Understanding ADHD: Toward an Innovative Therapeutic Intervention
Abstract
1. Introduction
2. Pathophysiology and Causes
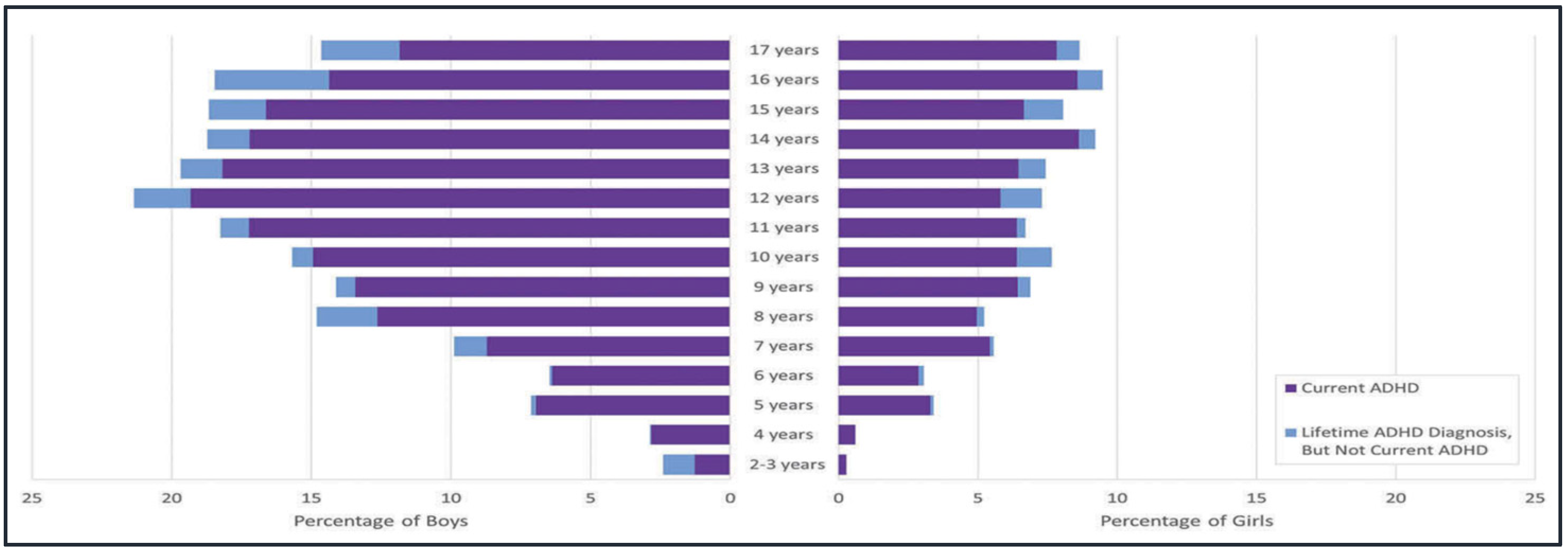
3. Clinical Manifestations
4. Diagnosis
5. Clinical Management
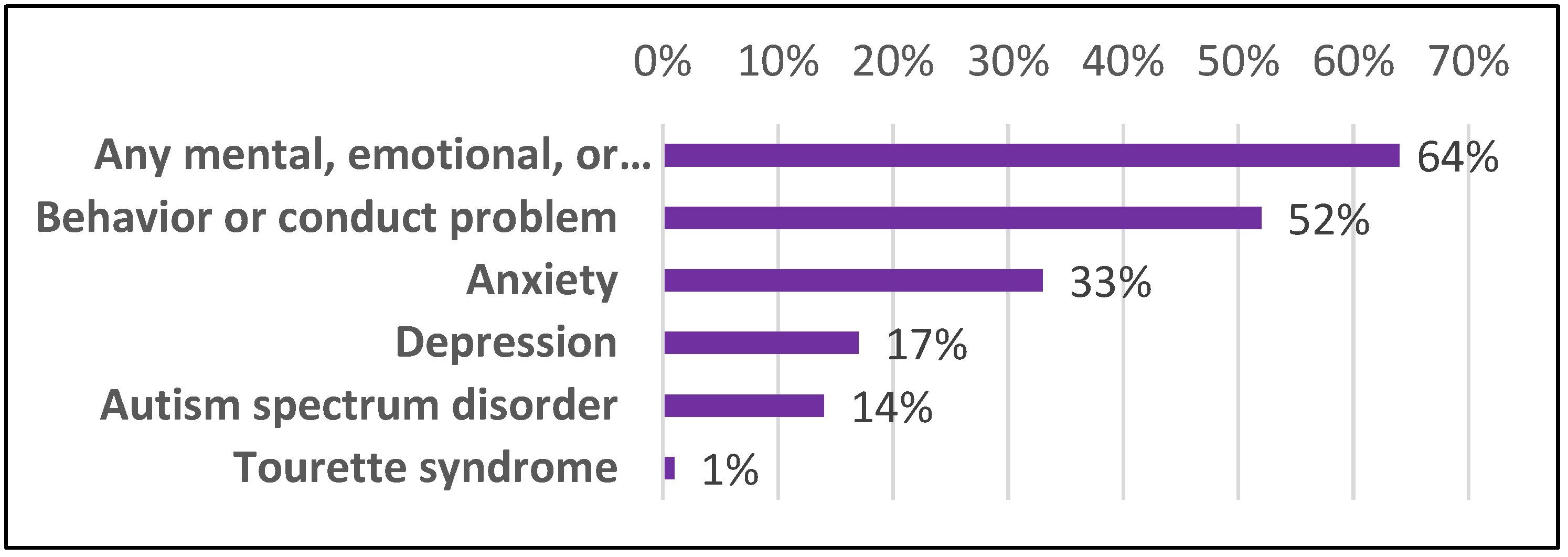
6. Societal Impact
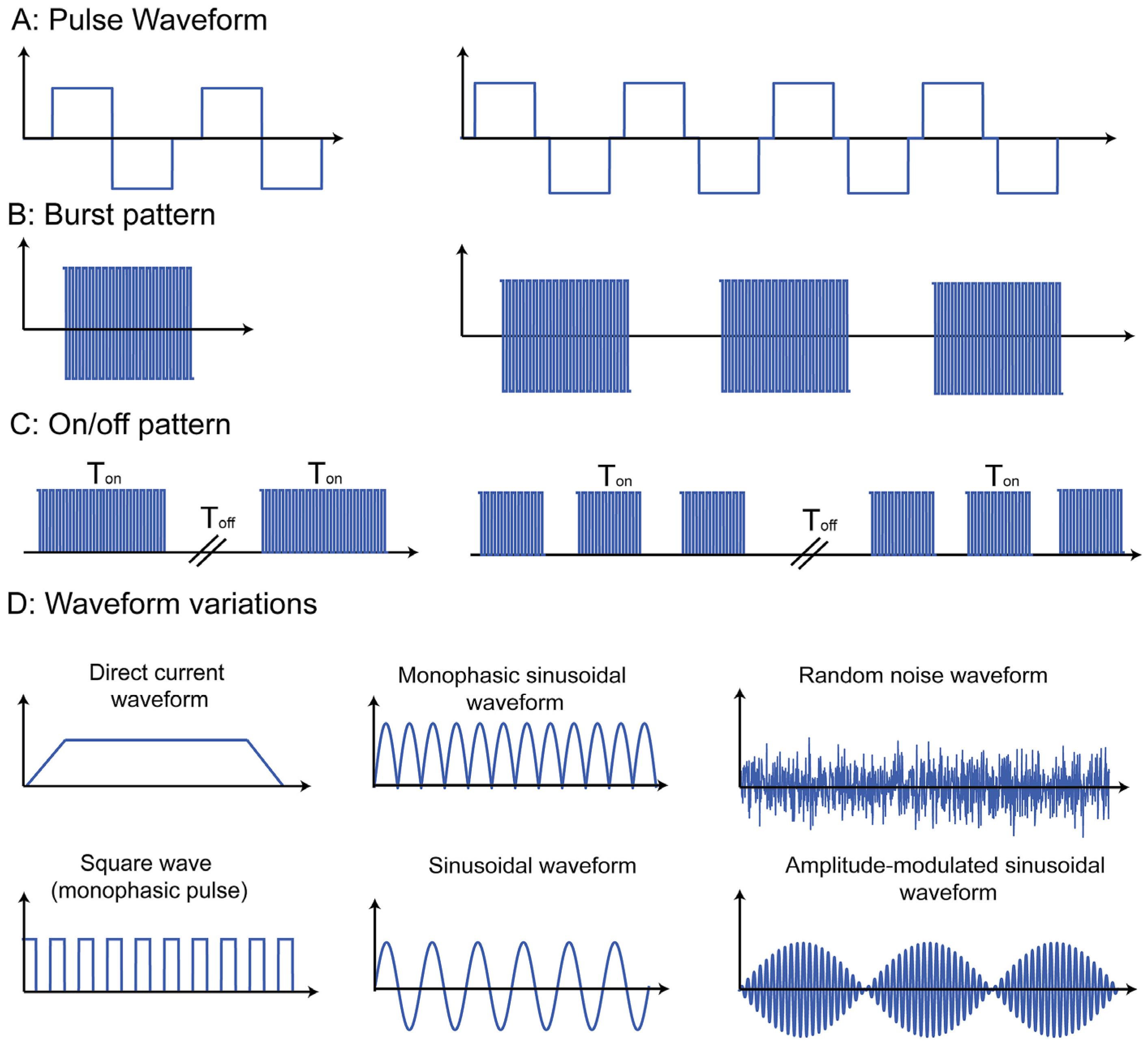
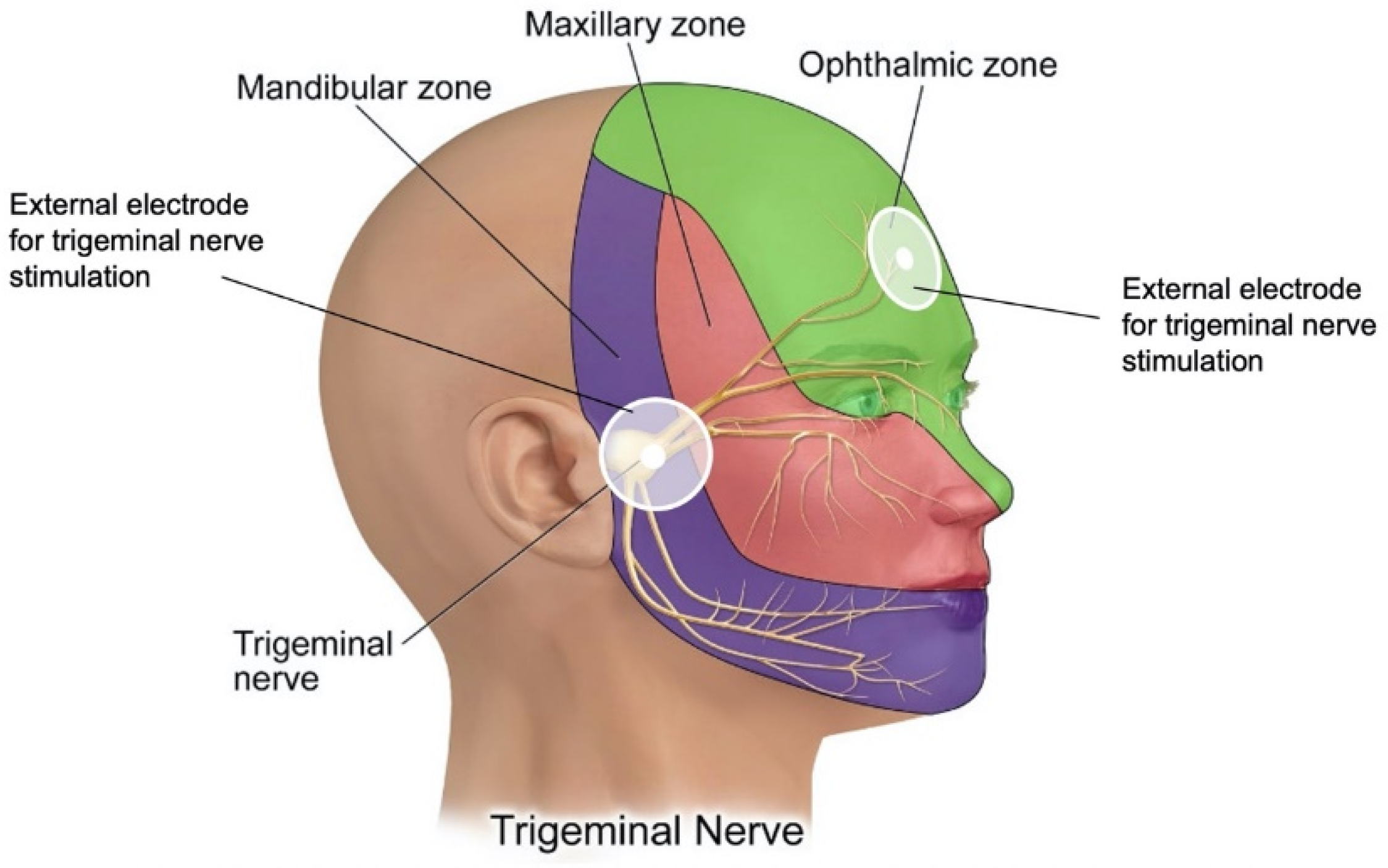
7. Innovations in Therapeutic Intervention
8. Conclusions
9. Future Prospects
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Data Availability Statement
Conflicts of Interest
References
- Mahone, E.M.; Denckla, M.B. Attention-deficit/hyperactivity disorder: A historical neuropsychological perspective. J. Int. Neuropsychol. Soc. 2017, 23, 91–929. [Google Scholar] [CrossRef] [PubMed]
- Force, D.-T. Neurodevelopmental Disorders. In Diagnostic and Statistical Manual of Mental Disorders DSM-5; American Psychiatric Association: Philadelphia, PA, USA, 2013. [Google Scholar]
- Silver, M.D.L.; Michele Novotni, P.D. What Causes ADHD? Is ADHD Genetic? Yes and No. 2019. Available online: https://www.additudemag.com/is-adhd-hereditary-yes-and-no/ (accessed on 29 April 2021).
- Kessler, R.C.; Adler, L.; Barkley, R.; Biederman, J.; Conners, C.K.; Demler, O.; Faraone, S.V.; Greenhill, L.L.; Howes, M.J.; Secnik, K.; et al. The prevalence and correlates of adult ADHD in the United States: Results from the national comorbidity survey replication. Am. J. Psychiatry 2006, 163, 716–723. [Google Scholar] [CrossRef]
- Danielson, M.L.; Bitsko, R.H.; Ghandour, R.M.; Holbrook, J.R.; Kogan, M.D.; Blumberg, S.J. Prevalence of parent-reported ADHD diagnosis and associated treatment among, U.S. children and adolescents, 2016. J. Clin. Child Adolesc. Psychol. 2018, 47, 199–212. [Google Scholar] [CrossRef] [PubMed]
- McGough, J.J.; Loo, S.K.; Sturm, A.; Cowen, J.; Leuchter, A.F.; Cook, I.A. An eight-week, open-trial, pilot feasibility study of trigeminal nerve stimulation in youth with attention-deficit/hyperactivity disorder. Brain Stimul. Basic Transl. Clin. Res. Neuromodulation 2015, 8, 299–304. [Google Scholar] [CrossRef] [PubMed]
- Daneshparvar, M.; Mostafavi, S.A.; Jeddi, M.Z.; Yunesian, M.; Mesdaghinia, A.; Mahvi, A.H.; Akhondzadeh, S. The role of lead exposure on attention-deficit/ hyperactivity disorder in children: A systematic review. Iran. J. Psychiatry 2016, 11, 1–14. [Google Scholar]
- Faraone, S.V.; Larsson, H. Genetics of attention deficit hyperactivity disorder. Mol. Psychiatry 2019, 24, 562–575. [Google Scholar] [CrossRef] [PubMed]
- Franke, B.; Faraone, S.V.; Asherson, P.; Buitelaar, J.; Bau, C.H.D.; Ramos-Quiroga, J.A.; Mick, E.; Grevet, E.H.; Johansson, S.; Haavik, J.; et al. The genetics of attention deficit/hyperactivity disorder in adults, a review. Mol. Psychiatry 2012, 17, 960–987. [Google Scholar] [CrossRef]
- Bruxel, E.M.; Salatino‐Oliveira, A.; Akutagava‐Martins, G.C.; Tovo‐Rodrigues, L.; Genro, J.P.; Zeni, C.P.; Polanczyk, G.V.; Chazan, R.; Schmitz, M.; Arcos‐Burgos, M.; et al. LPHN3 and attention-deficit/hyperactivity disorder: A susceptibility and pharmacogenetic study. Genes Brain Behav. 2015, 14, 419–427. [Google Scholar] [CrossRef]
- Langner, I.; Garbe, E.; Banaschewski, T.; Mikolajczyk, R.T. Twin and sibling studies using health insurance data: The example of attention deficit/hyperactivity disorder (ADHD). PLoS ONE 2013, 8, e62177. [Google Scholar] [CrossRef]
- Miller, M.; Musser, E.D.; Young, G.S.; Olson, B.; Steiner, R.D.; Nigg, J.T. Sibling recurrence risk and cross-aggregation of attention-deficit/hyperactivity disorder and autism spectrum DIsorder. JAMA Pediatrics 2019, 173, 147–152. [Google Scholar] [CrossRef]
- Grimm, O.; Kittel-Schneider, S.; Reif, A. Recent developments in the genetics of attention-deficit hyperactivity disorder. Psychiatry Clin. Neurosci. 2018, 72, 654–672. [Google Scholar] [CrossRef]
- Demontis, D.; Walters, R.K.; Martin, J.; Mattheisen, M.; Als, T.D.; Agerbo, E.; Baldursson, G.; Belliveau, R.; Bybjerg-Grauholm, J.; Bækvad-Hansen, M.; et al. Discovery of the first genome-wide significant risk loci for attention deficit/hyperactivity disorder. Nat. Genet. 2019, 51, 63–75. [Google Scholar] [CrossRef] [PubMed]
- Lange, M.; Norton, W.; Coolen, M.; Chaminade, M.; Merker, S.; Proft, F.; Schmitt, A.; Vernier, P.; Lesch, K.P.; Bally-Cuif, L. The ADHD-susceptibility gene lphn3.1 modulates dopaminergic neuron formation and locomotor activity during zebrafish development. Mol. Psychiatry 2012, 17, 946–954. [Google Scholar] [CrossRef]
- Kappel, D.B.; Schuch, J.B.; Rovaris, D.L.; da Silva, B.S.; Müller, D.; Breda, V.; Teche, S.P.; Riesgo, R.S.; Schüler-Faccini, L.; Rohde, L.A.; et al. ADGRL3 rs6551665 as a common vulnerability factor underlying attention-deficit/hyperactivity disorder and autism spec-trum disorder. NeuroMolecular Med. 2019, 21, 60–67. [Google Scholar] [CrossRef] [PubMed]
- Acosta, M.T.; Velez, J.I.; Bustamante, M.L.; Balog, J.Z.; Arcos-Burgos, M.; Muenke, M. A two-locus genetic interaction between LPHN3 and 11q predicts ADHD severity and long-term outcome. Transl. Psychiatry 2011, 1, e17. [Google Scholar] [CrossRef]
- Sonuga-Barke, E.J.S. Causal models of attention-deficit/hyperactivity disorder: From common simple deficits to multiple developmental pathways. Biol. Psychiatry 2005, 57, 123–1238. [Google Scholar] [CrossRef]
- Rubio, B.; Boes, A.D.; Laganiere, S.; Rotenberg, A.; Jeurissen, D.; Pascual-Leone, A. Noninvasive brain stimulation in pediatric attention-deficit hyperactivity disorder (ADHD): A review. J. Child Neurol. 2016, 31, 784–796. [Google Scholar] [CrossRef] [PubMed]
- Păsărelu, C.R.; Andersson, G.; Dobrean, A. Attention-deficit/hyperactivity disorder mobile apps: A systematic review. Int. J. Med. Inform. 2020, 138, 04133. [Google Scholar] [CrossRef]
- Samrén, E.B.; Van Duijn, C.M.; Lieve Christiaens, G.C.M.; Hofman, A.; Lindhout, D. Antiepileptic drug regimens and major congenital abnormalities in the offspring. Ann. Neurol. 2001, 46, 739–746. [Google Scholar] [CrossRef]
- Verrall, C.E.; Blue, G.M.; Loughran-Fowlds, A.; Kasparian, N.; Gecz, J.; Walker, K.; Dunwoodie, S.L.; Cordina, R.; Sholler, G.; Badawi, N.; et al. ‘Big issues’ in neurodevelopment for children and adults with congenital heart disease. Open Heart 2019, 6, e000998. [Google Scholar] [CrossRef]
- Christensen, J.; Pedersen, L.; Sun, Y.; Dreier, J.W.; Brikell, I.; Dalsgaard, S. Association of prenatal exposure to valproate and other antiepileptic drugs with risk for attention-deficit/hyperactivity disorder in offspring. JAMA Netw. Open 2019, 2, e186606. [Google Scholar] [CrossRef]
- Nigg, J.T. Attention-deficit/hyperactivity disorder:Endophenotypes, structure, and etiological pathways. Curr. Dir. Psychol. Sci. 2010, 19, 24–29. [Google Scholar] [CrossRef]
- Donzelli, G.; Carducci, A.; Llopis-Gonzalez, A.; Verani, M.; Llopis-Morales, A.; Cioni, L.; Morales-Suárez-Varela, M. The association between lead and attention-deficit/hyperactivity disorder: A systematic review. Int. J. Environ. Res. Public Health 2019, 16, 382. [Google Scholar] [CrossRef] [PubMed]
- Wilens, T.E.; Biederman, J.; Faraone, S.V.; Martelon, M.; Westerberg, D.; Spencer, T.J. Presenting ADHD symptoms, subtypes, and comorbid disorders in clinically referred adults with ADHD. J. Clin. Psychiatry 2009, 70, 1557–1562. [Google Scholar] [CrossRef] [PubMed]
- Kelly, B.D. Attention-deficit hyperactivity disorder: A clinical review of the concept, diagnosis and management. Irish J. Psychol. Med. 2018, 35, 157–161. [Google Scholar] [CrossRef] [PubMed]
- Felt, B.T.; Biermann, B.; Christner, J.G.; Kochhar, P.; Van Harrison, R. Diagnosis and Management of ADHD in Children. Am. Fam. Physician 2014, 90, 456–464. [Google Scholar] [PubMed]
- Wehmeier, P.M.; Schacht, A.; Barkley, R.A. Social and emotional impairment in children and adolescents with ADHD and the impact on quality of life. J. Adolesc. Health 2010, 46, 209–217. [Google Scholar] [CrossRef]
- DuPaul, G.J.; Gormley, M.J.; Laracy, S.D. Comorbidity of LD and ADHD: Implications of DSM-5 for assessment and treatment. J. Learn. Disabil. 2012, 46, 43–51. [Google Scholar] [CrossRef]
- Turgay, A.; Ansari, R. Major depression with ADHD: In children and adolescents. Psychiatry 2006, 3, 20–32. [Google Scholar]
- Haft, S.L.; Chen, T.; LeBlanc, C.; Tencza, F.; Hoeft, F. Impact of mentoring on socio-emotional and mental health outcomes of youth with learning disabilities and attention-deficit hyperactivity disorder. Child Adolesc. Ment. Health 2019, 24, 318–328. [Google Scholar] [CrossRef]
- Liu, X.; Liu, Z.Z.; Liu, B.P.; Sun, S.H.; Jia, C.X. Associations between sleep problems and ADHD symptoms among adolescents: Findings from the Shandong adolescent behavior and health cohort (SABHC). Sleep 2019, 43, zsz294. [Google Scholar] [CrossRef]
- Ironside, S.; Davidson, F.; Corkum, P. Circadian motor activity affected by stimulant medication in children with attention-deficit/hyperactivity disorder. J. Sleep Res. 2010, 19, 546–551. [Google Scholar] [CrossRef]
- Katzman, M.A.; Bilkey, T.S.; Chokka, P.R.; Fallu, A.; Klassen, L.J. Adult ADHD and comorbid disorders: Clinical implications of a dimensional approach. BMC Psychiatry 2017, 17, 1–15. [Google Scholar] [CrossRef]
- Shaw, P.; Stringaris, A.; Nigg, J.; Leibenluft, E. Emotion dysregulation in attention deficit hyperactivity disorder. Am. J. Psychiatry 2014, 171, 276–293. [Google Scholar] [CrossRef]
- Canela, C.; Buadze, A.; Dube, A.; Eich, D.; Liebrenz, M. Skills and compensation strategies in adult ADHD—A qualitative study. PLoS ONE 2017, 12, e0184964. [Google Scholar] [CrossRef] [PubMed]
- Wilens, T.E.; Spencer, T.J. Understanding attention-deficit/hyperactivity disorder from childhood to adulthood. Postgrad. Med. 2010, 122, 97–109. [Google Scholar] [CrossRef] [PubMed]
- Doernberg, E.; Hollander, E. Neurodevelopmental disorders (ASD and ADHD): DSM-5, ICD-10, and ICD-11. CNS Spectrums 2016, 21, 295–299. [Google Scholar] [CrossRef] [PubMed]
- Lahey, B.B.; Pelham, W.E.; Chronis, A.; Massetti, G.; Kipp, H.; Ehrhardt, A.; Lee, S.S. Predictive validity of ICD-10 hyperkinetic disorder relative to DSM-IV attention-deficit/hyperactivity disorder among younger children. J. Child Psychol. Psychiatry 2006, 47, 472–479. [Google Scholar] [CrossRef]
- DuPaul, G.J.; Power, T.J.; Anastopoulos, A.D.; Reid, R. ADHD Rating Scale–5 for Children and Adolescents: Checklists, Norms, and Clinical Interpretation; Guilford Publications: New York, NY, USA, 2016. [Google Scholar]
- Hulvershorn, L.A. How is ADHD Diagnosed? Available online: https://www.bbrfoundation.org/ask-an-expert/how-is-adhd-diagnosed (accessed on 29 April 2021).
- Brunkhorst-Kanaan, N.; Verdenhalven, M.; Kittel-Schneider, S.; Vainieri, I.; Reif, A.; Grimm, O. The quantified behavioral test—A confirmatory test. in the diagnostic process. of adult ADHD? Front. Psychiatry 2020, 11, 216. [Google Scholar] [CrossRef] [PubMed]
- Hirsch, O.; Christiansen, H. Factorial structure and validity of the quantified behavior test. Plus (Qb+©). Assessment 2017, 24, 1037–1049. [Google Scholar] [CrossRef] [PubMed]
- Emser, T.S.; Johnston, B.A.; Steele, J.D.; Kooij, S.; Thorell, L.; Christiansen, H. Assessing ADHD symptoms in children and adults: Evaluating the role of objective measures. Behav. Brain Funct. 2018, 14, 11. [Google Scholar] [CrossRef] [PubMed]
- Wolraich, M.L. NICHQ Vanderbilt Assessment Scales. In National Institute for Children’s Health Quality; American Academy of Pediatrics and National Initiative for Children’s Healthcare Quality: Boston, MA, USA, 2002. [Google Scholar]
- Swanson, J.M. SNAP-IV-C Rating Scale; University of California: Irvine, CA, USA, 1983. [Google Scholar]
- Ustun, B.; Adler, L.A.; Rudin, C.; Faraone, S.V.; Spencer, T.J.; Berglund, P.; Gruber, M.J.; Kessler, R.C. The World Health Organization adult attention-deficit/hyperactivity disorder self-report screening scale for DSM-5. JAMA Psychiatry 2017, 74, 520–526. [Google Scholar] [CrossRef]
- Foster, N. Gordon Diagnostic System. In Encyclopedia of Child. Behavior and Development; Goldstein, S., Naglieri, J.A., Eds.; Springer: Boston, MA, USA, 2011; p. 707. [Google Scholar]
- McCandless, S.; O’Laughlin, L. The clinical utility of the behavior rating inventory of executive function (BRIEF) in the diagnosis of ADHD. J. Atten. Disord. 2007, 10, 381–389. [Google Scholar] [CrossRef] [PubMed]
- Gevensleben, H.; Holl, B.; Albrecht, B.; Vogel, C.; Schlamp, D.; Kratz, O.; Studer, P.; Rothenberger, A.; Moll, G.H.; Heinrich, H. Is neurofeedback an efficacious treatment for ADHD? A randomised controlled clinical trial. J. Child Psychol. Psychiatry 2009, 50, 780–789. [Google Scholar] [CrossRef] [PubMed]
- War, M.F. The wender Utah rating scale: An ais in the retrospective diagnosis of childhood attention deficit hyperactivity disorder. Am. J. Psychiatry 1993, 150, 885–890. [Google Scholar]
- Conners, C.K.; Sitarenios, G.; Parker, J.D.; Epstein, J.N. The revised Conners’ Parent Rating Scale (CPRS-R): Factor structure, reliability, and criterion validity. J. Abnorm. Child Psychol. 1998, 26, 257–268. [Google Scholar] [CrossRef] [PubMed]
- Hulvershorn, L.A.; Mennes, M.; Castellanos, F.X.; Di Martino, A.; Milham, M.P.; Hummer, T.A.; Roy, A.K. Abnormal amygdala functional connectivity associated with emotional lability in children with attention-deficit/hyperactivity disorder. J. Am. Acad. Child Adolesc. Psychiatry 2014, 53, 351–361. [Google Scholar] [CrossRef] [PubMed]
- Parikh, A.R.; Baker, S.E. Adult ADHD: Pharmacologic treatment in the DSM-5 era. Curr. Psychiatry 2016, 15, 18–25. [Google Scholar]
- Joel, L.; Young, M.; David, M.; Goodman, W. Adult attention-deficit/hyperactivity disorder diagnosis, management, and treatment in the DSM-5 Era. Prim Care Companion CNS Disord. 2016, 18. [Google Scholar] [CrossRef]
- Millichap, J.G.; Millichap, J.J. Biological markers in diagnosis of ADHD: EEG theta/beta ratio in diagnosis of ADHD. Pediatric Neurol. Briefs 2014, 28, 5–6. [Google Scholar] [CrossRef]
- Kux, L. Medical Devices; Neurological Devices; Classification of the Neuropsychiatric Interpretive Electroencephalograph Assessment Aid. In Federal Register 79 FR 9083; Food and Drug Administration Department of Health and Human Services: Washington, DC, USA, 2019; Volume 21, p. 3. [Google Scholar]
- Stone, L. De Novo Classification Request For Neuropsychiatric EEG-Based Assessment Aid For ADHD (NEBA) System. US FDA, 2001. Available online: https://www.fda.gov/ (accessed on 29 April 2021).
- Arns, M.; Keith, C.C.; Kraemer, H.C. A decade of EEG theta/beta ratio research in ADHD: A meta-analysis. J. Atten. Disord. 2011, 17, 374–383. [Google Scholar] [CrossRef]
- Lenartowicz, A.; Loo, S.K. Use of EEG to diagnose ADHD. Curr. Psychiatry Rep. 2014, 16, 498. [Google Scholar] [CrossRef]
- Clarke, A.R.; Barry, R.J.; McCarthy, R.; Selikowitz, M. Age and sex effects in the EEG: Differences in two subtypes of.attention-de®cit/hyperactivity disorder. Clin. Neurophysiol. 2001, 112, 815–826. [Google Scholar] [CrossRef]
- Chronis-Tuscano, A.; Molina, B.S.; Pelham, W.E.; Applegate, B.; Dahlke, A.; Overmyer, M.; Lahey, B.B. Very early predictors of adolescent depression and suicide attempts in children with attention-deficit/hyperactivity disorder. Arch. Gen. Psychiatry 2010, 67, 1044–1051. [Google Scholar] [CrossRef] [PubMed]
- Bagwell, C.L.; Molina, B.S.; Pelham, W.E., Jr.; Hoza, B. Attention-deficit hyperactivity disorder and problems in peer relations: Predictions from childhood to Adolescence. J. Am. Acad. Child Adolesc. Psychiatry 2001, 40, 1285–1292. [Google Scholar] [CrossRef]
- Johnston, C.; Mash, E.J. Families of children with attention-deficit/hyperactivity disorder: Review and recommendations for future research. Clin. Child Fam. Psychol. Rev. 2001, 3, 183–207. [Google Scholar] [CrossRef]
- Visser, L.; Linkersdörfer, J.; Hasselhorn, M. The role of ADHD symptoms in the relationship between academic achievement and psychopathological symptoms. Res.Dev. Disabil. 2020, 97, 103552. [Google Scholar] [CrossRef] [PubMed]
- Pelham, W.E., III; Page, T.F.; Altszuler, A.R.; Gnagy, E.M.; Molina, B.S.; Pelham, W.E., Jr. The long-term financial outcome of children diagnosed with ADHD. J. Consult. Clin. Psychol. 2020, 88, 160–171. [Google Scholar] [CrossRef]
- Mueller, A.K.; Fuermaier, A.B.; Koerts, J.; Tucha, L. Stigma in attention deficit hyperactivity disorder. Atten. Deficit Hyperact. Disord. 2012, 4, 101–114. [Google Scholar] [CrossRef]
- Martinez-Raga, J.; Ferreros, A.; Knecht, C.; de Alvaro, R.; Carabal, E. Attention-deficit hyperactivity disorder medication use: Factors involved in prescribing, safety aspects and outcomes. Ther. Adv. Drug Saf. 2017, 8, 87–99. [Google Scholar] [CrossRef]
- Wei, Y.J.J.; Zhu, Y.; Liu, W.; Bussing, R.; Winterstein, A.G. Prevalence of and factors associated with long-term concurrent use of stimulants and opioids among adults with attention-deficit/hyperactivity disorder. JAMA Netw. Open 2018, 1, e181152. [Google Scholar] [CrossRef]
- Humphreys, K.L.; Eng, T.; Lee, S.S. Stimulant medication and substance use outcomes: A meta-analysis. JAMA Psychiatry 2013, 70, 740–749. [Google Scholar] [CrossRef]
- Huss, M.; Chen, W.; Ludolph, A.G. Guanfacine extended release: A new pharmacological treatment option in Europe. Clin. Drug Investig. 2016, 36, 1–25. [Google Scholar] [CrossRef]
- Sultan, M.A.; Pastrana, C.S.; Pajer, K.A. Shared care models in the treatment of pediatric attention-deficit/hyperactivity disorder (ADHD): Are they effective? Health Serv. Res. Manag. Epidemiol. 2018, 5, 2333392818762886. [Google Scholar] [CrossRef]
- Spiel, C.F.; Evans, S.W.; Langberg, J.M. Evaluating the content of individualized education programs and 504 plans of young adolescents with attention deficit/hyperactivity disorder. Sch. Psychol. Q. 2014, 29, 452–468. [Google Scholar] [CrossRef]
- Tandon, M.; Tillman, R.; Agrawal, A.; Luby, J. Trajectories of ADHD severity over 10 years from childhood into adulthood. Atten. Deficit Hyperact. Disord. 2016, 8, 121–130. [Google Scholar] [CrossRef]
- Caye, A.; Swanson, J.; Thapar, A.; Sibley, M.; Arseneault, L.; Hechtman, L.; Arnold, L.E.; Niclasen, J.; Moffitt, T.; Rohde, L.A. Life span studies of ADHD-conceptual challenges and predictors of persistence and outcome. Curr. Psychiatry Rep. 2016, 18, 111. [Google Scholar] [CrossRef]
- Hamre, H.J.; Witt, C.M.; Kienle, G.S.; Meinecke, C.; Glockmann, A.; Ziegler, R.; Willich, S.N.; Kiene, H. Anthroposophic therapy for attention deficit hyperactivity: A two-year prospective study in outpatients. Int. J. Gen. Med. 2010, 3, 239–253. [Google Scholar] [CrossRef] [PubMed][Green Version]
- Majorek, M.; Tüchelmann, T.; Heusser, P. Therapeutic Eurythmy—Movement therapy for children with attention deficit hyperactivity disorder (ADHD): A pilot study. Complementary Ther. Nurs. Midwifery 2004, 10, 46–53. [Google Scholar] [CrossRef]
- Board, A.E. Inaccurate Gender Stereotypes Hindering Treatment. ADHD in Women 2017/04/17 2020/04/08. Available online: https://www.additudemag.com/inaccurate-gender-stereotypes-hindering-treatment/ (accessed on 29 April 2021).
- Broadbent, E. It’s (Always Been) a Scary Time for Women with ADHD. ADHD in Women 2019/05/24 2020/04/08. Available online: https://www.additudemag.com/adhd-gender-roles-shame/ (accessed on 29 April 2021).
- Bener, A.; Qahtani, R.A.; Abdelaal, I. The prevalence of ADHD among primary school children in an Arabian society. J. Atten. Disord. 2006, 10, 77–82. [Google Scholar] [CrossRef] [PubMed]
- Archer, D. The ADHD Advantage: What You Thought Was a Diagnosis May Be Your Greatest Strength; Penguin Random House: New York, NY, USA, 2015. [Google Scholar]
- Karunwi, O.; Wilson, A.N.; Kotanen, C.; Guiseppi-Elie, A. Engineering the abio-bio interface to enable more than moore in functional bioelectronics. J. Electrochem. Soc. 2013, 160, B60–B65. [Google Scholar] [CrossRef]
- Foreman, D.M. Attention deficit hyperactivity disorder: Legal and ethical aspects. Arch. Dis. Child. 2006, 91, 192–194. [Google Scholar] [CrossRef] [PubMed]
- Gaston v. District of Columbia. Available online: https://scholar.google.com/scholar_case?case=2584641615744859567&q=Gaston+v.+District+of+Columbia&hl=en&as_sdt=6,41&as_vis=1 (accessed on 29 April 2021).
- Kessler, Z. ADHD Diagnosis: Prison or Freedom Pass? ADHD from A to Zoe 2012 2012/08/31 2020/04/08. Available online: https://blogs.psychcentral.com/adhd-zoe/2012/08/adhd-diagnosis-prison-or-freedom-pass/ (accessed on 29 April 2021).
- Majid, A. Electroceuticals: Advances in Electrostimulation Therapies; SpringerNature: Basingstoke, UK, 2017; p. 346. [Google Scholar]
- Stuyver, T.; Danovich, D.; Joy, J.; Shaik, S. External electric field effects on chemical structure and reactivity. WIREs Comput. Mol. Sci. 2020, 10, e1438. [Google Scholar] [CrossRef]
- Abasi, S.; Aggas, J.R.; Venkatesh, N.; Vallavanatt, I.G.; Guiseppi-Elie, A. Design, fabrication and testing of an electrical cell stimulation and recording apparatus (ECSARA) for cells in electroculture. Biosens. Bioelectron. 2020, 147, 111793. [Google Scholar] [CrossRef]
- Wong, H.C.; Zaman, R. Neurostimulation in treating ADHD. Psychiatr. Danub. 2019, 31, 265–275. [Google Scholar] [PubMed]
- Roth, Y.; Amir, A.; Levkovitz, Y.; Zangen, A. Three-dimensional distribution of the electric field induced in the brain by transcranial magnetic stimulation using figure-8 and deep h-coils. J. Clin. Neurophysiol. 2007, 24, 31–38. [Google Scholar] [CrossRef] [PubMed]
- Bikson, M.; Esmaeilpour, Z.; Adair, D.; Kronberg, G.; Tyler, W.J.; Antal, A.; Datta, A.; Sabel, B.A.; Nitsche, M.A.; Loo, C.; et al. Transcranial electrical stimulation nomenclature. Brain Stimul. 2019, 12, 1349–1366. [Google Scholar] [CrossRef]
- Bush, G.; Frazier, J.A.; Rauch, S.L.; Seidman, L.J.; Whalen, P.J.; Jenike, M.A. Anterior cingulate cortex dysfunction in attention-deficit/hyperactivity disorder revealed by fMRI and the counting stroop. Biol. Psychiatry 1999, 45, 1542–1552. [Google Scholar] [CrossRef]
- Cook, I.A.; Espinoza, R.; Leuchter, A.F. Neuromodulation for depression: Invasive and noninvasive (deep brain stimulation, transcranial magnetic stimulation, trigeminal nerve stimulation). Neurosurg. Clin. N. Am. 2014, 25, 103–116. [Google Scholar] [CrossRef] [PubMed]
- Nickel, K.; Tebartz van Elst, L.; Manko, J.; Unterrainer, J.; Rauh, R.; Klein, C.; Endres, D.; Kaller, C.P.; Mader, I.; Riedel, A.; et al. Inferior frontal gyrus volume loss distinguishes between autism and (comorbid) attention-deficit/hyperactivity disorder—A freeSurfer analysis in children. Front. Psychiatry 2018, 9, 521. [Google Scholar]
- Blausen Medical. Medical Gallery of Blausen Medical 2014. WikiJ. Med. 2014, 1, 1–79. [Google Scholar]
- Degiorgio, C.; Cook, I.A.; Ekchian, L.; Neurosigma Inc. Extracranial Implantable Devices, Systems and Methods for the Treatment of Neurological Disorders. U.S. Patent Application No. 12/898,696, 17 February 2015. [Google Scholar]
- Wyrwich, K.W.; Shaffer, S.; Gries, K.; Auguste, P.; Mooney, K.H.; Prasad, S.; Bilder, D.A. Content validity of the ADHD rating scale (ADHD RS-IV) and adult ADHD self-report scale (ASRS) in Phenylketonuria. J. Inborn Errors Metab. Screen. 2016, 4, 2326409816639316. [Google Scholar]
- Rösler, M.; Retz, W.; Thome, J.; Schneider, M.; Stieglitz, R.D.; Falkai, P. Psychopathological rating scales for diagnostic use in adults with attention-deficit/hyperactivity disorder (ADHD). Eur. Arch. Psychiatry Clin.Neurosci. 2006, 256, i3–i11. [Google Scholar] [CrossRef] [PubMed]
- McGough, J.J.; Sturm, A.; Cowen, J.; Tung, K.; Salgari, G.C.; Leuchter, A.F.; Cook, I.A.; Sugar, C.A.; Loo, S.K. Double-blind, sham-controlled, pilot study of trigeminal nerve stimulation for attention-deficit/hyperactivity disorder. J. Am. Acad. Child Adolesc. Psychiatry 2019, 58, 403–411.e3. [Google Scholar]
- Loo, S.K.; Salgari, G.C.; Ellis, A.; Cowen, J.; Dillon, A.; McGough, J.J. Trigeminal nerve stimulation for attention-deficit/hyperactivity disorder: Cognitive and electroencephalographic predictors of treatment response. J. Am. Acad. Child Adolesc.Psychiatry 2020. [Google Scholar] [CrossRef] [PubMed]
- Shakour, E.; Badran, Y.; Shakour, G.; Shacour, R. Noninvasive Electric Brain Stimulation System. U.S. Patent Application No. 15/999,761, 19 August 2018. [Google Scholar]
- Sprich, S.E.; Burbridge, J.; Lerner, J.A.; Safren, S.A. Cognitive-behavioral therapy for ADHD in adolescents: Clinical considerations and a case series. Cogn. Behav. Pract. 2015, 22, 116–126. [Google Scholar] [CrossRef] [PubMed]
- Shrestha, M.; Lautenschleger, J.; Soares, N. Non-pharmacologic management of attention-deficit/hyperactivity disorder in children and adolescents: A review. Transl. Pediatrics 2020, 9, S114–S124. [Google Scholar] [CrossRef] [PubMed]
- Mueller, N. FDA Permits Marketing of First Game-Based Digital Therapeutic to Improve Attention Function in Children with ADHD; U.S. Food and Drug Administration: Washington, DC, USA, 2020. [Google Scholar]
- Davis, N.O.; Bower, J.; Kollins, S.H. Proof-of-concept study of an at-home, engaging, digital intervention for pediatric ADHD. PLoS ONE 2018, 13, e0189749. [Google Scholar] [CrossRef] [PubMed]
- Kollins, S.H.; DeLoss, D.J.; Cañadas, E.; Lutz, J.; Findling, R.L.; Keefe, R.S.; Epstein, J.N.; Cutler, A.J.; Faraone, S.V. A novel digital intervention for actively reducing severity of paediatric ADHD (STARS-ADHD): A randomised controlled trial. Lancet Digital Health 2020, 2, e168–e178. [Google Scholar] [CrossRef]
- Pandian, G.S.B.; Jain, A.; Raza, Q.; Sahu, K.K. Digital health interventions (DHI) for the treatment of attention deficit hyperactivity disorder (ADHD) in children—A comparative review of literature among various treatment and DHI. Psychiatry Res. 2021, 297, 113742. [Google Scholar] [CrossRef]
- Banaschewski, T.; Ruppert, S.; Tannock, R.; Albrecht, B.; Becker, A.; Uebel, H.; Sergeant, J.A.; Rothenberger, A. Colour perception in ADHD. J. Child Psychol. Psychiatry 2006, 47, 568–572. [Google Scholar] [CrossRef]
| Inattention Subtype | Hyperactivity-Impulsive Subtype |
|---|---|
| (1) Lack of close attention to details, or makes careless mistakes | (1) Restlessness, fidgeting or squirming in seat |
| (2) Struggle to maintain focus on tasks | (2) Often interrupts others |
| (3) Pattern of losing necessary objects | (3) Difficulty remaining still, remaining seated |
| (4) Does not seem to listen when addressed | (4) Often blurts out answers |
| (5) Difficulty keeping track of assignments/tasks | (5) Struggles to stay quiet, talks excessively |
| (6) Repeatedly avoids tasks that demand intellectual efforts | (6) Unable to stay on task |
| (7) Difficulty following through on instructions and/or finishing tasks | (7) Often active when and where it is not appropriate |
| (8) Difficulty organizing and/or prioritizing tasks and activities | (8) Difficulty with quietly participating in leisure activities |
| (9) Easily distractible | (9) Feel as though they are “always on the go” or “driven by a motor” |
| (10) Forgetful with daily tasks and activities | (10) Difficulty with waiting their turn |
Publisher’s Note: MDPI stays neutral with regard to jurisdictional claims in published maps and institutional affiliations. |
© 2021 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Camp, A.; Pastrano, A.; Gomez, V.; Stephenson, K.; Delatte, W.; Perez, B.; Syas, H.; Guiseppi-Elie, A. Understanding ADHD: Toward an Innovative Therapeutic Intervention. Bioengineering 2021, 8, 56. https://doi.org/10.3390/bioengineering8050056
Camp A, Pastrano A, Gomez V, Stephenson K, Delatte W, Perez B, Syas H, Guiseppi-Elie A. Understanding ADHD: Toward an Innovative Therapeutic Intervention. Bioengineering. 2021; 8(5):56. https://doi.org/10.3390/bioengineering8050056
Chicago/Turabian StyleCamp, Allyson, Amanda Pastrano, Valeria Gomez, Kathleen Stephenson, William Delatte, Brianna Perez, Hunter Syas, and Anthony Guiseppi-Elie. 2021. "Understanding ADHD: Toward an Innovative Therapeutic Intervention" Bioengineering 8, no. 5: 56. https://doi.org/10.3390/bioengineering8050056
APA StyleCamp, A., Pastrano, A., Gomez, V., Stephenson, K., Delatte, W., Perez, B., Syas, H., & Guiseppi-Elie, A. (2021). Understanding ADHD: Toward an Innovative Therapeutic Intervention. Bioengineering, 8(5), 56. https://doi.org/10.3390/bioengineering8050056








