructose | 14.1 | Agitated | 3 days | [123] |
| G. xylinus (BRC 5) | Glucose | 15.3 | Fed-batch/agitated | 2 days | [124] |
| G. xylinus (MCRC 12334) | TS-Glu | 10.38 | Static | 7 days | [125] |
| A. xylinum (ATCC 700178) | CSL-Fru | 13 | Agitated | 5 days | [126] |
| G. xylinus (ATCC, 23770) | (Fiber sludge) Hydrolysates | 6.23 | Static | 14 days | [127] |
| G. xylinus (PTCC 1734) | Syrup | 43.5 | Static | 14 days | [128] |
| Acetobacter xylinum ssp. sucrofermentans BPR2001 | Fructose | 8.7 | Static | 44h | [129] |
| Gluconacetobacter xylinus IFO 13773 | Glucose | 10.1 | Static/agitated | 7 days | [130] |
| Acetobacter sp. V6 | Glucose | 4.16 | agitated | 8 days | [131] |
| Acetobacter sp. A9 | Glucose | 15.2 | agitated | 8 days | [132] |
| Gluconacetobacter xylinus IFO 13773 | Sugar cane molasses | 5.76 | Static/agitated | 7 days | [133] |
| Co-culture of Gluconacetobacter sp. st-60–12 | Sucrose | 4.2 | agitated | 3days | [134] |
| and Lactobacillus mali JCM1116 | |||||
| G. hansenii PJK (KCTC 10505 BP) | Glucose | 2.5 | Static | 3days | [76] |
| A. xylinum 0416 | Pineapple waste medium | 28.3 | Rotary disc reactor | 4 days | [135] |
| A. xylinum strain DA | Glucose | 0.15 | Five-stage horizontal | 68 h | K Toda, J Koizumi, T Asakura—1994 |
| flow reactor | |||||
| A. xylinum subsp. Sucrofermentans BPR2001 | Corn steep liquor-fructose (CSL-Fru) | 3.8 | Airlift reactor | 67h | [129] |
| medium | |||||
| G. persimmonis GH-2 | Galactose + Sucrose | 7.67 | Static | 14 days | [136] |
| Galactose + Lactose, | 6.89 | ||||
| Galactose + Maltose, | 6.28 | ||||
| Galactose + Fructose | 5.82 | ||||
| Molasses + HS medium | 5.75 | ||||
| Watermelon + HS medium | 5.98 | ||||
| Orange juice + HS medium | 6.18 | ||||
| Muskmelon + HS medium | 8.08 | ||||
| Coconut water + HS medium |
| Production Method | Description | Advantage | Disadvantage | |
|---|---|---|---|---|
| Static culture | -All media ingredients are mixed together at the early stage | -Simple process | -Laborious and time consuming | All references can be found in [122] |
| -Production occurs in tray | -Does not require complex instruments | -Fermentation condition cannot be controlled or monitored | ||
| -Production occurs at air-liquid medium interface | -Cellulose formed as pellicle, sometimes as reticulated cellulose | |||
| slurry | ||||
| -Not applicable for large-scale production | ||||
| Static intermittent fed batch technology | Definite amount of fresh media provided over growing | Simple process | -Fermentation condition cannot be monitored | |
| pellicle in intermittent time periods | -Highly enhanced production as compared to | -Cellulose formed as pellicle, sometimes as reticulated cellulose | ||
| standard static method | slurry | |||
| -Can be applied for large scale production | ||||
| Cell-free extract technology | Mechanical/thermal/enzymatic cell lysis releases all the | Simple process | No control over fermentation parameters | |
| necessary enzymes required for BNC production directly | -Can be applied for large scale production in | |||
| into the media | short time | |||
| -Better yield | ||||
| Agitated culture | -Reciprocal shaking at about 90–100 rpm | -Applicable for large scale production | -Cellulose not formed in pellicle form but as irregular shape | |
| -Agitation allows cells to grow more rapidly | -Surmount many limitations in static culture | sphere-like cellulose particle | ||
| including diffusion, controllability and scale-up | -Agitation often result in culture mutation resulting in low | |||
| productivity | ||||
| -Problem with culture instability which demonstrated by loss of | ||||
| ability to make cellulose | ||||
| Bioreactor based production e.g., Rotary disc | New alternative using concept of Rotating Biological | -High productivity | ||
| reactor, Air lift reactor | Contactor (RBC) | -Less labor needed | -No disadvantage (if culture conditions are properly maintained | |
| -It used discs that alternately soak the organisms in nutrient | -Easy scale-up | and suitable medium is used then high productivity can be | ||
| medium and expose them to air | achieved) |
| Mode of Modification | BC Strain and Drug Model | Intrinsic Feature | Final Application | DD Route | Reference |
|---|---|---|---|---|---|
| In situ | Komagataeibacter xylinus (K. xylinus) strain DSM 14666 (doxycycline) | Fleece-like appearance | Wound dressing and dental therapies | Transmucosal delivery | [158] |
| In situ | Komagataeibacter xylinus X-2 (graphene oxide) | Bead-like spheres with BC/GO porous structure | General carrier | Potentially for transdermal and transmucosal drug delivery | [159] |
| In situ | Gluconacetobacter xylinus (ATCC 10,245) | Pockets | Drug carrier | For transdermal and transmucosal drug delivery | [138] |
| In situ | Acetobacter xylinum (ŁOCK 0805) | 3D microfibres | Dressers for wounds, burns and ulcers | Transdermal | [161] |
| In situ | Gluconacetobacter xylinus (hydroxyapatite Ca5(PO4)3OH (HA) | Nanotextured fibrils | Varied applications | Mainly transdermal | [162] |
| In situ | Gluconacetobacter xylinus(magnetite nanoparticles (Fe3O4)) | Nanotextured fibrils | Blood vessels | Potentially for transdermal and transmucosal drug delivery | [163] |
| Ex situ (ExSUP) | Gluconacetobacter sacchari (ibuprofen and lidocaine) | 3D microfibrils | Drug carrier absorb exudates skin therapies | Transdermal | [16] |
| Ex situ (ExSUP) | Gluconacetobacter sacchari (glycerine) | 3D microfibrils | Skin therapy | Transdermal | [30] |
| Ex situ (ExSUP) | Acetobacter Xylinum (tetracycline diffusion) via irradiation | 3D microfibrils | Varied applications | Potentially for transdermal delivery | [167] |
| Ex situ (ExSUP) | Gluconacetobacter xylinus (ATCC No. 23769)(digluconate chlorhexidine) | 3D microfibrils | Varied applications | Potentially for transdermal delivery | [70] |
| Ex situ (ExSUP) | Acetobacter Xylinum 0416 (silver sulfadiazine) | Nano-spheres with 3D microfibrils of BC | Wound dressing for diabetic foot ulcer (DFU) | Transdermal delivery | [169] |
| Ex situ (ExSUP) | Komagataeibacter hansenii (2,3-dialdehyde + chlorhexidine) | Nano cavities with BC microfibrils | Bioabsorbable membrane/periodontal treatment | Potentially for transdermal and transmucosal drug delivery | [70] |
| Ex situ (ExSUP) | Gluconacetobacter xylinus (PTCC 1734)(carbon quantum dots-titanium dioxide (CQD-TiO2) | 3D microfibrils | Wound healing | Transdermal delivery | [170] |
| Ex situ (ExSUP) | Komagataeibacter xylinus (ATCC 23760)(Chitosan) (Ciprofloxacin) | 3D microfibrils | Wound treatments | Transdermal delivery | [171] |
| Ex situ (ExSUP) | Gluconacetobacter xylinus (alginate) | 3D microfibrils | Wound dressing | Transdermal delivery | [172] |
| Ex situ (ExSUP) | Komagataeibacter xylinus B-12068 P(3HB/4HB) | Nanotextured fibrils | Wound treatments | Transdermal delivery | [173] |
| Ex situ (ExSUP) | Gluconacetobacter sacchari (Silylation) | 3D nanotextured fibrils | Anti-bacterial activity | Transdermal delivery | [33] |
| Ex situ (ExSSuSol) | Acetobacter xylinum (Gelatin) | Spherical porous structure | Drug carriers | Transdermal and transmucosal drug delivery | [176] |
| Ex situ (ExSSuSol) | Acetobacter xylinum (CGMCC5173) (alfacalcidol via pickering emulsion method) | Spherical (bead-like) nanocrystals | Drug carriers | Transdermal and transmucosal drug delivery | [37] |
| Ex situ (ExSSuSol) | Acetobacter xylinum (Acrylic acid (AA)) | Sponge-like structure | Drug carriers | Potentially for transdermal and transmucosal drug delivery | [178] |
| Ex situ (ExSSuSol) | Glucanoacetobacter xylinus (MTCC7795) (cellulose-graft-poly(2-(methacryloyloxy)ethyltrimethyl ammonium chloride) (BC-g-PMTAC)) | Spherical (bead-like) nanocrystals | Drug carriers | Transdermal and transmucosal drug delivery | [177] |
| Hybrid pathway (In situ+Ex situ) | Gluconacetobacter xylinus (MgO) | Leaf-shaped nano-sheet structure | Clinical wound healing | Transdermal delivery | [147] |
Publisher’s Note: MDPI stays neutral with regard to jurisdictional claims in published maps and institutional affiliations. |
© 2021 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Mensah, A.; Chen, Y.; Christopher, N.; Wei, Q. Membrane Technological Pathways and Inherent Structure of Bacterial Cellulose Composites for Drug Delivery. Bioengineering 2022, 9, 3. https://doi.org/10.3390/bioengineering9010003
Mensah A, Chen Y, Christopher N, Wei Q. Membrane Technological Pathways and Inherent Structure of Bacterial Cellulose Composites for Drug Delivery. Bioengineering. 2022; 9(1):3. https://doi.org/10.3390/bioengineering9010003
Chicago/Turabian StyleMensah, Alfred, Yajun Chen, Narh Christopher, and Qufu Wei. 2022. "Membrane Technological Pathways and Inherent Structure of Bacterial Cellulose Composites for Drug Delivery" Bioengineering 9, no. 1: 3. https://doi.org/10.3390/bioengineering9010003
APA StyleMensah, A., Chen, Y., Christopher, N., & Wei, Q. (2022). Membrane Technological Pathways and Inherent Structure of Bacterial Cellulose Composites for Drug Delivery. Bioengineering, 9(1), 3. https://doi.org/10.3390/bioengineering9010003






