Back to the Cradle of Cytotherapy: Integrating a Century of Clinical Research and Biotechnology-Based Manufacturing for Modern Tissue-Specific Cellular Treatments in Switzerland
Abstract
1. Introduction
2. Genesis of Opotherapies and of Modern Cytotherapy: Drs. C.-E. Brown-Séquard and P. Niehans
3. Evolution and Standardization of Specific Therapeutic Preparations and of Cell Therapies in Switzerland during the 20th and 21st Centuries
4. Implementation of GMPs and Modern Regulatory Frameworks for Cell-Based Therapies in Switzerland
5. Safety and Quality as Paramount Attributes in the Modern Manufacture of Cell Therapy Products
6. The Swiss Progenitor Cell Transplantation Program and Two Decades of Clinical Cytotherapy Experience in Lausanne
7. Original Tissue-Specific Cytotherapeutic Concepts Enhanced by Biotechnological Manufacturing Processes and Modern Bioengineering Solutions
8. A Forward Return Back to Tissue-Specific Cell-Based Therapeutic Extracts for Individualized Regenerative Medicine
9. Conclusions
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Data Availability Statement
Acknowledgments
Conflicts of Interest
Abbreviations
| ACI | Autologous chondrocyte implantation |
| API | Active pharmaceutical ingredient |
| ATMP | Advanced therapy medicinal product |
| CEA | Cultured epithelial autograft |
| CDEA | Cultured dermal–epidermal autograft |
| CHUV | Centre Hospitalier Universitaire Vaudois |
| CPC | Cell production center |
| DMEM | Dulbecco’s modified Eagle medium |
| ECACC | European collection of authenticated cell cultures |
| EC | European Commission |
| EDQM | European Directorate for the Quality of Medicines and Healthcare |
| EMA | European Medicines Agency |
| ePBB | Equine progenitor biological bandage |
| FBS | Fetal bovine serum |
| FDA | US Food and Drug Administration |
| FIRDI | Food Industry Research and Development Institute |
| GMP | Good manufacturing practice |
| HPL | Human platelet lysate |
| HUG | Hôpitaux Universitaires de Genève |
| ICH | International Council for Harmonisation |
| ISO | International Organization for Standardization |
| MCB | Master cell bank |
| PBB | Progenitor biological bandage |
| PCB | Parental cell bank |
| PMDA | Pharmaceuticals and Medical Devices Agency |
| QC | Quality control |
| SPE | Sheep placental extract |
| TFDA | Taiwan Food and Drug Administration |
| TrSt | Standardized transplant product |
| WCB | Working cell bank |
References
- Kasser, S.; Applegate, L.; Hirt-Burri, N.; Jafari, P.; de Buys Roessingh, A.; Raffoul, W.; Berger, M. Acceptation of Folk Medicine and its “Secrets” in a Swiss Burn Centre. Ann. Burn. Fire Disasters 2019, 32, 227–233. [Google Scholar]
- Piccione, F.; Grecomoro, G.; Pinto, B.; Sanfilippo, A. Immune Therapy of Osteoarthritis: An Open Assessment of Clinical Results with Heterologous Antibodies to Articular Tissue (’Serocytol’). Pharmatherapeutica 1986, 4, 577–584. [Google Scholar]
- Kempenaers, C.; Simenon, G.; Vander Elst, M.; Fransolet, L.; Mingard, P.; De Maertelaer, V.; Appelboom, T.; Mendlewicz, J. Effect of an Antidiencephalon Immune Serum on Pain and Sleep in Primary Fibromyalgia. Neuropsychobiology 1994, 30, 66–72. [Google Scholar] [CrossRef] [PubMed]
- Lefrère, J.J.; Berche, P. Doctor Brown-Sequard’s Therapy. Ann. Endocrinol. 2010, 71, 69–75. [Google Scholar] [CrossRef]
- Brown-Sequard, C.E. Note on the Effects Produced on Man by Subcutaneous Injections of a Liquid Obtained from the Testis of Animals. Lancet 1889, 2, 105–107. [Google Scholar] [CrossRef]
- Brown-Sequard, C.E. Expérience Démontrant la Puissance Dynamogénique chez l’Homme d’un Liquide Extrait de Testicules d’Animaux. Arch. Phys. Norm. Pathol. 1889, 21, 651–656. [Google Scholar]
- Hersant, B.; SidAhmed-Mezi, M.; Niddam, J.; La Padula, S.; Noel, W.; Ezzedine, K.; Rodriguez, A.-M.; Meningaud, J.P. Efficacy of Autologous Platelet-rich Plasma Combined with Hyaluronic Acid on Skin Facial Rejuvenation: A Prospective Study. J. Am. Acad. Dermatol. 2017, 77, 584–586. [Google Scholar] [CrossRef]
- Bardelli, S.; Astori, G.; Sürder, D.; Tallone, T.; Terzic, A.; Soldati, G.; Moccetti, T. Stem Cell Update: Highlights from the 2010 Lugano Stem Cell Meeting. J. Cardiovasc. Transl. Res. 2010, 4, 192–199. [Google Scholar] [CrossRef]
- Hohlfeld, J.; de Buys Roessingh, A.; Hirt-Burri, N.; Chaubert, P.; Gerber, S.; Scaletta, C.; Hohlfeld, P.; Applegate, L.A. Tissue Engineered Fetal Skin Constructs for Paediatric Burns. Lancet 2005, 366, 840–842. [Google Scholar] [CrossRef]
- Mumme, M.; Barbero, A.; Miot, S.; Wixmerten, A.; Feliciano, S.; Wolf, F.; Asnaghi, A.M.; Baumhoer, D.; Bieri, O.; Kretzschmar, M.; et al. Nasal Chondrocyte-based Engineered Autologous Cartilage Tissue for Repair of Articular Cartilage Defects: An Observational First-in-human Trial. Lancet 2016, 388, 1985–1994. [Google Scholar] [CrossRef]
- Abdel-Sayed, P.; Michetti, M.; Scaletta, C.; Flahaut, M.; Hirt-Burri, N.; Roessingh, A.d.B.; Raffoul, W.; Applegate, L.A. Cell Therapies for Skin Regeneration: An Overview of 40 Years of Experience in Burn Units. Swiss Med. Wkly. 2019, 149, w20079. [Google Scholar] [CrossRef] [PubMed]
- Green, H.; Kehinde, O.; Thomas, J. Growth of Cultured Human Epidermal Cells into Multiple Epithelia Suitable for Grafting. Proc. Natl. Acad. Sci. USA 1979, 76, 5665–5668. [Google Scholar] [CrossRef] [PubMed]
- Déglise, B.; Benathan, M.; Frenk, E.; Krupp, S. Preliminary Results of Burn Treatment Using an Autograft of Cultured Epidermis. Schweiz. Med. Wochenschr. 1987, 117, 1380–1383. [Google Scholar]
- Green, H. The Birth of Therapy with Cultured Cells. BioEssays 2008, 30, 897–903. [Google Scholar] [CrossRef] [PubMed]
- Abdel-Sayed, P.; Hirt-Burri, N.; de Buys Roessingh, A.; Raffoul, W.; Applegate, L.A. Evolution of Biological Bandages as First Cover for Burn Patients. Adv. Wound Care 2019, 8, 555–564. [Google Scholar] [CrossRef]
- Chemali, M.; Laurent, A.; Scaletta, C.; Waselle, L.; Simon, J.-P.; Michetti, M.; Brunet, J.-F.; Flahaut, M.; Hirt-Burri, N.; Raffoul, W.; et al. Burn Center Organization and Cellular Therapy Integration: Managing Risks and Costs. J. Burn Care Res. 2021, 42, 911–924. [Google Scholar] [CrossRef]
- Applegate, L.A.; Weber, D.; Simon, J.-P.; Scaletta, C.; Hirt-Burri, N.; de Buys Roessingh, A.S.; Raffoul, W. Organ Donation and Whole-cell Bioprocessing in the Swiss Fetal Progenitor Cell Transplantation Platform. In Organ Donation and Organ Donors; Saidi, R.F., Ed.; Nova Science Publishers: New York, NY, USA, 2013; pp. 125–147. [Google Scholar]
- Laurent, A.; Hirt-Burri, N.; Scaletta, C.; Michetti, M.; de Buys Roessingh, A.S.; Raffoul, W.; Applegate, L.A. Holistic Approach of Swiss Fetal Progenitor Cell Banking: Optimizing Safe and Sustainable Substrates for Regenerative Medicine and Biotechnology. Front. Bioeng. Biotechnol. 2020, 8, 557758. [Google Scholar] [CrossRef] [PubMed]
- Ramelet, A.-A.; Hirt-Burri, N.; Raffoul, W.; Scaletta, C.; Pioletti, D.; Offord, E.; Mansourian, R.; Applegate, L.A. Chronic Wound Healing by Fetal Cell Therapy May be Explained by Differential Gene Profiling Observed in Fetal Versus Old Skin Cells. Exp. Gerontol. 2009, 44, 208–218. [Google Scholar] [CrossRef] [PubMed]
- Applegate, L.A.; Scaletta, C.; Hirt-Burri, N.; Raffoul, W.; Pioletti, D. Whole-Cell Bioprocessing of Human Fetal Cells for Tissue Engineering of Skin. Ski. Pharmacol. Physiol. 2009, 22, 63–73. [Google Scholar] [CrossRef] [PubMed]
- De Buys Roessingh, A.; Hirt-Burri, N.; Raffoul, W.; Scaletta, C.; Applegate, L.A. A Decade after Foetal Skin Progenitor Cell Therapy in Pediatric Burn Treatment. J. Regen. Med. 2015, 4, 1. [Google Scholar] [CrossRef]
- Hirt-Burri, N.; Ramelet, A.-A.; Raffoul, W.; de Buys Roessingh, A.; Scaletta, C.; Pioletti, D.; Applegate, L.A. Biologicals and Fetal Cell Therapy for Wound and Scar Management. ISRN Dermatol. 2011, 2011, 549870. [Google Scholar] [CrossRef]
- Al-Dourobi, K.; Laurent, A.; Deghayli, L.; Flahaut, M.; Abdel-Sayed, P.; Scaletta, C.; Michetti, M.; Waselle, L.; Simon, J.-P.; El Ezzi, O.; et al. Retrospective Evaluation of Progenitor Biological Bandage Use: A Complementary and Safe Therapeutic Management Option for Prevention of Hypertrophic Scarring in Pediatric Burn Care. Pharmaceuticals 2021, 14, 201. [Google Scholar] [CrossRef] [PubMed]
- Dimitropoulos, G.; Jafari, P.; de Buys Roessingh, A.; Hirt-Burri, N.; Raffoul, W.; Applegate, L.A. Burn Patient Care Lost in Good Manufacturing Practices? Ann. Burn. Fire Disasters 2016, 29, 111–115. [Google Scholar]
- European Parliament and Council. Regulation (EC) No 1394/2007 on Advanced Therapy Medicinal Products and amending Directive 2001/83/EC and Regulation (EC) No 726/2004. Off. J. Eur. Union 2007, L 324, 121–137. Available online: http://data.europa.eu/eli/reg/2007/1394/oj (accessed on 1 November 2021).
- Laurent, A.; Lin, P.; Scaletta, C.; Hirt-Burri, N.; Michetti, M.; de Buys Roessingh, A.S.; Raffoul, W.; She, B.-R.; Applegate, L.A. Bringing Safe and Standardized Cell Therapies to Industrialized Processing for Burns and Wounds. Front. Bioeng. Biotechnol. 2020, 8, 581. [Google Scholar] [CrossRef]
- Brown-Sequard, C.E. On a New Therapeutic Method Consisting in the Use of Organic Liquids Extracted from Glands and Other Organs. BMJ 1893, 1, 1145–1147. [Google Scholar] [CrossRef] [PubMed]
- Rengachary, S.S.; Colen, C.; Guthikonda, M. Charles-Edouard Brown-Séquard: An Eccentric Genius. Neurosurgery 2008, 62, 954–964. [Google Scholar] [CrossRef] [PubMed]
- American Cancer Society. Unproven Methods of Cancer Management. Fresh Cell Therapy. CA A Cancer J. Clin. 1991, 41, 126–128. [Google Scholar] [CrossRef] [PubMed]
- Aldag, C.; Nogueira Teixeira, D.; Leventhal, P.S. Skin Rejuvenation Using Cosmetic Products Containing Growth Factors, Cytokines, and Matrikines: A Review of the Literature. Clin. Cosmet. Investig. Dermatol. 2016, 9, 411–419. [Google Scholar] [CrossRef]
- Gold, M.H.; Biron, J. A Novel Skin Cream Containing a Mixture of Human Growth Factors and Cytokines for the Treatment of Adverse Events Associated with Photodynamic Therapy. J. Drugs Dermatol. 2006, 5, 796–798. [Google Scholar]
- Gold, M.H.; Goldman, M.P.; Biron, J. Efficacy of Novel Skin Cream Containing a Mixture of Human Growth Factors and Cytokines for Skin Rejuvenation. J. Drugs Dermatol. 2007, 6, 197–201. [Google Scholar]
- Office Fédéral de la Santé Publique et Institut Suisse des Produits Thérapeutiques Swissmedic. Mesures de Lutte Contre les Offres Thérapeutiques Illégales de Cellules Fraîches et de Préparations Non Autorisées à Base de Cellules Fraîches. Swissmedic J. 2015, 3, 194–199. [Google Scholar]
- Federal Assembly of the Swiss Confederation. Federal Law on Medication and Medical Devices (Law on Therapeutic Products) SR 812.21; Switzerland’s Federal Council: Bern, Switzerland, 2000. Available online: https://fedlex.data.admin.ch/eli/cc/2001/422 (accessed on 1 November 2021).
- Federal Assembly of the Swiss Confederation. Federal Law on the Transplantation of Organs, Tissues, and Cells (Law on Trans-Plantation) SR 810.21; Switzerland’s Federal Council: Bern, Switzerland, 2004. Available online: https://fedlex.data.admin.ch/eli/cc/2007/279 (accessed on 1 September 2021).
- Foglia, R.P.; DiPreta, J.; Statter, M.B.; Donahoe, P.K. Fetal Allograft Survival in Immunocompetent Recipients is Age Dependent and Organ Specific. Ann. Surg. 1986, 204, 402–410. [Google Scholar] [CrossRef]
- Crombleholme, T.M.; Langer, J.C.; Harrison, M.R.; Zanjani, E.D. Transplantation of Fetal Cells. Am. J. Obstet. Gynecol. 1991, 164, 218–230. [Google Scholar] [CrossRef]
- Brittberg, M.; Lindahl, A.; Nilsson, A.; Ohlsson, C.; Isaksson, O.; Peterson, L. Treatment of Deep Cartilage Defects in the Knee with Autologous Chondrocyte Transplantation. N. Engl. J. Med. 1994, 331, 889–895. [Google Scholar] [CrossRef] [PubMed]
- Vacanti, J.P.; Langer, R. Tissue Engineering: The Design and Fabrication of Living Replacement Devices for Surgical Reconstruction and Transplantation. Lancet 1999, 354, S32–S34. [Google Scholar] [CrossRef]
- Meuli, M.; Hartmann-Fritsch, F.; Hüging, M.; Marino, D.; Saglini, M.; Hynes, S.; Neuhaus, K.; Manuel, E.; Middelkoop, E.; Reichmann, E.; et al. A Cultured Autologous Dermo-epidermal Skin Substitute for Full-thickness Skin Defects: A Phase I, Open, Prospective Clinical Trial in Children. Plast. Reconstr. Surg. 2019, 144, 188–198. [Google Scholar] [CrossRef] [PubMed]
- Darwiche, S.; Scaletta, C.; Raffoul, W.; Pioletti, D.P.; Applegate, L.A. Epiphyseal Chondroprogenitors Provide a Stable Cell Source for Cartilage Cell Therapy. Cell Med. 2012, 4, 23–32. [Google Scholar] [CrossRef] [PubMed]
- Grognuz, A.; Scaletta, C.; Farron, A.; Raffoul, W.; Applegate, L.A. Human Fetal Progenitor Tenocytes for Regenerative Medicine. Cell Transplant. 2016, 25, 463–479. [Google Scholar] [CrossRef]
- Laurent, A.; Darwiche, S.E.; Hirt-Burri, N.; Scaletta, C.; Michetti, M.; Laurent, P.; Raffoul, W.; de Buys Roessingh, A.S.; Applegate, L.A. Banking Progenitor Cells for Hippiatric Regenerative Medicine: Optimized Establishment of Safe and Consistent Cell Sources for Standardized Veterinary Therapeutic Protocols. Am. J. Biomed. Sci. Res. 2020, 8, 252–271. [Google Scholar] [CrossRef]
- Laurent, A.; Abdel-Sayed, P.; Ducrot, A.; Hirt-Burri, N.; Scaletta, C.; Jaccoud, S.; Nuss, K.; de Buys Roessingh, A.; Raffoul, W.; Pioletti, D.; et al. Development of Standardized Fetal Progenitor Cell Therapy for Cartilage Regenerative Medicine: Industrial Transposition and Preliminary Safety in Xenogeneic Transplantation. Biomolecules 2021, 11, 250. [Google Scholar] [CrossRef]
- Laurent, A.; Abdel-Sayed, P.; Grognuz, A.; Scaletta, C.; Hirt-Burri, N.; Michetti, M.; de Buys Roessingh, A.S.; Raffoul, W.; Kronen, P.; Nuss, K.; et al. Industrial Development of Standardized Fetal Progenitor Cell Therapy for Tendon Regenerative Medicine: Preliminary Safety in Xenogeneic Transplantation. Biomedicines 2021, 9, 380. [Google Scholar] [CrossRef]
- Liu, J.; Luo, S.; Yang, J.; Ren, F.; Zhao, Y.; Luo, H.; Ge, K.; Zhang, H. The Protective Effect of Sheep Placental Extract on Concanavalin A-induced Liver Injury in Mice. Molecules 2018, 24, 28. [Google Scholar] [CrossRef]
- Firan, F.C.; Romila, A.; Onose, G. Current Synthesis and Systematic Review of Main Effects of Calf Blood Deproteinized Medicine (Actovegin®) in Ischemic Stroke. Int. J. Mol. Sci. 2020, 21, 3181. [Google Scholar] [CrossRef]
- Belikan, P.; Nauth, L.; Färber, L.C.; Abel, F.; Langendorf, E.; Drees, P.; Rommens, P.M.; Ritz, U.; Mattyasovszky, S.G. Intra-muscular Injection of Combined Calf Blood Compound (CFC) and Homeopathic Drug Tr14 Accelerates Muscle Regeneration In Vivo. Int. J. Mol. Sci. 2020, 21, 2112. [Google Scholar] [CrossRef] [PubMed]
- Stern, P. The Effect of Specific Brain Phospholipid on the Rat’s Allergic Encephalomyelitis. Arzneim. Forsch. 1975, 25, 753–755. [Google Scholar]
- Brodie, A.; El-Taji, O.; Jour, I.; Foley, C.; Hanbury, D. A Retrospective Study of Immunotherapy Treatment with Uro-Vaxom (OM-89®) for Prophylaxis of Recurrent Urinary Tract Infections. Curr. Urol. 2020, 14, 130–134. [Google Scholar] [CrossRef]
- Zhang, M.; Luan, H.; Zhang, Q.; Wang, L.; Lv, Y.-M.; He, F.; Chen, Y.; Zeng, H.-B.; Yao, Y.; Liu, Q. Prevention of Infection in Immunosuppressive Patients with Autoimmune Nephrosis by Using an Immunostimulating Bacterial Lysate Broncho-Vaxom. Hum. Vaccines Immunother. 2012, 8, 1802–1807. [Google Scholar] [CrossRef][Green Version]
- Hunsberger, J.; Harrysson, O.; Shirwaiker, R.; Starly, B.; Wysk, R.; Cohen, P.; Allickson, J.; Yoo, J.; Atala, A. Manufacturing Road Map for Tissue Engineering and Regenerative Medicine Technologies. Stem Cells Transl. Med. 2015, 4, 130–135. [Google Scholar] [CrossRef]
- Hartmann-Fritsch, F.; Marino, D.; Reichmann, E. About ATMPs, SOPs and GMP: The Hurdles to Produce Novel Skin Grafts for Clinical Use. Transfus. Med. Hemother. 2016, 43, 344–352. [Google Scholar] [CrossRef] [PubMed]
- De Wilde, S.; Veltrop-Duits, L.; Hoozemans-Strik, M.; Ras, T.; Blom-Veenman, J.; Guchelaar, H.-J.; Zandvliet, M.; Meij, P. Hurdles in Clinical Implementation of Academic Advanced Therapy Medicinal Products: A National Evaluation. Cytotherapy 2016, 18, 797–805. [Google Scholar] [CrossRef]
- Pearce, K.F.; Hildebrandt, M.; Greinix, H.; Scheding, S.; Koehl, U.; Worel, N.; Apperley, J.; Edinger, M.; Hauser, A.; Mischak-Weissinger, E.; et al. Regulation of Advanced Therapy Medicinal Products in Europe and the Role of Academia. Cytotherapy 2014, 16, 289–297. [Google Scholar] [CrossRef] [PubMed]
- Moiemen, N.; Schiestl, C.; Hartmann-Fritsch, F.; Neuhaus, K.; Reichmann, E.; Löw, A.; Stenger, C.; Böttcher-Haberzeth, S.; Meuli, M. First Time Compassionate Use of Laboratory Engineered Autologous Zurich Skin in a Massively Burned Child. Burn. Open 2021, 5, 113–117. [Google Scholar] [CrossRef]
- Schiestl, C.; Meuli, M.; Vojvodic, M.; Pontiggia, L.; Neuhaus, D.; Brotschi, B.; Reichmann, E.; Böttcher-Haberzeth, S.; Neuhaus, K. Expanding Into the Future: Combining a Novel Dermal Template with Distinct Variants of Autologous Cultured Skin Substitutes in Massive Burns. Burn. Open 2021, 5, 145–153. [Google Scholar] [CrossRef]
- Bicudo, E.; Brass, I.; Carmichael, P.; Farid, S. The UK’s Emerging Regulatory Framework for Point-of-care Manufacture: Insights from a Workshop on Advanced Therapies. Cell Gene Ther. Insights 2021, 7, 1005–1015. [Google Scholar]
- Laurent, A.; Simon, J.-P.; Hirt-Burri, N.; Raffoul, W.; Applegate, L.A.; de Buys Roessingh, A.S. GMP-grade Allogeneic Musculoskeletal Primary Progenitor Cell Types: Standardized Candidates for General or Pharmacopeial Monograph Elaboration. J. Transl. Sci. 2020, 7, 1–3. [Google Scholar] [CrossRef]
- Abbaspanah, B.; Momeni, M.; Ebrahimi, M.; Mousavi, S.H. Advances in Perinatal Stem Cells Research: A Precious Cell Source for Clinical Applications. Regen. Med. 2018, 13, 595–610. [Google Scholar] [CrossRef] [PubMed]
- Bhattacharya, N. Fetal Cell/Tissue Therapy in Adult Disease: A New Horizon in Regenerative Medicine. Clin. Exp. Obstet. Gynecol. 2004, 31, 167–173. [Google Scholar]
- Clarkson, E.D. Fetal Tissue Transplantation for Patients with Parkinson’s Disease: A Database of Published Clinical Results. Drugs Aging 2001, 18, 773–785. [Google Scholar] [CrossRef] [PubMed]
- Gaggi, G.; Izzicupo, P.; Di Credico, A.; Sancilio, S.; Di Baldassarre, A.; Ghinassi, B. Spare Parts from Discarded Materials: Fetal Annexes in Regenerative Medicine. Int. J. Mol. Sci. 2019, 20, 1573. [Google Scholar] [CrossRef]
- Kaviani, A.; Guleserian, K.; Perry, T.E.; Jennings, R.W.; Ziegler, M.M.; Fauza, D.O. Fetal Tissue Engineering from Amniotic Fluid. J. Am. Coll. Surg. 2003, 196, 592–597. [Google Scholar] [CrossRef]
- Kaviani, A.; Perry, T.; Rvi, E.; Barnes, C.M.; Oh, J.-T.; Ziegler, M.M.; Fishman, S.J.; Fauza, D.O. The placenta as a Cell Source in Fetal Tissue Engineering. J. Pediatr. Surg. 2002, 37, 995–999. [Google Scholar] [CrossRef] [PubMed]
- Hayflick, L.; Moorhead, P.S. The Serial Cultivation of Human Diploid Cell Strains. Exp. Cell Res. 1961, 25, 585–621. [Google Scholar] [CrossRef]
- Jacobs, J.P.; Jones, C.M.; Baille, J.P. Characteristics of a Human Diploid Cell Designated MRC-5. Nat. Cell Biol. 1970, 227, 168–170. [Google Scholar] [CrossRef] [PubMed]
- Olshansky, S.J.; Hayflick, L. The Role of the WI-38 Cell Strain in Saving Lives and Reducing Morbidity. AIMS Public Health 2017, 4, 127–138. [Google Scholar] [CrossRef]
- Laurent, A.; Abdel-Sayed, P.; Hirt-Burri, N.; Scaletta, C.; Michetti, M.; de Buys Roessingh, A.; Raffoul, W.; Applegate, L.A. Evolution of Diploid Progenitor Lung Cell Applications: From Optimized Biotechnological Substrates to Potential Active Pharmaceutical Ingredients in Respiratory Tract Regenerative Medicine. Cells 2021, 10, 2526. [Google Scholar] [CrossRef] [PubMed]
- Laurent, A.; Scaletta, C.; Abdel-Sayed, P.; Michetti, M.; Flahaut, M.; Simon, J.-P.; Roessingh, A.d.B.; Raffoul, W.; Hirt-Burri, N.; Applegate, L.A. Optimized Manufacture of Lyophilized Dermal Fibroblasts for Next-Generation Off-the-Shelf Progenitor Biological Bandages in Topical Post-Burn Regenerative Medicine. Biomedicines 2021, 9, 1072. [Google Scholar] [CrossRef] [PubMed]
- Laurent, A.; Scaletta, C.; Hirt-Burri, N.; Raffoul, W.; de Buys Roessingh, A.S.; Applegate, L.A. Swiss Fetal Transplantation Program and Non-enzymatically Isolated Primary Progenitor Cell Types for Regenerative Medicine. In Stem Cells and Good Manufacturing Practices: Methods and Protocols; Kursad, T., Ed.; Springer Science+Business Media: New York, NY, USA, 2020. [Google Scholar] [CrossRef]
- Hayflick, L. How and Why we Age. Exp. Gerontol. 1998, 33, 639–653. [Google Scholar] [CrossRef]
- Tyrrell, D.; Buckland, F.; Bynoe, M.; Hayflick, L. The Cultivation in Human-embryo Cells of a Virus (D.C.) Causing Colds in Man. Lancet 1962, 280, 320–322. [Google Scholar] [CrossRef]
- Hayflick, L. Living Forever and Dying in the Attempt. Exp. Gerontol. 2003, 38, 1231–1241. [Google Scholar] [CrossRef] [PubMed]
- Hayflick, L. Antecedents of Cell Aging Research. Exp. Gerontol. 1989, 24, 355–365. [Google Scholar] [CrossRef]
- Beskow, L.M. Lessons from HeLa cells: The Ethics and Policy of Biospecimens. Annu. Rev. Genom. Hum. Genet. 2016, 17, 395–417. [Google Scholar] [CrossRef] [PubMed]
- Hirt-Burri, N.; de Buys Roessingh, A.S.; Scaletta, C.; Gerber, S.; Pioletti, D.P.; Applegate, L.A.; Hohlfeld, J. Human Muscular Fetal Cells: A Potential Cell Source for Muscular Therapies. Pediatr. Surg. Int. 2007, 24, 37–47. [Google Scholar] [CrossRef][Green Version]
- Quintin, A.; Schizas, C.; Scaletta, C.; Jaccoud, S.; Gerber, S.; Osterheld, M.C.; Jullierat, L.; Applegate, L.A.; Pioletti, D.P. Isolation and In Vitro Chondrogenic Potential of Human Foetal Spine Cells. J. Cell. Mol. Med. 2009, 13, 2559–2569. [Google Scholar] [CrossRef]
- Montjovent, M.-O.; Burri, N.; Mark, S.; Federici, E.; Scaletta, C.; Zambelli, P.-Y.; Hohlfeld, P.; Leyvraz, P.-F.; Applegate, L.A.; Pioletti, D.P. Fetal Bone Cells for Tissue Engineering. Bone 2004, 35, 1323–1333. [Google Scholar] [CrossRef] [PubMed][Green Version]
- Hausherr, T.C.; Nuss, K.; Thein, E.; Applegate, L.A.; Pioletti, D.P. Human Bone Progenitor Cells for Clinical Application: What Kind of Immune Reaction Does Fetal Xenograft Tissue Trigger in Immunocompetent Rats? Cell Transplant. 2017, 26, 879–890. [Google Scholar] [CrossRef] [PubMed]
- Mpandi, M.; Schmutz, P.; Legrand, E.; Duc, R.; Geinoz, J.; Henzelin-Nkubana, C.; Giorgia, S.; Clerc, O.; Genoud, D.; Weber, T. Partitioning and Inactivation of Viruses by the Caprylic Acid Precipitation Followed by a Terminal Pasteurization in the Manufacturing Process of Horse Immunoglobulins. Biologicals 2007, 35, 335–341. [Google Scholar] [CrossRef] [PubMed]
- Jeannerat, A.; Peneveyre, C.; Armand, F.; Chiappe, D.; Hamelin, R.; Scaletta, C.; Hirt-Burri, N.; Roessingh, A.d.B.; Raffoul, W.; Applegate, L.A.; et al. Hypoxic Incubation Conditions for Optimized Manufacture of Tenocyte-Based Active Pharmaceutical Ingredients of Homologous Standardized Transplant Products in Tendon Regenerative Medicine. Cells 2021, 10, 2872. [Google Scholar] [CrossRef] [PubMed]
- Laurent-Applegate, L.A. Preparation of Parental Cell Bank from Foetal Tissue. European Patent No. 2,732,030,B1, 27 September 2017. [Google Scholar]
- Bari, E.; Ferrarotti, I.; Torre, M.L.; Corsico, A.G.; Perteghella, S. Mesenchymal Stem/Stromal Cell Secretome for Lung Regeneration: The Long Way Through “Pharmaceuticalization” for the Best Formulation. J. Control. Release 2019, 309, 11–24. [Google Scholar] [CrossRef]
- El Baradie, K.B.Y.; Nouh, M.; O’Brien III, F.; Liu, Y.; Fulzele, S.; Eroglu, A.; Hamrick, M.W. Freeze-Dried Extracellular Vesicles From Adipose-Derived Stem Cells Prevent Hypoxia-Induced Muscle Cell Injury. Front. Cell Dev. Biol. 2020, 8, 181. [Google Scholar] [CrossRef]
- Soejima, K.; Shimoda, K.; Kashimura, T.; Yamaki, T.; Kono, T.; Sakurai, H.; Nakazawa, H. Wound Dressing Material Containing Lyophilized Allogeneic Cultured Cells. Cryobiology 2013, 66, 210–214. [Google Scholar] [CrossRef] [PubMed]
- Ahangar, P.; Mills, S.J.; Cowin, A.J. Mesenchymal Stem Cell Secretome as an Emerging Cell-Free Alternative for Improving Wound Repair. Int. J. Mol. Sci. 2020, 21, 7038. [Google Scholar] [CrossRef] [PubMed]
- Bari, E.; Perteghella, S.; Catenacci, L.; Sorlini, M.; Croce, S.; Mantelli, M.; Avanzini, M.A.; Sorrenti, M.; Torre, M.L. Freeze-dried and GMP-compliant Pharmaceuticals Containing Exosomes for Acellular Mesenchymal Stromal Cell Immunomodulant Therapy. Nanomedicine 2019, 14, 753–765. [Google Scholar] [CrossRef] [PubMed]
- Huang, C.-P.; Yang, C.-Y.; Shyr, C.-R. Utilizing Xenogeneic Cells as a Therapeutic Agent for Treating Diseases. Cell Transplant. 2021, 30, 9636897211011995. [Google Scholar] [CrossRef] [PubMed]
- World Medical Association (WMA). Declaration of Helsinki: Ethical Principles for Medical Research Involving Human Subjects. JAMA 2013, 310, 233–238. [Google Scholar] [CrossRef]
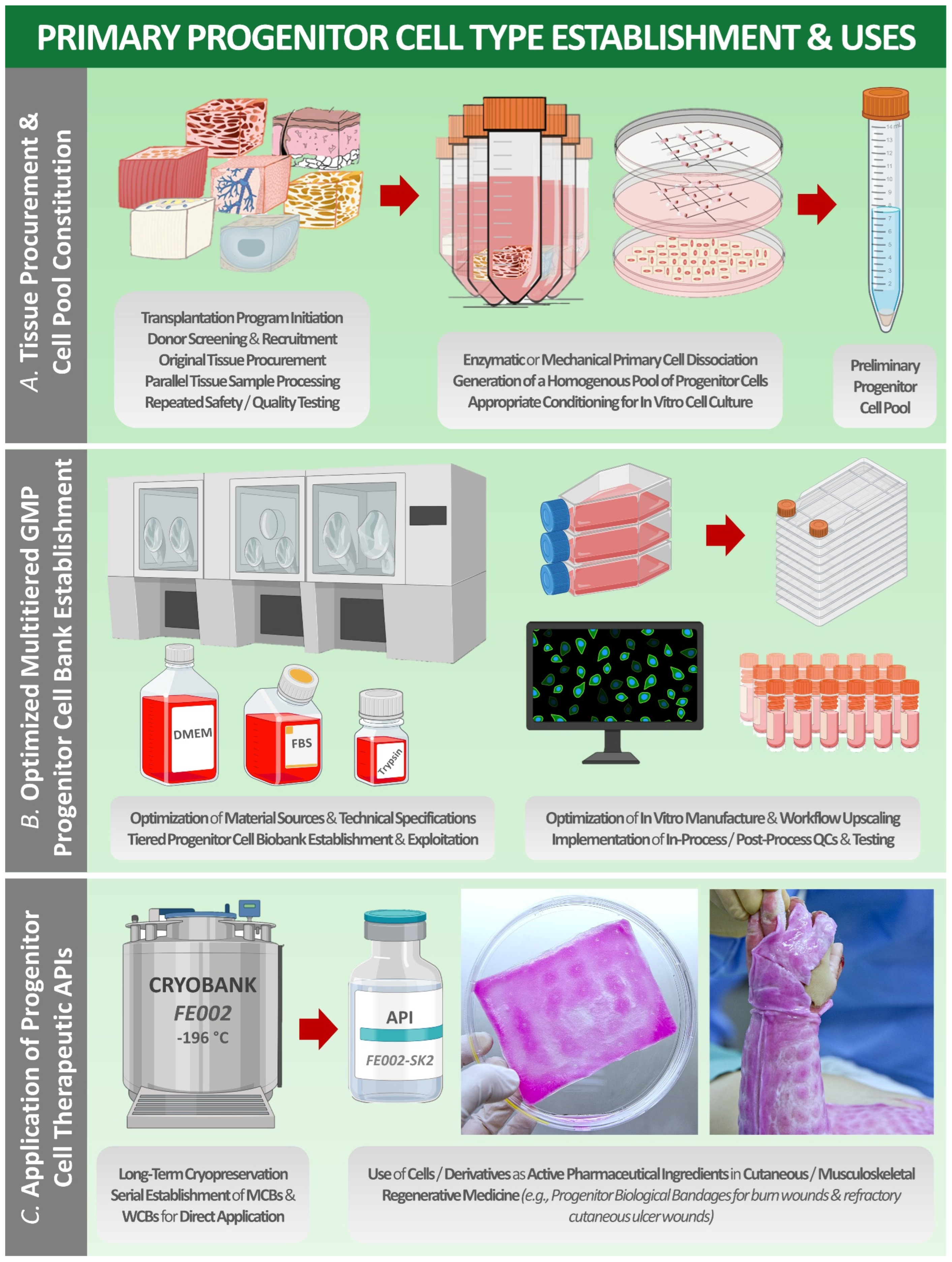

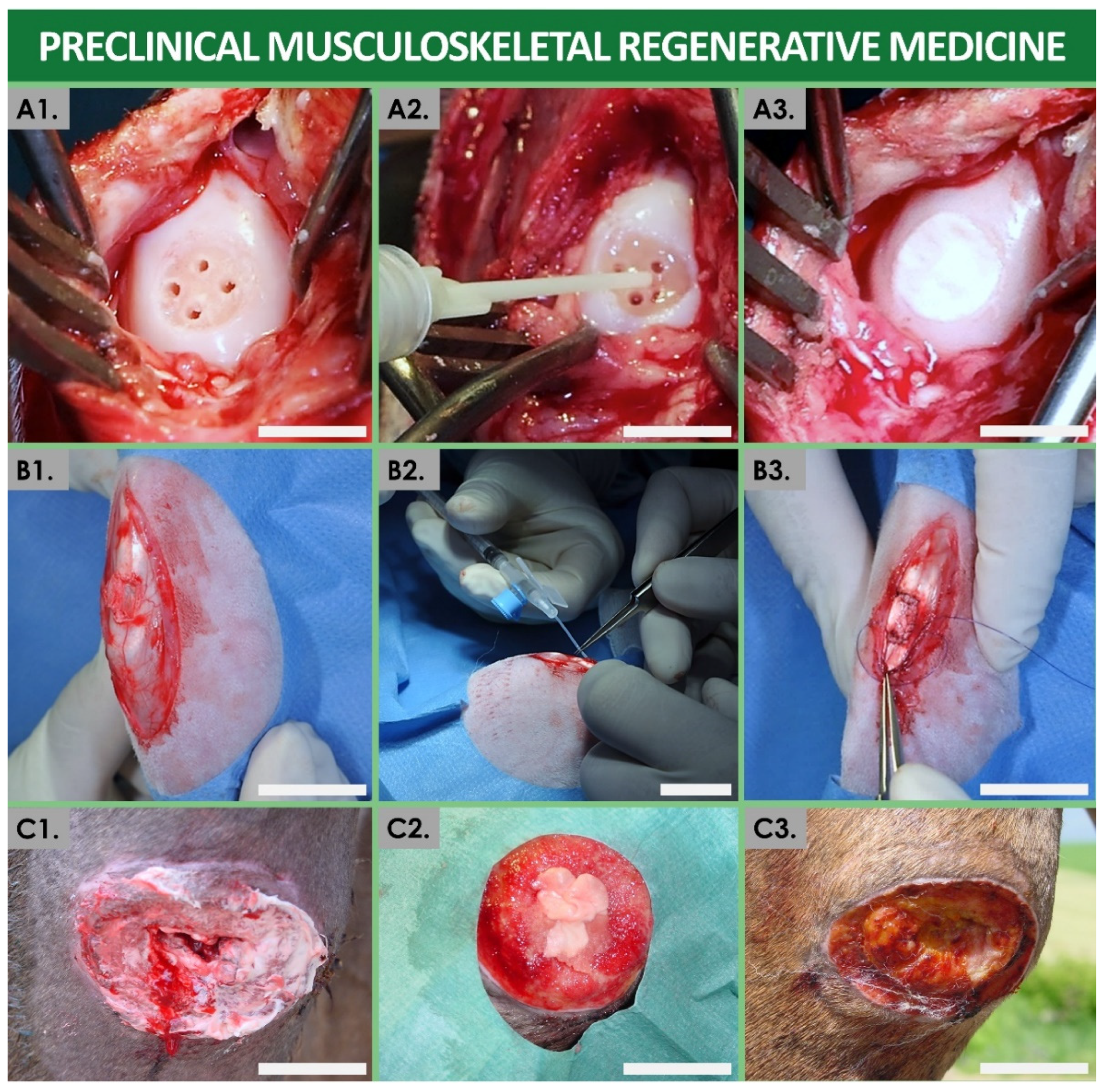
| Preparation Type/Name | Technical Description | Therapeutic Rationale, Examples, and Known Swiss Manufacturers |
|---|---|---|
| Sheep placental extracts (SPEs) | Processed ovine placenta (by hydrolysis or mechanical separation) for obtention of complex protein extract solutions. | Use of ovine starting material enables facilitated access to perinatal tissues, which have extensive history of use in Western and Asian medicine. Such extracts are used for protective and immunomodulatory effects in various product categories. No therapeutic SPE preparations have been approved in Switzerland, yet unlicensed use has been documented in several private practices for mesotherapy (or as probable substitutes for original “living cell therapy”) [33,46]. |
| Placental isotherapy | Formulation of patient-specific placental tissues into appropriate homeopathic preparations. | Placental isotherapy was commonly used until recently in Switzerland for various postpartum affections. Following medical prescription, thorough safety testing, and pharmaceutical magistral preparation, these products were dispensed to specific patients. Such preparations were notably available in Switzerland from Serolab SA. |
| Serocytol® | Equine immunobiologic products. Specific porcine tissues were transplanted to immunize horses, and the collected equine immunoglobulins were used to treat corresponding tissue-specific human affections. | The use of tissue-specific equine immunoglobulins was widely adopted in Switzerland since the 1930s, when Dr. Jean Thomas elaborated and democratized the practice of serocytotherapy. Specific porcine organs and tissues were transplanted in horses to generate immunoglobulins, which were then used as APIs in human medicine to treat affections of the corresponding organs and tissues. Several dozen pharmaceutical preparations (for oral, injectable, or rectal administration) based on this therapeutic principle were registered as therapeutic products in Switzerland by Serolab SA until 2020 [2,3]. |
| Actovegin® | Deproteinized calf serum extract, in semisolid or liquid preparations. | Actovegin® or equivalent products are highly used in injection form for circulatory affections and within professional athletic circles, for promotion of tissular repair and performance amelioration [47,48]. Actovegin® is a registered therapeutic product, owned by the global Switzerland-based Takeda Pharmaceutical Company. |
| GM-1 | Sialic-acid-containing glycosphingolipids, extracted and purified from mammalian nervous tissue. | Several neurotrophic and neuroprotective properties of GM-1 have been investigated, demonstrating potential roles and applications in neurodegenerative conditions. GM-1 has been produced by the global Switzerland-based TRB Chemedica SA. A similar preparation known under the appellation “Gricertine” was commercially available in Swiss pharmacies in the 1980s, that was presented as a central nervous system stimulant or protector, based on research around specific brain phospholipids [49]. |
| Uro-Vaxom® and Broncho-Vaxom® | Immunotherapy products containing complex bacterial cell lysates, formulated in dry oral form. | Such registered therapeutic products are used in the prevention of recurrent urinary or respiratory tract infections, respectively. They stimulate the immune system against potential pathogens [50,51]. These therapeutic products are registered and manufactured in Switzerland by OM Pharma SA. |
| Legal/Regulatory Texts in Switzerland | Legal/Regulatory Texts in the European Union |
|---|---|
| Federal law on the transplantation of organs, tissues, and cells (Law on Transplantation, 2004) | Directive 2004/23/EC of the European Parliament and of the Council of 31 March 2004 on setting standards of quality and safety for the donation, procurement, testing, processing, preservation, storage, and distribution of human tissues and cells (2004) |
| Federal law on medication and medical devices (Law on Therapeutic Products, LPTh, 2000) | Directive 2001/83/EC of the European Parliament and of the Council of 6 November 2001 on the Community Code relating to medicinal products for human use (2001) |
| Federal ordinance on authorizations in the domain of therapeutic products (OAMéd, 2018) | Regulation (EC) No. 1394/2007 on Advanced Therapy Medicinal Products and amending Directive 2001/83/EC and Regulation (EC) No. 726/2004 (2007) |
| Academic/Nonprofit Research Centers | Cell Therapy Interests | Industrial Partners |
|---|---|---|
| Lausanne University Hospital, Lausanne Burn Center | Skin (autologous and allogeneic solutions for burn wounds, donor site wounds, cutaneous ulcers) | ELANIX Sàrl |
| Lausanne University Hospital, Orthopedics and Traumatology Service | Cartilage (autologous chondrocyte implantation) | NA |
| University Hospital Basel, Department of Orthopedics and Traumatology | Cartilage (autologous chondrocyte implantation) | NA |
| Pediatric Burn Center, University Children’s Hospital Zurich | Skin (autologous solutions for burn wounds) | Wyss Zurich Regenerative Medicine Technologies Platform; CUTISS Ltd. |
| Swiss Stem Cell Foundation | Adipose stem cells (esthetics) | Technopark Zurich; Günter Leifheit Stem Cell Institute |
| Tissue Type | Progenitor Cell Type Examples | Application Types | Considered Therapeutic Applications | Selected References |
|---|---|---|---|---|
| Skin | FE002-SK2 1 | Manufacturing: industrial GMP manufacturing transposition. Clinical trials: severe burns, refractory cutaneous ulcers, donor-site wounds. | Cutaneous wounds, burns, scars, grafting sites. | [9,19,23,26,70] |
| Cartilage | FE002-Cart 2 | Manufacturing: industrial cell banking and product manufacturing. Preclinical studies: safety of transplantation in a caprine model. | Prevention of cartilage degeneration such as osteoarthritis. Treatment of critical cartilage lesions. | [44] |
| Tendon | FE002-Ten 3 | Manufacturing: industrial cell banking and optimized API manufacturing. Preclinical studies: safety of transplantation in a lagomorph model. | Treatment of subcritical defects such as tears, or of volumetric tissue loss. | [45,82] |
| Bone | FE002-Bone | Manufacturing: optimized cell banking and manufacturing. Preclinical studies: safety of transplantation in murine and rat models. | Treatment of subcritical bone fissures. Treatment of critical bone lesions. | [79,80] |
| Muscle | FE002-Mu | Manufacturing: optimized cell banking and manufacturing. Preclinical studies: safety of transplantation in a murine model. | Treatment of subcritical defects such as tears, or of volumetric tissue loss. | [77] |
| Intervertebral disc | FE002-Disc | Manufacturing: optimized cell banking and manufacturing. | Treatment of critical intervertebral disc lesions. | Unpublished results |
| Lung | FE002-Lu | Manufacturing: optimized cell banking and manufacturing. | Prevention and/or treatment of inflammatory respiratory tract affections. | Unpublished results |
Publisher’s Note: MDPI stays neutral with regard to jurisdictional claims in published maps and institutional affiliations. |
© 2021 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Laurent, A.; Abdel-Sayed, P.; Scaletta, C.; Laurent, P.; Laurent, E.; Michetti, M.; de Buys Roessingh, A.; Raffoul, W.; Hirt-Burri, N.; Applegate, L.A. Back to the Cradle of Cytotherapy: Integrating a Century of Clinical Research and Biotechnology-Based Manufacturing for Modern Tissue-Specific Cellular Treatments in Switzerland. Bioengineering 2021, 8, 221. https://doi.org/10.3390/bioengineering8120221
Laurent A, Abdel-Sayed P, Scaletta C, Laurent P, Laurent E, Michetti M, de Buys Roessingh A, Raffoul W, Hirt-Burri N, Applegate LA. Back to the Cradle of Cytotherapy: Integrating a Century of Clinical Research and Biotechnology-Based Manufacturing for Modern Tissue-Specific Cellular Treatments in Switzerland. Bioengineering. 2021; 8(12):221. https://doi.org/10.3390/bioengineering8120221
Chicago/Turabian StyleLaurent, Alexis, Philippe Abdel-Sayed, Corinne Scaletta, Philippe Laurent, Elénie Laurent, Murielle Michetti, Anthony de Buys Roessingh, Wassim Raffoul, Nathalie Hirt-Burri, and Lee Ann Applegate. 2021. "Back to the Cradle of Cytotherapy: Integrating a Century of Clinical Research and Biotechnology-Based Manufacturing for Modern Tissue-Specific Cellular Treatments in Switzerland" Bioengineering 8, no. 12: 221. https://doi.org/10.3390/bioengineering8120221
APA StyleLaurent, A., Abdel-Sayed, P., Scaletta, C., Laurent, P., Laurent, E., Michetti, M., de Buys Roessingh, A., Raffoul, W., Hirt-Burri, N., & Applegate, L. A. (2021). Back to the Cradle of Cytotherapy: Integrating a Century of Clinical Research and Biotechnology-Based Manufacturing for Modern Tissue-Specific Cellular Treatments in Switzerland. Bioengineering, 8(12), 221. https://doi.org/10.3390/bioengineering8120221








