Assessing the Asynchrony Event Based on the Ventilation Mode for Mechanically Ventilated Patients in ICU
Abstract
1. Introduction
2. Methodology
2.1. Patient Data
2.2. Time-Varying Elastance Model
2.3. Asynchrony Detection
2.4. Data Analysis
3. Results
4. Discussion
4.1. The Importance of Real-Time Assessment in AEs and
4.2. Limitations
5. Conclusions
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Data Availability Statement
Acknowledgments
Conflicts of Interest
References
- Murias, G.; Lucangelo, U.; Blanch, L. Patient-ventilator asynchrony. Curr. Opin. Crit. Care 2016, 22, 53–59. [Google Scholar] [CrossRef]
- Pan, Q.; Jia, M.; Liu, Q.; Zhang, L.; Pan, J.; Lu, F.; Zhang, Z.; Fang, L.; Ge, H. Identifying Patient–Ventilator Asynchrony on a Small Dataset Using Image-Based Transfer Learning. Sensors 2021, 21, 4149. [Google Scholar] [CrossRef] [PubMed]
- Khirani, S.; Polese, G.; Aliverti, A.; Appendini, L.; Nucci, G.; Pedotti, A.; Colledan, M.; Lucianetti, A.; Baconnier, P.; Rossi, A. On-line monitoring of lung mechanics during spontaneous breathing: A physiological study. Respir. Med. 2010, 104, 463–471. [Google Scholar] [CrossRef] [PubMed][Green Version]
- Damanhuri, N.S.; Ariffin, N.N.N.; Othman, N.A.; Chiew, Y.S.; Ralib, A.M.; Noor, M.B.M.; Docherty, P.D.; Chase, J.G. Assessing SB effort via a non-invasive model-based method in mechanically ventilated patients in malaysian ICU hospital. Indones. J. Electr. Eng. Comput. Sci. 2019, 15, 1232–1240. [Google Scholar] [CrossRef]
- Sinderby, C.; Liu, S.; Colombo, D.; Camarotta, G.; Slutsky, A.S.; Navalesi, P.; Beck, J. An automated and standardized neural index to quantify patient-ventilator interaction. Crit. Care 2013, 17, 1–9. [Google Scholar] [CrossRef]
- De Wit, M.; Miller, K.B.; Green, D.A.; Ostman, H.E.; Gennings, C.; Epstein, S.K. Ineffective triggering predicts increased duration of mechanical ventilation. Crit. Care Med. 2009, 37, 2740–2745. [Google Scholar] [PubMed]
- Thille, A.W.; Rodriguez, P.; Cabello, B.; Lellouche, F.; Brochard, L. Patient-ventilator asynchrony during assisted mechanical ventilation. Intensive Care Med. 2006, 32, 1515–1522. [Google Scholar] [CrossRef] [PubMed]
- Blanch, L.; Villagra, A.; Sales, B.; Montanya, J.; Lucangelo, U.; Luján, M.; García-Esquirol, O.; Chacón, E.; Estruga, A.; Oliva, J.C. Asynchronies during mechanical ventilation are associated with mortality. Intensive Care Med. 2015, 41, 633–641. [Google Scholar] [CrossRef]
- Zhang, L.; Mao, K.; Duan, K.; Fang, S.; Lu, Y.; Gong, Q.; Lu, F.; Jiang, Y.; Jiang, L.; Fang, W. Detection of patient-ventilator asynchrony from mechanical ventilation waveforms using a two-layer long short-term memory neural network. Comput. Biol. Med. 2020, 120, 103721. [Google Scholar] [CrossRef]
- Colombo, D.; Cammarota, G.; Alemani, M.; Carenzo, L.; Barra, F.L.; Vaschetto, R.; Slutsky, A.S.; Della Corte, F.; Navalesi, P. Efficacy of ventilator waveforms observation in detecting patient–ventilator asynchrony. Crit. Care Med. 2011, 39, 2452–2457. [Google Scholar] [CrossRef]
- Epstein, S.K. How often does patient-ventilator asynchrony occur and what are the consequences? Respir. Care 2011, 56, 25–38. [Google Scholar] [CrossRef] [PubMed]
- Loo, N.L.; Chiew, Y.S.; Tan, C.P.; Mat-Nor, M.B.; Ralib, A.M. A machine learning approach to assess magnitude of asynchrony breathing. Biomed. Signal Process. Control 2021, 66, 102505. [Google Scholar] [CrossRef]
- Al-Hetari, H.Y.; Kabir, M.N.; Al-Rumaima, M.A.; Al-Naggar, N.Q.; Alginahi, Y.M.; Hasan, M.M. A Mathematical Model of Lung Functionality using Pressure Signal for Volume-Controlled Ventilation. In Proceedings of the 2020 IEEE International Conference on Automatic Control and Intelligent Systems (I2CACIS), Shah Alam, Malaysia, 20 June 2020; pp. 135–140. [Google Scholar]
- Chanques, G.; Kress, J.P.; Pohlman, A.; Patel, S.; Poston, J.; Jaber, S.; Hall, J.B. Impact of ventilator adjustment and sedation–analgesia practices on severe asynchrony in patients ventilated in assist-control mode. Crit. Care Med. 2013, 41, 2177–2187. [Google Scholar] [CrossRef] [PubMed]
- Chiew, Y.S.; Pretty, C.; Docherty, P.D.; Lambermont, B.; Shaw, G.M.; Desaive, T.; Chase, J.G. Time-varying respiratory system elastance: A physiological model for patients who are spontaneously breathing. PLoS ONE 2015, 10, e0114847. [Google Scholar] [CrossRef] [PubMed]
- Poole, S.F.; Chiew, Y.S.; Redmond, D.P.; Davidson, S.M.; Damanhuri, N.S.; Pretty, C.; Docherty, P.D.; Desaive, T.; Shaw, G.M.; Chase, J.G. Real-time breath-to-breath asynchrony event detection using time-varying respiratory elastance model. IFAC Proc. Vol. 2014, 47, 5629–5634. [Google Scholar] [CrossRef]
- Chiew, Y.S.; Chase, J.G.; Arunachalam, G.; Tan, C.P.; Loo, N.L.; Chiew, Y.W.; Ralib, A.M.; Nor, M.B.M. Clinical application of respiratory elastance (CARE trial) for mechanically ventilated respiratory failure patients: A model-based study. IFAC-PapersOnLine 2018, 51, 209–214. [Google Scholar] [CrossRef]
- Docherty, P.D.; Chase, J.G.; David, T. Characterisation of the iterative integral parameter identification method. Med. Biol. Eng. Comput. 2012, 50, 127–134. [Google Scholar] [CrossRef]
- Hann, C.E.; Chase, J.G.; Lin, J.; Lotz, T.; Doran, C.V.; Shaw, G.M. Integral-based parameter identification for long-term dynamic verification of a glucose–insulin system model. Comput. Methods Programs Biomed. 2005, 77, 259–270. [Google Scholar] [CrossRef]
- Kim, K.T.; Redmond, D.P.; Morton, S.E.; Howe, S.L.; Chiew, Y.S.; Chase, J.G. Quantifying patient effort in spontaneously breathing patient using negative component of dynamic Elastance. IFAC-PapersOnLine 2017, 50, 5486–5491. [Google Scholar] [CrossRef]
- Chiew, Y.S.; Tan, C.P.; Chase, J.G.; Chiew, Y.W.; Desaive, T.; Ralib, A.M.; Nor, M.B.M. Assessing mechanical ventilation asynchrony through iterative airway pressure reconstruction. Comput. Methods Programs Biomed. 2018, 157, 217–224. [Google Scholar] [CrossRef]
- Lee, J.W.W.; Chiew, Y.S.; Wang, X.; Tan, C.P.; Mat Nor, M.B.; Damanhuri, N.S.; Chase, J.G. Stochastic Modelling of Respiratory System Elastance for Mechanically Ventilated Respiratory Failure Patients. Ann. Biomed. Eng. 2021, 49, 1–16. [Google Scholar] [CrossRef] [PubMed]
- Damanhuri, N.S.; Chiew, Y.S.; Othman, N.A.; Docherty, P.D.; Pretty, C.G.; Shaw, G.M.; Desaive, T.; Chase, J.G. Assessing respiratory mechanics using pressure reconstruction method in mechanically ventilated spontaneous breathing patient. Comput. Methods Programs Biomed. 2016, 130, 175–185. [Google Scholar] [CrossRef] [PubMed]
- Chiew, Y.S.; Pretty, C.G.; Shaw, G.M.; Chiew, Y.W.; Lambermont, B.; Desaive, T.; Chase, J.G. Feasibility of titrating PEEP to minimum elastance for mechanically ventilated patients. Pilot Feasibility Stud. 2015, 1, 1–10. [Google Scholar] [CrossRef]
- Chiew, Y.S.; Chase, J.G.; Shaw, G.M.; Sundaresan, A.; Desaive, T. Model-based PEEP optimisation in mechanical ventilation. Biomed. Eng. Online 2011, 10, 1–16. [Google Scholar] [CrossRef]
- Carvalho, A.R.S.; Jandre, F.C.; Pino, A.V.; Bozza, F.A.; Salluh, J.; Rodrigues, R.; Ascoli, F.O.; Giannella-Neto, A. Positive end-expiratory pressure at minimal respiratory elastance represents the best compromise between mechanical stress and lung aeration in oleic acid induced lung injury. Crit. Care 2007, 11, 1–13. [Google Scholar] [CrossRef] [PubMed]
- Guérin, C.; Richard, J.-C. Comparison of 2 correction methods for absolute values of esophageal pressure in subjects with acute hypoxemic respiratory failure, mechanically ventilated in the ICU. Respir. Care 2012, 57, 2045–2051. [Google Scholar]
- Damanhuri, N.S.; Chiew, Y.S.; Docherty, P.D.; Othman, N.A.; Shaw, G.M.; Desaive, T.; Chase, J.G. Negative Lung Elastance in Mechanically Ventilated Spontaneously Breathing Patient. IFAC-PapersOnLine 2017, 50, 15179–15184. [Google Scholar] [CrossRef]




| Patient No | Gender | Age | Clinical Diagnosis |
|---|---|---|---|
| 1 | Female | 64 | Pneumonia |
| 2 | Female | 34 | Pneumonia |
| 3 | Male | 43 | Pneumonia |
| 4 | Male | 74 | Pneumonia |
| 5 | Male | 48 | ARDS |
| 6 | Female | 43 | Thyroid |
| 7 | Male | 52 | CA Lung and SVC Obstruction |
| 8 | Male | 64 | Respiratory Failure, HAP, ESRF |
| 9 | Female | 66 | Septic shock 2° to HAP with Bronchospasms |
| 10 | Female | 63 | Septic shock |
| Patient No | Day | Ventilation Mode | Breathing Cycle | No of AEs | AI % | AUC
Median [IQR] (cmH2O·s/L) | PEEP (cmH2O) |
|---|---|---|---|---|---|---|---|
| 1 | 1 | SIMV VCV | 1370 | 14 | 1.02 | 27.59 [21.98–33.00] | 3–5 |
| 2 | SIMV VCV | 1853 | 254 | 13.71 | 21.97 [15.36–27.78] | 12–19 | |
| 2 | 1 | SIMV PCV | 1469 | 32 | 2.18 | 36.15 [32.27–38.22] | 8–9 |
| 2 | SIMV PCV | 1816 | 43 | 2.37 | 32.75 [27.50–34.52] | 15–17 | |
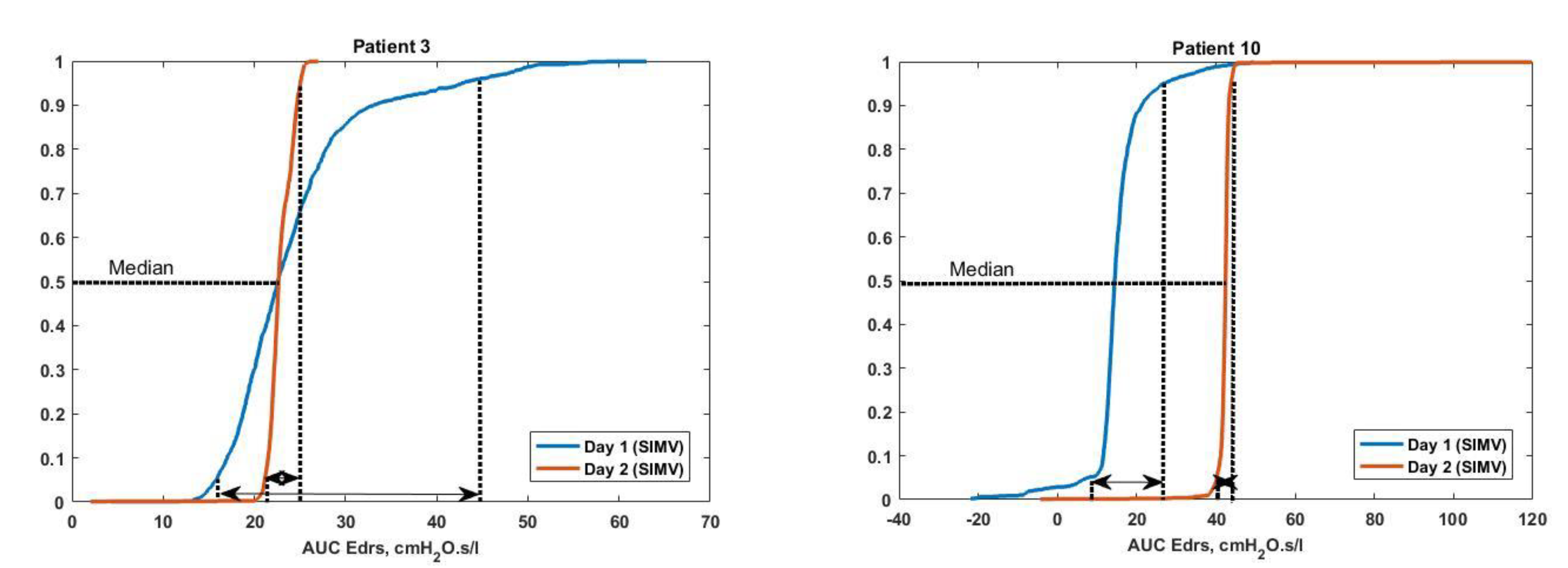
| 3 | 1 | SIMV VCV | 1321 | 124 | 9.39 | 22.58 [19.37–26.73] | 9–10 |
| 2 | SIMV VCV | 1380 | 0 | 0 | 22.79 [22.11–24.26] | 10–11 | |
| 4 | 1 | SIMV VCV | 1461 | 94 | 6.43 | 22.02 [20.11–26.12] | 8–18 |
| 2 | SIMV VCV | 1349 | 129 | 9.56 | 16.16 [13.82–1860] | 6–15 | |
| 5 | 1 | SIMV VCV | 1473 | 6 | 0.41 | 30.67 [27.86–34.02] | 11–12 |
| 2 | SIMV VCV | 1389 | 115 | 8.28 | 13.79 [11.28–15.83] | 10–12 | |
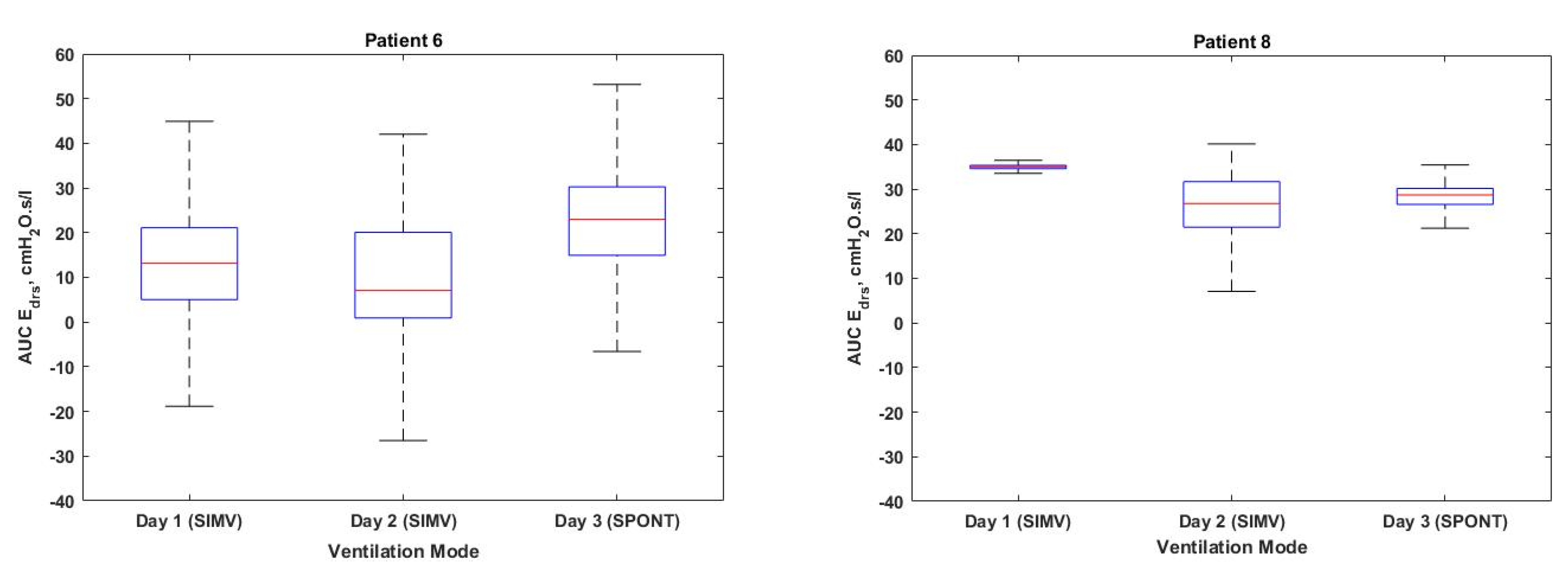
| 6 | 1 | SIMV VCV | 1418 | 452 | 31.88 | 12.10 [3.90–20.25] | 12–14 |
| 2 | SIMV VCV | 1261 | 509 | 40.36 | 7.11 [0.92–20.09] | 12–13 | |
| 3 | SPONT PAV | 1564 | 75 | 4.80 | 23.32 [15.72–30.28] | 10–12 | |
| 7 | 1 | SIMV PCV | 1258 | 58 | 4.61 | 25.88 [20.37–2897] | 8–17 |
| 2 | SIMV PCV | 1077 | 405 | 37.60 | 19.47 [14.08–35.48] | 10–14 | |
| 8 | 1 | SIMV VCV | 1240 | 0 | 0 | 35.01 [34.56–35.44] | 12–13 |
| 2 | SIMV VCV | 1258 | 20 | 1.59 | 24.89 [19.22–30.90] | 12–13 | |
| 3 | SPONT PAV | 1602 | 3 | 0.19 | 28.57 [26.33–29.81] | 8–10 | |
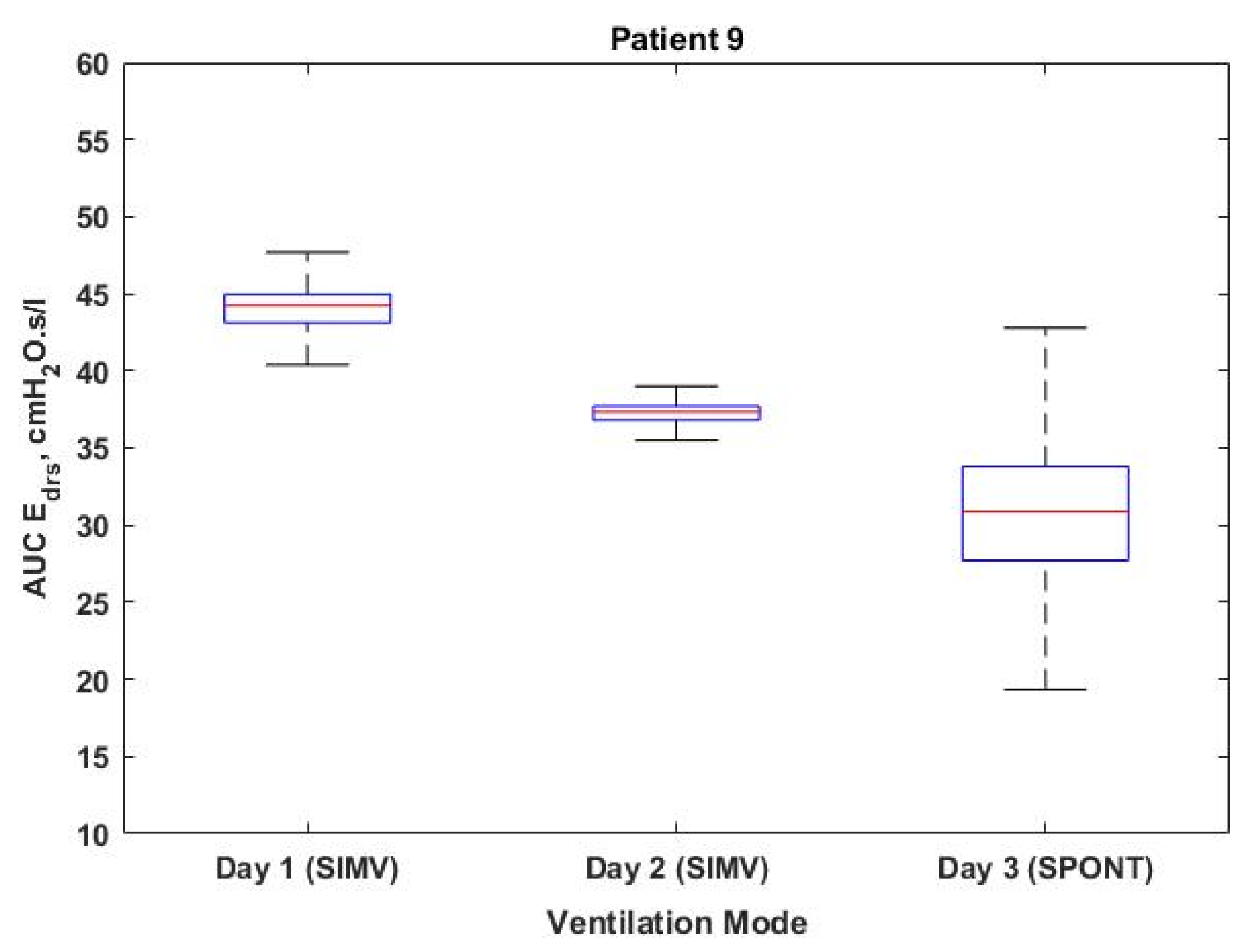
| 9 | 1 | SIMV VCV | 1188 | 0 | 0 | 44.16 [43.10–44.92] | 12–13 |
| 2 | SIMV VCV | 1160 | 12 | 1.03 | 37.34 [36.82–37.71] | 12–13 | |
| 3 | SPONT PAV | 1456 | 3 | 0.21 | 32.04 [28.59–35.19] | 12–13 | |
| 10 | 1 | SIMV PCV | 1645 | 127 | 7.72 | 14.26 [12.88–16.25] | 16–31 |
| 2 | SIMV PCV | 1314 | 2 | 0.15 | 42.45 [41.91–42.86] | 10–11 |
Publisher’s Note: MDPI stays neutral with regard to jurisdictional claims in published maps and institutional affiliations. |
© 2021 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Muhamad Sauki, N.S.; Damanhuri, N.S.; Othman, N.A.; Chiew Meng, B.C.; Chiew, Y.S.; Mat Nor, M.B. Assessing the Asynchrony Event Based on the Ventilation Mode for Mechanically Ventilated Patients in ICU. Bioengineering 2021, 8, 222. https://doi.org/10.3390/bioengineering8120222
Muhamad Sauki NS, Damanhuri NS, Othman NA, Chiew Meng BC, Chiew YS, Mat Nor MB. Assessing the Asynchrony Event Based on the Ventilation Mode for Mechanically Ventilated Patients in ICU. Bioengineering. 2021; 8(12):222. https://doi.org/10.3390/bioengineering8120222
Chicago/Turabian StyleMuhamad Sauki, Nur Sa’adah, Nor Salwa Damanhuri, Nor Azlan Othman, Belinda Chong Chiew Meng, Yeong Shiong Chiew, and Mohd Basri Mat Nor. 2021. "Assessing the Asynchrony Event Based on the Ventilation Mode for Mechanically Ventilated Patients in ICU" Bioengineering 8, no. 12: 222. https://doi.org/10.3390/bioengineering8120222
APA StyleMuhamad Sauki, N. S., Damanhuri, N. S., Othman, N. A., Chiew Meng, B. C., Chiew, Y. S., & Mat Nor, M. B. (2021). Assessing the Asynchrony Event Based on the Ventilation Mode for Mechanically Ventilated Patients in ICU. Bioengineering, 8(12), 222. https://doi.org/10.3390/bioengineering8120222






