Distinct Methodologies to Produce Capped Mesoporous Silica with Hydroxyapatite and the Influence in Intracellular Signaling as Cytotoxicity on Human Umbilical Vein Endothelial Cells
Abstract
:1. Introduction
2. Materials and Methods
2.1. Synthesis of Hydroxyapatite-Coated Mesoporous Silica Particles
2.1.1. Method 1—SBA-15/HAP-1
2.1.2. Method 2—SBA-15/HAP-2
2.2. Elemental Analysis (EA)
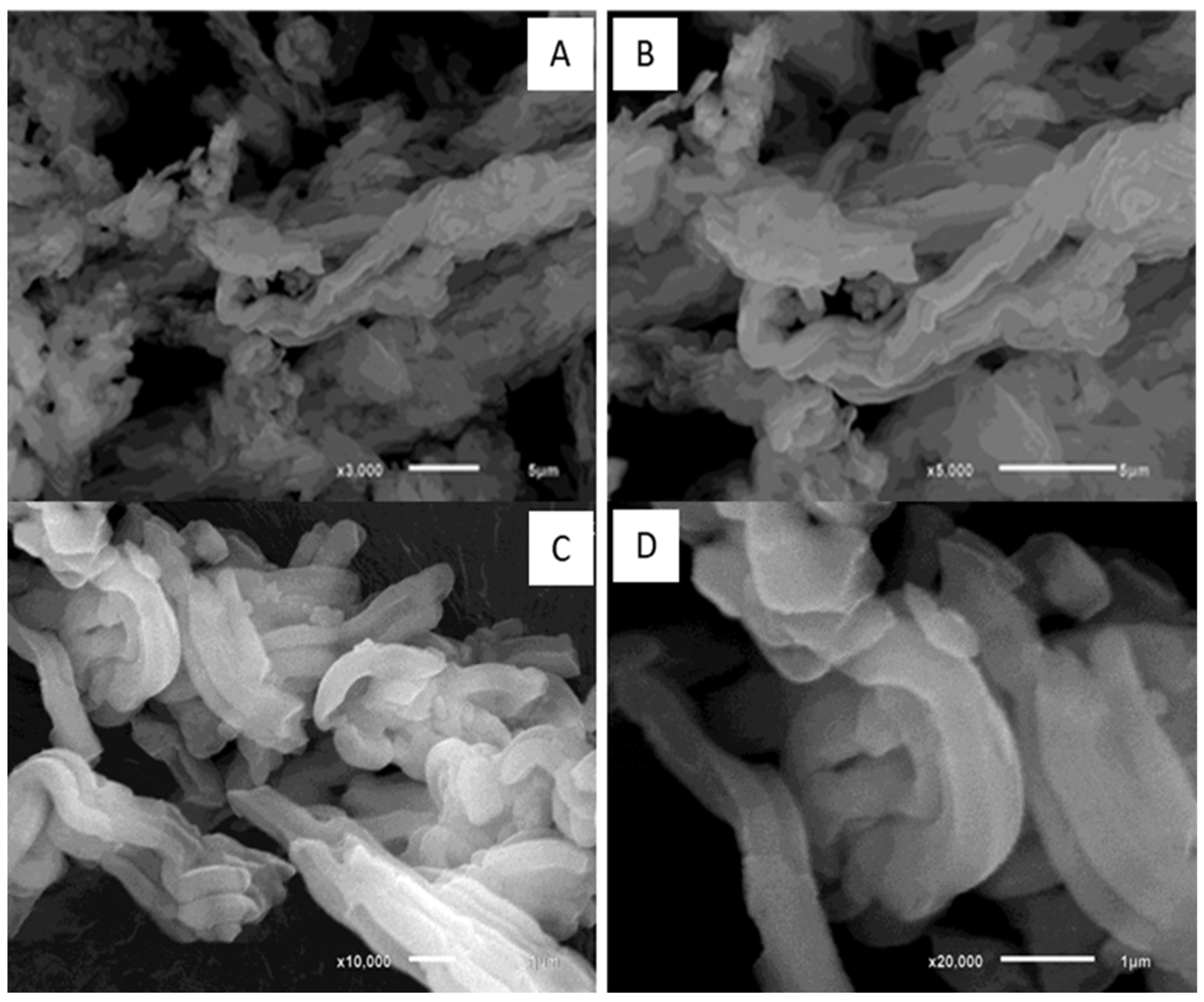
2.3. Scanning Electron Microscopy (SEM) Analysis
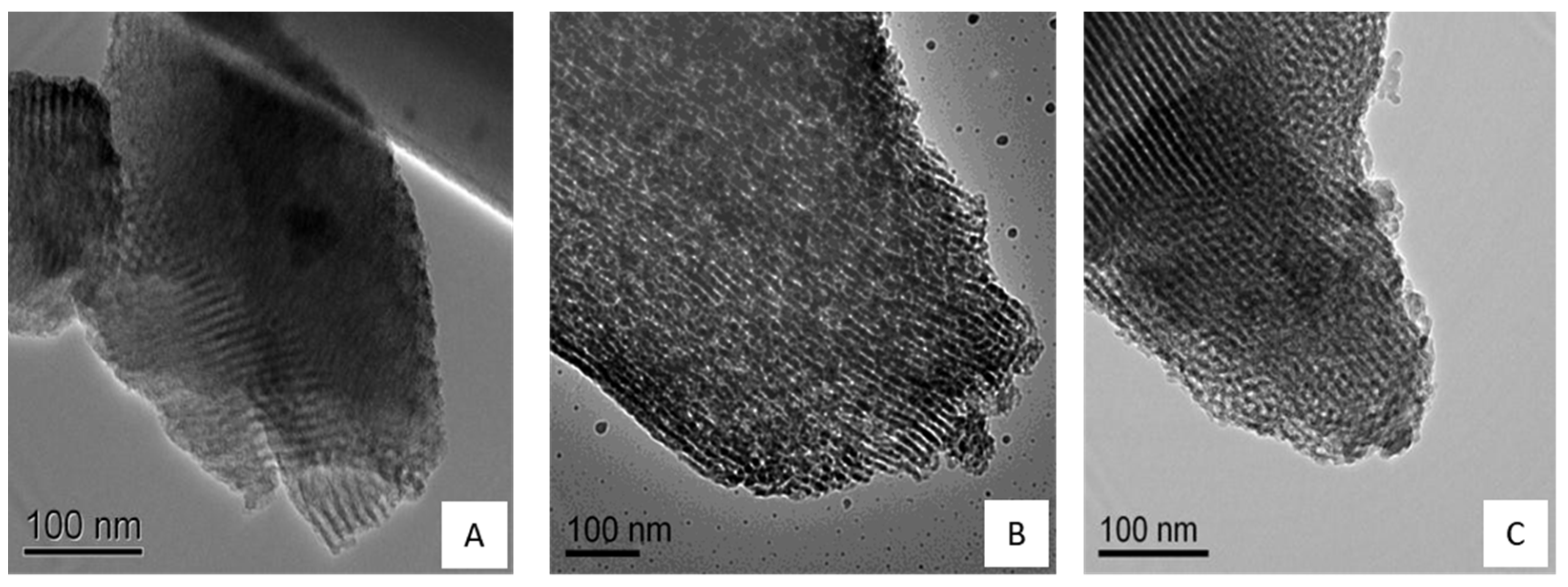
2.4. Transmission Electron Microscopy (TEM) Analysis
2.5. Small-Angle X-ray Diffraction (SXRD) and Wide-Angle X-ray Diffraction (WXRD) Analysis
2.6. Fourier Transform Infrared (FTIR) Analysis
2.7. Biological Characterization
2.7.1. In Vitro Studies
2.7.2. Cell Culture
Human Melanocytes
Human Fibroblast
Human Endothelial Cells
2.7.3. Proliferation Assay
2.8. Intracellular Signaling—Immunoblotting
2.9. Statistical Analysis
3. Results and Discussion
3.1. Synthesis and Characterization of Hydroxyapatite-Coated Mesoporous Silica Nanoparticles
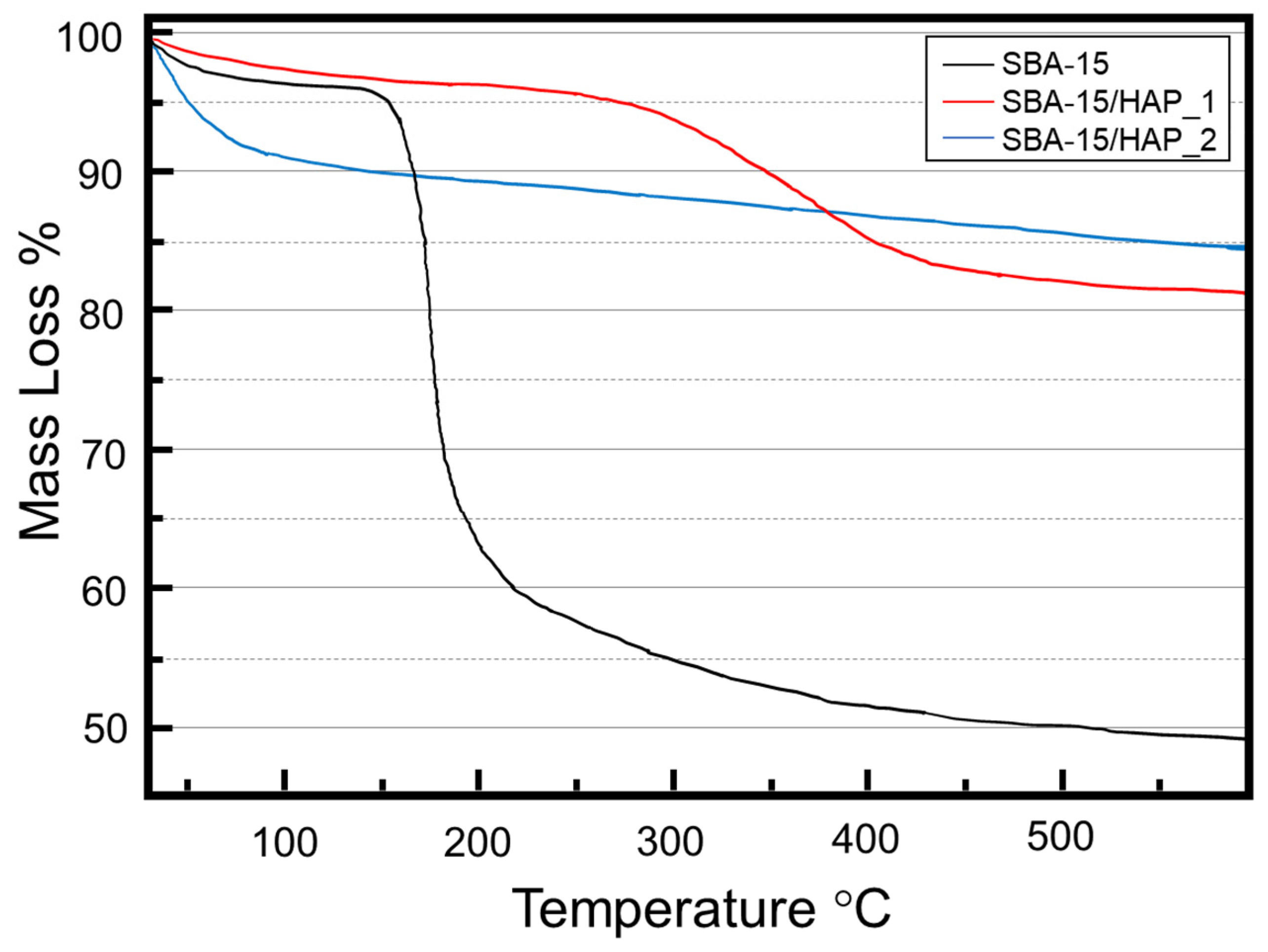
3.2. Elemental Analysis (EA)
3.3. Characterization of the Mesoporous Silica and the Hydroxyapatite Capped Mesoporous Silica Structure
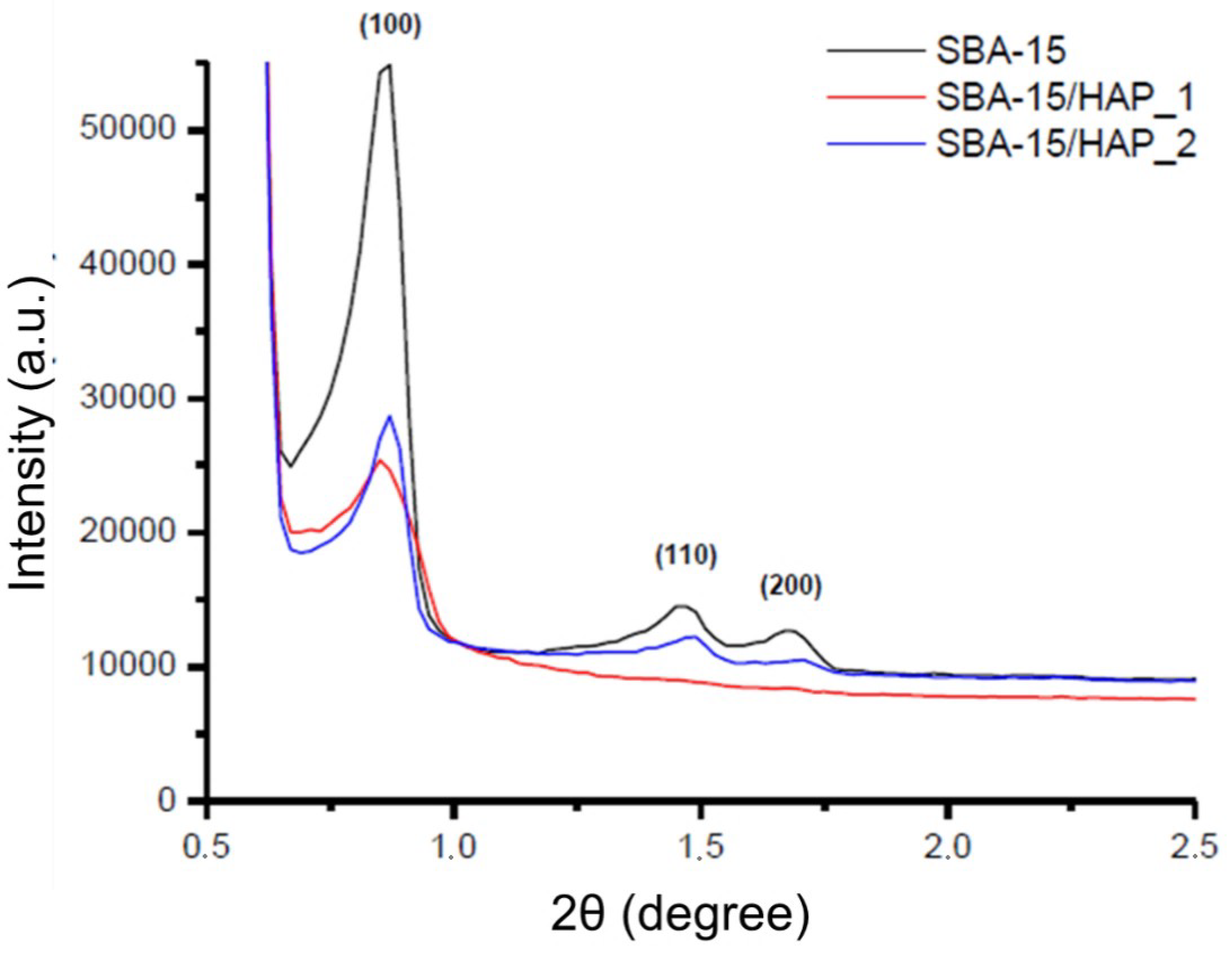
3.4. Characterization of the Hydroxyapatite Coating of Mesoporous Silica Nanoparticles with Small-Angle X-ray Diffraction (SXRD) and Wide-Angle X-ray Diffraction (WXRD)
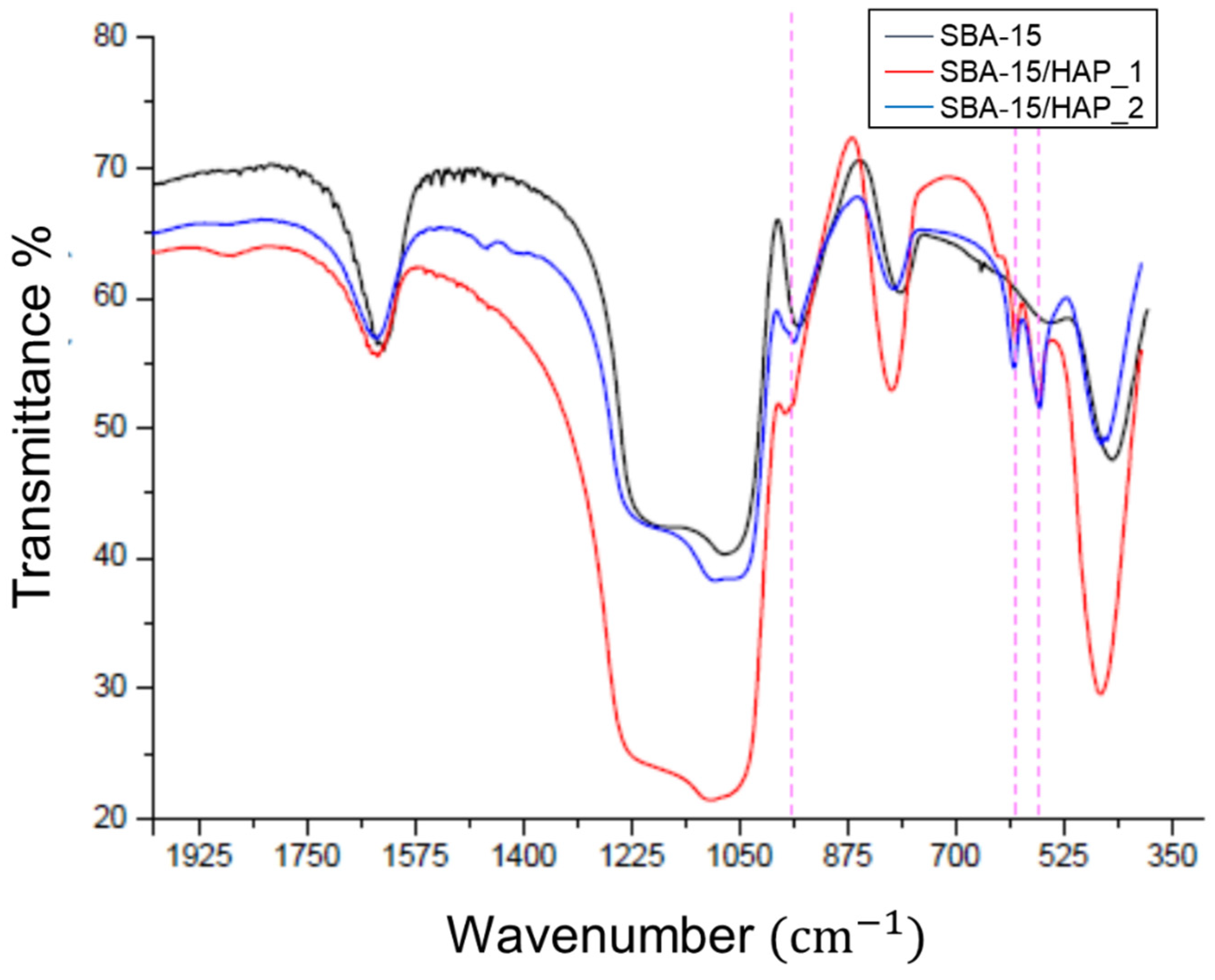
3.5. FTIR Analysis
3.6. Biological Characterization
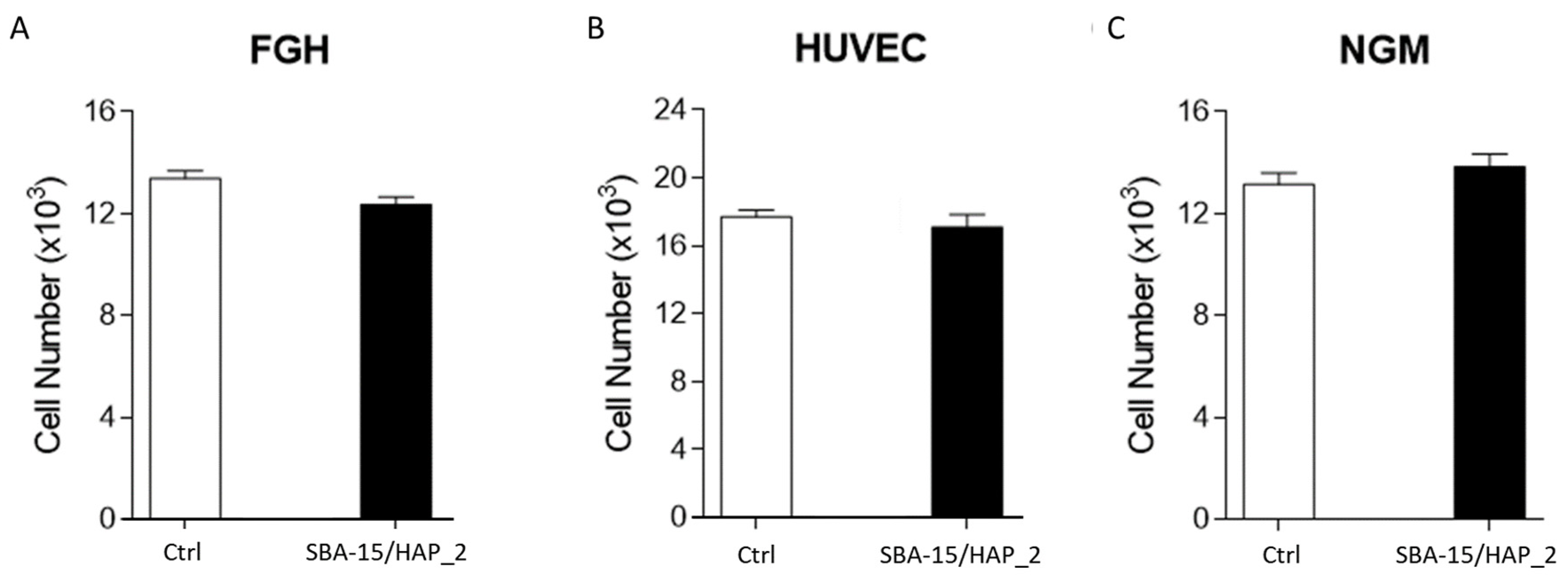
3.7. Cell Viability—Proliferation
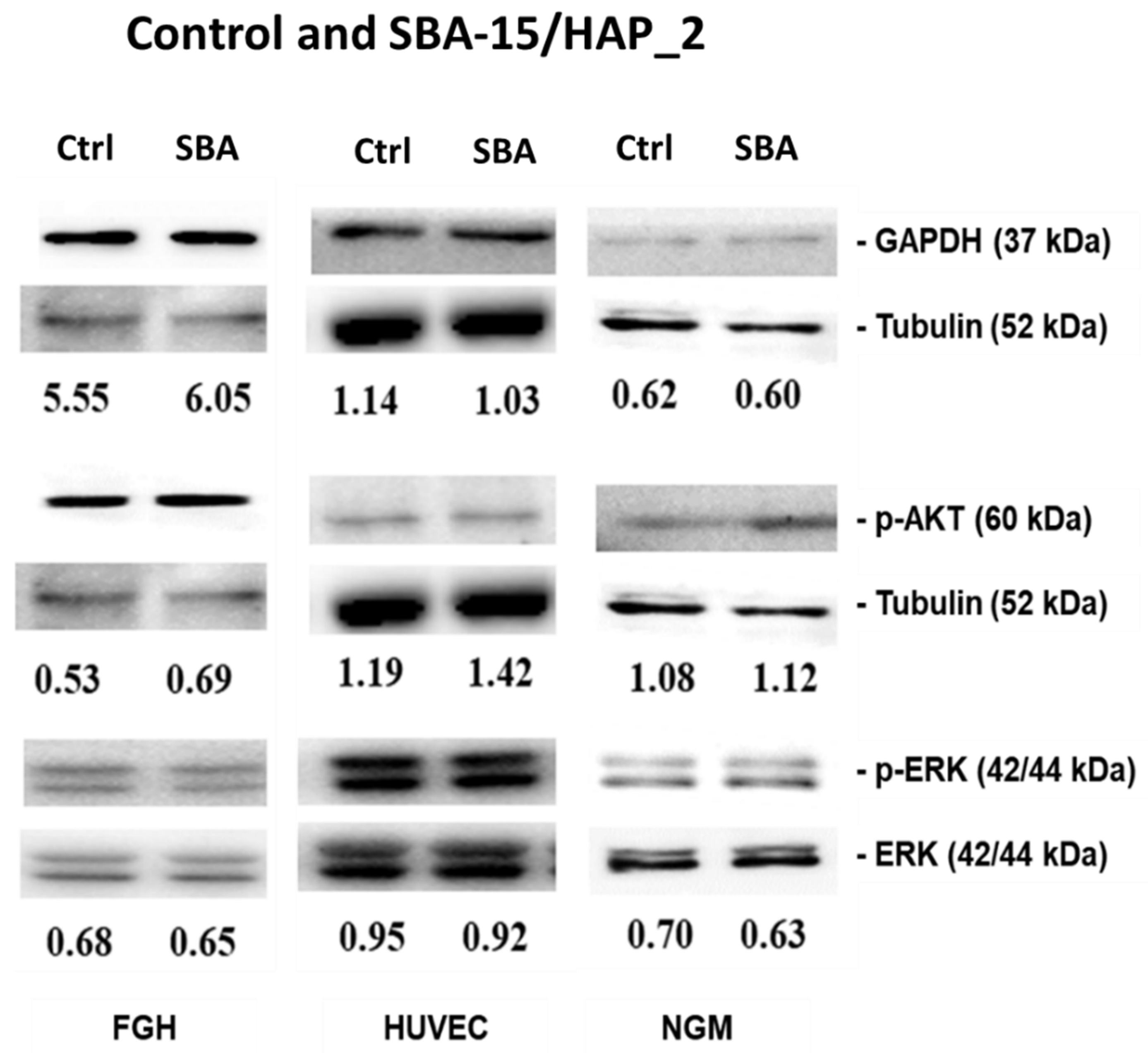
3.8. Immunoblotting
4. Conclusions
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Data Availability Statement
Acknowledgments
Conflicts of Interest
References
- Nakamura, T.; Sugihara, F.; Matsushita, H.; Yoshioka, Y.; Mizukami, S.; Kikuchi, K. Mesoporous silica nanoparticles for 19F magnetic resonance imaging, fluorescence imaging, and drug delivery. Chem. Sci. 2014, 6, 1986–1990. [Google Scholar] [CrossRef] [Green Version]
- Cheng, C.-A.; Deng, T.; Lin, F.-C.; Cai, Y.; Zink, J.I. Supramolecular Nanomachines as Stimuli-Responsive Gatekeepers on Mesoporous Silica Nanoparticles for Antibiotic and Cancer Drug Delivery. Theranostics 2019, 9, 3341–3364. [Google Scholar] [CrossRef] [PubMed]
- Thi, T.T.H.; Nguyen, T.N.Q.; Hoang, D.T.; Nguyen, D.H. Functionalized mesoporous silica nanoparticles and biomedical applications. Mater. Sci. Eng. C 2019, 99, 631–656. [Google Scholar]
- McCarthy, C.; Ahern, R.J.; Dontireddy, R.; Ryan, K.; Crean, A. Mesoporous silica formulation strategies for drug dissolution enhancement: A review. Expert Opin. Drug Deliv. 2016, 13, 93–108. [Google Scholar] [CrossRef] [PubMed]
- Maleki, A.; Kettiger, H.; Schoubben, A.; Rosenholm, J.M.; Ambrogi, V.; Hamidi, M. Mesoporous silica materials: From physico-chemical properties to enhanced dissolution of poorly water-soluble drugs. J. Control. Release 2017, 262, 329–347. [Google Scholar] [CrossRef] [PubMed]
- Kumar, D.; Chirravuri, S.S.; Shastri, N.R. Impact of surface area of silica particles on dissolution rate and oral bioavailability of poorly water soluble drugs: A case study with aceclofenac. Int. J. Pharm. 2014, 461, 459–468. [Google Scholar] [CrossRef]
- Liu, H.-J.; Xu, P. Smart Mesoporous Silica Nanoparticles for Protein Delivery. Nanomaterials 2019, 9, 511. [Google Scholar] [CrossRef] [Green Version]
- Iturrioz-Rodríguez, N.; Correa-Duarte, M.A.; Fanarraga, M.L. Controlled drug delivery systems for cancer based on mesoporous silica nanoparticles. Int. J. Nanomed. 2019, 14, 3389–3401. [Google Scholar] [CrossRef] [Green Version]
- Xu, C.; Lei, C.; Yu, C. Mesoporous Silica Nanoparticles for Protein Protection and Delivery. Front. Chem. 2019, 7, 290. [Google Scholar] [CrossRef] [Green Version]
- Lee, C.-H.; Cheng, S.-H.; Wang, Y.-J.; Chen, Y.-C.; Chen, N.-T.; Souris, J.; Chen, C.-T.; Mou, C.-Y.; Yang, C.-S.; Lo, L.-W. Near-Infrared Mesoporous Silica Nanoparticles for Optical Imaging: Characterization and In Vivo Biodistribution. Adv. Funct. Mater. 2009, 19, 215–222. [Google Scholar] [CrossRef]
- Cheng, S.-H.; Li, F.-C.; Souris, J.S.; Yang, C.-S.; Tseng, F.-G.; Lee, H.-S.; Chen, C.-T.; Dong, C.-Y.; Lo, L.-W. Visualizing Dynamics of Sub-Hepatic Distribution of Nanoparticles Using Intravital Multiphoton Fluorescence Microscopy. ACS Nano 2012, 6, 4122–4131. [Google Scholar] [CrossRef]
- Slowing, I.; Trewyn, B.G.; Lin, V.S.-Y. Effect of Surface Functionalization of MCM-41-Type Mesoporous Silica Nanoparticles on the Endocytosis by Human Cancer Cells. J. Am. Chem. Soc. 2006, 128, 14792–14793. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Chung, T.-H.; Wu, S.-H.; Yao, M.; Lu, C.-W.; Lin, Y.-S.; Hung, Y.; Mou, C.-Y.; Chen, Y.-C.; Huang, D.-M. The effect of surface charge on the uptake and biological function of mesoporous silica nanoparticles in 3T3-L1 cells and human mesenchymal stem cells. Biomaterials 2007, 28, 2959–2966. [Google Scholar] [CrossRef] [PubMed]
- Lu, F.; Wu, S.-H.; Hung, Y.; Mou, C.-Y. Size Effect on Cell Uptake in Well-Suspended, Uniform Mesoporous Silica Nanoparticles. Small 2009, 5, 1408–1413. [Google Scholar] [CrossRef] [PubMed]
- Chen, Y.-P.; Chen, H.-A.; Hung, Y.; Chien, F.-C.; Chen, P.; Mou, C.-Y. Surface charge effect in intracellular localization of mesoporous silicananoparticles as probed by fluorescent ratiometric pH imaging. RSC Adv. 2011, 2, 968–973. [Google Scholar] [CrossRef]
- Chou, C.-C.; Chen, W.; Hung, Y.; Mou, C.-Y. Molecular Elucidation of Biological Response to Mesoporous Silica Nanoparticles in Vitro and in Vivo. ACS Appl. Mater. Interfaces 2017, 9, 22235–22251. [Google Scholar] [CrossRef]
- Thangaraja, A.; Savitha, V.; Jegatheesan, K. Preparation and Characterization of Polyethylene Glycol Coated Silica Nanoparticles for Drug Delivery Application. Int. J. Nanotechnol. Appl. 2010, 4, 31–38. [Google Scholar]
- Cauda, V.; Argyo, C.; Bein, T. Impact of different PEGylation patterns on the long-term bio-stability of colloidal mesoporous silica nanoparticles. J. Mater. Chem. 2010, 20, 8693–8699. [Google Scholar] [CrossRef]
- Dos Santos, S.N.; dos Reis, S.R.R.; Pires, L.P.; Helal-Neto, E.; Sancenon, F.; Barja-Fidalgo, T.C.; de Mattos, R.M.; Nasciutti, L.E.; Martinez-Manez, R.; Santos-Oliveira, R. Avoiding the mononuclear phagocyte system using human albumin for mesoporous silica nanoparticle system. Microporous Mesoporous Mater. 2017, 251, 181–189. [Google Scholar] [CrossRef]
- Sopyan, I.; Mel, M.; Ramesh, S.; Khalid, K.A. Porous hydroxyapatite for artificial bone applications. Sci. Technol. Adv. Mater. 2007, 8, 116–123. [Google Scholar] [CrossRef] [Green Version]
- Hao, X.; Hu, X.; Zhang, C.; Chen, S.; Li, Z.; Yang, X.; Liu, H.; Jia, G.; Liu, D.; Ge, K.; et al. Hybrid Mesoporous Silica-Based Drug Carrier Nanostructures with Improved Degradability by Hydroxyapatite. ACS Nano 2015, 9, 9614–9625. [Google Scholar] [CrossRef]
- Hosseinpour, S.; Walsh, L.J.; Xu, C. Biomedical application of mesoporous silica nanoparticles as delivery systems: A biological safety perspective. J. Mater. Chem. B 2020, 8, 9863–9876. [Google Scholar] [CrossRef]
- Niculescu, V.C. Mesoporous Silica Nanoparticles for Bio-Applications. Front. Mater. 2020, 7, 36. [Google Scholar] [CrossRef] [Green Version]
- Wang, Y.; Zhao, Q.; Han, N.; Bai, L.; Li, J.; Che, E.; Hu, L.; Zhang, Q.; Jiang, T.; Wang, S. Mesoporous silica nanoparticles in drug delivery and biomedical applications. Nanomed. Nanotechnol. Biol. Med. 2015, 11, 313–327. [Google Scholar] [CrossRef] [PubMed]
- Jafari, S.; Derakhshankhah, H.; Alaei, L.; Fattahi, A.; Varnamkhasti, B.S.; Saboury, A.A. Mesoporous silica nanoparticles for therapeutic/diagnostic applications. Biomed. pharmacother. 2019, 109, 1100–1111. [Google Scholar] [CrossRef] [PubMed]
- Portilho, F.L.; Helal-Neto, E.; Cabezas, S.S.; Pinto, S.R.; Dos Santos, S.N.; Pozzo, L.; Sancenon, F.; Martinez-Manez, R.; Santos-Oliveira, R. Magnetic core mesoporous silica nanoparticles doped with dacarbazine and labelled with 99mTc for early and differential detection of metastatic melanoma by single photon emission computed tomography. Artif. Cells Nanomed. Biotechnol. 2018, 46, 1080–1087. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Kaliaraj, R.; Gandhi, S.; Sundaramurthi, D.; Sethuraman, S.; Krishnan, U.M. A biomimetic mesoporous silica–polymer composite scaffold for bone tissue engineering. J. Porous Mater. 2018, 25, 397–406. [Google Scholar] [CrossRef]
- Ricci-Junior, E.; Siqueira, L.B.D.O.D.; Rodrigues, R.A.S.; Sancenón, F.; Martínez-Máñez, R.; de Moraes, J.A.; Santos-Oliveira, R. Nanocarriers as phototherapeutic drug delivery system: Appraisal of three different nanosystems in an in vivo and in vitro exploratory study. Photodiagnosis Photodyn. Ther. 2018, 21, 43–49. [Google Scholar] [CrossRef]
- Jiang, W.; Kim, B.Y.; Rutka, J.T.; Chan, W.C. Nanoparticle-mediated cellular response is size-dependent. Nat. Nanotechnol. 2008, 3, 145–150. [Google Scholar] [CrossRef]
- Alexis, F.; Pridgen, E.; Molnar, L.K.; Farokhzad, O.C. Factors Affecting the Clearance and Biodistribution of Polymeric Nanoparticles. Mol. pharm. 2008, 5, 505–515. [Google Scholar] [CrossRef] [Green Version]
- Pasqua, L.; Cundari, S.; Ceresa, C.; Cavaletti, G. Recent Development, Applications, and Perspectives of Mesoporous Silica Particles in Medicine and Biotechnology. Curr. Med. Chem. 2009, 16, 3054–3063. [Google Scholar] [CrossRef]
- Pinto, S.R.; Helal-Neto, E.; Paumgartten, F.; Felzenswalb, I.; Araujo-Lima, C.F.; Martínez-Máñez, R.; Santos-Oliveira, R. Cytotoxicity, genotoxicity, transplacental transfer and tissue disposition in pregnant rats mediated by nanoparticles: The case of magnetic core mesoporous silica nanoparticles. Artif. Cells Nanomed. Biotechnol. 2018, 46, 527–538. [Google Scholar] [CrossRef] [Green Version]
- Chen, L.; Liu, J.; Zhang, Y.; Zhang, G.; Kang, Y.; Chen, A.; Feng, X.; Shao, L. The toxicity of silica nanoparticles to the immune system. Nanomedicine 2018, 13, 1939–1962. [Google Scholar] [CrossRef] [Green Version]
- Bhavsar, D.; Patel, V.; Sawant, K. Systematic investigation of in vitro and in vivo safety, toxicity and degradation of mesoporous silica nanoparticles synthesized using commercial sodium silicate. Microporous Mesoporous Mater. 2019, 284, 343–352. [Google Scholar] [CrossRef]
- Díaz, A.; López, T.; Manjarrez, J.; Basaldella, E.; Martínez-Blanes, J.; Odriozola, J. Growth of hydroxyapatite in a biocompatible mesoporous ordered silica. Acta Biomater. 2006, 2, 173–179. [Google Scholar] [CrossRef] [PubMed]
- Jaffe, E.A.; Nachman, R.L.; Becker, C.G.; Minick, C.R. Culture of human endothelial cells derived from umbilical veins. Identification by morphologic and immunologic criteria. J. Clin. Investig. 1973, 52, 2745–2756. [Google Scholar] [CrossRef]
- Nunes, S.S.; Outeiro-Bernstein, M.A.; Juliano, L.; Vardiero, F.; Nader, H.B.; Woods, A.; Legrand, C.; Morandi, V. Syndecan-4 contributes to endothelial tubulogenesis through interactions with two motifs inside the pro-angiogenic N-terminal domain of thrombospondin-1. J. Cell. physiol. 2008, 214, 828–837. [Google Scholar] [CrossRef]
- Pei, L.; Kurumada, K.; Tanigaki, M.; Hiro, M.; Susa, K. Effect of drying on the mesoporous structure of sol–gel derived silica with PPO–PEO–PPO template block copolymer. J. Colloid Interface Sci. 2005, 284, 222–227. [Google Scholar] [CrossRef]
- Morsi, R.E.; Mohamed, R.S. Nanostructured mesoporous silica: Influence of the preparation conditions on the physical-surface properties for efficient organic dye uptake. R. Soc. Open Sci. 2018, 5, 172021. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Awoke, Y.; Chebude, Y.; Díaz, I. Controlling Particle Morphology and Pore Size in the Synthesis of Ordered Mesoporous Materials. Molecules 2020, 25, 4909. [Google Scholar] [CrossRef]
- Prokopowicz, M.; Szewczyk, A.; Skwira, A.; Sadej, R.; Walker, G. Biphasic composite of calcium phosphate-based mesoporous silica as a novel bone drug delivery system. Drug Deliv. Transl. Res. 2020, 10, 455–470. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Szewczyk, A.; Skwira, A.; Ginter, M.; Tajer, D.; Prokopowicz, M. Microwave-Assisted Fabrication of Mesoporous Silica-Calcium Phosphate Composites for Dental Application. Polymers 2020, 13, 53. [Google Scholar] [CrossRef] [PubMed]
- Ye, H.; Liu, X.Y.; Hong, H. Characterization of sintered titanium/hydroxyapatite biocomposite using FTIR spectroscopy. J. Mater. Sci. Mater. Med. 2009, 20, 843–850. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Zhao, C.X.; Yu, L.; Middelberg, A.P. Magnetic mesoporous silica nanoparticles end-capped with hydroxyapatite for pH-responsive drug release. J. Mater. Chem. B 2013, 1, 4828–4833. [Google Scholar] [CrossRef]
- Wigner, P.; Zielinski, K.; Michlewska, S.; Danielska, P.; Marczak, A.; Ricci, E.J.; Santos-Oliveira, R.; Szwed, M. Disturbance of cellular homeostasis as a molecular risk evaluation of human endothelial cells exposed to nanoparticles. Sci. Rep. 2021, 11, 1–16. [Google Scholar] [CrossRef]
- Bhanumathi, R.; Manivannan, M.; Thangaraj, R.; Kannan, S. Drug-Carrying Capacity and Anticancer Effect of the Folic Acid- and Berberine-Loaded Silver Nanomaterial To Regulate the AKT-ERK Pathway in Breast Cancer. ACS Omega 2018, 3, 8317–8328. [Google Scholar] [CrossRef] [Green Version]
- Shukla, R.K.; Kumar, A.; Vallabani, N.V.S.; Pandey, A.K.; Dhawan, A. Titanium dioxide nanoparticle-induced oxidative stress triggers DNA damage and hepatic injury in mice. Nanomedicine 2014, 9, 1423–1434. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Cho, Y.-M.; Mizuta, Y.; Akagi, J.I.; Toyoda, T.; Sone, M.; Ogawa, K. Size-dependent acute toxicity of silver nanoparticles in mice. J. Toxicol. pathol. 2018, 31, 73–80. [Google Scholar] [CrossRef] [Green Version]
- Wang, Q.; Huang, J.Y.; Li, H.Q.; Chen, Z.; Zhao, A.Z.J.; Wang, Y.; Zhang, K.Q.; Sun, H.T.; Al-Deyab, S.S.; Lai, Y.K. TiO2 nanotube platforms for smart drug delivery: A review. Int. J. Nanomed. 2016, 11, 4819–4834. [Google Scholar]
- Gonzalez, G.; Sagarzazu, A.; Cordova, A.; Gomes, M.E.; Salas, J.; Contreras, L.; Noris-Suarez, K.; Lascano, L. Comparative study of two silica mesoporous materials (SBA-16 and SBA-15) modified with a hydroxyapatite layer for clindamycin controlled delivery. Microporous Mesoporous Mater. 2018, 256, 251–265. [Google Scholar] [CrossRef]
- Chen, Y.; Wang, J.; Zhu, X.; Fan, Y.; Zhang, X. Adsorption and Release Behaviors of Vascular Endothelial Growth Factor on Porous Hydroxyapatite Ceramic Under Competitive Conditions. J. Biomater. Tissue Eng. 2014, 4, 155–161. [Google Scholar] [CrossRef]
- Zhao, J.; Bu, D.; Zhang, N.; Tian, D.; Ma, L.; Yang, H. Cytotoxicity of mesoporous silica modified by amino and carboxyl groups on vascular endothelial cells. Environ. Toxicol. 2021, 36, 1422–1433. [Google Scholar] [CrossRef] [PubMed]
- Duan, J.; Yu, Y.; Yü, Y.; Li, Y.; Huang, P.; Zhou, X.; Peng, S.; Sun, Z. Silica nanoparticles enhance autophagic activity, disturb endothelial cell homeostasis and impair angiogenesis. Part. Fibre Toxicol. 2014, 11, 50. [Google Scholar] [CrossRef] [Green Version]
- Øvrevik, J.; Låg, M.; Schwarze, P.; Refsnes, M. p38 and Src-ERK1/2 Pathways Regulate Crystalline Silica-Induced Chemokine Release in Pulmonary Epithelial Cells. Toxicol. Sci. 2004, 81, 480–490. [Google Scholar] [CrossRef] [Green Version]
- Tai, G.; Liu, G.; Li, Q.; Ni, W.; Zhang, N.; Zheng, X.; Wang, Y.; Shao, D. Cytotoxicity of various types of gold-mesoporous silica nanoparticles in human breast cancer cells. Int. J. Nanomed. 2015, 10, 6075–6087. [Google Scholar] [CrossRef] [Green Version]
- Kim, K.-A.; Kim, Y.-H.; Seo, M.S.; Lee, W.K.; Kim, S.W.; Kim, H.; Lee, K.-H.; Shin, I.-C.; Han, J.-S.; Kim, H.J.; et al. Mechanism of silica-induced ROS generation in Rat2 fibroblast cells. Toxicol. Lett. 2002, 135, 185–191. [Google Scholar] [CrossRef]
- Sun, X.; Zhang, J.; Wang, Z.; Liu, B.; Zhu, S.; Zhu, L.; Peng, B. Licorice isoliquiritigenin-encapsulated mesoporous silica nanoparticles for osteoclast inhibition and bone loss prevention. Theranostics 2019, 9, 5183–5199. [Google Scholar] [CrossRef] [PubMed]
- Chauhan, S.; Manivasagam, G.; Kumar, P.; Ambasta, R.K. Cellular Toxicity of Mesoporous Silica Nanoparticle in SHSY5Y and BMMNCs Cell. Pharm. Nanotechnol. 2019, 6, 245–252. [Google Scholar] [CrossRef] [PubMed]



| Sample | Si (%) | Ca (%) | P (%) | Atomic Ratio Added Si:Ca:P | Atomic Ratio Founded Si:Ca:P | Atomic Ratio Ca:P |
|---|---|---|---|---|---|---|
| SBA-15 | 38 | - | - | - | - | - |
| SBA-15/HAP_1 | 40 | 4.1 | 2 | 1.0:1.0:0.6 | 1.00:0.10:0.05 | 2.05 |
| SBA-15/HAP_2 | 25 | 12 | 6.1 | 1.0:1.0:0.6 | 1.00:0.48:0.24 | 1.97 |
| HAP | - | 35 | 18 | -:1.0:0.6 | -:1.00:0.51 | 1.94 |
Publisher’s Note: MDPI stays neutral with regard to jurisdictional claims in published maps and institutional affiliations. |
© 2021 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
da Silva de Barros, A.O.; Rebêlo Alencar, L.M.; Alexis, F.; Santos-Oliveira, R. Distinct Methodologies to Produce Capped Mesoporous Silica with Hydroxyapatite and the Influence in Intracellular Signaling as Cytotoxicity on Human Umbilical Vein Endothelial Cells. Bioengineering 2021, 8, 125. https://doi.org/10.3390/bioengineering8090125
da Silva de Barros AO, Rebêlo Alencar LM, Alexis F, Santos-Oliveira R. Distinct Methodologies to Produce Capped Mesoporous Silica with Hydroxyapatite and the Influence in Intracellular Signaling as Cytotoxicity on Human Umbilical Vein Endothelial Cells. Bioengineering. 2021; 8(9):125. https://doi.org/10.3390/bioengineering8090125
Chicago/Turabian Styleda Silva de Barros, Aline Oliveira, Luciana Magalhães Rebêlo Alencar, Frank Alexis, and Ralph Santos-Oliveira. 2021. "Distinct Methodologies to Produce Capped Mesoporous Silica with Hydroxyapatite and the Influence in Intracellular Signaling as Cytotoxicity on Human Umbilical Vein Endothelial Cells" Bioengineering 8, no. 9: 125. https://doi.org/10.3390/bioengineering8090125
APA Styleda Silva de Barros, A. O., Rebêlo Alencar, L. M., Alexis, F., & Santos-Oliveira, R. (2021). Distinct Methodologies to Produce Capped Mesoporous Silica with Hydroxyapatite and the Influence in Intracellular Signaling as Cytotoxicity on Human Umbilical Vein Endothelial Cells. Bioengineering, 8(9), 125. https://doi.org/10.3390/bioengineering8090125







