A Loss of Nuclear—Cytoskeletal Interactions in Vascular Smooth Muscle Cell Differentiation Induced by a Micro-Grooved Collagen Substrate Enabling the Modeling of an In Vivo Cell Arrangement
Abstract
1. Introduction
2. Materials and Methods
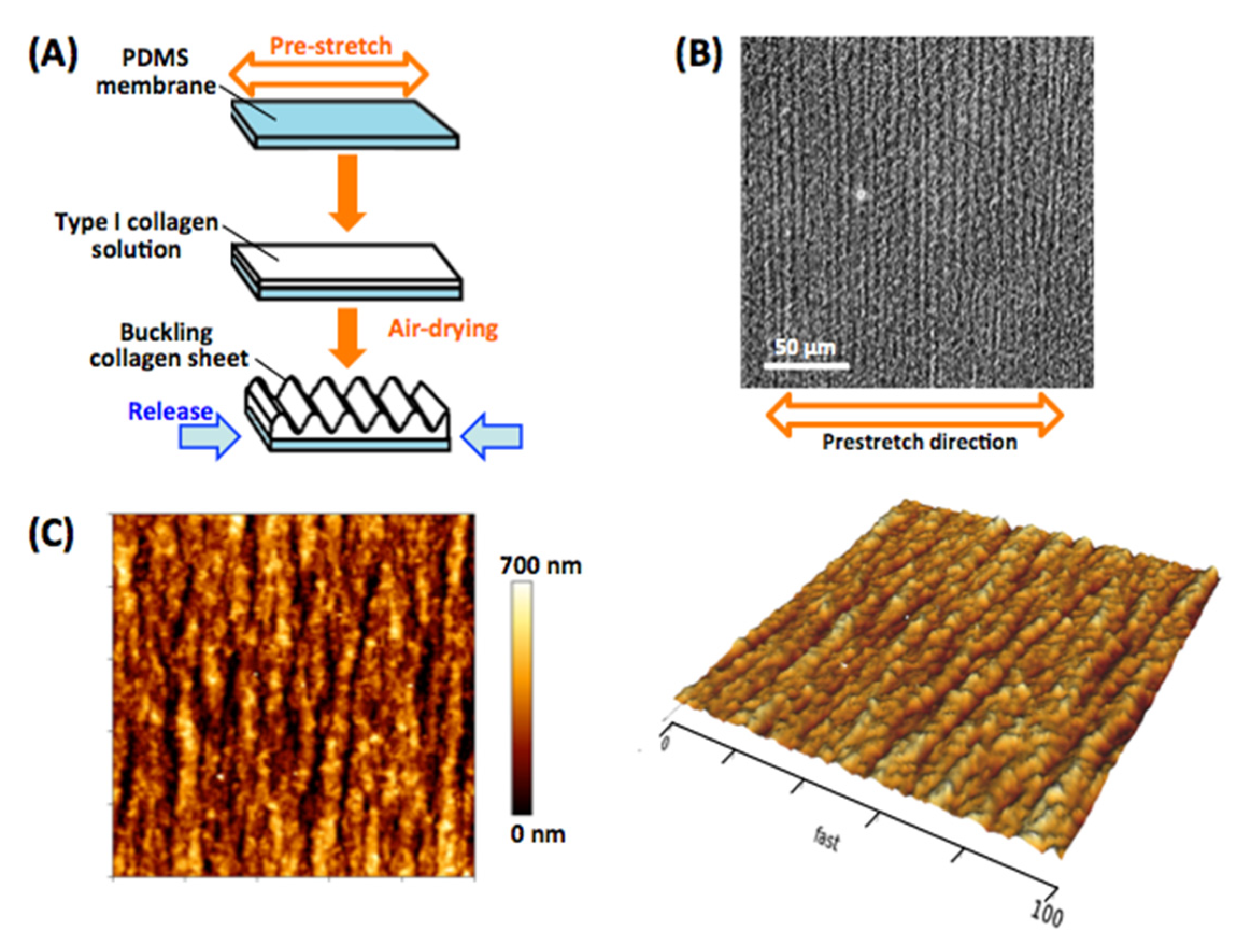
2.1. Fabrication of the Micro-Grooved Collagen Substrate
2.2. AFM Analyses of Substrate Surface Topography and Stiffness
2.3. Cell Culture
2.4. The Immunofluorescence Assay
2.5. Confocal Fluorescence Microscopy
2.6. The EdU Cell Proliferation Assay
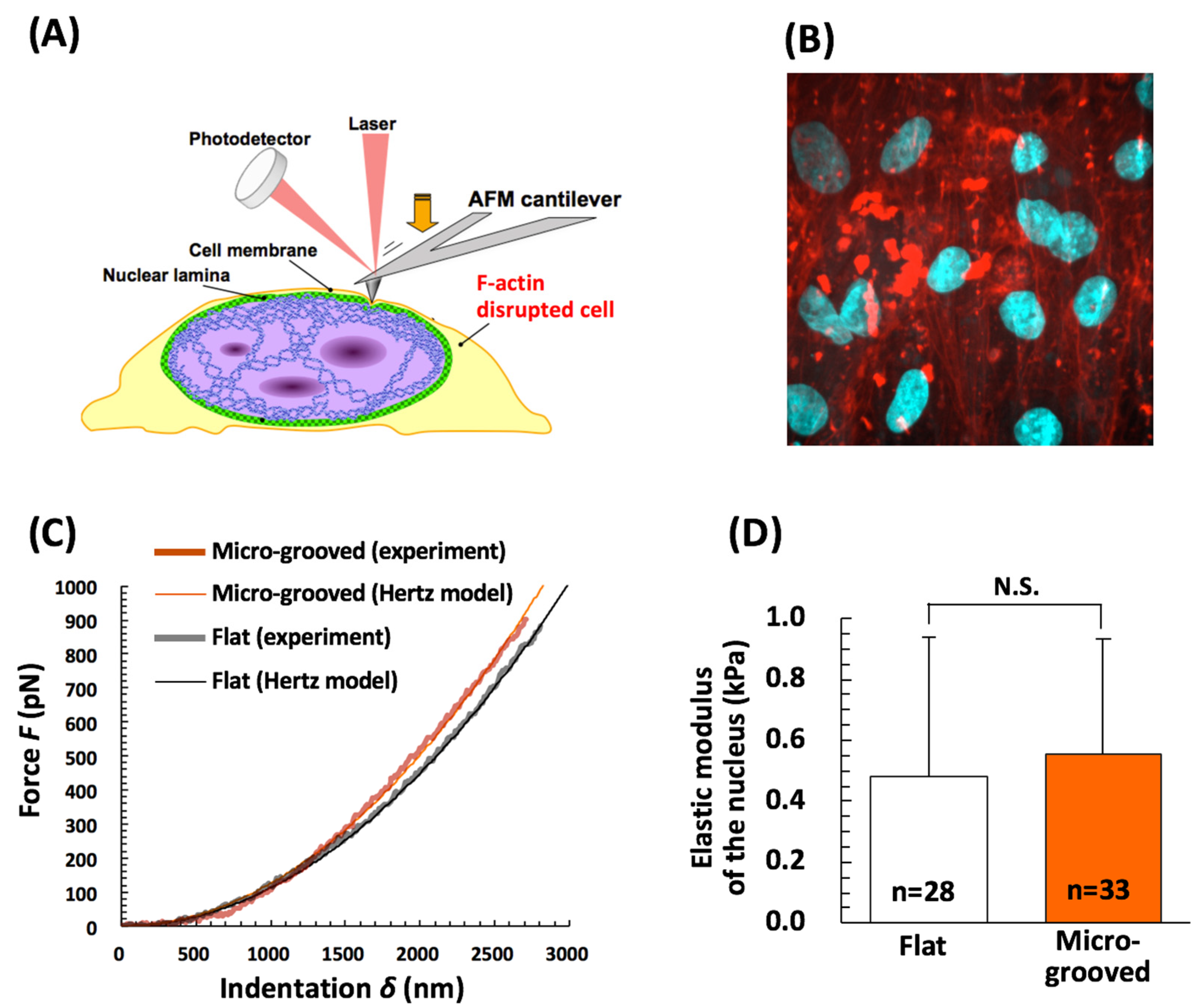
2.7. AFM Indentation Analysis of the Cell Nucleus
2.8. In Situ Measurement of Nucleus Deformation during Stretching
3. Results
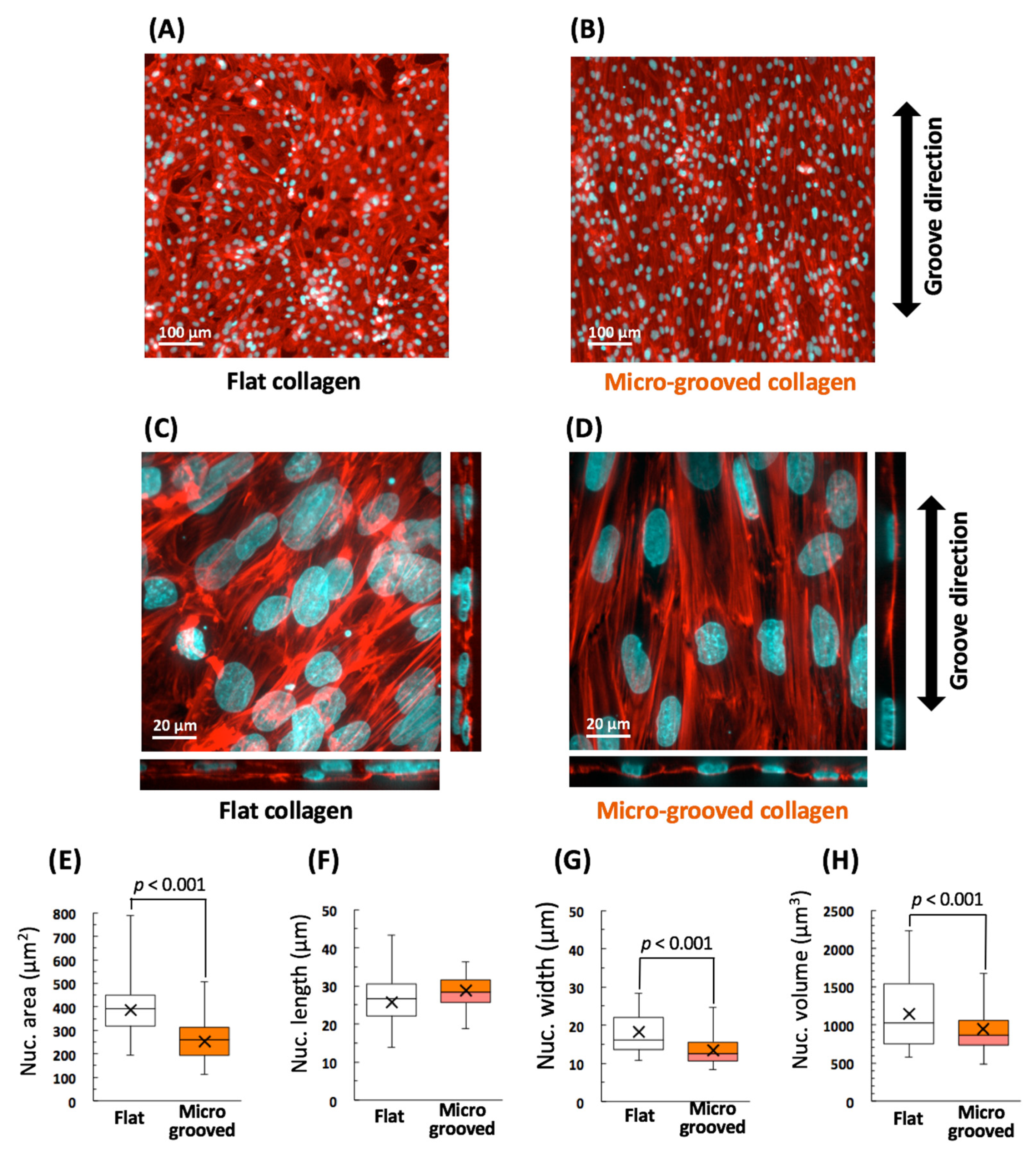
3.1. Significant Alignment and Morphological Changes of VSMCs on the Micro-Grooved Collagen
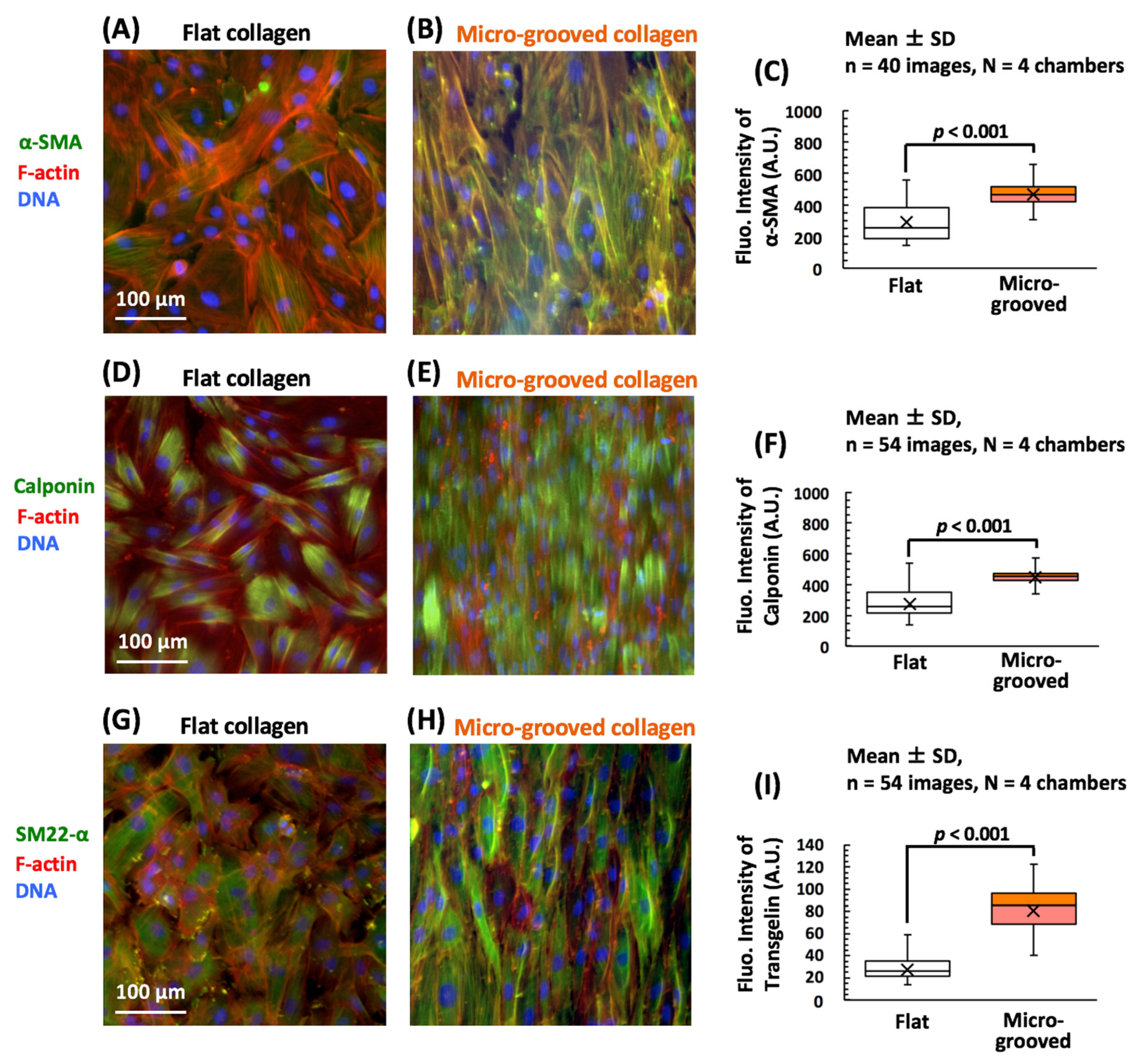
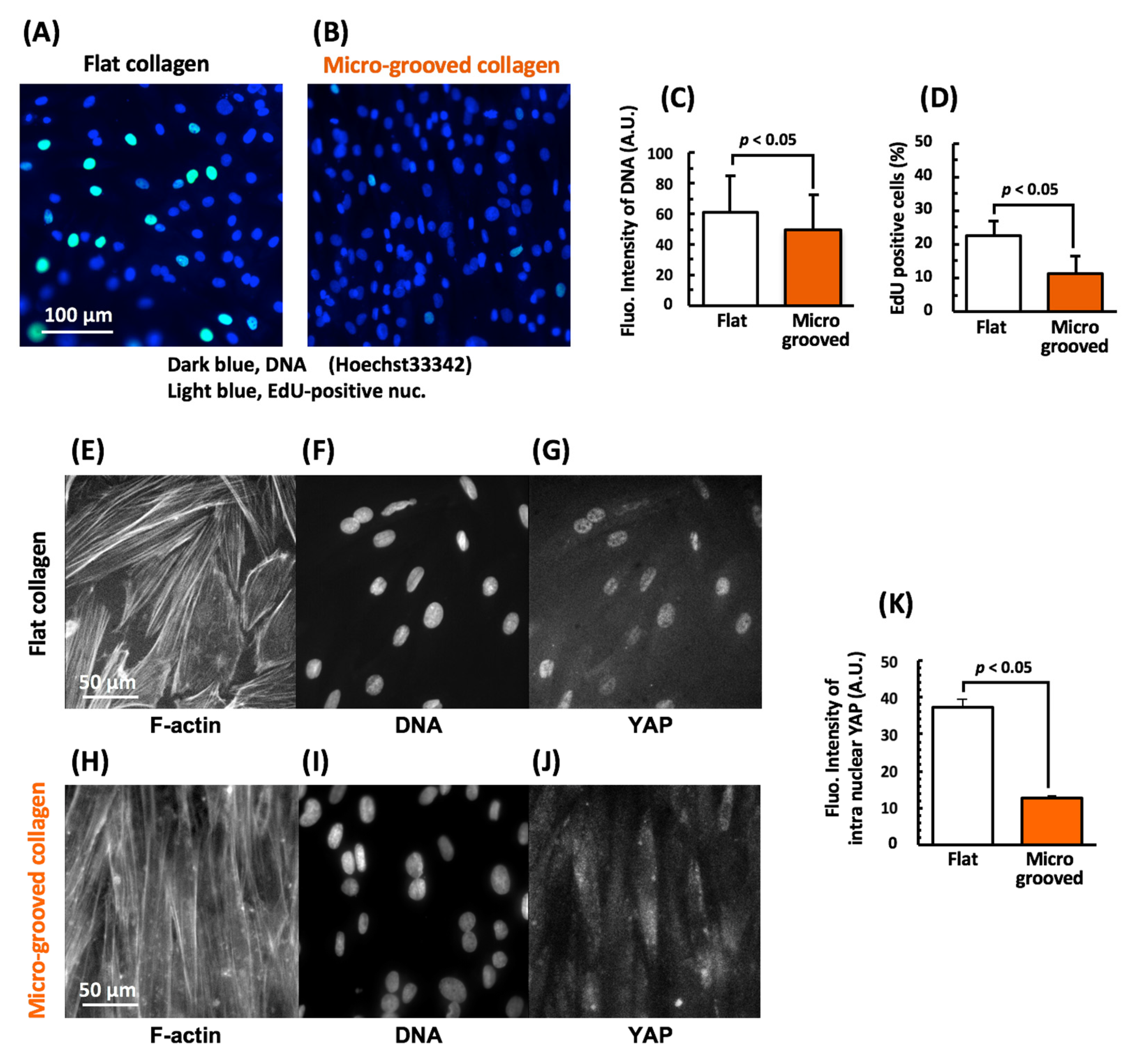
3.2. Cultivation on the Micro-Grooved Collagen Induces VSMC Differentiation with Proliferation Inhibition Involving YAP Signaling
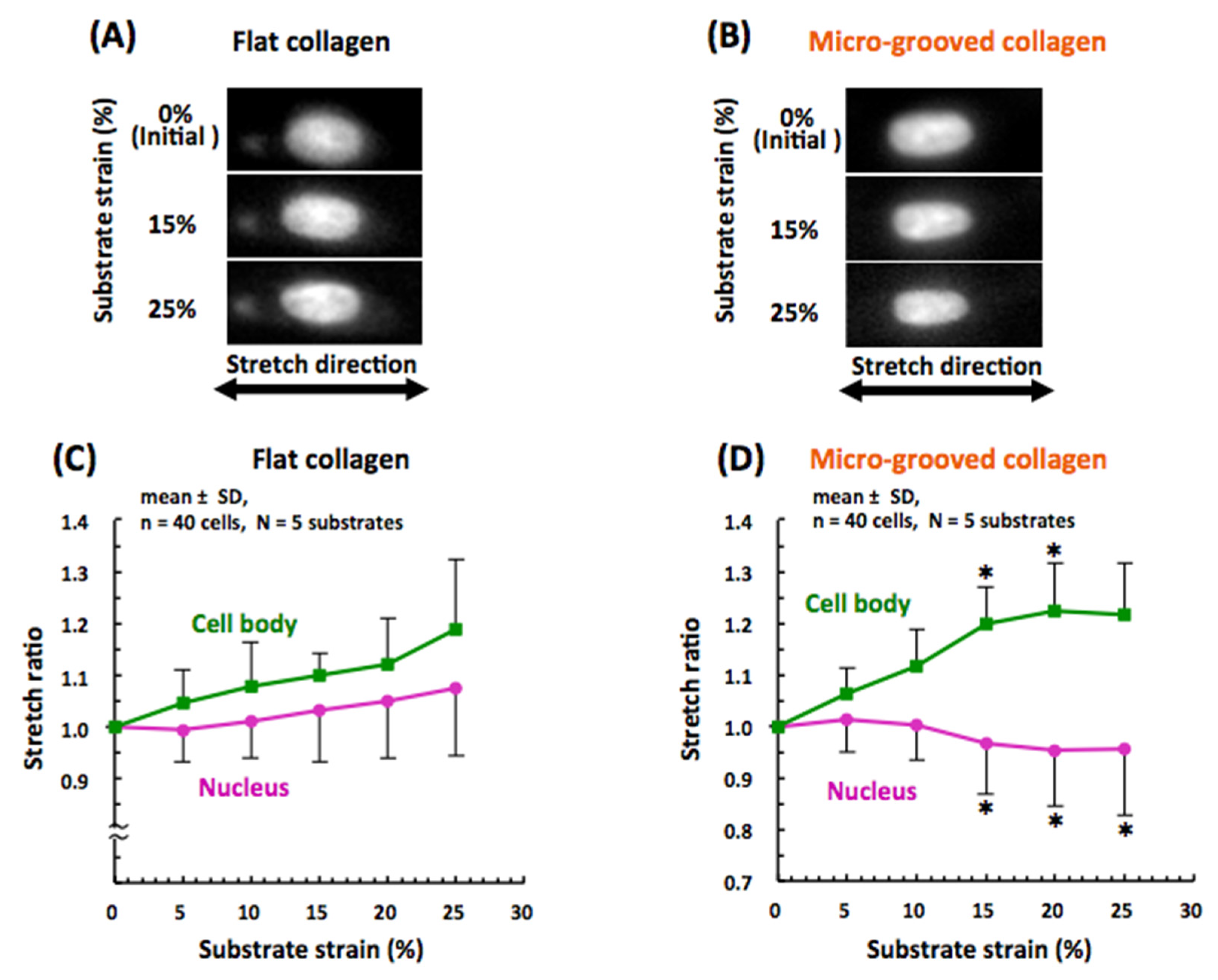
3.3. Nuclear Mechanical Deformation Is Dramatically Smaller in Differentiated VSMCs on Micro-Grooved Collagen during Macroscopic Stretch
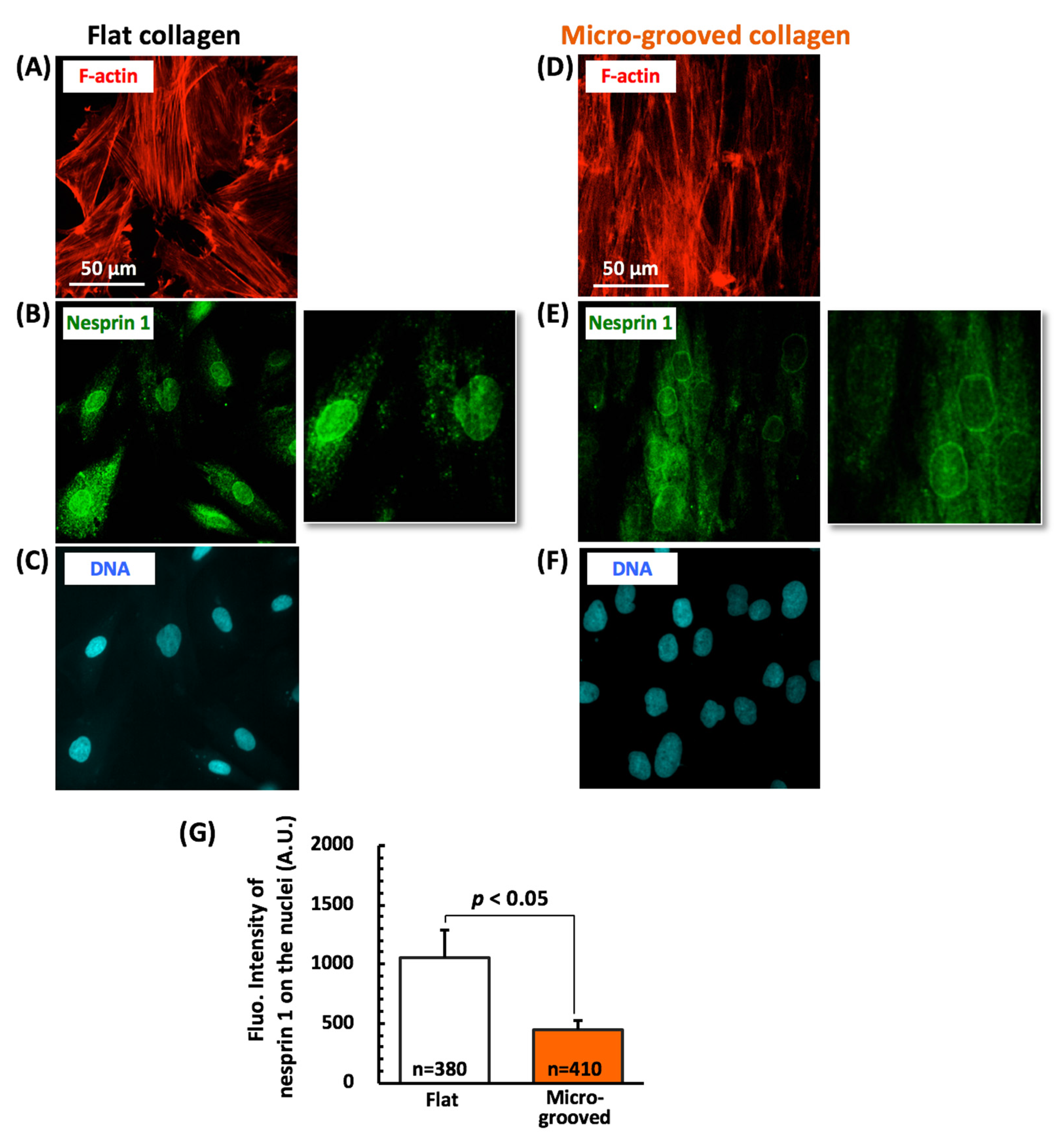
4. Discussion
Supplementary Materials
Funding
Institutional Review Board Statement
Informed Consent Statement
Data Availability Statement
Acknowledgments
Conflicts of Interest
References
- Wolinsky, H. Long-term effects of hypertention on the rat aortic wall and relation to concurrent aging changes. Circ. Res. 1972, 30, 301–309. [Google Scholar] [CrossRef] [PubMed]
- Noon, J.P.; Rice, P.J.; Baldessarini, R.J. Calcium leakage as a cause of the high resting tension in vascular smooth muscle from the spontaneously hypertensive rat. Proc. Natl. Acad. Sci. USA 1978, 75, 1605–1607. [Google Scholar] [CrossRef]
- Matsumoto, T.; Hayashi, K. Mechanical and dimensional adaptation of rat aorta to hypertension. J. Biomech. Eng. 1994, 116, 278–283. [Google Scholar] [CrossRef] [PubMed]
- Campbell, G.; Campbell, J. The Vascular Smooth Muscle; Schwartz, S., Mecham, R., Eds.; Academic Press: Cambridge, MA, USA, 1995; pp. 503–522. [Google Scholar]
- Kocher, O.; Gabbiani, F.; Gabbiani, G.; Reidy, M.A.; Cokay, M.S.; Peters, H. Phenotypic features of smooth muscle cells during the evolution of experimental carotid artery intimal thickening. Biochem. Morphol. Stud. Lab. Investig. 1991, 65, 459–470. [Google Scholar]
- Engler, A.; Bacakova, L.; Newman, C.; Hategan, A.; Griffin, M.; Discher, D. Substrate compliance versus ligand density in cell on gel responses. Biophys. J. 2004, 86, 617–628. [Google Scholar] [CrossRef]
- Peyton, S.R.; Raub, C.B.; Keschrumrus, V.P.; Putnam, A.J. The use of poly(ethylene glycol) hydrogels to investigate the impact of ECM chemistry and mechanics on smooth muscle cells. Biomaterials 2006, 27, 4881–4893. [Google Scholar] [CrossRef]
- Shi, Y.; Dong, Y.; Duan, Y.; Jiang, X.; Chen, C.; Deng, L. Substrate stiffness influences TGF-β1-induced differentiation of bronchial fibroblasts into myofibroblasts in airway remodeling. Mol. Med. Rep. 2013, 7, 419–424. [Google Scholar] [CrossRef] [PubMed]
- Bettinger, C.J.; Langer, R.; Borenstein, J.T. Engineering substrate topography at the micro- and nanoscale to control cell function. Angew. Chem. Int. Ed. Engl. 2009, 48, 5406–5415. [Google Scholar] [CrossRef]
- Thakar, R.G.; Cheng, Q.; Patel, S.; Chu, J.; Nasir, M.; Liepmann, D.; Komvopoulos, K.; Li, S. Cell-shape regulation of smooth muscle cell proliferation. Biophys. J. 2009, 96, 3423–3432. [Google Scholar] [CrossRef]
- Taneja, V.; Vertegel, A.; Langan, E.M.; Laberge, M. Influence of topography of an endovascular stent material on smooth muscle cell response. Ann. Vasc. Surg. 2011, 25, 675–685. [Google Scholar] [CrossRef]
- Chang, S.; Song, S.; Lee, J.; Yoon, J.; Park, J.; Choi, S.; Park, J.K.; Choi, K.; Choi, C. Phenotypic modulation of primary vascular smooth muscle cells by short-term culture on micropatterned substrate. PLoS ONE 2014, 9, e88089. [Google Scholar]
- Abagnale, G.; Steger, M.; Nguyen, V.H.; Hersch, N.; Sechi, A.; Joussen, S.; Denecke, B.; Merkel, R.; Hoffmann, B.; Dreser, A.; et al. Surface topography enhances differentiation of mesenchymal stem cells towards osteogenic and adipogenic lineages. Biomaterials 2015, 61, 316–326. [Google Scholar] [CrossRef]
- Nagayama, K.; Uchida, K.; Sato, A. A novel micro-grooved collagen substrate for inducing vascular smooth muscle differentiation through cell tissue arrangement and nucleus remodeling. J. Mech. Behav. Biomed. Mater. 2019, 90, 295–305. [Google Scholar] [CrossRef]
- Luxton, G.W.; Gomes, E.R.; Folker, E.S.; Vintinner, E.; Gundersen, G.G. Linear arrays of nuclear envelope proteins harness retrograde actin flow for nuclear movement. Science 2010, 329, 956–959. [Google Scholar] [CrossRef] [PubMed]
- Wang, N.; Tytell, J.; Ingber, D. Mechanotransduction at a distance: Mechanically coupling the extracellular matrix with the nucleus. Nat. Rev. Mol. Cell Biol. 2009, 10, 75–82. [Google Scholar] [CrossRef] [PubMed]
- Nagayama, K.; Yamazaki, S.; Yahiro, Y.; Matsumoto, T. Estimation of the mechanical connection between apical stress fibers and the nucleus in vascular smooth muscle cells cultured on a substrate. J. Biomech. 2014, 47, 1422–1429. [Google Scholar] [CrossRef] [PubMed]
- Dorban, P.B. Mechanical properties of arteries. In The Mechanics of the Circulation; Caro, C.G., Pedley, T.J., Schroter, R.C., Seed, W.A., Eds.; Oxford University Press: Oxford, UK, 1978; pp. 397–460. [Google Scholar]
- Halder, G.; Dupont, S.; Piccolo, S. Transduction of mechanical and cytoskeletal cues by YAP and TAZ. Nat. Rev. Mol. Cell Biol. 2012, 13, 591–600. [Google Scholar] [CrossRef] [PubMed]
- Wang, X.; Hu, G.; Gao, X.; Wang, Y.; Zhang, W.; Harmon, E.Y.; Zhi, X.; Xu, Z.; Lennartz, M.R.; Barroso, M.; et al. The Induction of Yes-Associated Protein Expression After Arterial Injury Is Crucial for Smooth Muscle Phenotypic Modulation and Neointima Formation. Arterioscler. Thromb. Vasc. Biol. 2012, 32, 2662–2669. [Google Scholar] [CrossRef]
- Yamashiro, Y.; Thang, B.Q.; Ramirez, K.; Shin, S.J.; Kohata, T.; Ohata, S.; Nguyen, T.A.V.; Ohtsuki, S.; Nagayama, K.; Yanagisawa, H. Matrix mechanotransduction mediated by thrombospondin-1/integrin/YAP in the vascular remodeling. Proc. Natl. Acad. Sci. USA 2020, 117, 9896–9905. [Google Scholar] [CrossRef]
- Hertz, H. Über die Berührung fester elastischer Körper. J. Für Die Reine Und Angew. Math. 1881, 92, 156–171. [Google Scholar]
- Nagayama, K.; Nagano, Y.; Sato, N.; Matsumoto, T. Effect of actin filament distribution on tensile properties of smooth muscle cells obtained from rat thoracic aortas. J. Biomech. 2006, 39, 293–301. [Google Scholar] [CrossRef]
- Nagayama, K.; Morishima, N.; Matsumoto, T. Effects of Three-Dimensional Culture and Cyclic Stretch Stimulation on Expression of Contractile Proteins in Freshly Isolated Rat Aortic Smooth Muscle Cells. J. Biomech. Sci. Eng. 2009, 4, 286–297. [Google Scholar] [CrossRef]
- Zeng, B.; Tong, S.; Ren, X.; Xia, H. Cardiac cell proliferation assessed by EdU, a novel analysis of cardiac regeneration. Cytotechnology 2016, 68, 763–770. [Google Scholar] [CrossRef][Green Version]
- Deguchi, S.; Maeda, K.; Ohashi, T.; Sato, M. Flow-induced hardening of endothelial nucleus as an intracellular stress-bearing organelle. J. Biomech. 2005, 38, 1751–1759. [Google Scholar] [CrossRef]
- Nagayama, K.; Adachi, A.; Matsumoto, T. Heterogeneous response of traction force at focal adhesions of vascular smooth muscle cells subjected to macroscopic stretch on a micropillar substrate. J. Biomech. 2011, 44, 2699–2705. [Google Scholar] [CrossRef] [PubMed]
- Mack, C.P.; Somlyo, A.V.; Hautmann, M.; Somlyo, A.P.; Owens, G.K. Smooth muscle differentiation marker gene expression is regulated by RhoA-mediated actin polymerization. J. Biol. Chem. 2001, 276, 341–347. [Google Scholar] [CrossRef] [PubMed]
- Sugita, S.; Matsumoto, T. Multiphoton microscopy observations of 3D elastin and collagen fiber microstructure changes during pressurization in aortic media. Biomech. Model. Mechanobiol. 2017, 16, 763–773. [Google Scholar] [CrossRef] [PubMed]
- Matsumoto, T.; Sugita, S.; Nagayama, K. Tensile Properties of Smooth Muscle Cells, Elastin, and Collagen Fibers, Vascular Engineering; Springer: Berlin/Heidelberg, Germany, 2016; pp. 27–140. [Google Scholar]
- Dupont, S.; Morsut, L.; Aragona, M.; Enzo, E.; Giulitti, S.; Cordenonsi, M.; Zanconato, F.; Le Digabel, J.; Forcato, M.; Bicciato, S.; et al. Role of YAP/TAZ in Mechanotransduction. Nature 2011, 474, 179–183. [Google Scholar] [CrossRef] [PubMed]
- Pavel, M.; Renna, M.; Park, S.J.; Menzies, F.M.; Ricketts, T.; Füllgrabe, J.; Ashkenazi, A.; Frake, R.A.; Lombarte, A.C.; Bento, C.F.; et al. Contact inhibition controls cell survival and proliferation via YAP/TAZ-autophagy axis. Nat. Commun. 2018, 9, 2961. [Google Scholar] [CrossRef]
- Sansores-Garcia, L.; Bossuyt, W.; Wada, K.; Yonemura, S.; Tao, C.; Sasaki, H.; Halder, G. Modulating F-actin organization induces organ growth by affecting the Hippo pathway. EMBO J. 2011, 30, 2325–2335. [Google Scholar] [CrossRef]
- Kim, D.H.; Khatau, S.B.; Feng, Y.; Walcott, S.; Sun, S.X.; Longmore, G.D.; Wirtz, D. Actin cap associated focal adhesions and their distinct role in cellular mechanosensing. Sci Rep. 2012, 2, 555. [Google Scholar] [CrossRef]
- Nagayama, K.; Yahiro, Y.; Matsumoto, T. Apical and Basal Stress Fibers have Different Roles in Mechanical Regulation of the Nucleus in Smooth Muscle Cells Cultured on a Substrate. Cell. Mol. Bioeng. 2013, 6, 473–481. [Google Scholar] [CrossRef]
- Wang, Y.; Cao, W.; Cui, J.; Yu, Y.; Zhao, Y.; Shi, J.; Wu, J.; Xia, Z.; Yu, B.; Liu, J. Arterial Wall Stress Induces Phenotypic Switching of Arterial Smooth Muscle Cells in Vascular Remodeling by Activating the YAP/TAZ Signaling Pathway. Cell Physiol. Biochem. 2018, 51, 842–853. [Google Scholar] [CrossRef]
- Caille, N.; Tardy, Y.; Meister, J.J. Assessment of strain field in endothelial cells subjected to uniaxial deformation of their substrate. Ann. Biomed. Eng. 1998, 26, 409–416. [Google Scholar] [CrossRef] [PubMed]
- Gilchrist, C.L.; Witvoet-Braam, S.W.; Guilak, F.; Setton, L.A. Measurement of intracellular strain on deformable substrates with texture correlation. J. Biomech. 2007, 40, 786–794. [Google Scholar] [CrossRef] [PubMed]
- Mayer, C.R.; Arsenovic, P.T.; Bathula, K.; Denis, K.B.; Conway, D.E. Characterization of 3D Printed Stretching Devices for Imaging Force Transmission in Live-Cells. Cell Mol. Bioeng. 2019, 12, 289–300. [Google Scholar] [CrossRef] [PubMed]
- Driscoll, T.P.; Cosgrove, B.D.; Heo, S.J.; Shurden, Z.E.; Mauck, R. Cytoskeletal to Nuclear Strain Transfer Regulates YAP Signaling in Mesenchymal Stem Cells. Biophys. J. 2015, 108, 2783–2793. [Google Scholar] [CrossRef]
Publisher’s Note: MDPI stays neutral with regard to jurisdictional claims in published maps and institutional affiliations. |
© 2021 by the author. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Nagayama, K. A Loss of Nuclear—Cytoskeletal Interactions in Vascular Smooth Muscle Cell Differentiation Induced by a Micro-Grooved Collagen Substrate Enabling the Modeling of an In Vivo Cell Arrangement. Bioengineering 2021, 8, 124. https://doi.org/10.3390/bioengineering8090124
Nagayama K. A Loss of Nuclear—Cytoskeletal Interactions in Vascular Smooth Muscle Cell Differentiation Induced by a Micro-Grooved Collagen Substrate Enabling the Modeling of an In Vivo Cell Arrangement. Bioengineering. 2021; 8(9):124. https://doi.org/10.3390/bioengineering8090124
Chicago/Turabian StyleNagayama, Kazuaki. 2021. "A Loss of Nuclear—Cytoskeletal Interactions in Vascular Smooth Muscle Cell Differentiation Induced by a Micro-Grooved Collagen Substrate Enabling the Modeling of an In Vivo Cell Arrangement" Bioengineering 8, no. 9: 124. https://doi.org/10.3390/bioengineering8090124
APA StyleNagayama, K. (2021). A Loss of Nuclear—Cytoskeletal Interactions in Vascular Smooth Muscle Cell Differentiation Induced by a Micro-Grooved Collagen Substrate Enabling the Modeling of an In Vivo Cell Arrangement. Bioengineering, 8(9), 124. https://doi.org/10.3390/bioengineering8090124




