Genitourinary Tissue Engineering: Reconstruction and Research Models
Abstract
1. Introduction
2. Male Genitourinary Tissue: Focus on the Urethra
2.1. Anatomy
2.2. Pathologies
2.3. Current Treatments
3. Female Genitourinary Tissue: Focus on the Vagina
3.1. Anatomy
3.2. Pathologies
3.3. Current Treatments
3.3.1. Non-Surgical Treatments
3.3.2. Surgical Treatments
4. Tissue Engineering
4.1. Synthetic Materials
4.2. Natural Materials
4.3. Tissue Engineering for Urethral Reconstruction
4.4. Tissue Engineering for Vaginal Reconstruction
5. The Self-Assembly Approach
5.1. Self-Assembly to Reconstruct Human Tissues
5.2. Self-Assembly Approaches to Produce Research Models
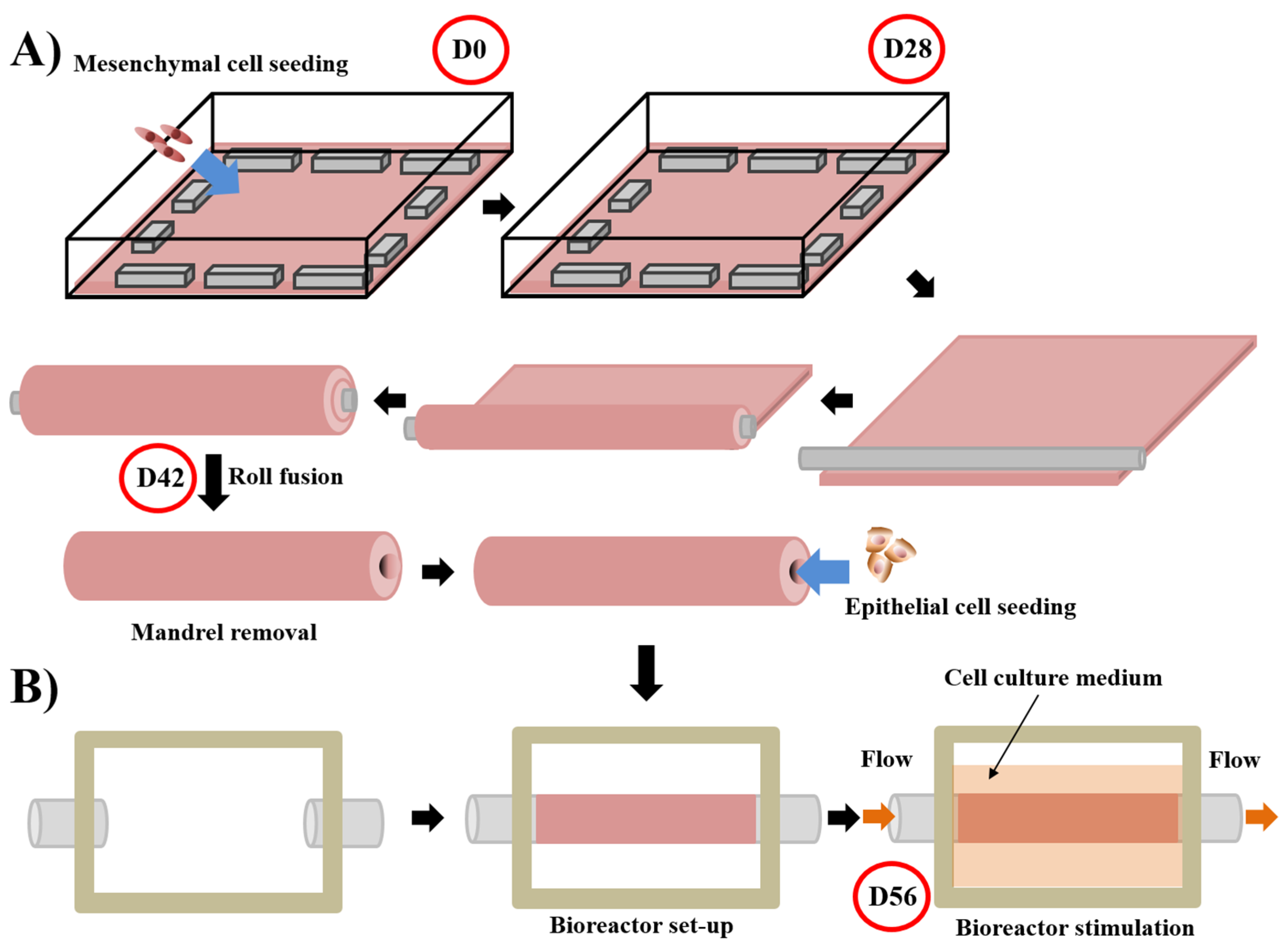
5.3. Self-Assembly Protocol for Urethral Substitute Model
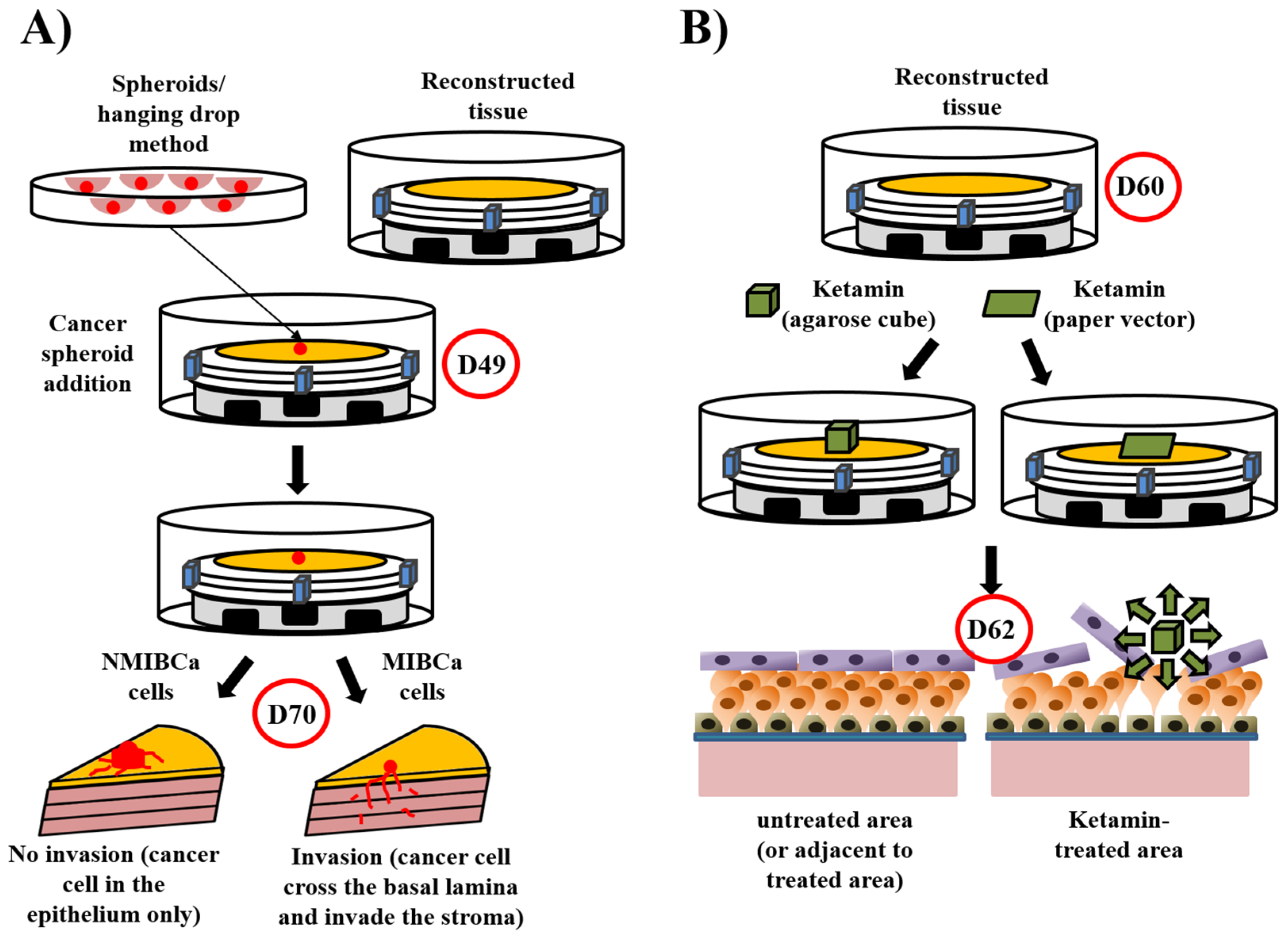
5.4. Disease Models Derived from the Urological Substitute Model
5.5. Self-Assembly Protocol for Vaginal Substitute Model
5.6. Disease Models Derived from the Vaginal Substitute Model
6. Perspectives
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Data Availability Statement
Acknowledgments
Conflicts of Interest
References
- Tyson, M.D., 2nd; Barocas, D.A. Quality of life after radical cystectomy. Urol. Clin. N. Am. 2018, 45, 249–256. [Google Scholar] [CrossRef]
- Ahmadi, H.; Lee, C.T. Health-related quality of life with urinary diversion. Curr. Opin. Urol. 2015, 25, 562–569. [Google Scholar] [CrossRef] [PubMed]
- Castro, R.A.; Arruda, R.M.; Bortolini, M.A. Female urinary incontinence: Effective treatment strategies. Climacteric 2015, 18, 135–141. [Google Scholar] [CrossRef]
- Ofman, U.S. Preservation of function in genitourinary cancers: Psychosexual and psychosocial issues. Cancer Investig. 1995, 13, 125–131. [Google Scholar] [CrossRef] [PubMed]
- Orvis, B.R.; Lue, T.F. New therapy for impotence. Urol. Clin. N. Am. 1987, 14, 569–581. [Google Scholar] [CrossRef]
- Saba, I.; Jakubowska, W.; Bolduc, S.; Chabaud, S. Engineering tissues without the use of a synthetic scaffold: A twenty-year history of the self-assembly method. Biomed. Res. Int. 2018, 2018, 5684679. [Google Scholar] [CrossRef]
- Pederzoli, F.; Joice, G.; Salonia, A.; Bivalacqua, T.J.; Sopko, N.A. Regenerative and engineered options for urethroplasty. Nat. Rev. Urol. 2019, 16, 453–464. [Google Scholar] [CrossRef]
- Dalghi, M.G.; Montalbetti, N.; Carattino, M.D.; Apodaca, G. The urothelium: Life in a liquid environment. Physiol. Rev. 2020, 100, 1621–1705. [Google Scholar] [CrossRef]
- Blaschko, S.D.; Cunha, G.R.; Baskin, L.S. Molecular mechanisms of external genitalia development. Differentiation 2012, 84, 261–268. [Google Scholar] [CrossRef] [PubMed]
- Keays, M.A.; Dave, S. Current hypospadias management: Diagnosis, surgical management, and long-term patient-centred outcomes. Can. Urol. Assoc. J. 2017, 11, S48–S53. [Google Scholar] [CrossRef]
- Nelson, C.P.; Park, J.M.; Wan, J.; Bloom, D.A.; Dunn, R.L.; Wei, J.T. The increasing incidence of congenital penile anomalies in the United States. J. Urol. 2005, 174, 1573–1576. [Google Scholar] [CrossRef] [PubMed]
- Nordenvall, A.S.; Frisen, L.; Nordenstrom, A.; Lichtenstein, P.; Nordenskjold, A. Population based nationwide study of hypospadias in Sweden, 1973 to 2009: Incidence and risk factors. J. Urol. 2014, 191, 783–789. [Google Scholar] [CrossRef] [PubMed]
- Czeizel, A. Increasing trends in congenital malformations of male external genitalia. Lancet 1985, 1, 462–463. [Google Scholar] [CrossRef]
- Paulozzi, L.J.; Erickson, J.D.; Jackson, R.J. Hypospadias trends in two US surveillance systems. Pediatrics 1997, 100, 831–834. [Google Scholar] [CrossRef]
- Baskin, L.S.; Himes, K.; Colborn, T. Hypospadias and endocrine disruption: Is there a connection? Environ. Health Perspect. 2001, 109, 1175–1183. [Google Scholar] [CrossRef]
- Wilcox, D.; Snodgrass, W. Long-term outcome following hypospadias repair. World J. Urol. 2006, 24, 240–243. [Google Scholar] [CrossRef]
- Springer, A.; van den Heijkant, M.; Baumann, S. Worldwide prevalence of hypospadias. J. Pediatr. Urol. 2016, 12, 152.e1–152.e7. [Google Scholar] [CrossRef]
- Van der Zanden, L.F.; van Rooij, I.A.; Feitz, W.F.; Franke, B.; Knoers, N.V.; Roeleveld, N. Aetiology of hypospadias: A systematic review of genes and environment. Hum. Reprod. Update 2012, 18, 260–283. [Google Scholar] [CrossRef] [PubMed]
- Sagodi, L.; Kiss, A.; Kiss-Toth, E.; Barkai, L. Prevalence and possible causes of hypospadias. Orv. Hetil. 2014, 155, 978–985. [Google Scholar] [CrossRef]
- Schnack, T.H.; Zdravkovic, S.; Myrup, C.; Westergaard, T.; Christensen, K.; Wohlfahrt, J.; Melbye, M. Familial aggregation of hypospadias: A cohort study. Am. J. Epidemiol. 2008, 167, 251–256. [Google Scholar] [CrossRef]
- Pellerin, E.; Caneparo, C.; Chabaud, S.; Bolduc, S.; Pelletier, M. Endocrine-disrupting effects of bisphenols on urological cancers. Environ. Res. 2021, 195, 110485. [Google Scholar] [CrossRef]
- Huisma, F.; Thomas, M.; Armstrong, L. Severe hypospadias and its association with maternal-placental factors. Am. J. Med. Genet. A 2013, 161, 2183–2187. [Google Scholar] [CrossRef] [PubMed]
- Snodgrass, W.; Macedo, A.; Hoebeke, P.; Mouriquand, P.D. Hypospadias dilemmas: A round table. J. Pediatr. Urol. 2011, 7, 145–157. [Google Scholar] [CrossRef]
- Mouriquand, P.D.; Persad, R.; Sharma, S. Hypospadias repair: Current principles and procedures. Br. J. Urol. 1995, 76 (Suppl. S3), 9–22. [Google Scholar] [CrossRef] [PubMed]
- Manzoni, G.; Bracka, A.; Palminteri, E.; Marrocco, G. Hypospadias surgery: When, what and by whom? BJU Int. 2004, 94, 1188–1195. [Google Scholar] [CrossRef]
- Ortqvist, L.; Fossum, M.; Andersson, M.; Nordenstrom, A.; Frisen, L.; Holmdahl, G.; Nordenskjold, A. Long-term followup of men born with hypospadias: Urological and cosmetic results. J. Urol. 2015, 193, 975–981. [Google Scholar] [CrossRef] [PubMed]
- Rompre, M.P.; Nadeau, G.; Moore, K.; Ajjaouj, Y.; Braga, L.H.; Bolduc, S. Learning curve for TIP urethroplasty: A single-surgeon experience. Can. Urol. Assoc. J. 2013, 7, E789–E794. [Google Scholar] [CrossRef]
- Stein, M.J.; DeSouza, R.A. Anterior urethral stricture review. Transl. Androl. Urol. 2013, 2, 32–38. [Google Scholar] [CrossRef]
- Mangera, A.; Osman, N.; Chapple, C. Evaluation and management of anterior urethral stricture disease. F1000Research 2016, 5. [Google Scholar] [CrossRef][Green Version]
- Davis, N.F.; Quinlan, M.R.; Bhatt, N.R.; Browne, C.; MacCraith, E.; Manecksha, R.; Walsh, M.T.; Thornhill, J.A.; Mulvin, D. Incidence, cost, complications and clinical outcomes of iatrogenic urethral catheterization injuries: A prospective multi-institutional study. J. Urol. 2016, 196, 1473–1477. [Google Scholar] [CrossRef]
- Santucci, R.A.; Joyce, G.F.; Wise, M. Male urethral stricture disease. J. Urol. 2007, 177, 1667–1674. [Google Scholar] [CrossRef]
- McAninch, J.W. Urethral reconstruction: A continuing challenge. J. Urol. 2005, 173, 7. [Google Scholar] [CrossRef] [PubMed]
- Korneyev, I.; Ilyin, D.; Schultheiss, D.; Chapple, C. The first oral mucosal graft urethroplasty was carried out in the 19th century: The pioneering experience of Kirill Sapezhko (1857–1928). Eur. Urol. 2012, 62, 624–627. [Google Scholar] [CrossRef]
- Burger, R.A.; Muller, S.C.; el-Damanhoury, H.; Tschakaloff, A.; Riedmiller, H.; Hohenfellner, R. The buccal mucosal graft for urethral reconstruction: A preliminary report. J. Urol. 1992, 147, 662–664. [Google Scholar] [CrossRef]
- Dessanti, A.; Rigamonti, W.; Merulla, V.; Falchetti, D.; Caccia, G. Autologous buccal mucosa graft for hypospadias repair: An initial report. J. Urol. 1992, 147, 1081–1083; discussion 1083–1084. [Google Scholar] [CrossRef]
- Mundy, A.R. The long-term results of skin inlay urethroplasty. Br. J. Urol. 1995, 75, 59–61. [Google Scholar] [CrossRef]
- Dublin, N.; Stewart, L.H. Oral complications after buccal mucosal graft harvest for urethroplasty. BJU Int. 2004, 94, 867–869. [Google Scholar] [CrossRef] [PubMed]
- Aldaqadossi, H.A.; Shaker, H.; Youssof, H.; Kotb, Y.; Eladawy, M. Outcomes of staged lingual mucosal graft urethroplasty for redo hypospadias repair. J. Pediatr. Urol. 2019, 15, 519.e1–519.e7. [Google Scholar] [CrossRef]
- Spilotros, M.; Sihra, N.; Malde, S.; Pakzad, M.H.; Hamid, R.; Ockrim, J.L.; Greenwell, T.J. Buccal mucosal graft urethroplasty in men-risk factors for recurrence and complications: A third referral centre experience in anterior urethroplasty using buccal mucosal graft. Transl. Androl. Urol. 2017, 6, 510–516. [Google Scholar] [CrossRef]
- Markiewicz, M.R.; DeSantis, J.L.; Margarone, J.E., 3rd; Pogrel, M.A.; Chuang, S.K. Morbidity associated with oral mucosa harvest for urological reconstruction: An overview. J. Oral Maxillofac. Surg. 2008, 66, 739–744. [Google Scholar] [CrossRef]
- Nelson, C.P.; Bloom, D.A.; Kinast, R.; Wei, J.T.; Park, J.M. Patient-reported sexual function after oral mucosa graft urethroplasty for hypospadias. Urology 2005, 66, 1086–1089; discussion 1089–1090. [Google Scholar] [CrossRef]
- Kim, S.J.; Lee, J.; Park, C.H.; Park, J.Y.; Song, S.H.; Kim, K.S.; Kim, H.G. Urethral defect due to periurethral abscess treated with a tunica vaginalis flap: A case report. Medicine 2018, 97, e13249. [Google Scholar] [CrossRef]
- Hmida, W.; Othmen, M.B.; Bako, A.; Jaidane, M.; Mosbah, F. Penile skin flap: A versatile substitute for anterior urethral stricture. Int. Braz. J. Urol. 2019, 45, 1057–1063. [Google Scholar] [CrossRef] [PubMed]
- Shaterian, N.; Abdi, F.; Ghavidel, N.; Alidost, F. Role of cesarean section in the development of neonatal gut microbiota: A systematic review. Open Med. 2021, 16, 624–639. [Google Scholar] [CrossRef] [PubMed]
- Pendergrass, P.B.; Belovicz, M.W.; Reeves, C.A. Surface area of the human vagina as measured from vinyl polysiloxane casts. Gynecol. Obstet. Investig. 2003, 55, 110–113. [Google Scholar] [CrossRef] [PubMed]
- Squier, C.A.; Mantz, M.J.; Schlievert, P.M.; Davis, C.C. Porcine vagina ex vivo as a model for studying permeability and pathogenesis in mucosa. J. Pharm. Sci. 2008, 97, 9–21. [Google Scholar] [CrossRef] [PubMed]
- Thompson, I.O.; van der Bijl, P.; van Wyk, C.W.; van Eyk, A.D. A comparative light-microscopic, electron-microscopic and chemical study of human vaginal and buccal epithelium. Arch. Oral Biol. 2001, 46, 1091–1098. [Google Scholar] [CrossRef]
- Patton, D.L.; Thwin, S.S.; Meier, A.; Hooton, T.M.; Stapleton, A.E.; Eschenbach, D.A. Epithelial cell layer thickness and immune cell populations in the normal human vagina at different stages of the menstrual cycle. Am. J. Obstet. Gynecol. 2000, 183, 967–973. [Google Scholar] [CrossRef]
- Nilsson, K.; Risberg, B.; Heimer, G. The vaginal epithelium in the postmenopause—Cytology, histology and pH as methods of assessment. Maturitas 1995, 21, 51–56. [Google Scholar] [CrossRef]
- Boskey, E.R.; Telsch, K.M.; Whaley, K.J.; Moench, T.R.; Cone, R.A. Acid production by vaginal flora in vitro is consistent with the rate and extent of vaginal acidification. Infect. Immun. 1999, 67, 5170–5175. [Google Scholar] [CrossRef]
- Morris, M.; Nicoll, A.; Simms, I.; Wilson, J.; Catchpole, M. Bacterial vaginosis: A public health review. BJOG 2001, 108, 439–450. [Google Scholar] [CrossRef]
- Vliet, R.; Roelofs, L.A.; Rassouli-Kirchmeier, R.; de Gier, R.P.; Claahsen-van der Grinten, H.L.; Verhaak, C.; Hosman, A.J.; Beerendonk, C.C.; van Lindert, E.J.; Willemsen, M.A.; et al. Clinical outcome of cloacal exstrophy, current status, and a change in surgical management. Eur. J. Pediatr. Surg. 2015, 25, 87–93. [Google Scholar] [CrossRef]
- Kariyawasam, D.; Nguyen-Khoa, T.; Gonzalez Briceno, L.; Polak, M. Newborn screening for congenital adrenal hyperplasia in France. Med. Sci. 2021, 37, 500–506. [Google Scholar] [CrossRef]
- Breech, L.L.; Laufer, M.R. Mullerian anomalies. Obstet. Gynecol. Clin. N. Am. 2009, 36, 47–68. [Google Scholar] [CrossRef]
- Oppelt, P.; Renner, S.P.; Kellermann, A.; Brucker, S.; Hauser, G.A.; Ludwig, K.S.; Strissel, P.L.; Strick, R.; Wallwiener, D.; Beckmann, M.W. Clinical aspects of Mayer-Rokitansky-Kuester-Hauser syndrome: Recommendations for clinical diagnosis and staging. Hum. Reprod. 2006, 21, 792–797. [Google Scholar] [CrossRef] [PubMed]
- Aittomaki, K.; Eroila, H.; Kajanoja, P. A population-based study of the incidence of Mullerian aplasia in Finland. Fertil. Steril. 2001, 76, 624–625. [Google Scholar] [CrossRef]
- Amankwah, Y.A.; Haefner, H.K.; Brincat, C.A. Management of vulvovaginal strictures/shortened vagina. Clin. Obstet. Gynecol. 2010, 53, 125–133. [Google Scholar] [CrossRef] [PubMed]
- O’Boyle, A.L.; O’Boyle, J.D.; Calhoun, B.; Davis, G.D. Pelvic organ support in pregnancy and postpartum. Int. Urogynecol. J. Pelvic. Floor Dysfunct. 2005, 16, 69–72; discussion 72. [Google Scholar] [CrossRef] [PubMed]
- DeLancey, J.O.; Kearney, R.; Chou, Q.; Speights, S.; Binno, S. The appearance of levator ani muscle abnormalities in magnetic resonance images after vaginal delivery. Obstet. Gynecol. 2003, 101, 46–53. [Google Scholar] [CrossRef] [PubMed]
- DeLancey, J.O.; Morgan, D.M.; Fenner, D.E.; Kearney, R.; Guire, K.; Miller, J.M.; Hussain, H.; Umek, W.; Hsu, Y.; Ashton-Miller, J.A. Comparison of levator ani muscle defects and function in women with and without pelvic organ prolapse. Obstet. Gynecol. 2007, 109, 295–302. [Google Scholar] [CrossRef] [PubMed]
- Williams, B. Formation of an artificial vagina by Frank’s method. J. Obstet. Gynaecol. Br. Emp. 1957, 64, 241–242. [Google Scholar] [CrossRef]
- Ingram, J.M. The bicycle seat stool in the treatment of vaginal agenesis and stenosis: A preliminary report. Am. J. Obstet. Gynecol. 1981, 140, 867–873. [Google Scholar] [CrossRef]
- Bellati, F.; Calcagno, M.; Pastore, M.; Maffucci, D.; Celentano, C.; Boni, T.; Panici, P.B. Vaginal apex necrosis following use of the Frank method of dilation for vaginal agenesis due to Mayer-Rokitansky-Kuster-Hauser syndrome. Int. J. Gynaecol. Obstet. 2009, 107, 254. [Google Scholar] [CrossRef]
- Vecchietti, G. Creation of an artificial vagina in Rokitansky-Kuster-Hauser syndrome. Attual. Ostet. Ginecol. 1965, 11, 131–147. [Google Scholar] [PubMed]
- Tolhurst, D.E.; van der Helm, T.W. The treatment of vaginal atresia. Surg. Gynecol. Obstet. 1991, 172, 407–414. [Google Scholar] [PubMed]
- Panici, P.B.; Ruscito, I.; Gasparri, M.L.; Maffucci, D.; Marchese, C.; Bellati, F. Vaginal reconstruction with the Abbe-McIndoe technique: From dermal grafts to autologous in vitro cultured vaginal tissue transplant. Semin. Reprod. Med. 2011, 29, 45–54. [Google Scholar] [CrossRef] [PubMed]
- Langer, R.; Vacanti, J.P. Tissue engineering. Science 1993, 260, 920–926. [Google Scholar] [CrossRef]
- Boys, A.J.; Barron, S.L.; Tilev, D.; Owens, R.M. Building scaffolds for tubular tissue engineering. Front. Bioeng. Biotechnol. 2020, 8, 589960. [Google Scholar] [CrossRef]
- Ercolani, E.; Del Gaudio, C.; Bianco, A. Vascular tissue engineering of small-diameter blood vessels: Reviewing the electrospinning approach. J. Tissue Eng. Regen. Med. 2015, 9, 861–888. [Google Scholar] [CrossRef]
- Haghjooy Javanmard, S.; Anari, J.; Zargar Kharazi, A.; Vatankhah, E. In vitro hemocompatibility and cytocompatibility of a three-layered vascular scaffold fabricated by sequential electrospinning of PCL, collagen, and PLLA nanofibers. J. Biomater. Appl. 2016, 31, 438–449. [Google Scholar] [CrossRef]
- Muerza-Cascante, M.L.; Haylock, D.; Hutmacher, D.W.; Dalton, P.D. Melt electrospinning and its technologization in tissue engineering. Tissue Eng. Part B Rev. 2015, 21, 187–202. [Google Scholar] [CrossRef] [PubMed]
- Zhang, K.; Fu, Q.; Yoo, J.; Chen, X.; Chandra, P.; Mo, X.; Song, L.; Atala, A.; Zhao, W. 3D bioprinting of urethra with PCL/PLCL blend and dual autologous cells in fibrin hydrogel: An in vitro evaluation of biomimetic mechanical property and cell growth environment. Acta Biomater. 2017, 50, 154–164. [Google Scholar] [CrossRef]
- Abbas, T.O.; Yalcin, H.C.; Pennisi, C.P. From acellular matrices to smart polymers: Degradable scaffolds that are transforming the shape of urethral tissue engineering. Int. J. Mol. Sci. 2019, 20, 1763. [Google Scholar] [CrossRef]
- Nooeaid, P.; Salih, V.; Beier, J.P.; Boccaccini, A.R. Osteochondral tissue engineering: Scaffolds, stem cells and applications. J. Cell Mol. Med. 2012, 16, 2247–2270. [Google Scholar] [CrossRef] [PubMed]
- Jeong, S.I.; Kim, B.S.; Lee, Y.M.; Ihn, K.J.; Kim, S.H.; Kim, Y.H. Morphology of elastic poly(L-lactide-co-epsilon-caprolactone) copolymers and in vitro and in vivo degradation behavior of their scaffolds. Biomacromolecules 2004, 5, 1303–1309. [Google Scholar] [CrossRef]
- Ceonzo, K.; Gaynor, A.; Shaffer, L.; Kojima, K.; Vacanti, C.A.; Stahl, G.L. Polyglycolic acid-induced inflammation: Role of hydrolysis and resulting complement activation. Tissue Eng. 2006, 12, 301–308. [Google Scholar] [CrossRef]
- Zamuner, A.; Cavo, M.; Scaglione, S.; Messina, G.M.L.; Russo, T.; Gloria, A.; Marletta, G.; Dettin, M. Design of decorated self-assembling peptide hydrogels as architecture for mesenchymal stem cells. Materials 2016, 9, 727. [Google Scholar] [CrossRef]
- Tachibana, M.; Nagamatsu, G.R.; Addonizio, J.C. Ureteral replacement using collagen sponge tube grafts. J. Urol. 1985, 133, 866–869. [Google Scholar] [CrossRef]
- Lin, C.H.; Hsia, K.; Ma, H.; Lee, H.; Lu, J.H. In vivo performance of decellularized vascular grafts: A review article. Int. J. Mol. Sci. 2018, 19, 2101. [Google Scholar] [CrossRef]
- Guruswamy Damodaran, R.; Vermette, P. Tissue and organ decellularization in regenerative medicine. Biotechnol. Prog. 2018, 34, 1494–1505. [Google Scholar] [CrossRef]
- Zhang, Y.; Frimberger, D.; Cheng, E.Y.; Lin, H.K.; Kropp, B.P. Challenges in a larger bladder replacement with cell-seeded and unseeded small intestinal submucosa grafts in a subtotal cystectomy model. BJU Int. 2006, 98, 1100–1105. [Google Scholar] [CrossRef]
- Davis, N.F.; Cunnane, E.M.; O’Brien, F.J.; Mulvihill, J.J.; Walsh, M.T. Tissue engineered extracellular matrices (ECMs) in urology: Evolution and future directions. Surgeon 2018, 16, 55–65. [Google Scholar] [CrossRef]
- Badylak, S.F.; Lantz, G.C.; Coffey, A.; Geddes, L.A. Small intestinal submucosa as a large diameter vascular graft in the dog. J. Surg. Res. 1989, 47, 74–80. [Google Scholar] [CrossRef]
- Liao, J.; Xu, B.; Zhang, R.; Fan, Y.; Xie, H.; Li, X. Applications of decellularized materials in tissue engineering: Advantages, drawbacks and current improvements, and future perspectives. J. Mater. Chem. B 2020, 8, 10023–10049. [Google Scholar] [CrossRef]
- Dal Sasso, E.; Zamuner, A.; Filippi, A.; Romanato, F.; Palmosi, T.; Vedovelli, L.; Gregori, D.; Gomez Ribelles, J.L.; Russo, T.; Gloria, A.; et al. Covalent functionalization of decellularized tissues accelerates endothelialization. Bioact. Mater. 2021, 6, 3851–3864. [Google Scholar] [CrossRef]
- Chung, Y.G.; Tu, D.; Franck, D.; Gil, E.S.; Algarrahi, K.; Adam, R.M.; Kaplan, D.L.; Estrada, C.R., Jr.; Mauney, J.R. Acellular bi-layer silk fibroin scaffolds support tissue regeneration in a rabbit model of onlay urethroplasty. PLoS ONE 2014, 9, e91592. [Google Scholar] [CrossRef] [PubMed]
- Jia, W.; Tang, H.; Wu, J.; Hou, X.; Chen, B.; Chen, W.; Zhao, Y.; Shi, C.; Zhou, F.; Yu, W.; et al. Urethral tissue regeneration using collagen scaffold modified with collagen binding VEGF in a beagle model. Biomaterials 2015, 69, 45–55. [Google Scholar] [CrossRef]
- Pinnagoda, K.; Larsson, H.M.; Vythilingam, G.; Vardar, E.; Engelhardt, E.M.; Thambidorai, R.C.; Hubbell, J.A.; Frey, P. Engineered acellular collagen scaffold for endogenous cell guidance, a novel approach in urethral regeneration. Acta Biomater. 2016, 43, 208–217. [Google Scholar] [CrossRef] [PubMed]
- Atala, A.; Danilevskiy, M.; Lyundup, A.; Glybochko, P.; Butnaru, D.; Vinarov, A.; Yoo, J.J. The potential role of tissue-engineered urethral substitution: Clinical and preclinical studies. J. Tissue Eng. Regen. Med. 2017, 11, 3–19. [Google Scholar] [CrossRef] [PubMed]
- Chun, S.Y.; Kim, B.S.; Kwon, S.Y.; Park, S.I.; Song, P.H.; Yoo, E.S.; Kim, B.W.; Kwon, T.G.; Kim, H.T. Urethroplasty using autologous urethral tissue-embedded acellular porcine bladder submucosa matrix grafts for the management of long-segment urethral stricture in a rabbit model. J. Korean Med. Sci. 2015, 30, 301–307. [Google Scholar] [CrossRef] [PubMed]
- Li, C.; Xu, Y.M.; Liu, Z.S.; Li, H.B. Urethral reconstruction with tissue engineering and RNA interference techniques in rabbits. Urology 2013, 81, 1075–1080. [Google Scholar] [CrossRef]
- Sartoneva, R.; Haaparanta, A.M.; Lahdes-Vasama, T.; Mannerstrom, B.; Kellomaki, M.; Salomaki, M.; Sandor, G.; Seppanen, R.; Miettinen, S.; Haimi, S. Characterizing and optimizing poly-L-lactide-co-epsilon-caprolactone membranes for urothelial tissue engineering. J. R. Soc. Interface 2012, 9, 3444–3454. [Google Scholar] [CrossRef]
- Sun, D.; Yang, Y.; Wei, Z.; Xu, Y.; Zhang, X.; Hong, B. Engineering of pre-vascularized urethral patch with muscle flaps and hypoxia-activated hUCMSCs improves its therapeutic outcome. J. Cell Mol. Med. 2014, 18, 434–443. [Google Scholar] [CrossRef] [PubMed]
- Dorin, R.P.; Pohl, H.G.; De Filippo, R.E.; Yoo, J.J.; Atala, A. Tubularized urethral replacement with unseeded matrices: What is the maximum distance for normal tissue regeneration? World J. Urol. 2008, 26, 323–326. [Google Scholar] [CrossRef] [PubMed]
- Orlando, G.; Soker, S.; Stratta, R.J. Organ bioengineering and regeneration as the new Holy Grail for organ transplantation. Ann. Surg. 2013, 258, 221–232. [Google Scholar] [CrossRef] [PubMed]
- Jerman, U.D.; Kreft, M.E.; Veranic, P. Epithelial-mesenchymal interactions in urinary bladder and small intestine and how to apply them in tissue engineering. Tissue Eng. Part B Rev. 2015, 21, 521–530. [Google Scholar] [CrossRef] [PubMed]
- Raya-Rivera, A.; Esquiliano, D.R.; Yoo, J.J.; Lopez-Bayghen, E.; Soker, S.; Atala, A. Tissue-engineered autologous urethras for patients who need reconstruction: An observational study. Lancet 2011, 377, 1175–1182. [Google Scholar] [CrossRef]
- Zhang, Y.; McNeill, E.; Tian, H.; Soker, S.; Andersson, K.E.; Yoo, J.J.; Atala, A. Urine derived cells are a potential source for urological tissue reconstruction. J. Urol. 2008, 180, 2226–2233. [Google Scholar] [CrossRef] [PubMed]
- Bharadwaj, S.; Liu, G.; Shi, Y.; Markert, C.; Andersson, K.E.; Atala, A.; Zhang, Y. Characterization of urine-derived stem cells obtained from upper urinary tract for use in cell-based urological tissue engineering. Tissue Eng. Part A 2011, 17, 2123–2132. [Google Scholar] [CrossRef]
- Versteegden, L.R.M.; de Jonge, P.; IntHout, J.; van Kuppevelt, T.H.; Oosterwijk, E.; Feitz, W.F.J.; de Vries, R.B.M.; Daamen, W.F. Tissue engineering of the urethra: A systematic review and meta-analysis of preclinical and clinical studies. Eur. Urol. 2017, 72, 594–606. [Google Scholar] [CrossRef]
- Engel, O.; Ram-Liebig, G.; Reiß, P.; Schwaiger, B.; Pfalzgraf, D.; Dahlem, R.; Fisch, M. 15 Tissue—Engineered buccal mucosa urethroplasty. Outcome of our first 10 patients. J. Urol. 2012, 187, e6. [Google Scholar] [CrossRef]
- Fossum, M.; Skikuniene, J.; Orrego, A.; Nordenskjold, A. Prepubertal follow-up after hypospadias repair with autologous in vitro cultured urothelial cells. Acta Paediatr. 2012, 101, 755–760. [Google Scholar] [CrossRef]
- Bhargava, S.; Patterson, J.M.; Inman, R.D.; MacNeil, S.; Chapple, C.R. Tissue-engineered buccal mucosa urethroplasty-clinical outcomes. Eur. Urol. 2008, 53, 1263–1269. [Google Scholar] [CrossRef]
- Osman, N.I.; Patterson, J.M.; MacNeil, S.; Chapple, C.R. Long-term follow-up after tissue-engineered buccal mucosa urethroplasty. Eur. Urol. 2014, 66, 790–791. [Google Scholar] [CrossRef]
- Ramsay, S.; Ringuette-Goulet, C.; Langlois, A.; Bolduc, S. Clinical challenges in tissue-engineered urethral reconstruction. Transl. Androl. Urol. 2016, 5, 267–270. [Google Scholar] [CrossRef]
- Sartoneva, R.; Nordback, P.H.; Haimi, S.; Grijpma, D.W.; Lehto, K.; Rooney, N.; Seppanen-Kaijansinkko, R.; Miettinen, S.; Lahdes-Vasama, T. Comparison of poly(l-lactide-co-varepsilon-caprolactone) and poly(trimethylene carbonate) membranes for urethral regeneration: An in vitro and in vivo study. Tissue Eng. Part A 2018, 24, 117–127. [Google Scholar] [CrossRef]
- Kanatani, I.; Kanematsu, A.; Inatsugu, Y.; Imamura, M.; Negoro, H.; Ito, N.; Yamamoto, S.; Tabata, Y.; Ikada, Y.; Ogawa, O. Fabrication of an optimal urethral graft using collagen-sponge tubes reinforced with Copoly(L-lactide/epsilon-caprolactone) fabric. Tissue Eng. 2007, 13, 2933–2940. [Google Scholar] [CrossRef]
- Wang, D.J.; Li, M.Y.; Huang, W.T.; Lu, M.H.; Hu, C.; Li, K.; Qiu, J.G.; Gao, X. Repair of urethral defects with polylactid acid fibrous membrane seeded with adipose-derived stem cells in a rabbit model. Connect. Tissue Res. 2015, 56, 434–439. [Google Scholar] [CrossRef] [PubMed]
- Rapoport, H.S.; Fish, J.; Basu, J.; Campbell, J.; Genheimer, C.; Payne, R.; Jain, D. Construction of a tubular scaffold that mimics J-shaped stress/strain mechanics using an innovative electrospinning technique. Tissue Eng. Part C Methods 2012, 18, 567–574. [Google Scholar] [CrossRef]
- Lv, X.; Guo, Q.; Han, F.; Chen, C.; Ling, C.; Chen, W.; Li, B. Electrospun poly(l-lactide)/poly(ethylene glycol) scaffolds seeded with human amniotic mesenchymal stem cells for urethral epithelium repair. Int. J. Mol. Sci. 2016, 17, 1262. [Google Scholar] [CrossRef] [PubMed]
- Huang, J.W.; Lv, X.G.; Li, Z.; Song, L.J.; Feng, C.; Xie, M.K.; Li, C.; Li, H.B.; Wang, J.H.; Zhu, W.D.; et al. Urethral reconstruction with a 3D porous bacterial cellulose scaffold seeded with lingual keratinocytes in a rabbit model. Biomed. Mater. 2015, 10, 055005. [Google Scholar] [CrossRef]
- Lv, X.; Li, Z.; Chen, S.; Xie, M.; Huang, J.; Peng, X.; Yang, R.; Wang, H.; Xu, Y.; Feng, C. Structural and functional evaluation of oxygenating keratin/silk fibroin scaffold and initial assessment of their potential for urethral tissue engineering. Biomaterials 2016, 84, 99–110. [Google Scholar] [CrossRef]
- Xie, M.; Xu, Y.; Song, L.; Wang, J.; Lv, X.; Zhang, Y. Tissue-engineered buccal mucosa using silk fibroin matrices for urethral reconstruction in a canine model. J. Surg. Res. 2014, 188, 1–7. [Google Scholar] [CrossRef]
- Xie, M.; Song, L.; Wang, J.; Fan, S.; Zhang, Y.; Xu, Y. Evaluation of stretched electrospun silk fibroin matrices seeded with urothelial cells for urethra reconstruction. J. Surg. Res. 2013, 184, 774–781. [Google Scholar] [CrossRef]
- Vardar, E.; Engelhardt, E.M.; Larsson, H.M.; Mouloungui, E.; Pinnagoda, K.; Hubbell, J.A.; Frey, P. Tubular compressed collagen scaffolds for ureteral tissue engineering in a flow bioreactor system. Tissue Eng. Part A 2015, 21, 2334–2345. [Google Scholar] [CrossRef] [PubMed]
- Sayeg, K.; Freitas-Filho, L.G.; Waitzberg, A.F.; Arias, V.E.; Laks, M.; Egydio, F.M.; Oliveira, A.S. Integration of collagen matrices into the urethra when implanted as onlay graft. Int. Braz. J. Urol. 2013, 39, 414–423. [Google Scholar] [CrossRef][Green Version]
- Larsson, H.M.; Vythilingam, G.; Pinnagoda, K.; Vardar, E.; Engelhardt, E.M.; Sothilingam, S.; Thambidorai, R.C.; Kamarul, T.; Hubbell, J.A.; Frey, P. Fiber density of collagen grafts impacts rabbit urethral regeneration. Sci. Rep. 2018, 8, 10057. [Google Scholar] [CrossRef]
- Aufderklamm, S.; Vaegler, M.; Kelp, A.; Maurer, S.; Gustafsson, L.; Mundhenk, J.; Busch, S.; Daum, L.; Stenzl, A.; Amend, B.; et al. Collagen cell carriers seeded with human urothelial cells for urethral reconstructive surgery: First results in a xenograft minipig model. World J. Urol. 2017, 35, 1125–1132. [Google Scholar] [CrossRef]
- Micol, L.A.; Arenas da Silva, L.F.; Geutjes, P.J.; Oosterwijk, E.; Hubbell, J.A.; Feitz, W.F.; Frey, P. In-vivo performance of high-density collagen gel tubes for urethral regeneration in a rabbit model. Biomaterials 2012, 33, 7447–7455. [Google Scholar] [CrossRef] [PubMed]
- Guo, H.; Sa, Y.; Huang, J.; Wang, Z.; Wang, L.; Xie, M.; Lv, X. Urethral reconstruction with small intestinal submucosa seeded with oral keratinocytes and TIMP-1 siRNA transfected fibroblasts in a rabbit model. Urol. Int. 2016, 96, 223–230. [Google Scholar] [CrossRef]
- Orabi, H.; Safwat, A.S.; Shahat, A.; Hammouda, H.M. The use of small intestinal submucosa graft for hypospadias repair: Pilot study. Arab. J. Urol. 2013, 11, 415–420. [Google Scholar] [CrossRef] [PubMed]
- Feil, G.; Christ-Adler, M.; Maurer, S.; Corvin, S.; Rennekampff, H.O.; Krug, J.; Hennenlotter, J.; Kuehs, U.; Stenzl, A.; Sievert, K.D. Investigations of urothelial cells seeded on commercially available small intestine submucosa. Eur. Urol. 2006, 50, 1330–1337. [Google Scholar] [CrossRef] [PubMed]
- Zhang, Y.; Kropp, B.P.; Moore, P.; Cowan, R.; Furness, P.D., 3rd; Kolligian, M.E.; Frey, P.; Cheng, E.Y. Coculture of bladder urothelial and smooth muscle cells on small intestinal submucosa: Potential applications for tissue engineering technology. J. Urol. 2000, 164, 928–934; discussion 925–934. [Google Scholar] [CrossRef]
- Fiala, R.; Vidlar, A.; Vrtal, R.; Belej, K.; Student, V. Porcine small intestinal submucosa graft for repair of anterior urethral strictures. Eur. Urol. 2007, 51, 1702–1708; discussion 1708. [Google Scholar] [CrossRef] [PubMed]
- Palminteri, E.; Berdondini, E.; Colombo, F.; Austoni, E. Small intestinal submucosa (SIS) graft urethroplasty: Short-term results. Eur. Urol. 2007, 51, 1695–1701; discussion 1701. [Google Scholar] [CrossRef]
- Pusateri, C.R.; Doudt, A.D.; Gauerke, S.; McCammon, K.; Qin, X.; Ork, B.; Khoury, J.M.; May, A.D.; Zuckerman, J.M. Placental membrane grafts for urethral replacement in a rabbit model: A pilot study. World J. Urol. 2020, 38, 2133–2138. [Google Scholar] [CrossRef]
- Huang, J.W.; Xie, M.K.; Zhang, Y.; Wei, G.J.; Li, X.; Li, H.B.; Wang, J.H.; Zhu, W.D.; Li, C.; Xu, Y.M.; et al. Reconstruction of penile urethra with the 3-dimensional porous bladder acellular matrix in a rabbit model. Urology 2014, 84, 1499–1505. [Google Scholar] [CrossRef]
- Li, H.; Xu, Y.; Xie, H.; Li, C.; Song, L.; Feng, C.; Zhang, Q.; Xie, M.; Wang, Y.; Lv, X. Epithelial-differentiated adipose-derived stem cells seeded bladder acellular matrix grafts for urethral reconstruction: An animal model. Tissue Eng. Part A 2014, 20, 774–784. [Google Scholar] [CrossRef]
- Orabi, H.; AbouShwareb, T.; Zhang, Y.; Yoo, J.J.; Atala, A. Cell-seeded tubularized scaffolds for reconstruction of long urethral defects: A preclinical study. Eur. Urol. 2013, 63, 531–538. [Google Scholar] [CrossRef]
- Simoes, I.N.; Vale, P.; Soker, S.; Atala, A.; Keller, D.; Noiva, R.; Carvalho, S.; Peleteiro, C.; Cabral, J.M.; Eberli, D.; et al. Acellular urethra bioscaffold: Decellularization of whole urethras for tissue engineering applications. Sci. Rep. 2017, 7, 41934. [Google Scholar] [CrossRef]
- Caneparo, C.; Chabaud, S.; Bolduc, S. Reconstruction of vascular and urologic tubular grafts by tissue engineering. Processes 2021, 9, 513. [Google Scholar] [CrossRef]
- Chapple, C. Tissue engineering of the urethra: Where are we in 2019? World J. Urol. 2020, 38, 2101–2105. [Google Scholar] [CrossRef] [PubMed]
- Bouhout, S.; Chabaud, S.; Bolduc, S. Organ-specific matrix self-assembled by mesenchymal cells improves the normal urothelial differentiation in vitro. World J. Urol. 2016, 34, 121–130. [Google Scholar] [CrossRef] [PubMed]
- Wefer, J.; Sekido, N.; Sievert, K.D.; Schlote, N.; Nunes, L.; Dahiya, R.; Jonas, U.; Tanagho, E.A. Homologous acellular matrix graft for vaginal repair in rats: A pilot study for a new reconstructive approach. World J. Urol. 2002, 20, 260–263. [Google Scholar] [CrossRef]
- Wu, S.; Cheng, Z.; Liu, G.; Zhao, X.; Zhong, L.; Zhu, Y.; Zhu, J. Urothelial differentiation of human umbilical cord-derived mesenchymal stromal cells in vitro. Anal. Cell Pathol. 2013, 36, 63–69. [Google Scholar] [CrossRef]
- Morton, K.E.; Davies, D.; Dewhurst, J. The use of the fasciocutaneous flap in vaginal reconstruction. Br. J. Obstet. Gynaecol. 1986, 93, 970–973. [Google Scholar] [CrossRef]
- Hendren, W.H.; Atala, A. Use of bowel for vaginal reconstruction. J. Urol. 1994, 152, 752–755; discussion 756–757. [Google Scholar] [CrossRef]
- Wiser, W.L.; Bates, G.W. Management of agenesis of the vagina. Surg. Gynecol. Obstet. 1984, 159, 108–112. [Google Scholar]
- Morton, K.E.; Dewhurst, C.J. Human amnion in the treatment of vaginal malformations. Br. J. Obstet. Gynaecol. 1986, 93, 50–54. [Google Scholar] [CrossRef]
- Zhou, J.H.; Sun, J.; Yang, C.B.; Xie, Z.W.; Shao, W.Q.; Jin, H.M. Long-term outcomes of transvestibular vaginoplasty with pelvic peritoneum in 182 patients with Rokitansky’s syndrome. Fertil. Steril. 2010, 94, 2281–2285. [Google Scholar] [CrossRef] [PubMed]
- Ding, J.X.; Zhang, X.Y.; Chen, L.M.; Hua, K.Q. Vaginoplasty using acellular porcine small intestinal submucosa graft in two patients with Meyer-von-Rokitansky-Kuster-Hauser syndrome: A prospective new technique for vaginal reconstruction. Gynecol. Obstet. Investig. 2013, 75, 93–96. [Google Scholar] [CrossRef] [PubMed]
- Lin, W.C.; Chang, C.Y.; Shen, Y.Y.; Tsai, H.D. Use of autologous buccal mucosa for vaginoplasty: A study of eight cases. Hum. Reprod. 2003, 18, 604–607. [Google Scholar] [CrossRef]
- Li, F.Y.; Xu, Y.S.; Zhou, C.D.; Zhou, Y.; Li, S.K.; Li, Q. Long-term outcomes of vaginoplasty with autologous buccal micromucosa. Obstet. Gynecol. 2014, 123, 951–956. [Google Scholar] [CrossRef] [PubMed]
- De Filippo, R.E.; Yoo, J.J.; Atala, A. Engineering of vaginal tissue in vivo. Tissue Eng. 2003, 9, 301–306. [Google Scholar] [CrossRef] [PubMed]
- De Filippo, R.E.; Bishop, C.E.; Filho, L.F.; Yoo, J.J.; Atala, A. Tissue engineering a complete vaginal replacement from a small biopsy of autologous tissue. Transplantation 2008, 86, 208–214. [Google Scholar] [CrossRef] [PubMed]
- Panici, P.B.; Maffucci, D.; Ceccarelli, S.; Vescarelli, E.; Perniola, G.; Muzii, L.; Marchese, C. Autologous in vitro cultured vaginal tissue for vaginoplasty in women with Mayer-Rokitansky-Kuster-Hauser syndrome: Anatomic and functional results. J. Minim. Invasive Gynecol. 2015, 22, 205–211. [Google Scholar] [CrossRef]
- Nodale, C.; Vescarelli, E.; D’Amici, S.; Maffucci, D.; Ceccarelli, S.; Monti, M.; Benedetti Panici, P.; Romano, F.; Angeloni, A.; Marchese, C. Characterization of human vaginal mucosa cells for autologous in vitro cultured vaginal tissue transplantation in patients with MRKH syndrome. Biomed. Res. Int. 2014, 2014, 201518. [Google Scholar] [CrossRef] [PubMed]
- Zhang, J.K.; Du, R.X.; Zhang, L.; Li, Y.N.; Zhang, M.L.; Zhao, S.; Huang, X.H.; Xu, Y.F. A new material for tissue engineered vagina reconstruction: Acellular porcine vagina matrix. J. Biomed. Mater. Res. A 2017, 105, 1949–1959. [Google Scholar] [CrossRef]
- Raya-Rivera, A.M.; Esquiliano, D.; Fierro-Pastrana, R.; Lopez-Bayghen, E.; Valencia, P.; Ordorica-Flores, R.; Soker, S.; Yoo, J.J.; Atala, A. Tissue-engineered autologous vaginal organs in patients: A pilot cohort study. Lancet 2014, 384, 329–336. [Google Scholar] [CrossRef]
- Navarro, V.; Acien, M.I.; Acien, P. Classical mcindoe technique versus the mcindoe technique with a neovaginal paciena prosthesis((R)) and no skin graft. J. Clin. Med. 2020, 9, 3648. [Google Scholar] [CrossRef]
- Acien, P.; Nohales-Alfonso, F.J.; Sanchez-Ferrer, M.L.; Sanchez-Lozano, M.; Navarro-Lillo, V.; Acien, M. Clinical pilot study to evaluate the neovaginal PACIENA prosthesis(R) for vaginoplasty without skin grafts in women with vaginal agenesis. BMC Womens Health 2019, 19, 144. [Google Scholar] [CrossRef]
- Vatsa, R.; Bharti, J.; Roy, K.K.; Kumar, S.; Sharma, J.B.; Singh, N.; Singhal, S.; Meena, J. Evaluation of amnion in creation of neovagina in women with Mayer-Rokitansky-Kuster-Hauser syndrome. Fertil. Steril. 2017, 108, 341–345. [Google Scholar] [CrossRef]
- Zhang, X.; Qiu, J.; Ding, J.; Hua, K. Comparison of neovaginoplasty using acellular porcine small intestinal submucosa graft or Interceed in patients with Mayer-Rokitansky-Kuster-Hauser syndrome. Arch. Gynecol. Obstet. 2019, 300, 1633–1636. [Google Scholar] [CrossRef] [PubMed]
- Ma, Y.; Zhang, Y.; Chen, J.; Li, L.; Liu, X.; Zhang, L.; Ma, C.; Wang, Y.; Tian, W.; Song, X.; et al. Mesenchymal stem cell-based bioengineered constructs enhance vaginal repair in ovariectomized rhesus monkeys. Biomaterials 2021, 275, 120863. [Google Scholar] [CrossRef]
- Tian, Y.; Zhao, S.; Zheng, J.; Li, Z.; Hou, C.; Qi, X.; Kong, D.; Zhang, J.; Huang, X. A stereological study of 3D printed tissues engineered from rat vaginas. Ann. Transl. Med. 2020, 8, 1490. [Google Scholar] [CrossRef] [PubMed]
- Wang, W.; Chen, F.; Cheng, J.; Peng, S.; Ye, H. Effects of different vaginal mould use approaches after vaginoplasty with artificial dermis in patients with Mayer-Rokitansky-Kuster-Hauser syndrome. J. Int. Med. Res. 2021, 49, 300060521990519. [Google Scholar] [CrossRef]
- Orabi, H.; Saba, I.; Rousseau, A.; Bolduc, S. Novel three-dimensional autologous tissue-engineered vaginal tissues using the self-assembly technique. Transl. Res. 2017, 180, 22–36. [Google Scholar] [CrossRef]
- Jakubowska, W.; Chabaud, S.; Saba, I.; Galbraith, T.; Berthod, F.; Bolduc, S. Prevascularized tissue-engineered human vaginal mucosa: In vitro optimization and in vivo validation. Tissue Eng. Part A 2020, 26, 811–822. [Google Scholar] [CrossRef] [PubMed]
- Switzer, B.R.; Summer, G.K. Collagen synthesis in human skin fibroblasts: Effects of ascorbate, -ketoglutarate and ferrous ion on proline hydroxylation. J. Nutr. 1972, 102, 721–728. [Google Scholar] [CrossRef] [PubMed]
- Hata, R.; Senoo, H. L-ascorbic acid 2-phosphate stimulates collagen accumulation, cell proliferation, and formation of a three-dimensional tissuelike substance by skin fibroblasts. J. Cell Physiol. 1989, 138, 8–16. [Google Scholar] [CrossRef]
- L’Heureux, N.; Paquet, S.; Labbe, R.; Germain, L.; Auger, F.A. A completely biological tissue-engineered human blood vessel. FASEB J. 1998, 12, 47–56. [Google Scholar] [CrossRef]
- Michel, M.; L’Heureux, N.; Pouliot, R.; Xu, W.; Auger, F.A.; Germain, L. Characterization of a new tissue-engineered human skin equivalent with hair. Vitro Cell Dev. Biol. Anim. 1999, 35, 318–326. [Google Scholar] [CrossRef]
- Vermette, M.; Trottier, V.; Menard, V.; Saint-Pierre, L.; Roy, A.; Fradette, J. Production of a new tissue-engineered adipose substitute from human adipose-derived stromal cells. Biomaterials 2007, 28, 2850–2860. [Google Scholar] [CrossRef]
- Proulx, S.; d’Arc Uwamaliya, J.; Carrier, P.; Deschambeault, A.; Audet, C.; Giasson, C.J.; Guerin, S.L.; Auger, F.A.; Germain, L. Reconstruction of a human cornea by the self-assembly approach of tissue engineering using the three native cell types. Mol. Vis. 2010, 16, 2192–2201. [Google Scholar]
- Hayward, C.J.; Fradette, J.; Galbraith, T.; Rémy, C.J.; Guignard, R.; Gauvin, R.; Germain, L.; Auger, F.A. Harvesting the potential of the human umbilical cord: Isolation and characterisation of four cell types for tissue engineering applications. Cells Tissues Organs 2013, 197, 37–54. [Google Scholar] [CrossRef]
- Magnan, M.; Berthod, F.; Champigny, M.F.; Soucy, F.; Bolduc, S. In vitro reconstruction of a tissue-engineered endothelialized bladder from a single porcine biopsy. J. Pediatr. Urol. 2006, 2, 261–270. [Google Scholar] [CrossRef] [PubMed]
- Carrier, P.; Deschambeault, A.; Audet, C.; Talbot, M.; Gauvin, R.; Giasson, C.J.; Auger, F.A.; Guerin, S.L.; Germain, L. Impact of cell source on human cornea reconstructed by tissue engineering. Investig. Ophthalmol. Vis. Sci. 2009, 50, 2645–2652. [Google Scholar] [CrossRef] [PubMed]
- Saba, I.; Barat, C.; Chabaud, S.; Reyjon, N.; Leclerc, M.; Jakubowska, W.; Orabi, H.; Lachhab, A.; Pelletier, M.; Tremblay, M.J.; et al. Immunocompetent human 3D organ-specific hormone-responding vaginal mucosa model of HIV-1 infection. Tissue Eng. Part C Methods 2021, 27, 152–166. [Google Scholar] [CrossRef] [PubMed]
- Chabaud, S.; Rousseau, A.; Marcoux, T.L.; Bolduc, S. Inexpensive production of near-native engineered stromas. J. Tissue Eng. Regen. Med. 2017, 11, 1377–1389. [Google Scholar] [CrossRef]
- Larouche, D.; Paquet, C.; Fradette, J.; Carrier, P.; Auger, F.A.; Germain, L. Regeneration of skin and cornea by tissue engineering. Methods Mol. Biol. 2009, 482, 233–256. [Google Scholar] [CrossRef]
- Bouhout, S.; Perron, E.; Gauvin, R.; Bernard, G.; Ouellet, G.; Cattan, V.; Bolduc, S. In vitro reconstruction of an autologous, watertight, and resistant vesical equivalent. Tissue Eng. Part A 2010, 16, 1539–1548. [Google Scholar] [CrossRef] [PubMed]
- Chabaud, S.; Marcoux, T.L.; Deschenes-Rompre, M.P.; Rousseau, A.; Morissette, A.; Bouhout, S.; Bernard, G.; Bolduc, S. Lysophosphatidic acid enhances collagen deposition and matrix thickening in engineered tissue. J. Tissue Eng. Regen. Med. 2015, 9, E65–E75. [Google Scholar] [CrossRef] [PubMed]
- Galbraith, T.; Roy, V.; Bourget, J.M.; Tsutsumi, T.; Picard-Deland, M.; Morin, J.F.; Gauvin, R.; Ismail, A.A.; Auger, F.A.; Gros-Louis, F. Cell seeding on UV-C-treated 3D polymeric templates allows for cost-effective production of small-caliber tissue-engineered blood vessels. Biotechnol. J. 2019, 14, e1800306. [Google Scholar] [CrossRef]
- Magnan, M.; Levesque, P.; Gauvin, R.; Dube, J.; Barrieras, D.; El-Hakim, A.; Bolduc, S. Tissue engineering of a genitourinary tubular tissue graft resistant to suturing and high internal pressures. Tissue Eng. Part A 2009, 15, 197–202. [Google Scholar] [CrossRef]
- Imbeault, A.; Bernard, G.; Rousseau, A.; Morissette, A.; Chabaud, S.; Bouhout, S.; Bolduc, S. An endothelialized urothelial cell-seeded tubular graft for urethral replacement. Can. Urol. Assoc. J. 2013, 7, E4–E9. [Google Scholar] [CrossRef][Green Version]
- Cattan, V.; Bernard, G.; Rousseau, A.; Bouhout, S.; Chabaud, S.; Auger, F.A.; Bolduc, S. Mechanical stimuli-induced urothelial differentiation in a human tissue-engineered tubular genitourinary graft. Eur. Urol. 2011, 60, 1291–1298. [Google Scholar] [CrossRef]
- Vallieres, K.; Laterreur, V.; Tondreau, M.Y.; Ruel, J.; Germain, L.; Fradette, J.; Auger, F.A. Human adipose-derived stromal cells for the production of completely autologous self-assembled tissue-engineered vascular substitutes. Acta Biomater. 2015, 24, 209–219. [Google Scholar] [CrossRef]
- Le-Bel, G.; Guerin, L.P.; Carrier, P.; Mouriaux, F.; Germain, L.; Guerin, S.L.; Bazin, R. Grafting of an autologous tissue-engineered human corneal epithelium to a patient with limbal stem cell deficiency (LSCD). Am. J. Ophthalmol. Case Rep. 2019, 15, 100532. [Google Scholar] [CrossRef]
- Tremblay, C.; Ruel, J.; Bourget, J.M.; Laterreur, V.; Vallieres, K.; Tondreau, M.Y.; Lacroix, D.; Germain, L.; Auger, F.A. A new construction technique for tissue-engineered heart valves using the self-assembly method. Tissue Eng. Part C Methods 2014, 20, 905–915. [Google Scholar] [CrossRef]
- Proulx, M.; Mayrand, D.; Vincent, C.; Boisvert, A.; Aubin, K.; Trottier, V.; Fradette, J. Short-term post-implantation dynamics of in vitro engineered human microvascularized adipose tissues. Biomed. Mater. 2018, 13, 065013. [Google Scholar] [CrossRef] [PubMed]
- Morissette Martin, P.; Maux, A.; Laterreur, V.; Mayrand, D.; Gagné, V.L.; Moulin, V.J.; Fradette, J. Enhancing repair of full-thickness excisional wounds in a murine model: Impact of tissue-engineered biological dressings featuring human differentiated adipocytes. Acta Biomater. 2015, 22, 39–49. [Google Scholar] [CrossRef]
- Kawecki, F.; Galbraith, T.; Clafshenkel, W.P.; Fortin, M.; Auger, F.A.; Fradette, J. In vitro prevascularization of self-assembled human bone-like tissues and preclinical assessment using a rat calvarial bone defect model. Materials 2021, 14, 2023. [Google Scholar] [CrossRef]
- Germain, L.; Larouche, D.; Nedelec, B.; Perreault, I.; Duranceau, L.; Bortoluzzi, P.; Beaudoin Cloutier, C.; Genest, H.; Caouette-Laberge, L.; Dumas, A.; et al. Autologous bilayered self-assembled skin substitutes (SASSs) as permanent grafts: A case series of 14 severely burned patients indicating clinical effectiveness. Eur. Cell Mater. 2018, 36, 128–141. [Google Scholar] [CrossRef]
- Gibot, L.; Galbraith, T.; Huot, J.; Auger, F.A. Development of a tridimensional microvascularized human skin substitute to study melanoma biology. Clin. Exp. Metastasis 2013, 30, 83–90. [Google Scholar] [CrossRef]
- Bourland, J.; Fradette, J.; Auger, F.A. Tissue-engineered 3D melanoma model with blood and lymphatic capillaries for drug development. Sci. Rep. 2018, 8, 13191. [Google Scholar] [CrossRef] [PubMed]
- Roy, V.; Magne, B.; Vaillancourt-Audet, M.; Blais, M.; Chabaud, S.; Grammond, E.; Piquet, L.; Fradette, J.; Laverdiere, I.; Moulin, V.J.; et al. Human organ-specific 3D cancer models produced by the stromal self-assembly method of tissue engineering for the study of solid tumors. Biomed. Res. Int. 2020, 2020, 6051210. [Google Scholar] [CrossRef] [PubMed]
- Goyer, B.; Pereira, U.; Magne, B.; Larouche, D.; Kearns-Turcotte, S.; Rochette, P.J.; Martin, L.; Germain, L. Impact of ultraviolet radiation on dermal and epidermal DNA damage in a human pigmented bilayered skin substitute. J. Tissue Eng. Regen. Med. 2019, 13, 2300–2311. [Google Scholar] [CrossRef] [PubMed]
- Roy, V.; Lamontagne, R.; Talagas, M.; Touzel-Deschenes, L.; Khuong, H.T.; Saikali, S.; Dupre, N.; Gros-Louis, F. Biofabrication of a three dimensional human-based personalized neurofibroma model. Biotechnol. J. 2021, 16, e2000250. [Google Scholar] [CrossRef]
- Laplante, A.F.; Germain, L.; Auger, F.A.; Moulin, V. Mechanisms of wound reepithelialization: Hints from a tissue-engineered reconstructed skin to long-standing questions. FASEB J. 2001, 15, 2377–2389. [Google Scholar] [CrossRef][Green Version]
- Simon, F.; Bergeron, D.; Larochelle, S.; Lopez-Valle, C.A.; Genest, H.; Armour, A.; Moulin, V.J. Enhanced secretion of TIMP-1 by human hypertrophic scar keratinocytes could contribute to fibrosis. Burns 2012, 38, 421–427. [Google Scholar] [CrossRef] [PubMed]
- Bellemare, J.; Roberge, C.J.; Bergeron, D.; Lopez-Valle, C.A.; Roy, M.; Moulin, V.J. Epidermis promotes dermal fibrosis: Role in the pathogenesis of hypertrophic scars. J. Pathol. 2005, 206, 1–8. [Google Scholar] [CrossRef]
- Corriveau, M.P.; Boufaied, I.; Lessard, J.; Chabaud, S.; Senecal, J.L.; Grodzicky, T.; Chartier, S.; Raymond, Y.; Moulin, V.J. The fibrotic phenotype of systemic sclerosis fibroblasts varies with disease duration and severity of skin involvement: Reconstitution of skin fibrosis development using a tissue engineering approach. J. Pathol. 2009, 217, 534–542. [Google Scholar] [CrossRef]
- Jean, J.; Lapointe, M.; Soucy, J.; Pouliot, R. Development of an in vitro psoriatic skin model by tissue engineering. J. Dermatol. Sci. 2009, 53, 19–25. [Google Scholar] [CrossRef]
- Dakiw Piaceski, A.; Larouche, D.; Ghani, K.; Bisson, F.; Cortez Ghio, S.; Larochelle, S.; Moulin, V.J.; Caruso, M.; Germain, L. Translating the combination of gene therapy and tissue engineering for treating recessive dystrophic epidermolysis bullosa. Eur. Cell Mater. 2018, 35, 73–86. [Google Scholar] [CrossRef] [PubMed]
- Pare, B.; Touzel-Deschenes, L.; Lamontagne, R.; Lamarre, M.S.; Scott, F.D.; Khuong, H.T.; Dion, P.A.; Bouchard, J.P.; Gould, P.; Rouleau, G.A.; et al. Early detection of structural abnormalities and cytoplasmic accumulation of TDP-43 in tissue-engineered skins derived from ALS patients. Acta Neuropathol. Commun. 2015, 3, 5. [Google Scholar] [CrossRef] [PubMed]
- Goyer, B.; Theriault, M.; Gendron, S.P.; Brunette, I.; Rochette, P.J.; Proulx, S. Extracellular matrix and integrin expression profiles in fuchs endothelial corneal dystrophy cells and tissue model. Tissue Eng. Part A 2018, 24, 607–615. [Google Scholar] [CrossRef]
- Desjardins, P.; Couture, C.; Germain, L.; Guerin, S.L. Contribution of the WNK1 kinase to corneal wound healing using the tissue-engineered human cornea as an in vitro model. J. Tissue Eng. Regen. Med. 2019, 13, 1595–1608. [Google Scholar] [CrossRef]
- Gibot, L.; Galbraith, T.; Bourland, J.; Rogic, A.; Skobe, M.; Auger, F.A. Tissue-engineered 3D human lymphatic microvascular network for in vitro studies of lymphangiogenesis. Nat. Protoc. 2017, 12, 1077–1088. [Google Scholar] [CrossRef]
- Ouellet, G.; Dube, J.; Gauvin, R.; Laterreur, V.; Bouhout, S.; Bolduc, S. Production of an optimized tissue-engineered pig connective tissue for the reconstruction of the urinary tract. Tissue Eng. Part A 2011, 17, 1625–1633. [Google Scholar] [CrossRef] [PubMed]
- Bouhout, S.; Gauvin, R.; Gibot, L.; Aube, D.; Bolduc, S. Bladder substitute reconstructed in a physiological pressure environment. J. Pediatr. Urol. 2011, 7, 276–282. [Google Scholar] [CrossRef] [PubMed]
- Rousseau, A.; Fradette, J.; Bernard, G.; Gauvin, R.; Laterreur, V.; Bolduc, S. Adipose-derived stromal cells for the reconstruction of a human vesical equivalent. J. Tissue Eng. Regen. Med. 2015, 9, E135–E143. [Google Scholar] [CrossRef]
- Morissette, A.; Imbeault, A.; Cattan, V.; Bernard, G.; Taillon, G.; Chabaud, S.; Bolduc, S. Strategies to reconstruct a functional urethral substitute by self-assembly method. Procedia Eng. 2013, 59, 193–200. [Google Scholar] [CrossRef]
- Chabaud, S.; Saba, I.; Baratange, C.; Boiroux, B.; Leclerc, M.; Rousseau, A.; Bouhout, S.; Bolduc, S. Urothelial cell expansion and differentiation are improved by exposure to hypoxia. J. Tissue Eng. Regen. Med. 2017, 11, 3090–3099. [Google Scholar] [CrossRef] [PubMed]
- Takahashi, K.; Yamanaka, S. Induction of pluripotent stem cells from mouse embryonic and adult fibroblast cultures by defined factors. Cell 2006, 126, 663–676. [Google Scholar] [CrossRef] [PubMed]
- Bedard, P.; Gauvin, S.; Ferland, K.; Caneparo, C.; Pellerin, E.; Chabaud, S.; Bolduc, S. Innovative human three-dimensional tissue-engineered models as an alternative to animal testing. Bioengineering 2020, 7, 115. [Google Scholar] [CrossRef] [PubMed]
- Amling, C.L. Diagnosis and management of superficial bladder cancer. Curr. Probl. Cancer 2001, 25, 219–278. [Google Scholar] [CrossRef]
- Neely, L.A.; Rieger-Christ, K.M.; Neto, B.S.; Eroshkin, A.; Garver, J.; Patel, S.; Phung, N.A.; McLaughlin, S.; Libertino, J.A.; Whitney, D.; et al. A microRNA expression ratio defining the invasive phenotype in bladder tumors. Urol. Oncol. 2010, 28, 39–48. [Google Scholar] [CrossRef] [PubMed]
- Nepple, K.G.; O’Donnell, M.A. The optimal management of T1 high-grade bladder cancer. Can. Urol. Assoc. J. 2009, 3, S188–S192. [Google Scholar] [CrossRef]
- Shah, J.B.; McConkey, D.J.; Dinney, C.P. New strategies in muscle-invasive bladder cancer: On the road to personalized medicine. Clin. Cancer Res. 2011, 17, 2608–2612. [Google Scholar] [CrossRef]
- Ringuette Goulet, C.; Bernard, G.; Tremblay, S.; Chabaud, S.; Bolduc, S.; Pouliot, F. Exosomes induce fibroblast differentiation into cancer-associated fibroblasts through TGFbeta signaling. Mol. Cancer Res. 2018, 16, 1196–1204. [Google Scholar] [CrossRef] [PubMed]
- Goulet, C.R.; Champagne, A.; Bernard, G.; Vandal, D.; Chabaud, S.; Pouliot, F.; Bolduc, S. Cancer-associated fibroblasts induce epithelial-mesenchymal transition of bladder cancer cells through paracrine IL-6 signalling. BMC Cancer 2019, 19, 137. [Google Scholar] [CrossRef]
- Santi, A.; Kugeratski, F.G.; Zanivan, S. Cancer associated fibroblasts: The architects of stroma remodeling. Proteomics 2018, 18, e1700167. [Google Scholar] [CrossRef]
- Ringuette Goulet, C.; Bernard, G.; Chabaud, S.; Couture, A.; Langlois, A.; Neveu, B.; Pouliot, F.; Bolduc, S. Tissue-engineered human 3D model of bladder cancer for invasion study and drug discovery. Biomaterials 2017, 145, 233–241. [Google Scholar] [CrossRef]
- Le Dare, B.; Pelletier, R.; Morel, I.; Gicquel, T. History of Ketamine: An ancient molecule that is still popular today. Ann. Pharm. 2021. [Google Scholar] [CrossRef]
- Natoli, S. The multiple faces of ketamine in anaesthesia and analgesia. Drugs Context 2021, 10. [Google Scholar] [CrossRef] [PubMed]
- McMullen, E.P.; Lee, Y.; Lipsitz, O.; Lui, L.M.W.; Vinberg, M.; Ho, R.; Rodrigues, N.B.; Rosenblat, J.D.; Cao, B.; Gill, H.; et al. Strategies to prolong ketamine’s efficacy in adults with treatment-resistant depression. Adv. Ther. 2021. [Google Scholar] [CrossRef]
- Worrell, S.D.; Gould, T.J. Therapeutic potential of ketamine for alcohol use disorder. Neurosci. Biobehav. Rev. 2021, 126, 573–589. [Google Scholar] [CrossRef] [PubMed]
- Myers, F.A., Jr.; Bluth, M.H.; Cheung, W.W. Ketamine: A cause of urinary tract dysfunction. Clin. Lab. Med. 2016, 36, 721–744. [Google Scholar] [CrossRef] [PubMed]
- Wei, Y.B.; Yang, J.R.; Yin, Z.; Guo, Q.; Liang, B.L.; Zhou, K.Q. Genitourinary toxicity of ketamine. Hong Kong Med. J. 2013, 19, 341–348. [Google Scholar] [CrossRef] [PubMed]
- Bureau, M.; Pelletier, J.; Rousseau, A.; Bernard, G.; Chabaud, S.; Bolduc, S. Demonstration of the direct impact of ketamine on urothelium using a tissue engineered bladder model. Can. Urol. Assoc. J. 2015, 9, E613–E617. [Google Scholar] [CrossRef] [PubMed]
- Lange, D.; Bidnur, S.; Hoag, N.; Chew, B.H. Ureteral stent-associated complications—Where we are and where we are going. Nat. Rev. Urol. 2015, 12, 17–25. [Google Scholar] [CrossRef]
- Wang, X.; Shan, H.; Wang, J.; Hou, Y.; Ding, J.; Chen, Q.; Guan, J.; Wang, C.; Chen, X. Characterization of nanostructured ureteral stent with gradient degradation in a porcine model. Int. J. Nanomed. 2015, 10, 3055–3064. [Google Scholar] [CrossRef]
- Hermawan, H. Updates on the research and development of absorbable metals for biomedical applications. Prog. Biomater. 2018, 7, 93–110. [Google Scholar] [CrossRef]
- Paramitha, D.; Chabaud, S.; Bolduc, S.; Hermawan, H. Biological assessment of Zn-based absorbable metals for ureteral stent applications. Materials 2019, 12, 3325. [Google Scholar] [CrossRef]
- Costin, G.E.; Raabe, H.A.; Priston, R.; Evans, E.; Curren, R.D. Vaginal irritation models: The current status of available alternative and in vitro tests. Altern. Lab. Anim. 2011, 39, 317–337. [Google Scholar] [CrossRef]



| Type of Scaffold | Biomaterials | Ref Example | Advantages | Drawbacks |
|---|---|---|---|---|
| Synthetic | PLCL | [106] | - biocompatible - mechanical properties | - Degradation products |
| PLCL/Collagen | [107] | - low cost | - Poor differentiation of epithelial cells (except for cellularised collagen matrices; improved by functionalisation) | |
| PLA | [108] | - highly reproducible | -degradation rate (too low or too high) | |
| PU/mesh in PGA | [109] | - quickly available | -mechanical properties during or after degradation | |
| PLGA | [97] | - functionalisation | - poor angiogenesis | |
| PLLA | [110] | |||
| Natural | Cellulose | [111] | ||
| Silk Fibroin | [86,112,113,114] | |||
| Collagen | [78,88,115,116,117,118,119] | |||
| Acellular matrix | SIS | [81,120,121,122,123,124,125] | - Adequate microenvironment for cell proliferation and differentiation | - Immune risk (including DNA, prions) |
| Placental membrane | [126] | - Significant angiogenesis | - Unfavourable clinical experience | |
| BAMG | [127,128,129] | - Quality of the matrix | ||
| Urethra | [130] | |||
| Self-Assembly | None | [73,131,132] | - Excellent microenvironment with organ-specific cells - Mechanical properties | - time and cost to produce tissues |
| Type of Scaffolds | Biomaterials | Patients # | References |
|---|---|---|---|
| Synthetic | PGA | 4 | [149] |
| PLA (©PACIENA) | 9 | [150] | |
| 7 | [151] | ||
| Natural | Collagen IV and hyaluronic acid | 1 | [146] |
| 23 | [146] | ||
| Acellular matrix | Amnion | 50 | [152] |
| SIS | 65 (vs Interceed) | [153] | |
| Monkey | [154] | ||
| Acellular vaginal matrix | Rat | [155] | |
| Rat | [148] | ||
| Artificial dermis | 35 | [156] | |
| Self-Assembly | Mouse | [157,158] |
Publisher’s Note: MDPI stays neutral with regard to jurisdictional claims in published maps and institutional affiliations. |
© 2021 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Caneparo, C.; Brownell, D.; Chabaud, S.; Bolduc, S. Genitourinary Tissue Engineering: Reconstruction and Research Models. Bioengineering 2021, 8, 99. https://doi.org/10.3390/bioengineering8070099
Caneparo C, Brownell D, Chabaud S, Bolduc S. Genitourinary Tissue Engineering: Reconstruction and Research Models. Bioengineering. 2021; 8(7):99. https://doi.org/10.3390/bioengineering8070099
Chicago/Turabian StyleCaneparo, Christophe, David Brownell, Stéphane Chabaud, and Stéphane Bolduc. 2021. "Genitourinary Tissue Engineering: Reconstruction and Research Models" Bioengineering 8, no. 7: 99. https://doi.org/10.3390/bioengineering8070099
APA StyleCaneparo, C., Brownell, D., Chabaud, S., & Bolduc, S. (2021). Genitourinary Tissue Engineering: Reconstruction and Research Models. Bioengineering, 8(7), 99. https://doi.org/10.3390/bioengineering8070099






