The Resistance Force of the Anterior Cruciate Ligament during Pull Probing Is Related to the Mechanical Property
Abstract
1. Introduction
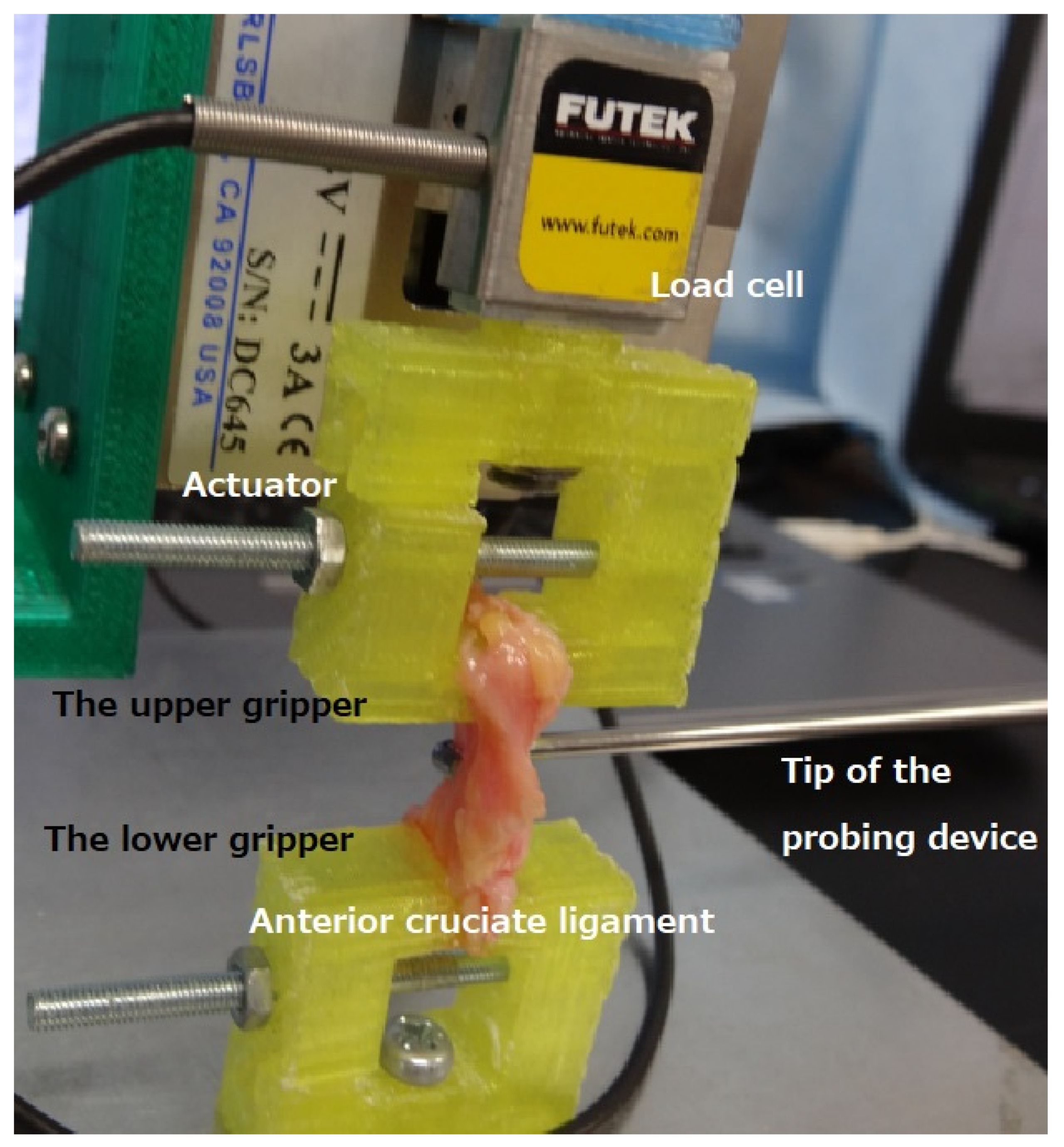
2. Materials and Methods
Statistical Analysis
3. Results
4. Discussion
5. Conclusions
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Data Availability Statement
Conflicts of Interest
References
- Varady, N.H.; Kernkamp, W.A.; Li, J.; Wang, L.; Koga, H.; Asnis, P.; Li, G. The biomechanical effect of tunnel placement on ACL graft forces in double-bundle ACL reconstruction d A 3D computational simulation. Int. J. Med. Robot. 2017, 13, e1840. [Google Scholar] [CrossRef] [PubMed]
- Noailles, T.; Boisrenoult, P.; Sanchez, M.; Beaufils, P.; Pujol, N. Torsional appearance of the anterior cruciate ligament explaining “ribbon” and double-bundle concepts: A cadaver-based study. Arthroscopy 2017, 33, 1703–1709. [Google Scholar] [CrossRef]
- Beyaz, S.; Güler, Ü.Ö.; Demir, Ş.; Yüksel, S.; Çınar, B.M.; Özkoç, G.; Akpınar, S. Tunnel widening after single- versus double-bundle anterior cruciate ligament reconstruction: A randomized 8-year follow-up study. Arch. Orthop. Trauma Surg. 2017, 137, 1547–1555. [Google Scholar] [CrossRef]
- Shino, K.; Mae, T.; Tachibana, Y. Anatomic ACL reconstruction: Rectangular tunnel/bone-patellar tendon-bone or triple-bundle/semitendinosus tendon grafting. J. Orthop. Sci. 2015, 20, 457–468. [Google Scholar] [CrossRef] [PubMed]
- Suzuki, T.; Shino, K.; Otsubo, H.; Suzuki, D.; Mae, T.; Fujimiya, M.; Yamashita, T.; Fujie, H. Biomechanical comparison between the rectangular-tunnel and the round-tunnel anterior cruciate ligament reconstruction procedures with a bone-patellar tendon-bone graft. Arthroscopy 2014, 30, 1294–1302. [Google Scholar] [CrossRef] [PubMed]
- Suzuki, T.; Shino, K.; Yamakawa, S.; Otsubo, H.; Suzuki, D.; Matsumura, T.; Fujimiya, M.; Fujie, H.; Yamashita, T. A biomechanical comparison of single-, double-, and triple-bundle anterior cruciate ligament reconstructions using a hamstring tendon graft. Arthroscopy 2019, 35, 896–905. [Google Scholar] [CrossRef] [PubMed]
- Kato, Y.; Maeyama, A.; Lertwanich, P.; Wang, J.H.; Ingham, S.J.; Kramer, S.; Martins, C.Q.A.; Smolinski, P.; Fu, F.H. Biomechanical comparison of different graft positions for single-bundle anterior cruciate ligament reconstruction. Knee Surg. Sports Traumatol. Arthrosc. 2013, 21, 816–823. [Google Scholar] [CrossRef]
- Mae, T.; Shino, K.; Matsumoto, N.; Hamada, M.; Yoneda, M.; Nakata, K. Anatomical two-bundle versus Rosenberg’s isometric bi-socket ACL reconstruction: A biomechanical comparison in laxity match pretension. Knee Surg. Sports Traumatol. Arthrosc. 2007, 15, 328–334. [Google Scholar] [CrossRef]
- Youssefzadeh, K.A.; Stein, S.M.; Orr Limpisvast, O. Anterior cruciate ligament repair using a knotless suture implant. Arthrosc. Tech. 2020, 9, e623–e626. [Google Scholar] [CrossRef]
- Hananouchi, T.; Uchida, S.; Hashimoto, Y.; Noboru, F.; Aoki, S.K. Comparison of labrum resistance force while pull-probing in vivo and cadaveric hips. Biomimetics 2021, 6, 35. [Google Scholar] [CrossRef]
- Ristaniemi, A.; Stenroth, L.; Mikkonen, S.; Korhonen, R.K. Comparison of elastic, viscoelastic and failure tensile material properties of knee ligaments and patellar tendon. J. Biomech. 2018, 79, 31–38. [Google Scholar] [CrossRef] [PubMed]
- Hananouchi, T. A Probing device for quantitatively measuring the mechanical properties of soft tissues during arthroscopy. J. Vis. Exp. 2020, 159, e60722. [Google Scholar] [CrossRef]
- Hananouchi, T.; Aoki, S.K. Quantitative evaluation of capsular and labral resistances in the hip joint using a probing device. Bio-Med. Mater. Eng. 2019, 30, 333–340. [Google Scholar] [CrossRef] [PubMed]
- Sawai, A.; Mitsuhashi, R.; Zaboronok, A.; Warashina, Y.; Mathis, B.J. Serum creatine kinase increases after acute strength training in college athletes with menstrual irregularities. Women 2021, 1, 71–79. [Google Scholar] [CrossRef]
- Hihara, H.; Tagaino, R.; Washio, J.; Laosuwan, K.; Wicaksono, D.P.; Izumita, K.; Koide, R.; Takahashi, N.; Sasaki, K. Effectiveness and safety of a new dental plaque removal device utilizing micro mist spray for removing oral biofilm in vitro. BMC Oral Health 2021, 21, 286. [Google Scholar] [CrossRef] [PubMed]
- Hertz, H. Uber die Berührung fester elastischer Körper. (On the contact of elastic solids). J. Reine Und Angew. Math. 1882, 92, 156–171. [Google Scholar]
- Hananouchi, T.; Chen, Y.; Jerban, S.; Teramoto, M.; Ma, Y.; Dorthe, E.W.; Chang, E.Y.; Du, J.; D’Lima, D.D. A useful combination of quantitative ultrashort echo time MR imaging and a probing device for biomechanical evaluation of articular cartilage. Biosensors 2021, 11, 52. [Google Scholar] [CrossRef]
- Cohen, J. A power primer. Psychol. Bull. 1992, 112, 155–159. [Google Scholar] [CrossRef]
- Yazdi, H.; Torkaman, A.; Ghahramani, M.; Moradi, A.; Nazarian, A.; Ghorbanhoseini, M. Short term results of anterior cruciate ligament augmentation in professional and amateur athletes. J. Orthop. Traumatol. 2017, 18, 171–176. [Google Scholar] [CrossRef]
- Butler, D.L.; Guan, Y.; Kay, M.D.; Cummings, J.F.; Feder, S.M.; Levy, M.S. Location-dependent variations in the material properties of the anterior cruciate ligament. J. Biomech. 1992, 25, 511–518. [Google Scholar] [CrossRef]
- Marieswaran, M.; Jain, I.; Garg, B.; Sharma, V.; Kalyanasundaram, D. A review on biomechanics of anterior cruciate ligament and materials for reconstruction. Appl. Bionics Biomech. 2018, 2018, 4657824. [Google Scholar] [CrossRef]
- Mae, T.; Shino, K.; Miyama, T.; Shinjo, H.; Ochi, T.; Yoshikawa, H.; Fujie, H. Single- versus two femoral socket anterior cruciate ligament reconstruction technique: Biomechanics analysis using a robotic simulator. Arthroscopy 2001, 17, 708–716. [Google Scholar] [CrossRef]
- Kim, Y.H.; Kim, J.S.; Yoon, S.H. A recession of posterior cruciate ligament in posterior cruciate-retaining total knee arthrosplasty. J. Arthroplast. 2008, 23, 999–1004. [Google Scholar] [CrossRef] [PubMed]
- Yagishita, K.; Muneta, T.; Ju, Y.J.; Morito, T.; Yamazaki, J.; Sekiya, I. High-flex posterior cruciate-retaining vs posterior cruciate-substituting designs in simultaneous bilateral total knee arthroplasty: A prospective, randomized study. J. Arthroplast. 2012, 27, 368–374. [Google Scholar] [CrossRef] [PubMed]
- Tsai, T.Y.; Liow, M.H.L.; Li, G.; Arauz, P.; Peng, Y.; Klemt, C.; Kwon, Y.M. Bi-cruciate retaining total knee arthroplasty does not restore native tibiofemoral articular contact kinematics during gait. J. Orthop. Res. 2019, 37, 1929–1937. [Google Scholar] [CrossRef] [PubMed]
- De Faoite, D.; Ries, C.; Foster, M.; Boese, C.K. Indications for bi-cruciate retaining total knee replacement: An international survey of 346 knee surgeons. PLoS ONE 2020, 15, e0234616. [Google Scholar] [CrossRef]
- Paxton, J.Z.; Donnelly, K.; Keatch, R.P.; Baar, K. Engineering the boneeligament interface using polyethylene glycol diacrylate incorporated with hydroxyapatite. Tissue Eng. 2009, 15, 1201–1209. [Google Scholar] [CrossRef]
- Li, H.; Fan, J.; Sun, L.; Liu, X.; Cheng, P.; Fan, H. Functional regeneration of ligament-bone interface using a triphasic silk-based graft. Biomaterials 2016, 106, 180–192. [Google Scholar] [CrossRef]
- Chami, G.; Ward, J.W.; Phillips, R.; Sherman, K.P. Haptic feedback can provide an objective assessment of arthroscopic skills. Clin. Orthop. Relat. Res. 2008, 466, 963–968. [Google Scholar] [CrossRef][Green Version]
| Parameters | First Phase (Strain; 6.7%) | Second Phase (Strain, 13.3%) | Third Phase (Strain; 16.7%) |
|---|---|---|---|
| Load (N) | 0.43 (SD; 0.15, Range; 0.25–0.71) | 1.08 (SD; 0.26, Range; 0.79–1.64) | 1.10 (SD; 0.22, Range; 1.10–1.79) |
| Stiffness (N/mm) | 0.43 (SD; 0.15, Range; 0.25–0.71) | 0.72 (SD; 0.18, Range; 0.52–1.09) | 0.55 (SD; 0.09, Range; 0.44–0.71) |
| Young’s modulus (MPa) | 0.21 (SD; 0.10, Range; 0.10–0.40) | 0.26 (SD; 0.09, Range; 0.15–0.48) | 0.26 (SD; 0.06, Range; 0.16–0.36) |
| Probing Force (Z direction (N)) | 0.43 (SD; 0.15, Range; 0.25–0.71) | 1.08 (SD; 0.26, Range; 0.79–1.64) | 1.39 (SD; 0.33, Range; 0.90–2.11) |
| Probing Force (Y direction (N)) | 0.48 (SD; 0.11, Range; 0.24–0.68) | 0.57 (SD; 0.12, Range; 0.32–0.71) | 0.62 (SD; 0.11, Range; 0.43–0.85) |
| Probing Force (resultant Z and Y (N)) | 0.66 (SD; 0.12, Range; 0.51–0.90) | 1.23 (SD; 0.25, Range; 0.25–0.71) | 1.53 (SD; 0.32, Range; 1.02–2.24) |
| Probing Force (X direction (N)) | 0.13 (SD; 0.07, Range; 0.02–0.25) | 0.15 (SD; 0.10, Range; 0.03–0.35) | 0.16 (SD; 0.11, Range; 0.03–0.37) |
| Probing Force (all resultant force) (N)) | 0.67 (SD; 0.13, Range; 0.53–0.93) | 1.24 (SD; 0.26, Range; 0.86–1.79) | 1.54 (SD; 0.33, Range; 1.03–2.26) |
| The amount of the increased force by the load cell while the pull probing (N) | 0.48 (SD; 0.12, Range; 0.27–0.71) | 0.54 (SD;0.16, Range; 0.29–0.73) | 0.54 (SD; 0.14, Range; 0.37–0.79) |
| Parameters | First Phase (Strain; 6.7%) | Second Phase (Strain; 13.3%) | Third Phase (Strain; 16.7%) |
|---|---|---|---|
| Stiffness and probing force (only Z) | −0.10 (p = 0.56) | 0.03 (p = 0.92) | 0.54 (p = 0.11) |
| Stiffness and probing force (resultant Z and Y) | −0.17 (p = 0.56) | 0.08 (p = 0.79) | 0.53 (p = 0.049) |
| Stiffness and probing force (all resultant force) | −0.16 (p = 0.60) | 0.14 (p = 0.63) | 0.56 (p = 0.045) |
| Young’s modulus and probing force (only Z) | −0.16 (p = 0.60) | −0.12 (p = 0.69) | 0.38 (p = 0.19) |
| Young’s modulus and probing force (resultant Z and Y) | −0.20 (p = 0.51) | −0.03 (p = 0.91) | 0.40 (p = 0.18) |
| Young’s modulus and probing force (all resultant force) | −0.16 (p = 0.58) | −0.03 (p = 0.91) | 0.42 (p = 0.14) |
Publisher’s Note: MDPI stays neutral with regard to jurisdictional claims in published maps and institutional affiliations. |
© 2021 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Hananouchi, T.; Suzuki, T.; Dorthe, E.W.; Du, J.; D’Lima, D.D. The Resistance Force of the Anterior Cruciate Ligament during Pull Probing Is Related to the Mechanical Property. Bioengineering 2022, 9, 4. https://doi.org/10.3390/bioengineering9010004
Hananouchi T, Suzuki T, Dorthe EW, Du J, D’Lima DD. The Resistance Force of the Anterior Cruciate Ligament during Pull Probing Is Related to the Mechanical Property. Bioengineering. 2022; 9(1):4. https://doi.org/10.3390/bioengineering9010004
Chicago/Turabian StyleHananouchi, Takehito, Tomoyuki Suzuki, Erik W. Dorthe, Jiang Du, and Darryl D. D’Lima. 2022. "The Resistance Force of the Anterior Cruciate Ligament during Pull Probing Is Related to the Mechanical Property" Bioengineering 9, no. 1: 4. https://doi.org/10.3390/bioengineering9010004
APA StyleHananouchi, T., Suzuki, T., Dorthe, E. W., Du, J., & D’Lima, D. D. (2022). The Resistance Force of the Anterior Cruciate Ligament during Pull Probing Is Related to the Mechanical Property. Bioengineering, 9(1), 4. https://doi.org/10.3390/bioengineering9010004







