Amphiphilic and Perfluorinated Poly(3-Hydroxyalkanoate) Nanocapsules for 19F Magnetic Resonance Imaging
Abstract
:1. Introduction
2. Materials and Methods
2.1. Materials
2.2. PHA(72)U(28) Biosynthesis
2.3. PEG-SH Synthesis
2.4. Synthesis of PHAU-g-(F)
2.5. Synthesis of PHA-g-(F; PEG)
2.6. Polymer Characterization
2.7. Preparation of Nanoparticles
2.8. Nanoparticles Characterization
2.9. 1H/19F MRI Imaging
3. Results and Discussion
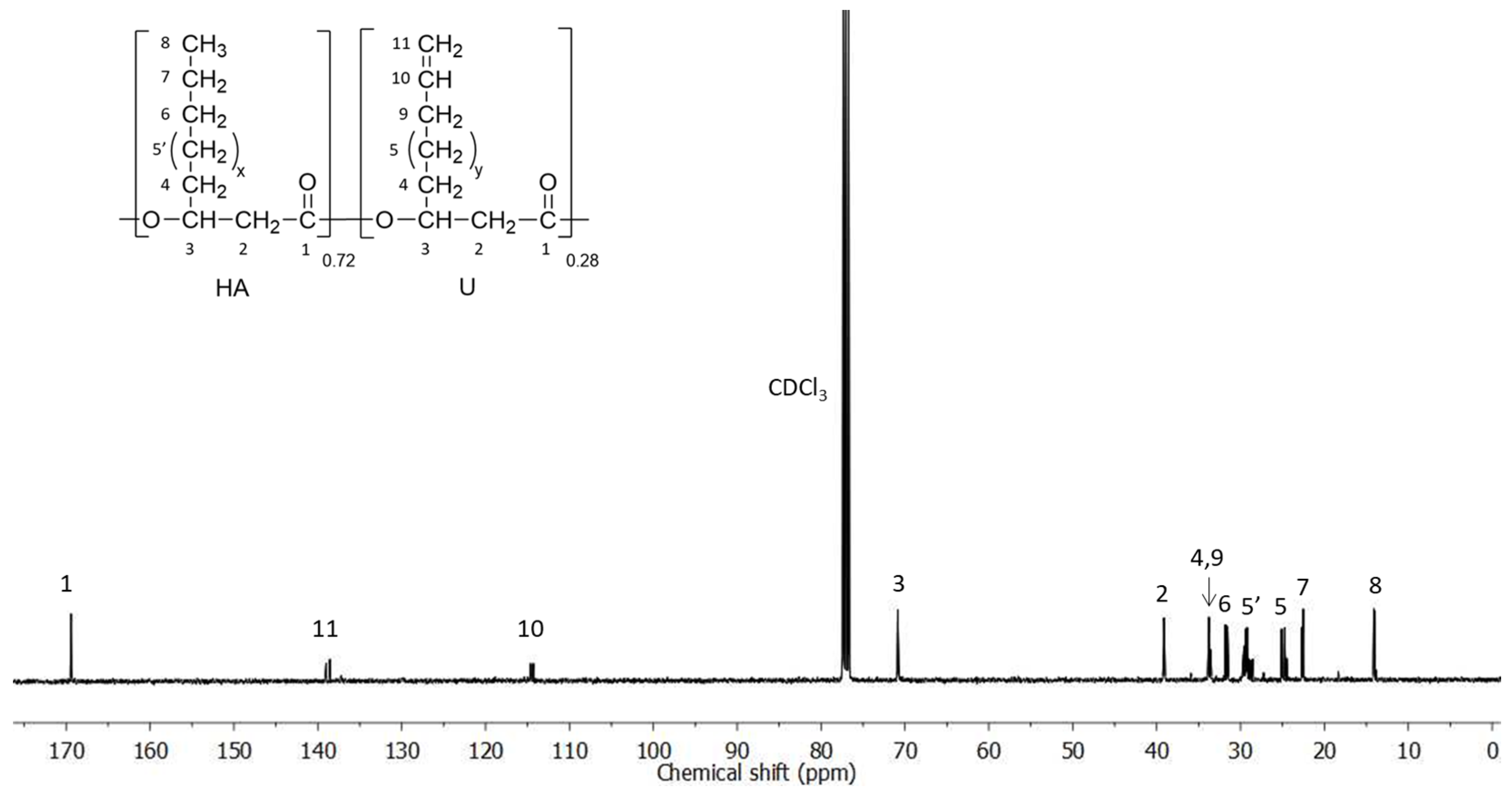
3.1. PHAU Production
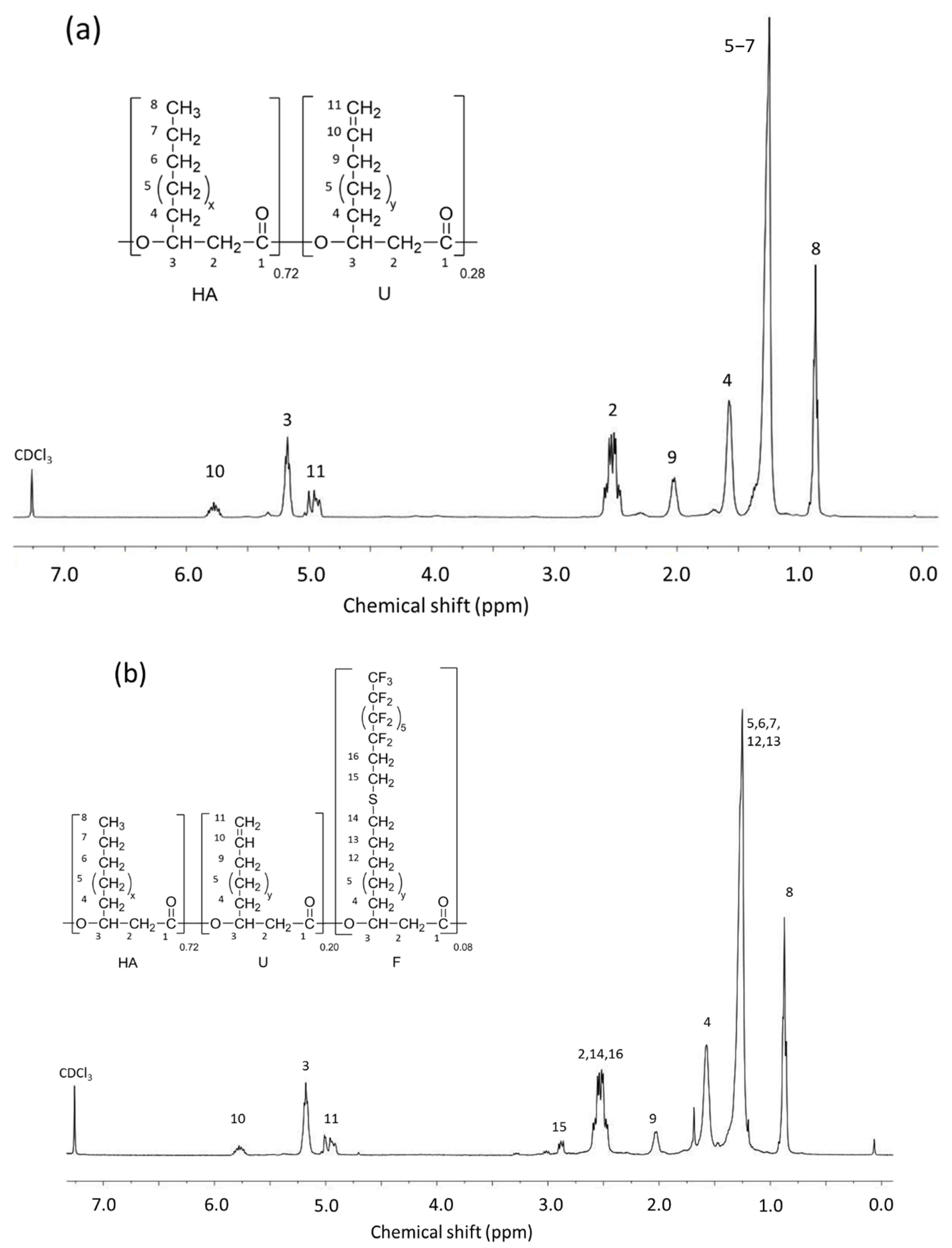
3.2. PHA-g-(F; PEG) Synthesis from PHAU
3.3. Synthesis and Characterization of the Nanoparticles
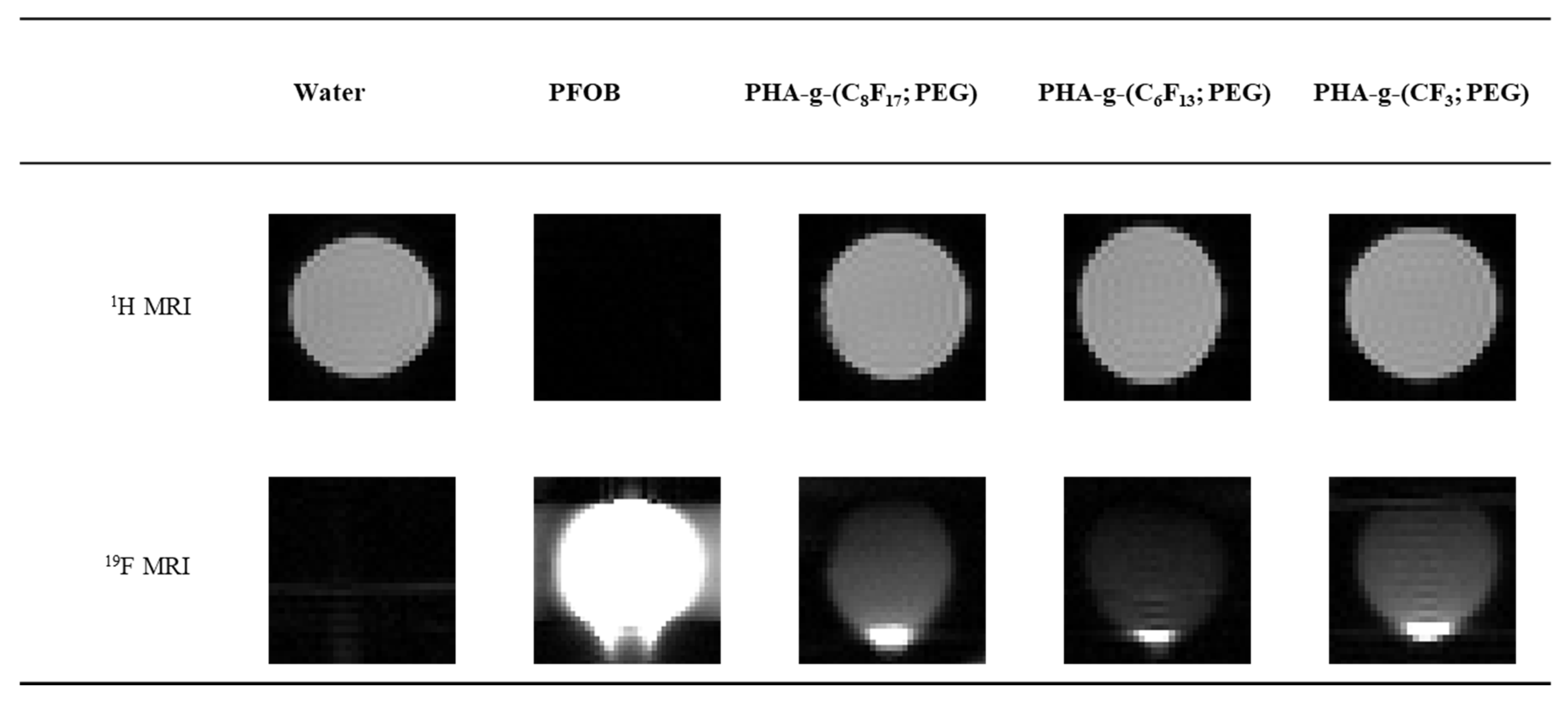
3.4. In Vitro 19F NMR
4. Conclusions
Supplementary Materials
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Data Availability Statement
Acknowledgments
Conflicts of Interest
References
- Bartusik, D.; Aebisher, D. 19F applications in drug development and imaging—A review. Biomed. Pharmacother. 2014, 68, 813–817. [Google Scholar] [CrossRef] [PubMed]
- Couch, M.J.; Ball, I.K.; Li, T.; Fox, M.S.; Biman, B.; Albert, M.S. 19F MRI of the Lungs Using Inert Fluorinated Gases: Challenges and New Developments. J. Magn. Reson. Imaging 2019, 49, 343–354. [Google Scholar] [CrossRef]
- Ahrens, E.T.; Helfer, B.M.; O’Hanlon, C.F.; Schirda, C. Clinical cell therapy imaging using a perfluorocarbon tracer and fluorine-19F MRI. Magn. Reson. Med. 2014, 72, 1696–1701. [Google Scholar] [CrossRef] [PubMed]
- Gutberlet, M.; Kaireit, T.F.; Voskrebenzev, A.; Lasch, F.; Freise, J.; Welte, T.; Wacker, F.; Hohlfeld, J.M.; Vogel-Claussen, J. Free-breathing Dynamic 19F Gas MR Imaging for Mapping of Regional Lung Ventilation in Patients with COPD. Radiology. 2017, 286, 1040–1051. [Google Scholar] [CrossRef] [Green Version]
- Cosco, D.; Fattal, E.; Fresta, M.; Tsapis, N. Perfluorocarbon-loaded micro and nanosystems for medical imaging: A state of the art. J. Fluor. Chem. 2015, 171, 18–26. [Google Scholar] [CrossRef]
- Giraudeau, C.; Flament, J.; Marty, B.; Boumezbeur, F.; Mériaux, S.; Robic, C.; Port, M.; Tsapis, N.; Fattal, E.; Giacomini, E.; et al. A new paradigm for high-sensitivity 19F magnetic resonance imaging of perfluorooctylbromide. Magn. Reson. Med. 2010, 63, 1119–1124. [Google Scholar] [CrossRef] [PubMed]
- Jirak, D.; Galisova, A.; Kolouchova, K.; Babuka, D.; Hruby, M. Fluorine polymer probes for magnetic resonance imaging: Quo vadis? Magn. Reson. Mater. Phys. Biol. Med. 2019, 32, 173–185. [Google Scholar] [CrossRef] [Green Version]
- Riess, J.G. Blood substitutes and other potential biomedical applications of Fuorinated colloids. J. Fluor. Chem. 2002, 114, 119–126. [Google Scholar] [CrossRef]
- Liu, M.-S.; Long, D.M. Perfluoroctylbromide as a Diagnostic Contrast Medium in Gastroenterography. Radiology. 1977, 122, 71–76. [Google Scholar] [CrossRef]
- Lowe, K.C. Engineering blood: Synthetic substitutes from fluorinated compounds. Tissue Eng. 2003, 9, 389–399. [Google Scholar] [CrossRef]
- Lozano, J.A.; Castro, J.A.; Rodrigo, I. Partial liquid ventilation with perfluorocarbons for treatment of ARDS in burns. Burns. 2001, 27, 635–642. [Google Scholar] [CrossRef]
- Wickline, S.A.; Hughes, M.; Ngo, F.C.; Hall, C.S.; Marsh, J.N.; Brown, P.A.; Allen, J.S.; McLean, M.D.; Scott, M.J.; Fuhrhop, R.W.; et al. Blood contrast enhancement with a novel, non-gaseous nanoparticle contrast agent. Acad. Radiol. 2002, 9 (Suppl. 2), S290–S293. [Google Scholar]
- Barnett, B.P.; Ruiz-Cabello, J.; Hota, P.; Liddell, R.; Walczak, P.; Howland, V.; Chacko, V.P.; Kraitchman, D.L.; Arepally, A.; Bulte, J.W.M. Fluorocapsules for Improved Function, Immunoprotection, and Visualization of Cellular Therapeutics with MR, US, and CT Imaging. Radiology. 2011, 258, 182–191. [Google Scholar] [CrossRef] [Green Version]
- Pisani, E.; Fattal, E.; Paris, J.; Ringard, C.; Rosilio, V.; Tsapis, N. Surfactant dependent morphology of polymeric capsules of perfluorooctyl bromide: Influence of polymer adsorption at the dichloromethane–water interface. J. Colloid Interface Sci. 2008, 326, 66–71. [Google Scholar] [CrossRef]
- Giraudeau, C.; Geffroy, F.; Mériaux, S.; Boumezbeur, F.; Robert, P.; Port, M.; Robic, C.; le Bihan, D.; Lethimonnier, F.; Valette, J. 19F molecular MR imaging for detection of brain tumor angiogenesis: In vivo validation using targeted PFOB nanoparticles. Angiogenesis 2013, 16, 171–179. [Google Scholar] [CrossRef] [PubMed]
- Diou, O.; Tsapis, N.; Giraudeau, C.; Valette, J.; Gueutin, C.; Bourasset, F.; Zanna, S.; Vauthier, C.; Fattal, E. Long-circulating perfluorooctyl bromide nanocapsules for tumor imaging by 19F MRI. Biomaterials 2012, 33, 5593–5602. [Google Scholar] [CrossRef]
- Boissenot, T.; Fattal, E.; Bordat, A.; Houvenagel, S.; Valette, J.; Chacun, H.; Gueutin, C.; Tsapis, N. Paclitaxel-loaded PEGylated nanocapsules of perfluorooctyl bromide as theranostic agents. Eur. J. Pharm. Biopharm. Off. J. Arbeitsgemeinschaft Pharm. Verfahrenstechnik EV 2016, 108, 136–144. [Google Scholar] [CrossRef] [PubMed]
- Boissenot, T.; Bordat, A.; Larrat, B.; Varna, M.; Chacun, H.; Paci, A.; Poinsignon, V.; Fattal, E.; Tsapis, N. Ultrasound-induced mild hyperthermia improves the anticancer efficacy of both Taxol® and paclitaxel-loaded nanocapsules. J. Control. Release Off. J. Control. Release Soc. 2017, 264, 219–227. [Google Scholar] [CrossRef]
- Rifai, N.A.; Desgranges, S.; Guillou-Buffello, D.L.; Giron, A.; Urbach, W.; Nassereddine, M.; Charara, J.; Contino-Pépin, C.; Taulier, N. Ultrasound-triggered delivery of paclitaxel encapsulated in an emulsion at low acoustic pressures. J. Mater. Chem. B. 2020, 8, 1640–1648. [Google Scholar] [CrossRef]
- Cheng, C.; Powell, K.T.; Khoshdel, E.; Wooley, K.L. Polydimethylsiloxane- (PDMS-) Grafted Fluorocopolymers by a “Grafting through” Strategy Based on Atom Transfer Radical (Co)polymerization. Macromolecules 2007, 40, 7195–7207. [Google Scholar] [CrossRef]
- Peng, H.; Thurecht, K.J.; Blakey, I.; Taran, E.; Whittaker, A.K. Effect of Solvent Quality on the Solution Properties of Assemblies of Partially Fluorinated Amphiphilic Diblock Copolymers. Macromolecules 2012, 45, 8681–8690. [Google Scholar] [CrossRef]
- Peng, H.; Blakey, I.; Dargaville, B.; Rasoul, F.; Rose, S.; Whittaker, A.K. Synthesis and Evaluation of Partly Fluorinated Block Copolymers as MRI Imaging Agents. Biomacromolecules 2009, 10, 374–381. [Google Scholar] [CrossRef] [PubMed]
- Kolouchova, K.; Sedlacek, O.; Jirak, D.; Babuka, D.; Blahut, J.; Kotek, J.; Vit, M.; Trousil, J.; Konefał, R.; Janouskova, O.; et al. Self-Assembled Thermoresponsive Polymeric Nanogels for 19F MR Imaging. Biomacromolecules 2018, 19, 3515–3524. [Google Scholar] [CrossRef] [PubMed]
- Mao, J.; Ni, P.; Mai, Y.; Yan, D. Multicompartment Micelles from Hyperbranched Star-Block Copolymers Containing Polycations and Fluoropolymer Segment. Langmuir 2007, 23, 5127–5134. [Google Scholar] [CrossRef]
- Srinivas, M.; Morel, P.A.; Ernst, L.A.; Laidlaw, D.H.; Ahrens, E.T. Fluorine-19 MRI for visualization and quantification of cell migration in a diabetes model. Magn. Reson. Med. 2007, 58, 725–734. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Ahrens, E.T.; Flores, R.; Xu, H.; Morel, P.A. In vivo imaging platform for tracking immunotherapeutic cells. Nat. Biotechnol. 2005, 23, 983–987. [Google Scholar] [CrossRef]
- Jamgotchian, L.; Vaillant, S.; Selingue, E.; Doerflinger, A.; Belime, A.; Vandamme, M.; Pinna, G.; Ling, W.L.; Gravel, E.; Mériaux, S.; et al. Tumor-targeted superfluorinated micellar probe for sensitive in vivo 19F-MRI. Nanoscale. 2021, 13, 2373–2377. [Google Scholar] [CrossRef] [PubMed]
- Houvenagel, S.; Picheth, G.; Dejean, C.; Brûlet, A.; Chennevière, A.; Couture, O.; Huang, N.; Moine, L.; Tsapis, N. End-chain fluorination of polyesters favors perfluorooctyl bromide encapsulation into echogenic PEGylated nanocapsules. Polym. Chem. 2017, 8, 2559–2570. [Google Scholar] [CrossRef]
- Sawada, H. Novel self-assembled molecular aggregates formed by fluoroalkyl end-capped oligomers and their application. J. Fluor. Chem. 2003, 121, 111–130. [Google Scholar] [CrossRef]
- Samanta, B.; Ofir, Y.; Patra, D.; Rotello, V.M. Self-assembly of fluorocarbon-coated FePt nanoparticles for controlling structure and wettability of surfaces. Soft Matter. 2009, 5, 1247–1250. [Google Scholar] [CrossRef] [Green Version]
- Astafyeva, K.; Somaglino, L.; Desgranges, S.; Berti, R.; Patinote, C.; Langevin, D.; Lazeyras, F.; Salomir, R.; Polidori, A.; Contino-Pépin, C.; et al. Perfluorocarbon nanodroplets stabilized by fluorinated surfactants: Characterization and potentiality as theranostic agents. J. Mater. Chem. B 2015, 3, 2892–2907. [Google Scholar] [CrossRef] [Green Version]
- Berger, R.; Resnati, G.; Metrangolo, P.; Weber, E.; Hulliger, J. Organic fluorine compounds: A great opportunity for enhanced materials properties. Chem. Soc. Rev. 2011, 40, 3496–3508. [Google Scholar] [CrossRef] [PubMed]
- Cametti, M.; Crousse, B.; Metrangolo, P.; Milani, R.; Resnati, G. The fluorous effect in biomolecular applications. Chem. Soc. Rev. 2011, 41, 31–42. [Google Scholar] [CrossRef] [PubMed]
- Krafft, M.P.; Riess, J.G. Chemistry, Physical Chemistry, and Uses of Molecular Fluorocarbon−Hydrocarbon Diblocks, Triblocks, and Related Compounds—Unique “Apolar” Components for Self-Assembled Colloid and Interface Engineering. Chem. Rev. 2009, 109, 1714–1792. [Google Scholar] [CrossRef]
- Steinbüchel, A.; Valentin, H.E. Diversity of bacterial polyhydroxyalkanoic acids. FEMS Microbiol. Lett. 1995, 128, 219–228. [Google Scholar] [CrossRef]
- Sudesh, K.; Abe, H.; Doi, Y. Synthesis, structure and properties of polyhydroxyalkanoates: Biological polyesters. Prog. Polym. Sci. 2000, 10, 1503–1555. [Google Scholar] [CrossRef]
- Lenz, R.W.; Marchessault, R.H. Bacterial polyesters: Biosynthesis, biodegradable plastics and biotechnology. Biomacromolecules 2005, 6, 1–8. [Google Scholar] [CrossRef]
- Zhang, J.; Shishatskaya, E.I.; Volova, T.G.; da Silva, L.F.; Chen, G.-Q. Polyhydroxyalkanoates (PHA) for therapeutic applications. Mater. Sci. Eng. C. 2018, 86, 144–150. [Google Scholar] [CrossRef] [Green Version]
- Rai, R.; Keshavarz, T.; Roether, J.A.; Boccaccini, A.R.; Roy, I. Medium chain length polyhydroxyalkanoates, promising new biomedical materials for the future. Mater. Sci. Eng. R Rep. 2011, 72, 29–47. [Google Scholar] [CrossRef]
- Koller, M. Biodegradable and Biocompatible Polyhydroxy-alkanoates (PHA): Auspicious Microbial Macromolecules for Pharmaceutical and Therapeutic Applications. Molecules 2018, 23, 362. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Williams, S.F.; Martin, D.P. Applications of polyhydroxyalkanoates (PHA) in medicine and pharmacy. Biopolym. Online 2005. [Google Scholar] [CrossRef]
- Hazer, B. Amphiphiles from Poly(3-hydroxyalkanoates). In Handbook Polyhydroxyalkanoates; CRC Press: Boca Raton, FL, USA, 2020. [Google Scholar]
- Bear, M.-M.; Renard, E.; Randriamahefa, S.; Langlois, V.; Guérin, P. Preparation of a bacterial polyester with carboxy groups in side chains. Comptes Rendus Académie Sci.-Ser. IIC-Chem. 2001, 4, 289–293. [Google Scholar] [CrossRef]
- Jain-Beuguel, C.; Li, X.; Houel-Renault, L.; Modjinou, T.; Simon-Colin, C.; Gref, R.; Renard, E.; Langlois, V. Water-Soluble Poly(3-hydroxyalkanoate) Sulfonate: Versatile Biomaterials Used as Coatings for Highly Porous Nano-Metal Organic Framework. Biomacromolecules 2019, 20, 3324–3332. [Google Scholar] [CrossRef]
- Babinot, J.; Renard, E.; le Droumaguet, B.; Guigner, J.-M.; Mura, S.; Nicolas, J.; Couvreur, P.; Langlois, V. Facile Synthesis of Multicompartment Micelles Based on Biocompatible Poly(3-hydroxyalkanoate). Macromol. Rapid Commun. 2013, 34, 362–368. [Google Scholar] [CrossRef] [PubMed]
- Simon-Colin, C.; Alain, K.; Raguénès, G.; Schmitt, S.; Kervarec, N.; Gouin, C.; Crassous, P.; Costa, B.; Guezennec, J.G. Biosynthesis of medium chain length poly(3-hydroxyalkanoates) (mcl PHAs) from cosmetic co-products by Pseudomonas raguenesii sp. nov., isolated from Tetiaroa, French Polynesia. Bioresour. Technol. 2009, 100, 6033–6039. [Google Scholar] [CrossRef] [PubMed]
- Lee, D.; Donkers, R.L.; DeSimone, J.M.; Murray, R.W. Voltammetry and Electron-Transfer Dynamics in a Molecular Melt of a 1.2 nm Metal Quantum Dot. J. Am. Chem. Soc. 2003, 125, 1182–1183. [Google Scholar] [CrossRef] [PubMed]
- Haggag, Y.A.; Osman, M.A.; El-Gizawy, S.A.; Goda, A.E.; Shamloula, M.M.; Faheem, A.M.; McCarron, P.A. Polymeric nano-encapsulation of 5-fluorouracil enhances anti-cancer activity and ameliorates side effects in solid Ehrlich Carcinoma-bearing mice. Biomed. Pharmacother. 2018, 105, 215–224. [Google Scholar] [CrossRef] [PubMed]






| Fluorinated Thiol | Chemical Structure | Abbreviation |
|---|---|---|
| Trifluoropropane-1-thiol |  | CF3 |
| Perfluorooctane-1-thiol | 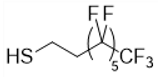 | C6F13 |
| Perfluorodecane-1-thiol |  | C8F17 |
| Copolymers | PEG (%) 1 | C8F17 (%) 1 | Contact Angle (°) |
|---|---|---|---|
| PHAU | - | - | 96.2 |
| PHAU-g-(C8F17) | - | 8 | 132.5 |
| PHA-g-(C8F17; PEG) | 20 | 8 | 106.3 |
Publisher’s Note: MDPI stays neutral with regard to jurisdictional claims in published maps and institutional affiliations. |
© 2021 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Le Gal, M.; Renard, E.; Simon-Colin, C.; Larrat, B.; Langlois, V. Amphiphilic and Perfluorinated Poly(3-Hydroxyalkanoate) Nanocapsules for 19F Magnetic Resonance Imaging. Bioengineering 2021, 8, 121. https://doi.org/10.3390/bioengineering8090121
Le Gal M, Renard E, Simon-Colin C, Larrat B, Langlois V. Amphiphilic and Perfluorinated Poly(3-Hydroxyalkanoate) Nanocapsules for 19F Magnetic Resonance Imaging. Bioengineering. 2021; 8(9):121. https://doi.org/10.3390/bioengineering8090121
Chicago/Turabian StyleLe Gal, Marion, Estelle Renard, Christelle Simon-Colin, Benoit Larrat, and Valérie Langlois. 2021. "Amphiphilic and Perfluorinated Poly(3-Hydroxyalkanoate) Nanocapsules for 19F Magnetic Resonance Imaging" Bioengineering 8, no. 9: 121. https://doi.org/10.3390/bioengineering8090121
APA StyleLe Gal, M., Renard, E., Simon-Colin, C., Larrat, B., & Langlois, V. (2021). Amphiphilic and Perfluorinated Poly(3-Hydroxyalkanoate) Nanocapsules for 19F Magnetic Resonance Imaging. Bioengineering, 8(9), 121. https://doi.org/10.3390/bioengineering8090121








