Is Culture Expansion Necessary in Autologous Mesenchymal Stromal Cell Therapy to Obtain Superior Results in the Management of Knee Osteoarthritis?—Meta-Analysis of Randomized Controlled Trials
Abstract
1. Introduction
2. Materials and Methods
2.1. Search Strategy
2.2. Inclusion Criteria
| Population: | Patients with knee osteoarthritis. |
| Intervention: | Culture-expanded MSC therapy. |
| Comparator: | Non-cultured MSC therapy. |
| Outcomes: | Visual Analog Score (VAS) for Pain, Western Ontario McMaster Universities Osteoarthritis Index (WOMAC), Lysholm Knee Scale (Lysholm), Knee Osteoarthritis Outcome Score (KOOS), and adverse events. |
| Study Design: | Randomized Controlled Trials. |
2.3. Exclusion Criteria
- RCTs on MSC based therapy for knee osteoarthritis without mention on the source of MSCs utilized in the study;
- In vitro studies involving stem cell therapy;
- Studies of observational nature and interventional studies without an appropriate comparison group;
- Studies conduction animal models of knee osteoarthritis investigating stem cell therapy;
- Review articles and in vitro studies involving stem cell therapy.
2.4. Data Extraction
- Study characteristics: year of publication, authors, country, level of evidence, number of patients enrolled;
- Baseline characteristics: mean age, gender proportions, Kellgren Lawrence grade of osteoarthritis, source of MSC utilized, intervention for both the groups, delivery method of MSCs, follow-up duration, and assessment parameters utilized;
- Efficacy Outcomes: VAS for pain, Functional outcomes like WOMAC score, Lysholm and KOOS score;
- Safety Outcomes: Adverse events in the included studies.
2.5. Risk of Bias and Quality Assessment
2.6. Statistical Analysis
3. Results
3.1. Search Results
3.2. Quality Assessment
3.3. Efficacy Outcomes
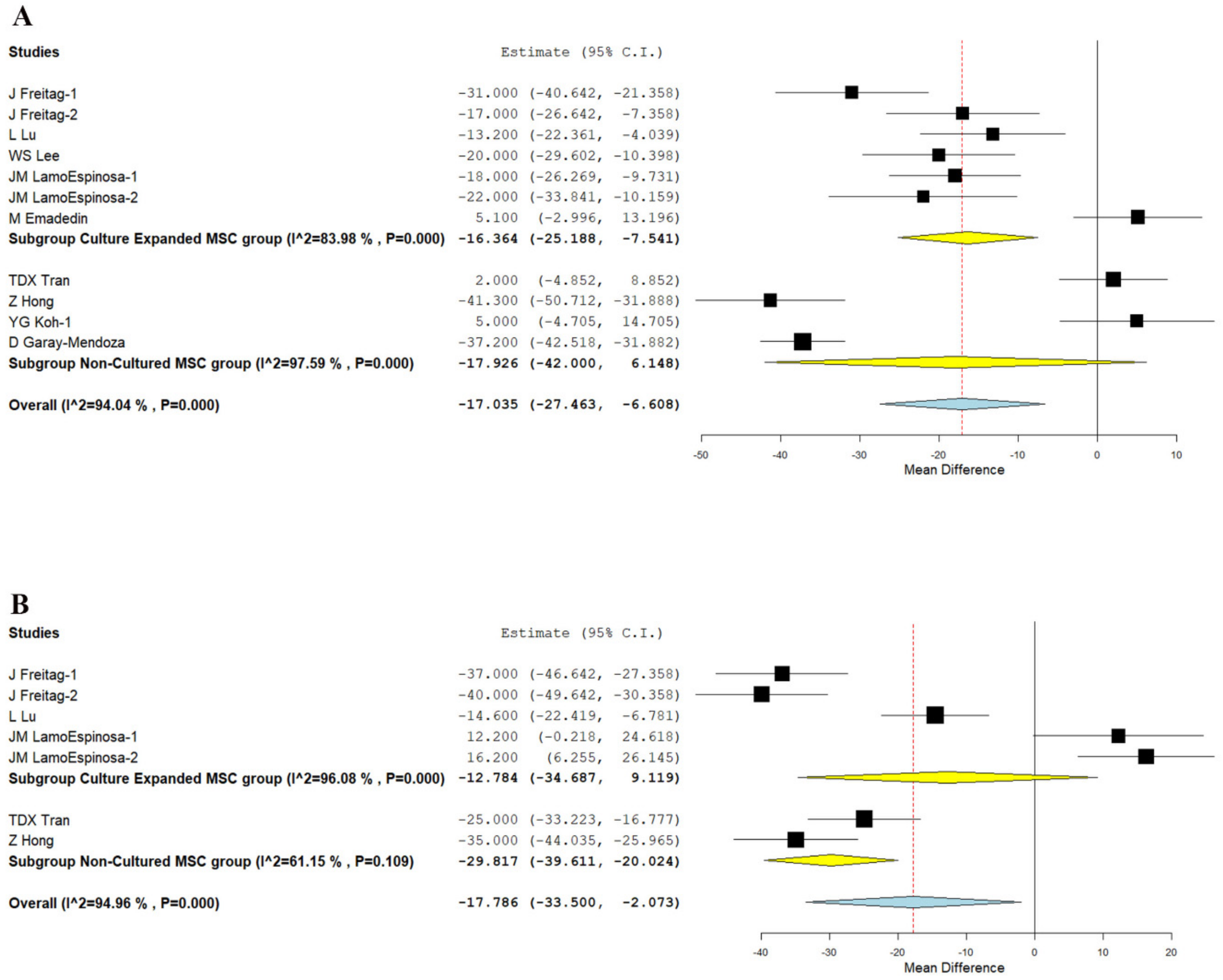
3.3.1. Visual Analog Scale for Pain
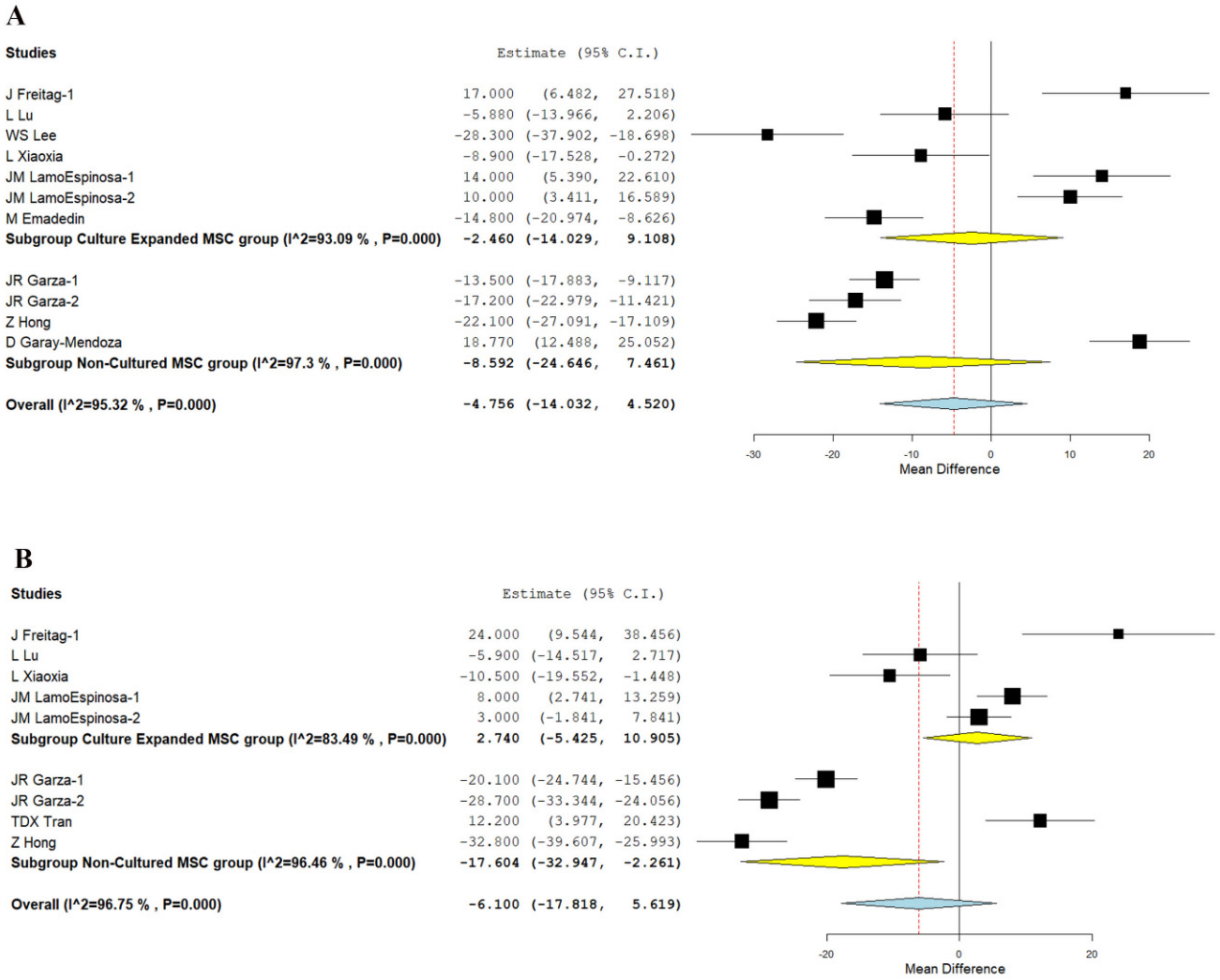
3.3.2. WOMAC Score
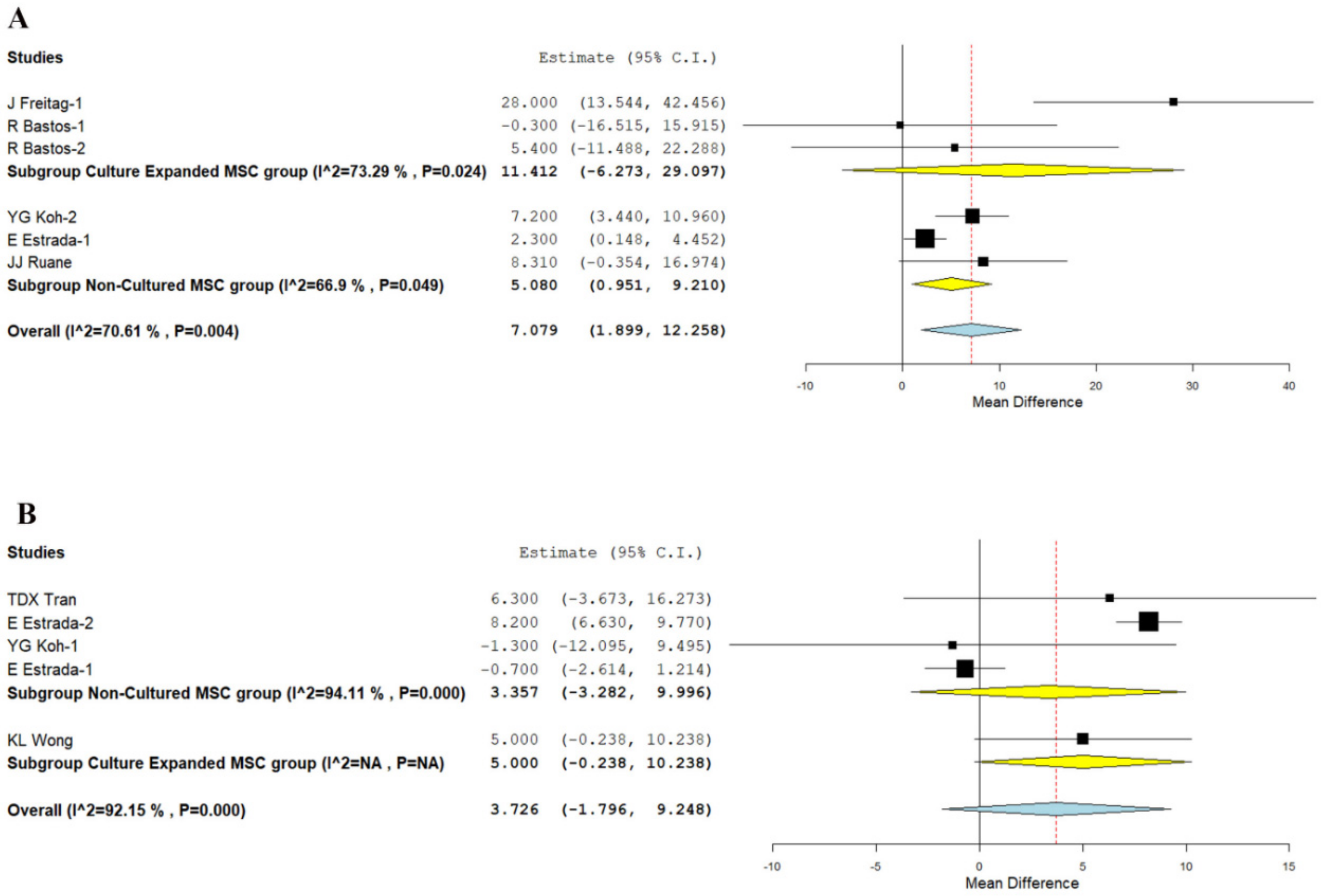
3.3.3. KOOS Score
3.3.4. Lysholm Knee Score
3.3.5. Safety
3.3.6. Sensitivity Analysis
3.3.7. Subgroup Analysis
3.3.8. Publications Bias
4. Discussion
4.1. Main Finding
- Although at six months, culture expanded MSCs showed significantly better VAS improvement (p < 0.001), it was not consistent at 1 year (p = 0.253). Non-cultured MSCs, on the other hand, demonstrated significant VAS improvement in the long term (p < 0.001), which was not noted in short term (p = 0.144).
- Similarly, adipose-derived non-cultured MSCs outperformed culture-expanded MSCs in both the short term (six months) and long term (12 months) in functional outcome parameters, such as WOMAC (p < 0.001, p = 0.025), Lysholm (p < 0.006), and KOOS (p < 0.003) scores, respectively, compared to their controls.
- No significant adverse events were noted in either culture expanded MSC (p = 0.485) or non-cultured MSC (p = 1.000) groups compared to their controls.
4.2. Limitations
5. Conclusions
Supplementary Materials
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Data Availability Statement
Conflicts of Interest
References
- Iijima, H.; Isho, T.; Kuroki, H.; Takahashi, M.; Aoyama, T. Effectiveness of mesenchymal stem cells for treating patients with knee osteoarthritis: A meta-analysis toward the establishment of effective regenerative rehabilitation. NPJ Regen. Med. 2018, 3, 15. [Google Scholar] [CrossRef]
- Nekanti, U.; Mohanty, L.; Venugopal, P.; Balasubramanian, S.; Totey, S.; Ta, M. Optimization and scale-up of Wharton’s jelly-derived mesenchymal stem cells for clinical applications. Stem Cell Res. 2010, 5, 244–254. [Google Scholar] [CrossRef]
- Caruso, S.R.; Orellana, M.D.; Mizukami, A.; Fernandes, T.R.; Fontes, A.M.; Suazo, C.A.T.; Oliveira, V.C.; Covas, D.T.; Swiech, K. Growth and functional harvesting of human mesenchymal stromal cells cultured on a microcarrier-based system. Biotechnol. Prog. 2014, 30, 889–895. [Google Scholar] [CrossRef] [PubMed]
- El-Kheir, W.A.; Gabr, H.; Awad, M.R.; Ghannam, O.; Barakat, Y.; Farghali, H.A.M.A.; El Maadawi, Z.M.; Ewes, I.; Sabaawy, H.E. Autologous Bone Marrow-Derived Cell Therapy Combined with Physical Therapy Induces Functional Improvement in Chronic Spinal Cord Injury Patients. Cell Transplant. 2014, 23, 729–745. [Google Scholar] [CrossRef] [PubMed]
- Vaquero, J.; Zurita, M.; Rico, M.A.; Aguayo, C.; Bonilla, C.; Marin, E.; Tapiador, N.; Sevilla, M.; Vazquez, D.; Carballido, J.; et al. Intrathecal administration of autologous mesenchymal stromal cells for spinal cord injury: Safety and efficacy of the 100/3 guideline. Cytotherapy 2018, 20, 806–819. [Google Scholar] [CrossRef] [PubMed]
- Bin Hassan, M.N.F.; Yazid, M.D.; Yunus, M.H.M.; Chowdhury, S.R.; Lokanathan, Y.; Idrus, R.B.H.; Ng, A.M.H.; Law, J.X. Large-Scale Expansion of Human Mesenchymal Stem Cells. Stem Cells Int. 2020, 2020, e9529465. [Google Scholar] [CrossRef]
- Jung, S.; Panchalingam, K.; Wuerth, R.D.; Rosenberg, L.; Behie, L.A. Large-scale production of human mesenchymal stem cells for clinical applications. Biotechnol. Appl. Biochem. 2012, 59, 106–120. [Google Scholar] [CrossRef]
- Centeno, C.J.; Busse, D.; Kisiday, J.; Keohan, C.; Freeman, M.; Karli, D. Increased knee cartilage volume in degenerative joint disease using percutaneously implanted, autologous mesenchymal stem cells. Pain Physician 2008, 11, 343–353. [Google Scholar]
- Spakova, T.; Plsikova, J.; Harvanova, D.; Lacko, M.; Stolfa, S.; Rosocha, J. Influence of Kartogenin on Chondrogenic Differentiation of Human Bone Marrow-Derived MSCs in 2D Culture and in Co-Cultivation with OA Osteochondral Explant. Molecules 2018, 23, 181. [Google Scholar] [CrossRef] [PubMed]
- Zhang, Y.; Hao, C.; Guo, W.; Peng, X.; Wang, M.; Yang, Z.; Li, X.; Zhang, X.; Chen, M.; Sui, X.; et al. Co-culture of hWJMSCs and pACs in double biomimetic ACECM oriented scaffold enhances mechanical properties and accelerates articular cartilage regeneration in a caprine model. Stem Cell Res. Ther. 2020, 11, 180. [Google Scholar] [CrossRef] [PubMed]
- Schmal, H.; Kowal, J.M.; Kassem, M.; Seidenstuecker, M.; Bernstein, A.; Böttiger, K.; Xiong, T.; Südkamp, N.P.; Kubosch, E.J. Comparison of Regenerative Tissue Quality following Matrix-Associated Cell Implantation Using Amplified Chondrocytes Compared to Synovium-Derived Stem Cells in a Rabbit Model for Cartilage Lesions. Stem Cells Int. 2018, 2018, e4142031. [Google Scholar] [CrossRef]
- Benz, K.; Stippich, C.; Freudigmann, C.; Mollenhauer, J.A.; Aicher, W.K. Maintenance of “stem cell” features of cartilage cell sub-populations during in vitro propagation. J. Transl. Med. 2013, 11, 27. [Google Scholar] [CrossRef] [PubMed]
- Yang, Y.-H.; Lee, A.J.; Barabino, G.A. Coculture-Driven Mesenchymal Stem Cell-Differentiated Articular Chondrocyte-Like Cells Support Neocartilage Development. Stem Cells Transl. Med. 2012, 1, 843–854. [Google Scholar] [CrossRef] [PubMed]
- Furlan, A.; Malmivaara, A.; Chou, R.; Maher, C.; Deyo, R.A.; Schoene, M.L.; Bronfort, G.; van Tulder, M. 2015 Updated Method Guideline for Systematic Reviews in the Cochrane Back and Neck Group. Spine 2015, 40, 1660–1673. [Google Scholar] [CrossRef] [PubMed]
- Moher, D.; Liberati, A.; Tetzlaff, J.; Altman, D.G.; The PRISMA Group. Preferred reporting items for systematic reviews and meta-analyses: The PRISMA Statement. PLoS Med. 2009, 6, e1000097. [Google Scholar] [CrossRef] [PubMed]
- Sterne, J.A.C.; Savović, J.; Page, M.J.; Elbers, R.G.; Blencowe, N.S.; Boutron, I.; Cates, C.J.; Cheng, H.-Y.; Corbett, M.S.; Eldridge, S.M.; et al. RoB 2: A revised tool for assessing risk of bias in randomised trials. BMJ 2019, 366, l4898. [Google Scholar] [CrossRef]
- Wallace, B.C.; Dahabreh, I.J.; Trikalinos, T.A.; Lau, J.; Trow, P.; Schmid, C.H. Closing the Gap between Methodologists and End-Users:Ras a Computational Back-End. J. Stat. Softw. 2012, 49, 1–15. [Google Scholar] [CrossRef]
- Higgins, J.P.T.; Thompson, S.G.; Deeks, J.J.; Altman, D.G. Measuring inconsistency in meta-analyses. BMJ 2003, 327, 557–560. [Google Scholar] [CrossRef]
- Estrada, E.; Décima, J.L.; Rodríguez, M.; Di Tomaso, M.; Roberti, J. Patient-Reported Outcomes after Platelet-Rich Plasma, Bone Marrow Aspirate, and Adipose-Derived Mesenchymal Stem Cell Injections for Symptomatic Knee Osteoarthritis. Clin. Med. Insights Arthritis Musculoskelet. Disord. 2020, 13, 1179544120931086. [Google Scholar] [CrossRef] [PubMed]
- Garay-Mendoza, D.; Villarreal-Martínez, L.; Garza-Bedolla, A.; Pérez-Garza, D.M.; Acosta-Olivo, C.; Vilchez-Cavazos, F.; Diaz-Hutchinson, C.; Gómez-Almaguer, D.; Jaime-Pérez, J.C.; Mancías-Guerra, C. The effect of intra-articular injection of autologous bone marrow stem cells on pain and knee function in patients with osteoarthritis. Int. J. Rheum. Dis. 2017, 21, 140–147. [Google Scholar] [CrossRef]
- Freitag, J.; Bates, D.; Wickham, J.; Shah, K.; Huguenin, L.; Tenen, A.; Paterson, K.; Boyd, R. Adipose-derived mesenchymal stem cell therapy in the treatment of knee osteoarthritis: A randomized controlled trial. Regen. Med. 2019, 14, 213–230. [Google Scholar] [CrossRef] [PubMed]
- Lamo-Espinosa, J.M.; Mora, G.; Blanco, J.F.; Granero-Moltó, F.; Nuñez-Córdoba, J.M.; Sánchez-Echenique, C.; Bondía, J.M.; Aquerreta, J.D.; Andreu, E.J.; Ornilla, E.; et al. Intra-articular injection of two different doses of autologous bone marrow mesenchymal stem cells versus hyaluronic acid in the treatment of knee osteoarthritis: Multicenter randomized controlled clinical trial (phase I/II). J. Transl. Med. 2016, 14, 246. [Google Scholar] [CrossRef] [PubMed]
- Garza, J.R.; Campbell, R.E.; Tjoumakaris, F.P.; Freedman, K.B.; Miller, L.S.; Maria, D.S.; Tucker, B.S. Clinical Efficacy of Intra-articular Mesenchymal Stromal Cells for the Treatment of Knee Osteoarthritis: A Double-Blinded Prospective Randomized Controlled Clinical Trial. Am. J. Sports Med. 2020, 48, 588–598. [Google Scholar] [CrossRef]
- Wong, K.L.; Lee, K.B.L.; Tai, B.C.; Law, P.; Lee, E.H.; Hui, J.H. Injectable Cultured Bone Marrow–Derived Mesenchymal Stem Cells in Varus Knees with Cartilage Defects Undergoing High Tibial Osteotomy: A Prospective, Randomized Controlled Clinical Trial with 2 Years’ Follow-up. Arthrosc. J. Arthrosc. Relat. Surg. 2013, 29, 2020–2028. [Google Scholar] [CrossRef]
- Lu, L.; Dai, C.; Zhang, Z.; Du, H.; Li, S.; Ye, P.; Fu, Q.; Zhang, L.; Wu, X.; Dong, Y.; et al. Treatment of knee osteoarthritis with intra-articular injection of autologous adipose-derived mesenchymal progenitor cells: A prospective, randomized, double-blind, active-controlled, phase IIb clinical trial. Stem Cell Res. Ther. 2019, 10, 143. [Google Scholar] [CrossRef]
- Emadedin, M.; Labibzadeh, N.; Liastani, M.G.; Karimi, A.; Jaroughi, N.; Bolurieh, T.; Hosseini, S.-E.; Baharvand, H.; Aghdami, N. Intra-articular implantation of autologous bone marrow–derived mesenchymal stromal cells to treat knee osteoarthritis: A randomized, triple-blind, placebo-controlled phase 1/2 clinical trial. Cytotherapy 2018, 20, 1238–1246. [Google Scholar] [CrossRef] [PubMed]
- Bastos, R.; Mathias, M.; Andrade, R.; Amaral, R.D.; Schott, V.; Balduino, A.; Bastos, R.; Oliveira, J.M.; Reis, R.L.; Rodeo, S.; et al. Intra-articular injection of culture-expanded mesenchymal stem cells with or without addition of platelet-rich plasma is effective in decreasing pain and symptoms in knee osteoarthritis: A controlled, double-blind clinical trial. Knee Surg. Sports Traumatol. Arthrosc. 2019, 28, 1989–1999. [Google Scholar] [CrossRef]
- Wakitani, S.; Imoto, K.; Yamamoto, T.; Saito, M.; Murata, N.; Yoneda, M. Human autologous culture expanded bone marrow mesenchymal cell transplantation for repair of cartilage defects in osteoarthritic knees. Osteoarthr. Cartil. 2002, 10, 199–206. [Google Scholar] [CrossRef] [PubMed]
- Tran, T.D.X.; Wu, C.-M.; Dubey, N.K.; Deng, Y.-H.; Su, C.-W.; Pham, T.T.; Le, P.B.T.; Sestili, P.; Deng, W.-P. Time- and Kellgren–Lawrence Grade-Dependent Changes in Intra-Articularly Transplanted Stromal Vascular Fraction in Osteoarthritic Patients. Cells 2019, 8, 308. [Google Scholar] [CrossRef]
- Lee, W.; Kim, H.J.; Kim, K.; Kim, G.B.; Jin, W. Intra-Articular Injection of Autologous Adipose Tissue-Derived Mesenchymal Stem Cells for the Treatment of Knee Osteoarthritis: A Phase IIb, Randomized, Placebo-Controlled Clinical Trial. Stem Cells Transl. Med. 2019, 8, 504–511. [Google Scholar] [CrossRef]
- Koh, Y.-G.; Choi, Y.-J. Infrapatellar fat pad-derived mesenchymal stem cell therapy for knee osteoarthritis. Knee 2012, 19, 902–907. [Google Scholar] [CrossRef] [PubMed]
- Koh, Y.-G.; Kwon, O.-R.; Kim, Y.-S.; Choi, Y.-J. Comparative Outcomes of Open-Wedge High Tibial Osteotomy with Platelet-Rich Plasma Alone or in Combination With Mesenchymal Stem Cell Treatment: A Prospective Study. Arthrosc. J. Arthrosc. Relat. Surg. 2014, 30, 1453–1460. [Google Scholar] [CrossRef]
- Hong, Z.; Chen, J.; Zhang, S.; Zhao, C.; Bi, M.; Chen, X.; Bi, Q. Intra-articular injection of autologous adipose-derived stromal vascular fractions for knee osteoarthritis: A double-blind randomized self-controlled trial. Int. Orthop. 2018, 43, 1123–1134. [Google Scholar] [CrossRef]
- Ruane, J.J.; Ross, A.; Zigmont, V.; McClure, D.; Gascon, G. A Single-Blinded Randomized Controlled Trial of Mesenchymal Stem Cell Therapy for the Treatment of Osteoarthritis of the Knee with Active Control. J. Stem Cells Regen. Med. 2021, 17, 3–17. [Google Scholar] [CrossRef] [PubMed]
- Lv, X.; Huang, C.; Yin, Z.; Hong, B.; Jiang, H.; Huang, X. Effectiveness of autologous bone marrow mesenchymal stem cell transplant for knee osteoarthritis. Chin. J. Cell Stem Cell 2015, 5, 28–32. [Google Scholar]
- Owen, M.; Friedenstein, A.J. Stromal Stem Cells: Marrow-Derived Osteogenic Precursors. Cell Mol. Biol. Vertebr. Hard Tissues 2007, 136, 42–60. [Google Scholar] [CrossRef]
- Friedenstein, A.J.; Chailakhjan, R.K.; Lalykina, K.S. The development of fibroblast colonies in monolayer cultures of guinea-pig bone marrow and spleen cells. Cell Prolif. 1970, 3, 393–403. [Google Scholar] [CrossRef]
- Haynesworth, S.; Goshima, J.; Goldberg, V.; Caplan, A. Characterization of cells with osteogenic potential from human marrow. Bone 1992, 13, 81–88. [Google Scholar] [CrossRef]
- Lazarus, H.M.; Haynesworth, S.E.; Gerson, S.L.; Rosenthal, N.S.; Caplan, A. Ex vivo expansion and subsequent infusion of human bone marrow-derived stromal progenitor cells (mesenchymal progenitor cells): Implications for therapeutic use. Bone Marrow Transplant. 1995, 16, 557–564. [Google Scholar]
- Pittenger, M.F.; Discher, D.E.; Péault, B.M.; Phinney, D.G.; Hare, J.M.; Caplan, A.I. Mesenchymal stem cell perspective: Cell biology to clinical progress. NPJ Regen. Med. 2019, 4, 22. [Google Scholar] [CrossRef]
- Pittenger, M.F. Mesenchymal Stem Cells from Adult Bone Marrow. In Mesenchymal Stem Cells; Humana Press: Totowa, NI, USA, 2008; Volume 449, pp. 27–44. [Google Scholar] [CrossRef]
- Sekiya, E.J.; Forte, A.; Kühn, T.I.B.D.B.; Janz, F.; Bydlowski, S.P.; Alves, A. Establishing a stem cell culture laboratory for clinical trials. Rev. Bras. Hematol. Hemoter. 2012, 34, 236–241. [Google Scholar] [CrossRef]
- Drela, K.; Stanaszek, L.; Nowakowski, A.; Kuczynska, Z.; Lukomska, B. Experimental Strategies of Mesenchymal Stem Cell Propagation: Adverse Events and Potential Risk of Functional Changes. Stem Cells Int. 2019, 2019, 7012692. [Google Scholar] [CrossRef] [PubMed]
- Antebi, B.; Ii, L.A.R.; Walker, K.P.; Asher, A.M.; Kamucheka, R.M.; Alvarado, L.; Mohammadipoor, A.; Cancio, L.C. Short-term physiological hypoxia potentiates the therapeutic function of mesenchymal stem cells. Stem Cell Res. Ther. 2018, 9, 265. [Google Scholar] [CrossRef] [PubMed]
- Maleki, M.; Ghanbarvand, F.; Behvarz, M.R.; Ejtemaei, M.; Ghadirkhomi, E. Comparison of Mesenchymal Stem Cell Markers in Multiple Human Adult Stem Cells. Int. J. Stem Cells 2014, 7, 118–126. [Google Scholar] [CrossRef] [PubMed]
- Penna, V.; Lipay, M.V.; Duailibi, M.T.; Duailibi, S.E. The likely role of proteolytic enzymes in unwanted differentiation of stem cells in culture. Futur. Sci. OA 2015, 1, FSO28. [Google Scholar] [CrossRef] [PubMed]
- Morris, C.; De Wreede, L.; Scholten, M.; Brand, R.; Van Biezen, A.; Sureda, A.; Dickmeiss, E.; Trneny, M.; Apperley, J.; Chiusolo, P.; et al. Should the standard dimethyl sulfoxide concentration be reduced? Results of a European Group for Blood and Marrow Transplantation prospective noninterventional study on usage and side effects of dimethyl sulfoxide. Transfusion 2014, 54, 2514–2522. [Google Scholar] [CrossRef]
- Mastrolia, I.; Foppiani, E.M.; Murgia, A.; Candini, O.; Samarelli, A.V.; Grisendi, G.; Veronesi, E.; Horwitz, E.M.; Dominici, M. Challenges in Clinical Development of Mesenchymal Stromal/Stem Cells: Concise Review. Stem Cells Transl. Med. 2019, 8, 1135–1148. [Google Scholar] [CrossRef] [PubMed]
- Bae, Y.-J.; Kwon, Y.-R.; Kim, H.J.; Lee, S.; Kim, Y.-J. Enhanced differentiation of mesenchymal stromal cells by three-dimensional culture and azacitidine. Blood Res. 2017, 52, 18–24. [Google Scholar] [CrossRef]
- Tan, C.; Shichinohe, H.; Wang, Z.; Hamauchi, S.; Abumiya, T.; Nakayama, N.; Kazumata, K.; Ito, T.; Kudo, K.; Takamoto, S.; et al. Feasibility and Efficiency of Human Bone Marrow Stromal Cell Culture with Allogeneic Platelet Lysate-Supplementation for Cell Therapy against Stroke. Stem Cells Int. 2016, 2016, 6104780. [Google Scholar] [CrossRef]
- Wuchter, P.; Vetter, M.; Saffrich, R.; Diehlmann, A.; Bieback, K.; Ho, A.D.; Horn, P. Evaluation of GMP-compliant culture media for in vitro expansion of human bone marrow mesenchymal stromal cells. Exp. Hematol. 2016, 44, 508–518. [Google Scholar] [CrossRef]
- Legzdina, D.; Romanauska, A.; Nikulshin, S.; Kozlovska, T.; Berzins, U. Characterization of Senescence of Culture-expanded Human Adipose-derived Mesenchymal Stem Cells. Int. J. Stem Cells 2016, 9, 124–136. [Google Scholar] [CrossRef] [PubMed]
- Kim, S.Y.; Im, K.; Park, S.N.; Kwon, J.; Kim, J.-A.; Choi, Q.; Hwang, S.M.; Han, S.-H.; Kwon, S.; Oh, I.-H.; et al. Asymmetric Aneuploidy in Mesenchymal Stromal Cells Detected by In Situ Karyotyping and Fluorescence In Situ Hybridization: Suggestions for Reference Values for Stem Cells. Stem Cells Dev. 2015, 24, 77–92. [Google Scholar] [CrossRef] [PubMed]
- Miura, M.; Miura, Y.; Padilla-Nash, H.M.; Molinolo, A.A.; Fu, B.; Patel, V.; Seo, B.; Sonoyama, W.; Zheng, J.J.; Baker, C.C.; et al. Accumulated Chromosomal Instability in Murine Bone Marrow Mesenchymal Stem Cells Leads to Malignant Transformation. Stem Cells 2006, 24, 1095–1103. [Google Scholar] [CrossRef] [PubMed]
- Jeong, J.-O.; Han, J.W.; Kim, J.M.; Cho, H.-J.; Park, C.; Lee, N.; Kim, D.-W.; Yoon, Y.-S. Malignant Tumor Formation after Transplantation of Short-Term Cultured Bone Marrow Mesenchymal Stem Cells in Experimental Myocardial Infarction and Diabetic Neuropathy. Circ. Res. 2011, 108, 1340–1347. [Google Scholar] [CrossRef]
- Wang, M.-Y.; Nestvold, J.; Rekdal, Ø.; Kvalheim, G.; Fodstad, Ø. A novel rat fibrosarcoma cell line from transformed bone marrow-derived mesenchymal stem cells with maintained in vitro and in vivo stemness properties. Exp. Cell Res. 2017, 352, 218–224. [Google Scholar] [CrossRef] [PubMed]
- Rubio, D.; Garcia-Castro, J.; Martín, M.C.; De La Fuente, R.; Cigudosa, J.C.; Lloyd, A.C.; Bernad, A. Spontaneous Human Adult Stem Cell Transformation. Cancer Res. 2005, 65, 3035–3039. [Google Scholar] [CrossRef]
- De La Fuente, R.; Bernad, A.; Garcia-Castro, J.; Martin, M.C.; Cigudosa, J.C. Retraction: Spontaneous Human Adult Stem Cell Transformation. Cancer Res. 2010, 70, 6682. [Google Scholar] [CrossRef]
- Hladik, D.; Höfig, I.; Oestreicher, U.; Beckers, J.; Matjanovski, M.; Bao, X.; Scherthan, H.; Atkinson, M.J.; Rosemann, M. Long-term culture of mesenchymal stem cells impairs ATM-dependent recognition of DNA breaks and increases genetic instability. Stem Cell Res. Ther. 2019, 10, 218. [Google Scholar] [CrossRef]
- Geraghty, R.J.; Capes-Davis, A.; Davis, J.M.; Downward, J.; Freshney, R.I.; Knezevic, I.; Lovell-Badge, R.; Masters, J.R.W.; Meredith, J.; Stacey, G.N.; et al. Guidelines for the use of cell lines in biomedical research. Br. J. Cancer 2014, 111, 1021–1046. [Google Scholar] [CrossRef]
- Verma, A.; Verma, M.; Singh, A. Animal tissue culture principles and applications. In Animal Biotechnology; Academic Press: Cambridge, MA, USA, 2020; pp. 269–293. [Google Scholar] [CrossRef]
- Langhans, S.A. Three-Dimensional In Vitro Cell Culture Models in Drug Discovery and Drug Repositioning. Front. Pharmacol. 2018, 9, 6. [Google Scholar] [CrossRef]
- Wikswo, J.P. The relevance and potential roles of microphysiological systems in biology and medicine. Exp. Biol. Med. 2014, 239, 1061–1072. [Google Scholar] [CrossRef] [PubMed]
- Marx, U.; Andersson, T.B.; Bahinski, A.; Beilmann, M.; Beken, S.; Cassee, F.R.; Cirit, M.; Daneshian, M.; Fitzpatrick, S.; Frey, O.; et al. Biology-inspired microphysiological system approaches to solve the prediction dilemma of substance testing. ALTEX 2016, 33, 272–321. [Google Scholar] [CrossRef]
- Hartung, T.; Gstraunthaler, G.; Coecke, S.; Lewis, D.; Blanck, O.; Balls, M. Good cell culture practice (GCCP)—An initiative for standardization and quality control of in vitro studies. The establishment of an ECVAM Task Force on GCCP. ALTEX 2001, 18, 75–78. [Google Scholar] [PubMed]
- Pamies, D. Advanced Good Cell Culture Practice for human primary, stem cell-derived and organoid models as well as microphysiological systems. ALTEX 2018, 35, 353–378. [Google Scholar] [CrossRef] [PubMed]
- Pamies, D.; Bal-Price, A.; Simeonov, A.; Tagle, D.; Allen, D.; Gerhold, D.; Yin, D.; Pistollato, F.; Inutsuka, T.; Sullivan, K.; et al. Good Cell Culture Practice for stem cells and stem-cell-derived models. ALTEX 2017, 34, 95–132. [Google Scholar] [CrossRef]
- Saha, C.N.; Bhattacharya, S. Intellectual property rights: An overview and implications in pharmaceutical industry. J. Adv. Pharm. Technol. Res. 2011, 2, 88–93. [Google Scholar] [CrossRef] [PubMed]
- OECD Guidance Document on Good In Vitro Method Practices (GIVIMP). Available online: https://www.oecd.org/env/guidance-document-on-good-in-vitro-method-practices-givimp-9789264304796-en.htm (accessed on 11 November 2021).
- Research C for BE and Regulatory Considerations for Human Cells, Tissues, and Cellular and Tissue-Based Products: Minimal Manipulation and Homologous Use. U.S. Food and Drug Administration. Available online: https://www.fda.gov/regulatory-information/search-fda-guidance-documents/regulatory-considerations-human-cells-tissues-and-cellular-and-tissue-based-products-minimal (accessed on 13 September 2021).
- Infectious Substances Shipping Guidelines (ISSG). Available online: https://www.iata.org/en/publications/store/infectious-substances-shipping-guidelines/ (accessed on 11 November 2021).
- Dangerous Goods Documentation. Available online: https://www.iata.org/en/programs/cargo/dgr/download/ (accessed on 11 November 2021).
- Environmental Health & Safety. University of Nevada, Reno. Chapter 15, Biosafety Manual: Packaging and Shipping Infectious Agents. Available online: https://www.unr.edu/ehs/policies-manuals/biosafety-manual/chapter-15 (accessed on 11 November 2021).
- Guidance for Industry- Characterization and Qualification of Cell Substrates and Other Biological Materials Used in the Production of Viral Vaccines for Infectious Disease Indications. Available online: https://www.fda.gov/media/78428/download (accessed on 4 December 2021).
- Tietje, C.; Brouder, A. (Eds.) International Conference On Harmonisation Of Technical Requirements For Registration Of Pharmaceuticals for Human Use. In Handbook of Transnational Economic Governance Regimes; Brill Nijhoff: Leiden, The Netherlands, 2010; pp. 1041–1053. [Google Scholar] [CrossRef]
- Park, Y.-B.; Ha, C.-W.; Lee, C.-H.; Yoon, Y.C.; Park, Y.G. Cartilage Regeneration in Osteoarthritic Patients by a Composite of Allogeneic Umbilical Cord Blood-Derived Mesenchymal Stem Cells and Hyaluronate Hydrogel: Results from a Clinical Trial for Safety and Proof-of-Concept with 7 Years of Extended Follow-Up. Stem Cells Transl. Med. 2016, 6, 613–621. [Google Scholar] [CrossRef] [PubMed]
- Gupta, P.K.; Chullikana, A.; Rengasamy, M.; Shetty, N.; Pandey, V.; Agarwal, V.; Wagh, S.Y.; Vellotare, P.K.; Damodaran, D.; Viswanathan, P.; et al. Efficacy and safety of adult human bone marrow-derived, cultured, pooled, allogeneic mesenchymal stromal cells (Stempeucel®): Preclinical and clinical trial in osteoarthritis of the knee joint. Arthritis Res. Ther. 2016, 18, 301. [Google Scholar] [CrossRef]

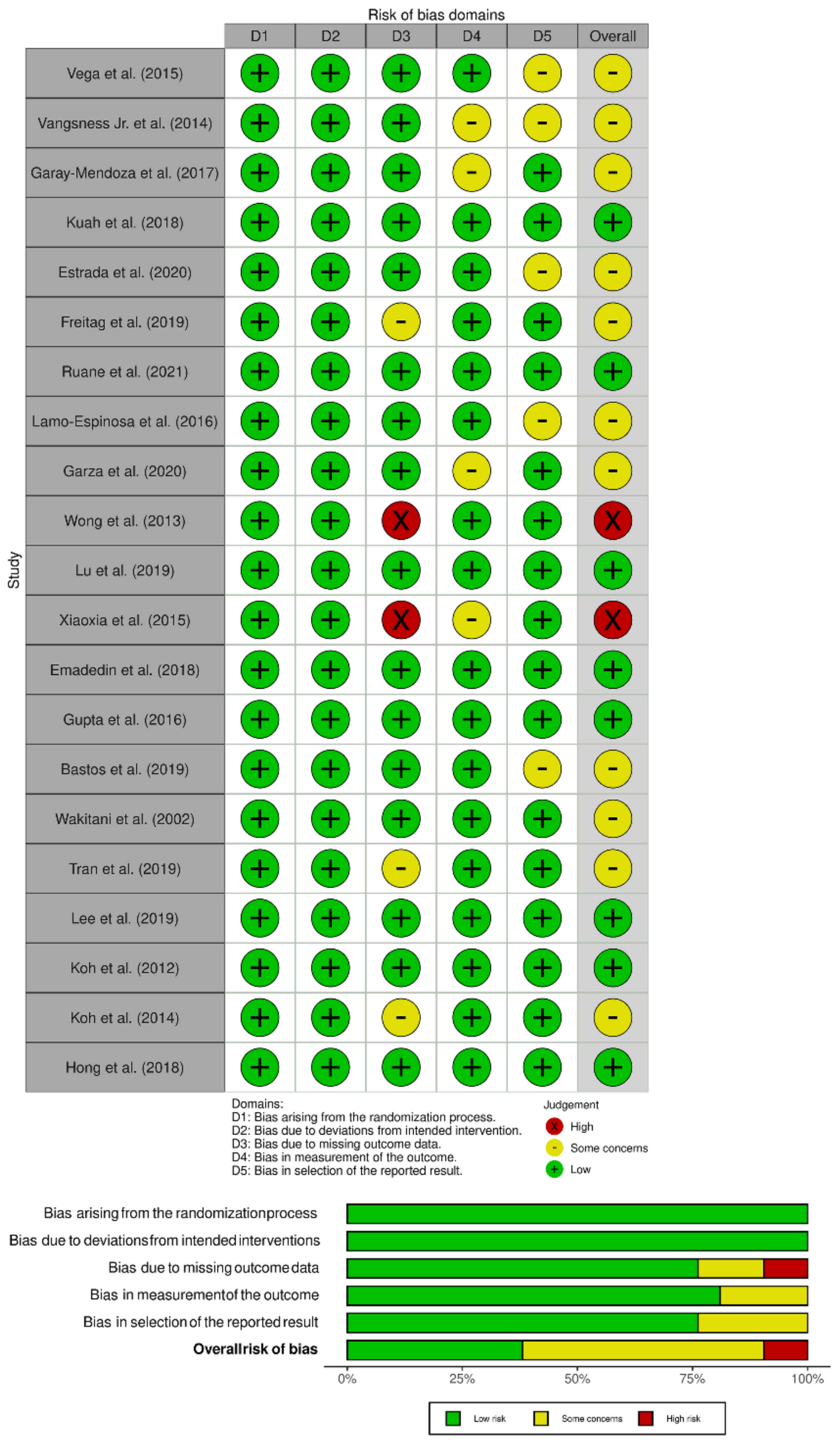


| Sl. No | Study | Year | Country | Nature of Study | Kellgren Lawrence Grade | Sample Size | Treatment/ Control | Mean Age (SD) | Male/Female | MSC Type | Culture Expanded/ Non-Cultured | Follow-Up (Months) | ||
|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|
| Treatment Group | Control Group | Treatment Group | Control Group | |||||||||||
| 1 | Garay-Mendoza et al. | 2017 | Mexico | RCT | NR | 61 | 30/31 | 55.57 ± 12.02 | 59.32 ± 10.85 | 07/23 | 09/22 | BM | NC | 6 |
| 2 | Estrada et al. | 2020 | Argentina | RCT | I, II, III | 89 | 60/29 | 61 ± 12 | 61 ± 12 | NR | NR | BM/AD | NC | 12 |
| 3 | Freitag et al. | 2019 | Australia | RCT | II, III | 30 | 20/10 | 54.6 ± 6.3 | 51.5 ± 6.1 | 11/09 | 01/09 | AD | CE | 12 |
| 4 | Ruane et al. | 2021 | USA | RCT | I, II, III | 32 | 17/15 | 58.06 ± 9.14 | 58.6 ± 8.05 | 09/08 | 10/05 | BM | NC | 12 |
| 5 | Lamo-Espinosa et al. | 2016 | Spain | RCT | II, III, IV | 30 | 20/10 | 65.9 | 60.3 | 12/08 | 07/03 | BM | CE | 12 |
| 6 | Garza et al. | 2020 | USA | RCT | II, III | 39 | 26/13 | 60.5 ± 7.9 | 57.1 ± 9.1 | 15/11 | 7/6 | AD | NC | 12 |
| 7 | Wong et al. | 2013 | Singapore | RCT | NR | 56 | 28/28 | 53 | 49 | 15/13 | 14/14 | BM | CE | 24 |
| 8 | Lu et al. | 2019 | China | RCT | I, II, III | 53 | 27/26 | 55.03 ± 9.19 | 59.64 ± 5.97 | 03/24 | 03/23 | AD | CE | 12 |
| 9 | Xiaoxia et al. | 2015 | China | RCT | I, II | 80 | 40/40 | 55.9 ± 8.1 | 55.1 ± 6.8 | 14/26 | 13/27 | BM | CE | 12 |
| 10 | Emadedin et al. | 2018 | Iran | RCT | II, III, IV | 43 | 19/24 | 51.7 ± 9.2 | 54.7 ± 5.3 | 12/07 | 15/09 | BM | CE | 6 |
| 11 | Bastos et al. | 2019 | Brazil | RCT | I, II, III, IV | 47 | 30/17 | 55.7 ± 7.8 | 55.9 ± 13.4 | 15/15 | 09/08 | BM | CE | 12 |
| 12 | Wakitani et al. | 2002 | Japan | I, II | 24 | 12/12 | NR | NR | NR | NR | BM | CE | 16 | |
| 13 | Tran et al. | 2019 | Taiwan | RCT | II, III | 33 | 15/18 | 58.2 ± 5.70 | 59.0 ± 6.04 | 03/12 | 05/13 | AD | NC | 24 |
| 14 | Lee et al. | 2019 | South Korea | RCT | II, III, IV | 24 | 12/12 | 62.2 ± 6.5 | 63.2 ± 4.2 | 03/09 | 03/09 | AD | CE | 6 |
| 15 | Koh et al. | 2012 | South Korea | RCT | IV | 50 | 25/25 | 54.2 ± 9.3 | 54.4 ± 11.3 | 08/17 | 08/17 | AD | NC | 16 |
| 16 | Koh et al. | 2014 | South Korea | RCT | I, II, III | 44 | 23/21 | 52.3 ± 4.9 | 54.2 ± 2.9 | 06/17 | 05/16 | AD | NC | 24 |
| 17 | Hong et al. | 2018 | China | RCT | II, III | 32 | 16/16 | 51 ± 5.95 | 53 ± 10.97 | 03/13 | 03/13 | AD | NC | 12 |
| Study | MSC Type | MSC Source | MSC Preparation | MSC Count (107 cells) | Treatment Group Intervention | Control Group Intervention | Outcome Measures |
|---|---|---|---|---|---|---|---|
| Garay-Mendoza et al. | BM | Auto | BMC | NA | 600 µg/day G-CSF for 3 consecutive days before the procedure + sIA Injection of MSC | Oral acetaminophen 500 mg every 8 h for 6 months | VAS, WOMAC |
| Estrada et al. | AD | Auto | BMC | NA | sIA Injection of bone marrow concentrate | sIA Injection of PRP | IKDC, Lysholm Score, KOOS |
| Estrada et al. | BM | Auto | SVF | NA | sIA Injection of lipoaspirate | sIA Injection of PRP | |
| Freitag et al. | AD | Auto | CE- ADMSC | 10 | sIA Injection of MSC ± 2nd injection at 6 months | Conservative management | VAS, WOMAC, KOOS, MRI assessment |
| Ruane et al. | BM | Auto | BMC | NA | sIA Injection of bone marrow concentrate + PRP | Gel-One® Cross-Linked Hyaluronate injection | VAS, KOOS |
| Lamo-Espinosa et al. | BM | Auto | CE-BMMSC | 1 | sIA Injection of MSC +60 mg HA | sIA Injection of 60 mg HA | VAS, WOMAC, MRI assessment |
| Garza et al. | AD | Auto | SVF | NA | sIA Injection of MSC | Placebo injection without cells | WOMAC, MRI assessment |
| Wong et al. | BM | Auto | CE-BMMSC | 1.46 | HTO + Microfracture + sIA Injection of MSC + 20 mg HA | HTO + Microfracture + sIA Injection of 20 mg HA | Tegner Score, Lysholm Score |
| Lu et al. | AD | Auto | CE- ADMSC | 5 | 2 IA Injection of MSC at 0, 3 weeks and sham injection at 1, 2 weeks | 4 IA Injection of 25 mg HA at 0, 1, 2, 3 weeks | VAS, WOMAC |
| Xiaoxia et al. | BM | Auto | CE-BMMSC | 3.82 | 3 × Monthly IA Injection of MSC + 20 mg HA | sIA Injection of 20 mg HA | Tegner Score, Lysholm Score |
| Emadedin et al. | BM | Auto | CE-BMMSC | 4 | sIA Injection of MSC | Placebo sIA Injection of Normal Saline | VAS, WOMAC |
| Bastos et al. | BM | Auto | CE-BMMSC | 4 | sIA Injection of MSC in 10 mL of PRP | sIA Injection of 4 mg Dexamethasone | KOOS, MRI assessment |
| Wakitani et al. | BM | Auto | CE-BMMSC | 1 | HTO + Microfracture + sIA Injection of MSC | HTO + Microfracture + Placebo injection | MRI assessment, HSS Knee rating scale |
| Tran et al. | AD | Auto | SVF | NA | Arthroscopic micro fracture + sIA Injection of MSC | Arthroscopic micro fracture | WOMAC, MRI assessment |
| Lee et al. | AD | Auto | CE- ADMSC | 10 | sIA Injection of MSC | Placebo injection with Normal Saline | WOMAC, MRI assessment |
| Koh et al. | AD | Auto | SVF | 0.189 | Arthroscopic debridement + sIA Injection of MSC + PRP | Arthroscopic debridement + PRP | VAS, Tegner Score, Lysholm Score |
| Koh et al. | AD | Auto | CE- ADMSC | 0.411 | HTO + sIA Injection of MSC + PRP | HTO + PRP | VAS, Lysholm Score |
| Hong et al. | AD | Auto | SVF | 0.745 | sIA Injection of MSC | sIA Injection of 40 mg HA | VAS, WOMAC, MRI assessment |
| Outcomes | Culture Expanded MSCs | Uncultured MSCs | ||||||||||
|---|---|---|---|---|---|---|---|---|---|---|---|---|
| Bone Marrow Derived MSCs | Adipose Derived MSCs | Bone Marrow Derived MSCs | Adipose Derived MSCs | |||||||||
| Estimate | 95% CI | p-Value | Estimate | 95% CI | p-Value | Estimate | 95% CI | p-Value | Estimate | 95% CI | p-Value | |
| VAS—6 months | −11.344 | −28.555, 5.867 | 0.196 | −21.319 | −31.512, −11.125 | <0.001 | −37.200 | −42.518, −31.882 | NA | −11.366 | −39.218,16.487 | 0.424 |
| VAS—12 months | 14.637 | 6.875, 22.399 | <0.001 | −30.328 | −47.004, −13.652 | <0.001 | NA | NA | NA | −29.817 | −39.611, −20.024 | <0.001 |
| WOMAC—6 months | 5.139 | −7.847, 18.124 | 0.438 | 5.303 | −17.114, 27.719 | 0.643 | 18.770 | 12.488, 25.052 | NA | −17.508 | −22.715, −12.302 | <0.001 |
| WOMAC—12 months | 0.967 | −7.659, 9.594 | 0.826 | 8.464 | −20.815, 37.742 | 0.571 | NA | NA | NA | −17.604 | −32.947, −2.261 | 0.025 |
| Lysholm Score—12 months | 5.000 | −0.238, 10.238 | NA | NA | NA | NA | −0.700 | −2.614, 1.214 | NA | 6.494 | 1.889, 11.100 | 0.006 |
| KOOS Score—12 months | 2.434 | −9.262, 14.130 | 0.683 | 28.000 | 13.544, 42.456 | NA | 3.780 | −1.295, 8.854 | 0.144 | 5.083 | 1.729, 8.437 | 0.003 |
| Adverse Events | 1.047 | 0.177, 6.200 | 0.960 | 0.377 | 0.061, 2.327 | 0.293 | NA | NA | NA | NA | NA | NA |
Publisher’s Note: MDPI stays neutral with regard to jurisdictional claims in published maps and institutional affiliations. |
© 2021 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Muthu, S.; Kartheek, R.R.; Jeyaraman, N.; Rajendran, R.L.; Khanna, M.; Jeyaraman, M.; Packkyarathinam, R.P.; Gangadaran, P.; Ahn, B.-C. Is Culture Expansion Necessary in Autologous Mesenchymal Stromal Cell Therapy to Obtain Superior Results in the Management of Knee Osteoarthritis?—Meta-Analysis of Randomized Controlled Trials. Bioengineering 2021, 8, 220. https://doi.org/10.3390/bioengineering8120220
Muthu S, Kartheek RR, Jeyaraman N, Rajendran RL, Khanna M, Jeyaraman M, Packkyarathinam RP, Gangadaran P, Ahn B-C. Is Culture Expansion Necessary in Autologous Mesenchymal Stromal Cell Therapy to Obtain Superior Results in the Management of Knee Osteoarthritis?—Meta-Analysis of Randomized Controlled Trials. Bioengineering. 2021; 8(12):220. https://doi.org/10.3390/bioengineering8120220
Chicago/Turabian StyleMuthu, Sathish, Randhi Rama Kartheek, Naveen Jeyaraman, Ramya Lakshmi Rajendran, Manish Khanna, Madhan Jeyaraman, Rathinavelpandian Perunchezhian Packkyarathinam, Prakash Gangadaran, and Byeong-Cheol Ahn. 2021. "Is Culture Expansion Necessary in Autologous Mesenchymal Stromal Cell Therapy to Obtain Superior Results in the Management of Knee Osteoarthritis?—Meta-Analysis of Randomized Controlled Trials" Bioengineering 8, no. 12: 220. https://doi.org/10.3390/bioengineering8120220
APA StyleMuthu, S., Kartheek, R. R., Jeyaraman, N., Rajendran, R. L., Khanna, M., Jeyaraman, M., Packkyarathinam, R. P., Gangadaran, P., & Ahn, B.-C. (2021). Is Culture Expansion Necessary in Autologous Mesenchymal Stromal Cell Therapy to Obtain Superior Results in the Management of Knee Osteoarthritis?—Meta-Analysis of Randomized Controlled Trials. Bioengineering, 8(12), 220. https://doi.org/10.3390/bioengineering8120220











