CRISPR/Cas9-Based Lateral Flow and Fluorescence Diagnostics
Abstract
1. Introduction
2. Materials and Methods
2.1. Nucleic Acids
2.2. Amplification
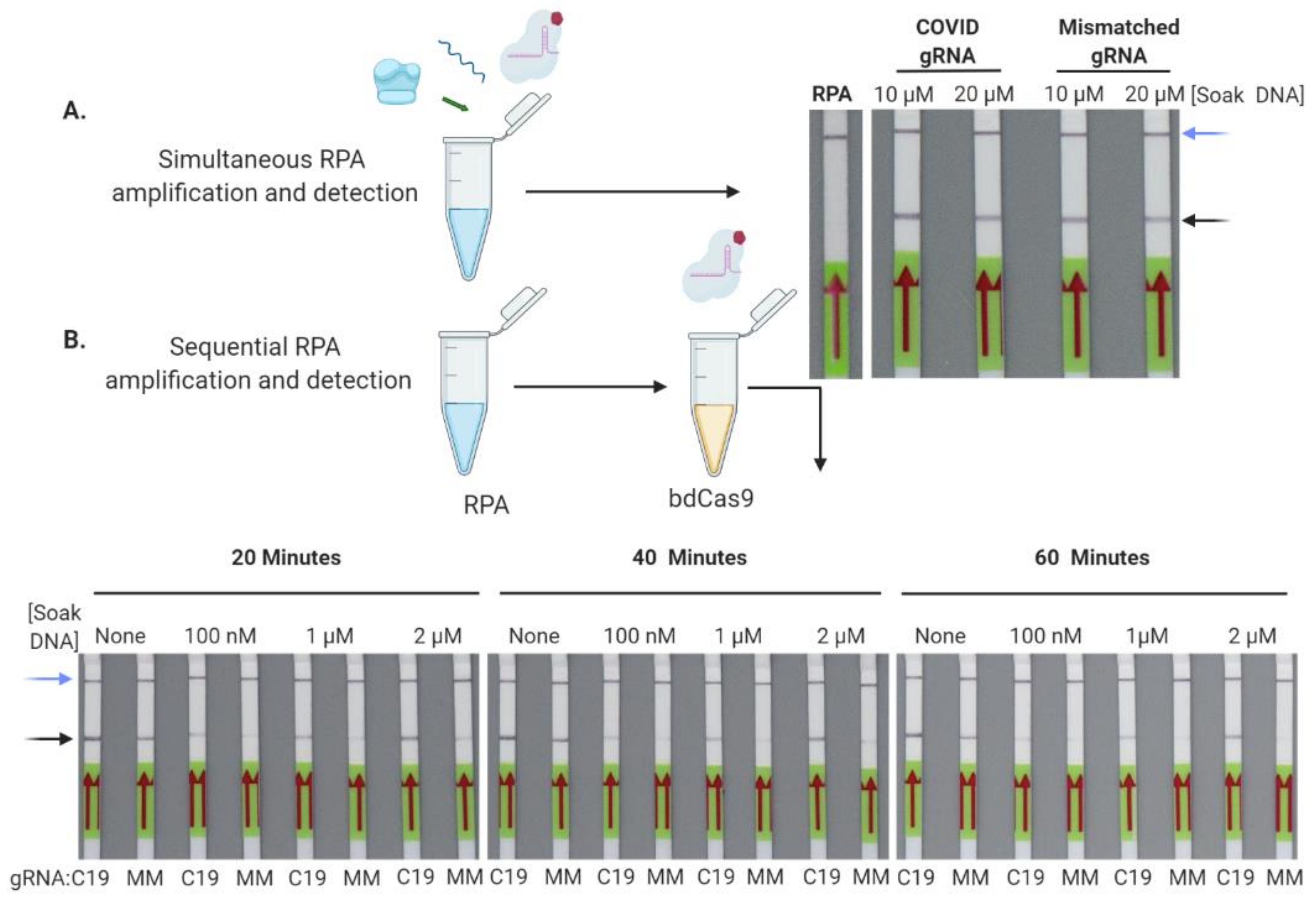
2.3. Soak DNA Oligonucleotides
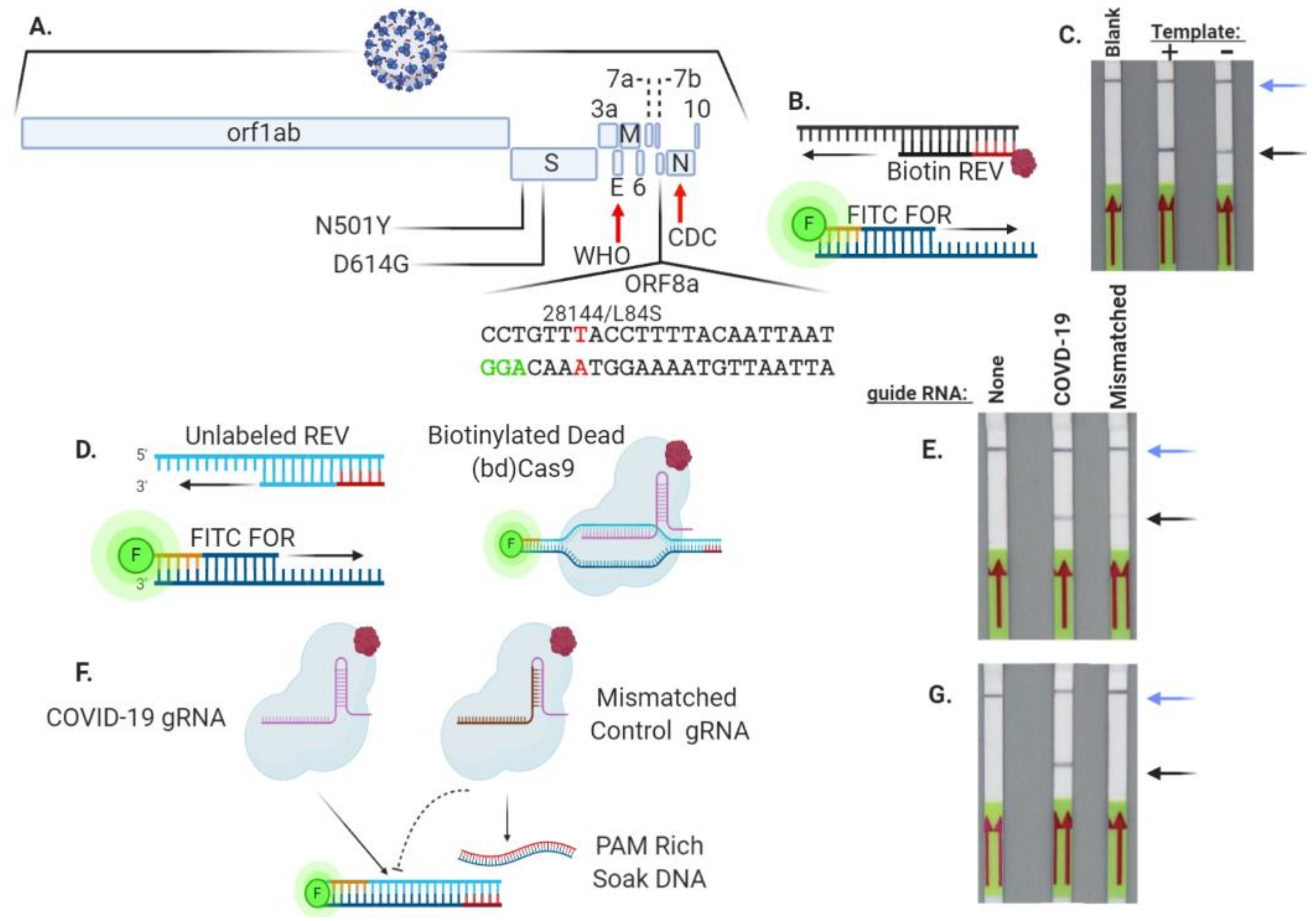
2.4. Guide RNAs
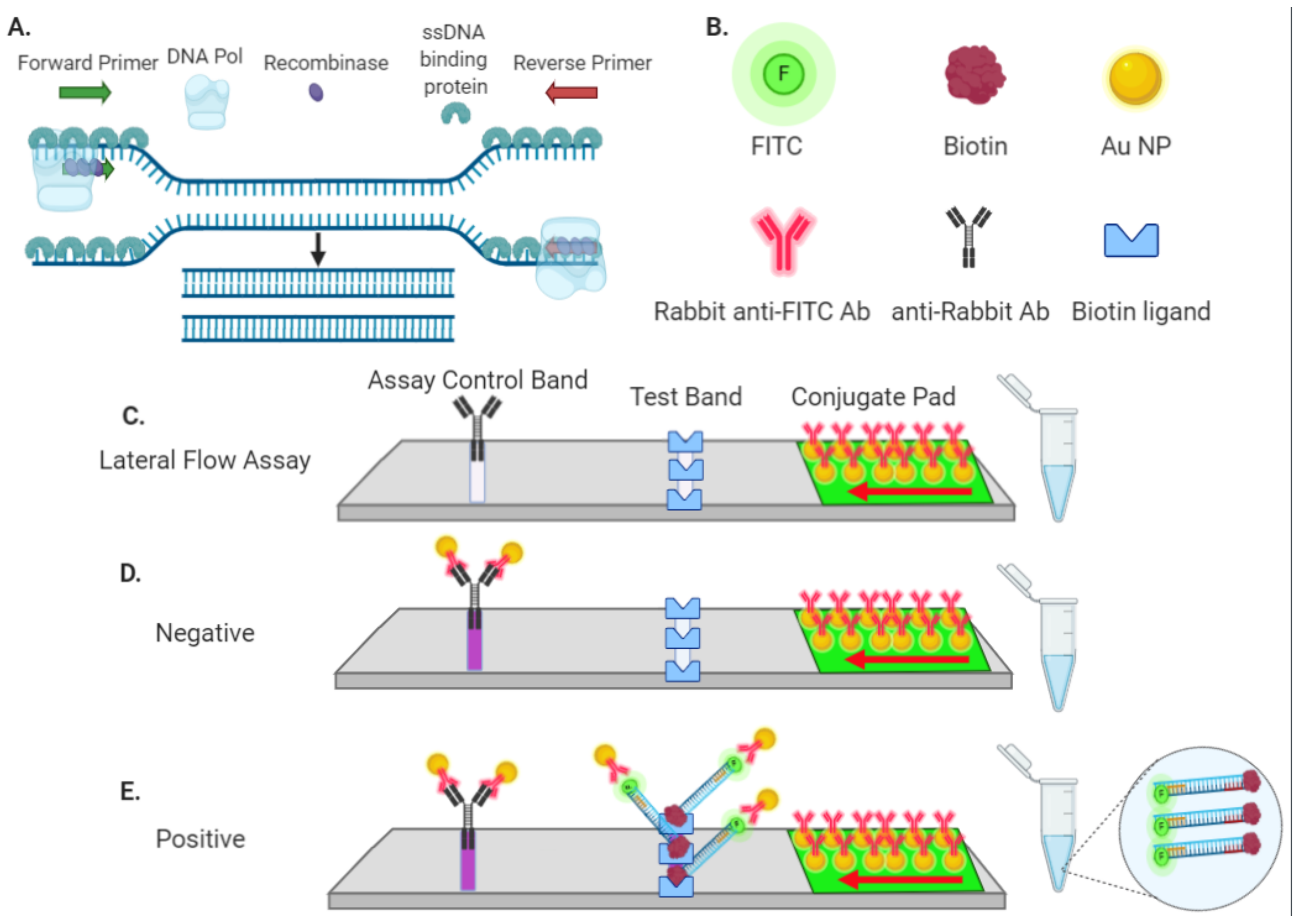
2.5. Biotinylated Cas9 and Lateral Flow
2.6. CRISPR/Cas9 Fluorescence Assay
2.7. RT-PCR
2.8. Genome Analysis
2.9. Graphing and Statistics
2.10. Images
3. Results
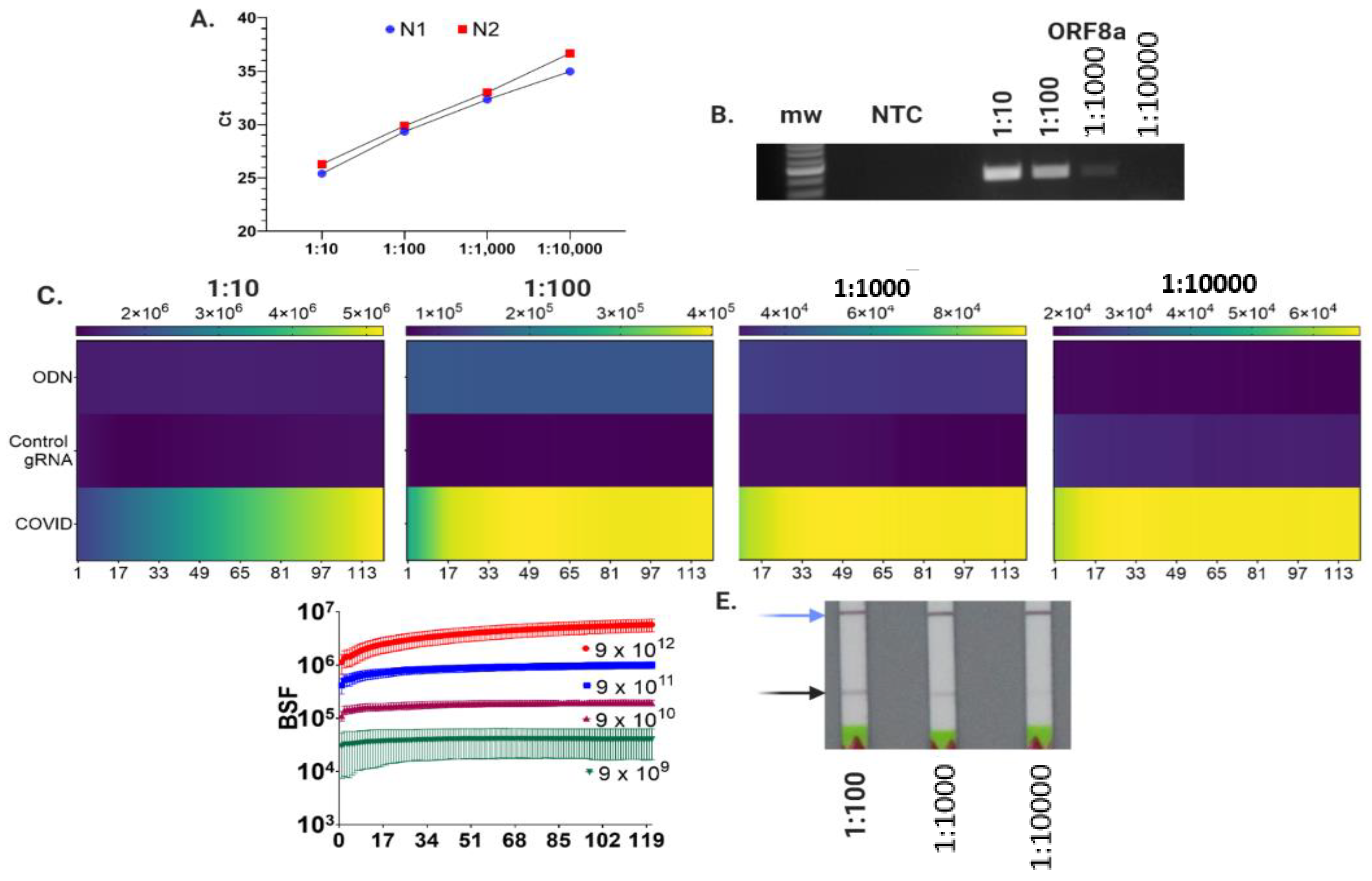
3.1. Rapid Nucleic Amplification and Lateral Flow Detection
3.2. Cas9 Allows for Specific SARS-Co-V2 ORF8a Sequence Detection
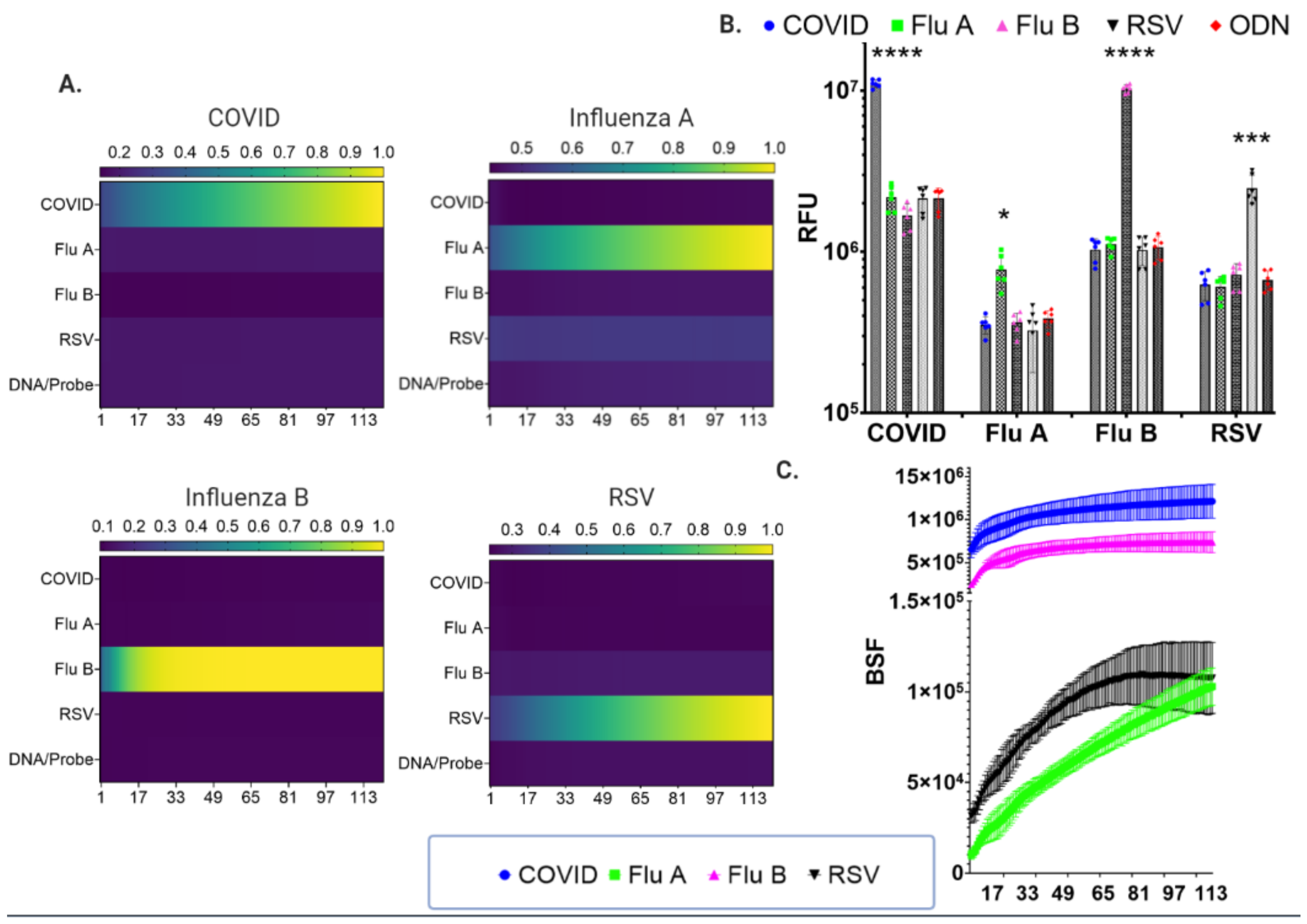
3.3. Cas9-Nuclease-Based Diagnostics for Single and Multiplexed Targets
3.4. LFA and Fluorescence Assay Validation with SARS-CoV-2 Genomic RNA
3.5. Cas9 Analysis of a SARS-CoV-2 Variant
4. Discussion
5. Conclusions
6. Patents
Supplementary Materials
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Data Availability Statement
Acknowledgments
Conflicts of Interest
References
- Liang, L.-L.; Tseng, C.-H.; Ho, H.J.; Wu, C.-Y. Covid-19 mortality is negatively associated with test number and government effectiveness. Sci. Rep. 2020, 10. [Google Scholar] [CrossRef] [PubMed]
- Zhang, W.; Du, R.H.; Li, B.; Zheng, X.S.; Yang, X.L.; Hu, B.; Wang, Y.Y.; Xiao, G.F.; Yan, B.; Shi, Z.L.; et al. Molecular and serological investigation of 2019-nCoV infected patients: Implication of multiple shedding routes. Emerg. Microbes Infect. 2020, 9, 386–389. [Google Scholar] [CrossRef]
- Usherwood, T.; Zhang, L.; Tripathi, A. The Path Forward for COVID-19 Diagnostics. Mol. Diagn. 2020, 24, 637–639. [Google Scholar] [CrossRef] [PubMed]
- Pray, I.W.; Ford, L.; Cole, D.; Lee, C.; Bigouette, J.P.; Abedi, G.R.; Bushman, D.; Delahoy, M.J.; Currie, D.; Cherney, B.; et al. Performance of an Antigen-Based Test for Asymptomatic and Symptomatic SARS-CoV-2 Testing at Two University Campuses - Wisconsin, September-October 2020. MMWR Morb. Mortal. Wkly. Rep. 2021, 69, 1642–1647. [Google Scholar] [CrossRef]
- Esbin, M.N.; Whitney, O.N.; Chong, S.; Maurer, A.; Darzacq, X.; Tjian, R. Overcoming the bottleneck to widespread testing: A rapid review of nucleic acid testing approaches for COVID-19 detection. RNA 2020, 26, 771–783. [Google Scholar] [CrossRef] [PubMed]
- Lobato, I.M.; O’Sullivan, C.K. Recombinase polymerase amplification: Basics, applications and recent advances. Trends Anal. Chem. 2018, 98, 19–35. [Google Scholar] [CrossRef] [PubMed]
- Wong, Y.P.; Othman, S.; Lau, Y.L.; Radu, S.; Chee, H.Y. Loop-mediated isothermal amplification (LAMP): A versatile technique for detection of micro-organisms. J. Appl. Microbiol. 2018, 124, 626–643. [Google Scholar] [CrossRef]
- Joung, J.; Ladha, A.; Saito, M.; Kim, N.G.; Woolley, A.E.; Segel, M.; Barretto, R.P.J.; Ranu, A.; Macrae, R.K.; Faure, G.; et al. Detection of SARS-CoV-2 with SHERLOCK One-Pot Testing. N. Engl. J. Med. 2020, 383, 1492–1494. [Google Scholar] [CrossRef] [PubMed]
- Joung, J.; Ladha, A.; Saito, M.; Segel, M.; Bruneau, R.; Huang, M.W.; Kim, N.G.; Yu, X.; Li, J.; Walker, B.D.; et al. Point-of-care testing for COVID-19 using SHERLOCK diagnostics. medRxiv 2020. [Google Scholar] [CrossRef]
- Kellner, M.J.; Koob, J.G.; Gootenberg, J.S.; Abudayyeh, O.O.; Zhang, F. SHERLOCK: Nucleic acid detection with CRISPR nucleases. Nat. Protoc. 2019, 14, 2986–3012. [Google Scholar] [CrossRef] [PubMed]
- Baerwald, M.R.; Goodbla, A.M.; Nagarajan, R.P.; Gootenberg, J.S.; Abudayyeh, O.O.; Zhang, F.; Schreier, A.D. Rapid and accurate species identification for ecological studies and monitoring using CRISPR-based SHERLOCK. Mol. Ecol. Resour. 2020, 20, 961–970. [Google Scholar] [CrossRef]
- Petri, K.; Pattanayak, V. SHERLOCK and DETECTR Open a New Frontier in Molecular Diagnostics. Cris. J. 2018, 1, 209–211. [Google Scholar] [CrossRef]
- Chen, J.S.; Ma, E.; Harrington, L.B.; Da Costa, M.; Tian, X.; Palefsky, J.M.; Doudna, J.A. CRISPR-Cas12a target binding unleashes indiscriminate single-stranded DNase activity. Science 2018, 360, 436–439. [Google Scholar] [CrossRef] [PubMed]
- Hajian, R.; Balderston, S.; Tran, T.; deBoer, T.; Etienne, J.; Sandhu, M.; Wauford, N.A.; Chung, J.Y.; Nokes, J.; Athaiya, M.; et al. Detection of unamplified target genes via CRISPR-Cas9 immobilized on a graphene field-effect transistor. Nat. Biomed. Eng. 2019, 3, 427–437. [Google Scholar] [CrossRef]
- Lee, H.; Choi, J.; Jeong, E.; Baek, S.; Kim, H.C.; Chae, J.H.; Koh, Y.; Seo, S.W.; Kim, J.S.; Kim, S.J. dCas9-mediated Nanoelectrokinetic Direct Detection of Target Gene for Liquid Biopsy. Nano Lett. 2018, 18, 7642–7650. [Google Scholar] [CrossRef] [PubMed]
- Yang, W.; Restrepo-Perez, L.; Bengtson, M.; Heerema, S.J.; Birnie, A.; van der Torre, J.; Dekker, C. Detection of CRISPR-dCas9 on DNA with Solid-State Nanopores. Nano Lett. 2018, 18, 6469–6474. [Google Scholar] [CrossRef]
- Ramachandran, A.; Huyke, D.A.; Sharma, E.; Sahoo, M.K.; Huang, C.; Banaei, N.; Pinsky, B.A.; Santiago, J.G. Electric field-driven microfluidics for rapid CRISPR-based diagnostics and its application to detection of SARS-CoV-2. Proc. Natl. Acad. Sci. USA 2020, 117, 29518–29525. [Google Scholar] [CrossRef]
- Wang, X.; Xiong, E.; Tian, T.; Cheng, M.; Lin, W.; Wang, H.; Zhang, G.; Sun, J.; Zhou, X. Clustered Regularly Interspaced Short Palindromic Repeats/Cas9-Mediated Lateral Flow Nucleic Acid Assay. ACS Nano 2020, 14, 2497–2508. [Google Scholar] [CrossRef] [PubMed]
- Pardee, K.; Green, A.A.; Takahashi, M.K.; Braff, D.; Lambert, G.; Lee, J.W.; Ferrante, T.; Ma, D.; Donghia, N.; Fan, M.; et al. Rapid, Low-Cost Detection of Zika Virus Using Programmable Biomolecular Components. Cell 2016, 165, 1255–1266. [Google Scholar] [CrossRef] [PubMed]
- Huang, M.; Zhou, X.; Wang, H.; Xing, D. Clustered Regularly Interspaced Short Palindromic Repeats/Cas9 Triggered Isothermal Amplification for Site-Specific Nucleic Acid Detection. Anal. Chem. 2018, 90, 2193–2200. [Google Scholar] [CrossRef] [PubMed]
- Guk, K.; Keem, J.O.; Hwang, S.G.; Kim, H.; Kang, T.; Lim, E.K.; Jung, J. A facile, rapid and sensitive detection of MRSA using a CRISPR-mediated DNA FISH method, antibody-like dCas9/sgRNA complex. Biosens. Bioelectron. 2017, 95, 67–71. [Google Scholar] [CrossRef]
- Wang, T.; Liu, Y.; Sun, H.H.; Yin, B.C.; Ye, B.C. An RNA-Guided Cas9 Nickase-Based Method for Universal Isothermal DNA Amplification. Angew Chem. Int. Ed. Engl. 2019, 58, 5382–5386. [Google Scholar] [CrossRef]
- Zhang, Y.; Qian, L.; Wei, W.; Wang, Y.; Wang, B.; Lin, P.; Liu, W.; Xu, L.; Li, X.; Liu, D.; et al. Paired Design of dCas9 as a Systematic Platform for the Detection of Featured Nucleic Acid Sequences in Pathogenic Strains. ACS Synth. Biol. 2017, 6, 211–216. [Google Scholar] [CrossRef] [PubMed]
- Zhou, W.; Hu, L.; Ying, L.; Zhao, Z.; Chu, P.K.; Yu, X.F. A CRISPR-Cas9-triggered strand displacement amplification method for ultrasensitive DNA detection. Nat. Commun. 2018, 9, 5012. [Google Scholar] [CrossRef]
- Koczula, K.M.; Gallotta, A. Lateral flow assays. Essays Biochem. 2016, 60, 111–120. [Google Scholar] [CrossRef]
- Qin, P.; Park, M.; Alfson, K.J.; Tamhankar, M.; Carrion, R.; Patterson, J.L.; Griffiths, A.; He, Q.; Yildiz, A.; Mathies, R.; et al. Rapid and Fully Microfluidic Ebola Virus Detection with CRISPR-Cas13a. ACS Sens. 2019; 4, 1048–1054. [Google Scholar] [CrossRef]
- Broughton, J.P.; Deng, X.; Yu, G.; Fasching, C.L.; Servellita, V.; Singh, J.; Miao, X.; Streithorst, J.A.; Granados, A.; Sotomayor-Gonzalez, A.; et al. CRISPR-Cas12-based detection of SARS-CoV-2. Nat. Biotechnol. 2020, 38, 870–874. [Google Scholar] [CrossRef] [PubMed]
- Huang, Z.; Tian, D.; Liu, Y.; Lin, Z.; Lyon, C.J.; Lai, W.; Fusco, D.; Drouin, A.; Yin, X.; Hu, T.; et al. Ultra-sensitive and high-throughput CRISPR-p owered COVID-19 diagnosis. Biosens. Bioelectron. 2020, 164, 112316. [Google Scholar] [CrossRef] [PubMed]
- Flower, T.G.; Buffalo, C.Z.; Hooy, R.M.; Allaire, M.; Ren, X.; Hurley, J.H. Structure of SARS-CoV-2 ORF8, a rapidly evolving coronavirus protein implicated in immune evasion. bioRxiv 2020. [Google Scholar] [CrossRef]
- Wang, R.; Chen, J.; Gao, K.; Hozumi, Y.; Yin, C.; Wei, G. Characterizing SARS-CoV-2 mutations in the United States. Res. Sq. 2020. [Google Scholar] [CrossRef]
- Huang, X.; Aguilar, Z.P.; Xu, H.; Lai, W.; Xiong, Y. Membrane-based lateral flow immunochromatographic strip with nanoparticles as reporters for detection: A review. Biosens. Bioelectron. 2016, 75, 166–180. [Google Scholar] [CrossRef]
- Mekler, V.; Minakhin, L.; Severinov, K. Mechanism of duplex DNA destabilization by RNA-guided Cas9 nuclease during target interrogation. Proc. Natl. Acad. Sci. USA 2017, 114, 5443–5448. [Google Scholar] [CrossRef]
- Plante, J.A.; Liu, Y.; Liu, J.; Xia, H.; Johnson, B.A.; Lokugamage, K.G.; Zhang, X.; Muruato, A.E.; Zou, J.; Fontes-Garfias, C.R.; et al. Spike mutation D614G alters SARS-CoV-2 fitness. Nature 2020. [Google Scholar] [CrossRef]
- Zhang, L.; Yang, J.-R.; Zhang, Z.; Lin, Z. Genomic variations of SARS-CoV-2 suggest multiple outbreak sources of transmission. MedRxiv 2020. [Google Scholar] [CrossRef]
- Hou, Y.J.; Chiba, S.; Halfmann, P.; Ehre, C.; Kuroda, M.; Dinnon, K.H., 3rd; Leist, S.R.; Schafer, A.; Nakajima, N.; Takahashi, K.; et al. SARS-CoV-2 D614G variant exhibits efficient replication ex vivo and transmission in vivo. Science 2020. [Google Scholar] [CrossRef] [PubMed]
- Tang, J.W.; Toovey, O.T.R.; Harvey, K.N.; Hui, D.D.S. Introduction of the South African SARS-CoV-2 variant 501Y.V2 into the UK. J. Infect. 2021. [Google Scholar] [CrossRef] [PubMed]
- Vakulskas, C.A.; Dever, D.P.; Rettig, G.R.; Turk, R.; Jacobi, A.M.; Collingwood, M.A.; Bode, N.M.; McNeill, M.S.; Yan, S.; Camarena, J.; et al. A high-fidelity Cas9 mutant delivered as a ribonucleoprotein complex enables efficient gene editing in human hematopoietic stem and progenitor cells. Nat. Med. 2018, 24, 1216–1224. [Google Scholar] [CrossRef] [PubMed]
- Paul, R.; Arif, A.A.; Adeyemi, O.; Ghosh, S.; Han, D. Progression of COVID-19 From Urban to Rural Areas in the United States: A Spatiotemporal Analysis of Prevalence Rates. J. Rural Health 2020, 36, 591–601. [Google Scholar] [CrossRef]
- Kaufman, B.G.; Whitaker, R.; Pink, G.; Holmes, G.M. Half of Rural Residents at High Risk of Serious Illness Due to COVID-19, Creating Stress on Rural Hospitals. J. Rural Health 2020, 36, 584–590. [Google Scholar] [CrossRef] [PubMed]
- Davoodi, N.M.; Healy, M.; Goldberg, E.M. Rural America’s Hospitals are Not Prepared to Protect Older Adults From a Surge in COVID-19 Cases. Gerontol. Geriatr. Med. 2020, 6. [Google Scholar] [CrossRef]
- Souch, J.M.; Cossman, J.S. A Commentary on Rural-Urban Disparities in COVID-19 Testing Rates per 100,000 and Risk Factors. J. Rural Health 2020. [Google Scholar] [CrossRef]
- Nickbakhsh, S.; Ho, A.; Marques, D.F.P.; McMenamin, J.; Gunson, R.N.; Murcia, P.R. Epidemiology of Seasonal Coronaviruses: Establishing the Context for the Emergence of Coronavirus Disease 2019. J. Infect. Dis. 2020, 222, 17–25. [Google Scholar] [CrossRef]
- Ivanov, I.E.; Wright, A.V.; Cofsky, J.C.; Aris, K.D.P.; Doudna, J.A.; Bryant, Z. Cas9 interrogates DNA in discrete steps modulated by mismatches and supercoiling. Proc. Natl. Acad. Sci. USA 2020, 117, 5853–5860. [Google Scholar] [CrossRef]
- Jones, D.L.; Leroy, P.; Unoson, C.; Fange, D.; Curic, V.; Lawson, M.J.; Elf, J. Kinetics of dCas9 target search in Escherichia coli. Science 2017, 357, 1420–1424. [Google Scholar] [CrossRef] [PubMed]
- Woo, C.H.; Jang, S.; Shin, G.; Jung, G.Y.; Lee, J.W. Sensitive fluorescence detection of SARS-CoV-2 RNA in clinical samples via one-pot isothermal ligation and transcription. Nat. Biomed. Eng. 2020, 4, 1168–1179. [Google Scholar] [CrossRef]
- Wu, X.; Cai, Y.; Huang, X.; Yu, X.; Zhao, L.; Wang, F.; Li, Q.; Gu, S.; Xu, T.; Li, Y.; et al. Co-infection with SARS-CoV-2 and Influenza A Virus in Patient with Pneumonia, China. Emerg Infect. Dis. 2020, 26, 1324–1326. [Google Scholar] [CrossRef] [PubMed]
- Waggoner, J.J.; Stittleburg, V.; Pond, R.; Saklawi, Y.; Sahoo, M.K.; Babiker, A.; Hussaini, L.; Kraft, C.S.; Pinsky, B.A.; Anderson, E.J.; et al. Triplex Real-Time RT-PCR for Severe Acute Respiratory Syndrome Coronavirus 2. Emerg Infect. Dis. 2020, 26, 1633–1635. [Google Scholar] [CrossRef]
- Knight, S.C.; Xie, L.; Deng, W.; Guglielmi, B.; Witkowsky, L.B.; Bosanac, L.; Zhang, E.T.; El Beheiry, M.; Masson, J.B.; Dahan, M.; et al. Dynamics of CRISPR-Cas9 genome interrogation in living cells. Science 2015, 350, 823–826. [Google Scholar] [CrossRef] [PubMed]
- Lu, X.; Wang, L.; Sakthivel, S.K.; Whitaker, B.; Murray, J.; Kamili, S.; Lynch, B.; Malapati, L.; Burke, S.A.; Harcourt, J.; et al. US CDC Real-Time Reverse Transcription PCR Panel for Detection of Severe Acute Respiratory Syndrome Coronavirus 2. Emerg Infect. Dis. 2020, 26. [Google Scholar] [CrossRef]
- Singanayagam, A.; Patel, M.; Charlett, A.; Lopez Bernal, J.; Saliba, V.; Ellis, J.; Ladhani, S.; Zambon, M.; Gopal, R. Duration of infectiousness and correlation with RT-PCR cycle threshold values in cases of COVID-19, England, January to May 2020. Euro Surveill. 2020, 25. [Google Scholar] [CrossRef]
- McNamara, R.P.; Caro-Vegas, C.; Landis, J.T.; Moorad, R.; Pluta, L.J.; Eason, A.B.; Thompson, C.; Bailey, A.; Villamor, F.C.S.; Lange, P.T.; et al. High-Density Amplicon Sequencing Identifies Community Spread and Ongoing Evolution of SARS-CoV-2 in the Southern United States. Cell Rep. 2020, 33, 108352. [Google Scholar] [CrossRef] [PubMed]
- Sevajol, M.; Subissi, L.; Decroly, E.; Canard, B.; Imbert, I. Insights into RNA synthesis, capping, and proofreading mechanisms of SARS-coronavirus. Virus Res. 2014, 194, 90–99. [Google Scholar] [CrossRef]
- Pereira, F. SARS-CoV-2 variants lacking a functional ORF8 may reduce accuracy of serological testing. J. Immunol Methods 2021, 488, 112906. [Google Scholar] [CrossRef]
- Gralinski, L.E.; Menachery, V.D. Return of the Coronavirus: 2019-nCoV. Viruses 2020, 12, 135. [Google Scholar] [CrossRef]
- Wu, X.; Scott, D.A.; Kriz, A.J.; Chiu, A.C.; Hsu, P.D.; Dadon, D.B.; Cheng, A.W.; Trevino, A.E.; Konermann, S.; Chen, S.; et al. Genome-wide binding of the CRISPR endonuclease Cas9 in mammalian cells. Nat. Biotechnol. 2014, 32, 670–676. [Google Scholar] [CrossRef]
- Jinek, M.; Chylinski, K.; Fonfara, I.; Hauer, M.; Doudna, J.A.; Charpentier, E. A programmable dual-RNA-guided DNA endonuclease in adaptive bacterial immunity. Science 2012, 337, 816–821. [Google Scholar] [CrossRef]
- Sternberg, S.H.; Redding, S.; Jinek, M.; Greene, E.C.; Doudna, J.A. DNA interrogation by the CRISPR RNA-guided endonuclease Cas9. Nature 2014, 507, 62–67. [Google Scholar] [CrossRef] [PubMed]
- Cong, L.; Ran, F.A.; Cox, D.; Lin, S.; Barretto, R.; Habib, N.; Hsu, P.D.; Wu, X.; Jiang, W.; Marraffini, L.A.; et al. Multiplex genome engineering using CRISPR/Cas systems. Science 2013, 229, 819–823. [Google Scholar] [CrossRef]
- Jiang, W.; Bikard, D.; Cox, D.; Zhang, F.; Marraffini, L.A. RNA-guided editing of bacterial genomes using CRISPR-Cas systems. Nat. Biotechnol. 2013, 31, 233–239. [Google Scholar] [CrossRef] [PubMed]
- Laamarti, M.; Alouane, T.; Kartti, S.; Chemao-Elfihri, M.W.; Hakmi, M.; Essabbar, A.; Laamarti, M.; Hlali, H.; Bendani, H.; Boumajdi, N.; et al. Large scale genomic analysis of 3067 SARS-CoV-2 genomes reveals a clonal geo-distribution and a rich genetic variations of hotspots mutations. PLoS ONE 2020, 15, e0240345. [Google Scholar] [CrossRef] [PubMed]
- Mitani, Y.; Lezhava, A.; Kawai, Y.; Kikuchi, T.; Oguchi-Katayama, A.; Kogo, Y.; Itoh, M.; Miyagi, T.; Takakura, H.; Hoshi, K.; et al. Rapid SNP diagnostics using asymmetric isothermal amplification and a new mismatch-suppression technology. Nat. Methods 2007, 4, 257–262. [Google Scholar] [CrossRef]
- Kilic, T.; Weissleder, R.; Lee, H. Molecular and Immunological Diagnostic Tests of COVID-19: Current Status and Challenges. iScience 2020, 23, 101406. [Google Scholar] [CrossRef] [PubMed]
- Whitman, J.D.; Hiatt, J.; Mowery, C.T.; Shy, B.R.; Yu, R.; Yamamoto, T.N.; Rathore, U.; Goldgof, G.M.; Whitty, C.; Woo, J.M.; et al. Evaluation of SARS-CoV-2 serology assays reveals a range of test performance. Nat. Biotechnol. 2020, 38, 1174–1183. [Google Scholar] [CrossRef] [PubMed]

| Identification | Sequence (5′-3′) | Function |
|---|---|---|
| SARS-Co-V2 FAM Forward | /56-FAM/GAATTGTGCGTGGATGAGGCTGG | FAM/FITC-labeled forward primer for SARS-Co-V2 amplification from synthetic or viral genome templates. |
| SARS-Co-V2 Biotinylated Reverse | /5Biosg/CAACACGAACGTCATGATACTC | Biotinylated-labeled reverse primer for SARS-Co-V2 amplification from synthetic template. |
| SARS-Co-V2 Unlabeled Reverse | CAACACGAACGTCATGATACTC | Reverse primer for SARS-Co-V2 amplification from synthetic template. |
| SARS-Co-V2 Genome Reverse | TTAGATGAAATCTAAAACAACACG | Reverse primer for SARS-Co-V2 amplification from genomic RNA template. |
| SARS-Co-V2 T SNP DNA PCR template. | GAATTGTGCGTGGATGAG GCTGGTTCTAAATCACCCA TTCAGTACATCGATATCGGT AATTATACAGTTTCCTGTTTA CCTTTTACAATTAATTGCCAG GAACCTAAATTGGGTAGTCTT GTAGTGCGTTGTTCGTTCTATG AAGACTTTTTAGAGTATCATG ACGTTCGTGTTG | Synthetic SARS-CoV-2 PCR template containing a thymine SNP at position 28144. |
| SARS-Co-V2 C SNP DNA PCR template. | GAATTGTGCGTGGATGAG GCTGGTTCTAAATCACCC ATTCAGTACATCGATATC GGTAATTATACAGTTTCC TGTTCACCTTTTACAATTA ATTGCCAGGAACCTAAAT TGGGTAGTCTTGTAGTGCG TTGTTCGTTCTATGAAGACT TTTTAGAGTATCATGACGTTCGTGTTG | Synthetic SARS-CoV-2 PCR template containing a cytosine SNP at position 28144. |
| Influenza A Forward | CAAGACCAATCYTGTCACCTCTGAC | Forward primer for Influenza A amplification from synthetic DNA. |
| Influenza A Reverse | GCATTYTGGACAAAVCGTCTACG | Reverse primer for Influenza A amplification from synthetic DNA. |
| Synthetic Influenza A DNA PCR template | GCTCTCATGGAATGGCTAAAGACA AGACCAATCTTGTCACCTCTGACTA AGGGGATTTTAGGATTTGTGTTCAC GCTCACCGTGCCCAGTGAGCGAGG ACTGCAGCGTAGACGCTTTGTCCAA AATGCCCTAAATGGGAATGGGGAC CCGAACAACATGG | Synthetic PCR template to generate Influenza A DNA amplicons. |
| Influenza B Forward | TCCTCAAYTCACTCTTCGAGCG | Forward primer for Influenza B amplification from synthetic DNA. |
| Influenza B Reverse | CGGTGCTCTTGACCAAATTGG | Reverse primer for Influenza B amplification from synthetic DNA. |
| Synthetic Influenza B DNA PCR template | TACAGTGGAGGATGAAGAAGATGG CCATCGGATCCTCAATTCACTCTTC GAGCGTCTTAATGAAGGACATTCA AAGCCAATTCGAGCAGCTGAAACT GCGGTGGGAGTCTTATCCCAATTTG GTCAAGAGCACCGACTATCACCAG AAGAGGGAGACAAT | Synthetic PCR template to generate Influenza B DNA amplicons. |
| RSV Forward | GGCAAATATGGAAACATACGTGAA | Forward primer for RSV amplification from synthetic DNA. |
| RSV Reverse | CATGGGCACCCATATTGTAA | Reverse primer for RSV from synthetic DNA. |
| Synthetic RSV DNA PCR template | GGCAAATATGGAAACATACGTG AACAAGCTTCACGAAGGCTCCA CATACACAGCTGCTGTTCAATAC AATGTCCTAGAAAAAGACGATG ACCCTGCATCACTTACAATATGG GTGCCCATGTTCCAATCA | Synthetic PCR template to generate RSV DNA amplicons. |
| Identification | Sequence (5′-3′) | Function |
|---|---|---|
| COVID T Probe: | /56-FAM/TCCTGTTTACCTTTTA CAATTAATTGCCAGGA/3IABkFQ/ | FAM fluorescent probe with Iowa Black quencher that recognizes SARS-CoV-2 with a thymine at position 28144. |
| COVID C Probe: | /56-FAM/TCCTGTTCACCTTTTA CAATTAATTGCCAGGA/3IABkFQ | FAM fluorescent probe with Iowa Black quencher that recognizes SARS-CoV-2 with a cytosine at position 28144. |
| Influenza A Probe: | /5TexRd-XN/CAGTCCTCGCTCACT GGGCACGGTGAGCGTGA/3IAbRQSp/ | Texas Red fluorescent probe with Iowa Black quencher that recognizes Influenza A sequence. |
| Influenza B Probe: | /5YAkYel/TCCCACCGCAGTTTCAG CTGCTCGAATTGGCT/3IABkFQ/ | Yakima Yellow fluorescent probe with Iowa Black quencher that recognizes Influenza B sequence. |
| RSV Probe: | /5Cy3/GCTCCACATACACAGCTG CTGTTCAATACAAT/3IAbRQSp/ | Cy3 fluorescent probe with Iowa Black quencher that recognizes RSV sequence. |
| RSV Probe: | /56-TAMN/GCTCCACATACACAGCTG CTGTTCAATACAAT/3IAbRQSp/ | TAMRA fluorescent probe with Iowa Black quencher that recognizes RSV sequence. |
| Identification | Sequence (5′-3′) | Function |
|---|---|---|
| PAM Soak Forward: | CGGGAGGGTGGGCGGGAGGGTGGG CGGGAGGGTGGGCGGGAGGGTGGG | PAM-rich ODN that acts as a bait for promiscuous Cas9 binding; sense/top/forward/strand. |
| PAM Soak Reverse: | CCCACCCTCCCGCCCACCCTCCCG CCCACCCTCCCGCCCACCCTCCCG | PAM-rich ODN that acts as a bait for promiscuous Cas9 binding; anti-sense/bottom/reverse/strand. |
| C Soak Forward: | GGAGGGTGGGGATTAATTGTAAA AGGTGAACGGGCGGGAGGGTGG | Bait ODN that contains SARS-CoV-2 sequences and a cytosine at position 28144; sense/top/forward/strand. In yellow is the anti-parralel guanine. |
| C Soak Reverse: | CCACCCTCCCGCCCGTTCACCTTT TACAATTAATCCCCACCCTCC | Bait ODN that contains SARS-CoV-2 sequences and a cytosine at position 28144; anti-sense/bottom/reverse/strand. |
| T Soak Forward: | GGAGGGTGGGGATTAATTGTAAA AGGTAAACGGGCGGGAGGGTGG | Bait ODN that contains SARS-CoV-2 sequences and a thymine at position 28144; sense/top/forward/strand. In yellow is the anti-parralel adenine. |
| T Soak Reverse: | CCACCCTCCCGCCCGTTTACCTTT TACAATTAATCCCCACCCTCC | Bait ODN that contains SARS-CoV-2 sequences and a thymine at position 28144; anti-sense/bottom/reverse/strand. |
| Identification. | Sequence (5′-3′) | Function |
|---|---|---|
| COVID-19 T sgRNA: | AUUAAUUGUAAAAGGUAAAC | Recognizes ORF8a that has a thymine at nucelotide position 28144. |
| COVID-19 C sgRNA: | AUUAAUUGUAAAAGGUGAAC | Recognizes ORF8a that has a cytosine at nucelotide position 28144. |
| Influenza A sgRNA: | CUCACCGUGCCCAGUGAGCG | Recognizes the influenza A amplicon. |
| Influenza B sgRNA: | AAUUCGAGCAGCUGAAACUG | Recognizes the influenza B amplicon. |
| RSV sgRNA: | UUGAACAGCAGCUGUGUAUG | Recognizes the influenza RSV amplicon. |
| Control sgRNA: | CACUGGCUGUCGCUUCUCAA | Irrelevant control sgRNA that has no homology to viral genomes. |
Publisher’s Note: MDPI stays neutral with regard to jurisdictional claims in published maps and institutional affiliations. |
© 2021 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (http://creativecommons.org/licenses/by/4.0/).
Share and Cite
Osborn, M.J.; Bhardwaj, A.; Bingea, S.P.; Knipping, F.; Feser, C.J.; Lees, C.J.; Collins, D.P.; Steer, C.J.; Blazar, B.R.; Tolar, J. CRISPR/Cas9-Based Lateral Flow and Fluorescence Diagnostics. Bioengineering 2021, 8, 23. https://doi.org/10.3390/bioengineering8020023
Osborn MJ, Bhardwaj A, Bingea SP, Knipping F, Feser CJ, Lees CJ, Collins DP, Steer CJ, Blazar BR, Tolar J. CRISPR/Cas9-Based Lateral Flow and Fluorescence Diagnostics. Bioengineering. 2021; 8(2):23. https://doi.org/10.3390/bioengineering8020023
Chicago/Turabian StyleOsborn, Mark J., Akshay Bhardwaj, Samuel P. Bingea, Friederike Knipping, Colby J. Feser, Christopher J. Lees, Daniel P. Collins, Clifford J. Steer, Bruce R. Blazar, and Jakub Tolar. 2021. "CRISPR/Cas9-Based Lateral Flow and Fluorescence Diagnostics" Bioengineering 8, no. 2: 23. https://doi.org/10.3390/bioengineering8020023
APA StyleOsborn, M. J., Bhardwaj, A., Bingea, S. P., Knipping, F., Feser, C. J., Lees, C. J., Collins, D. P., Steer, C. J., Blazar, B. R., & Tolar, J. (2021). CRISPR/Cas9-Based Lateral Flow and Fluorescence Diagnostics. Bioengineering, 8(2), 23. https://doi.org/10.3390/bioengineering8020023








