Biocompatible Customized 3D Bone Scaffolds Treated with CRFP, an Osteogenic Peptide
Abstract
1. Introduction
2. Materials and Methods
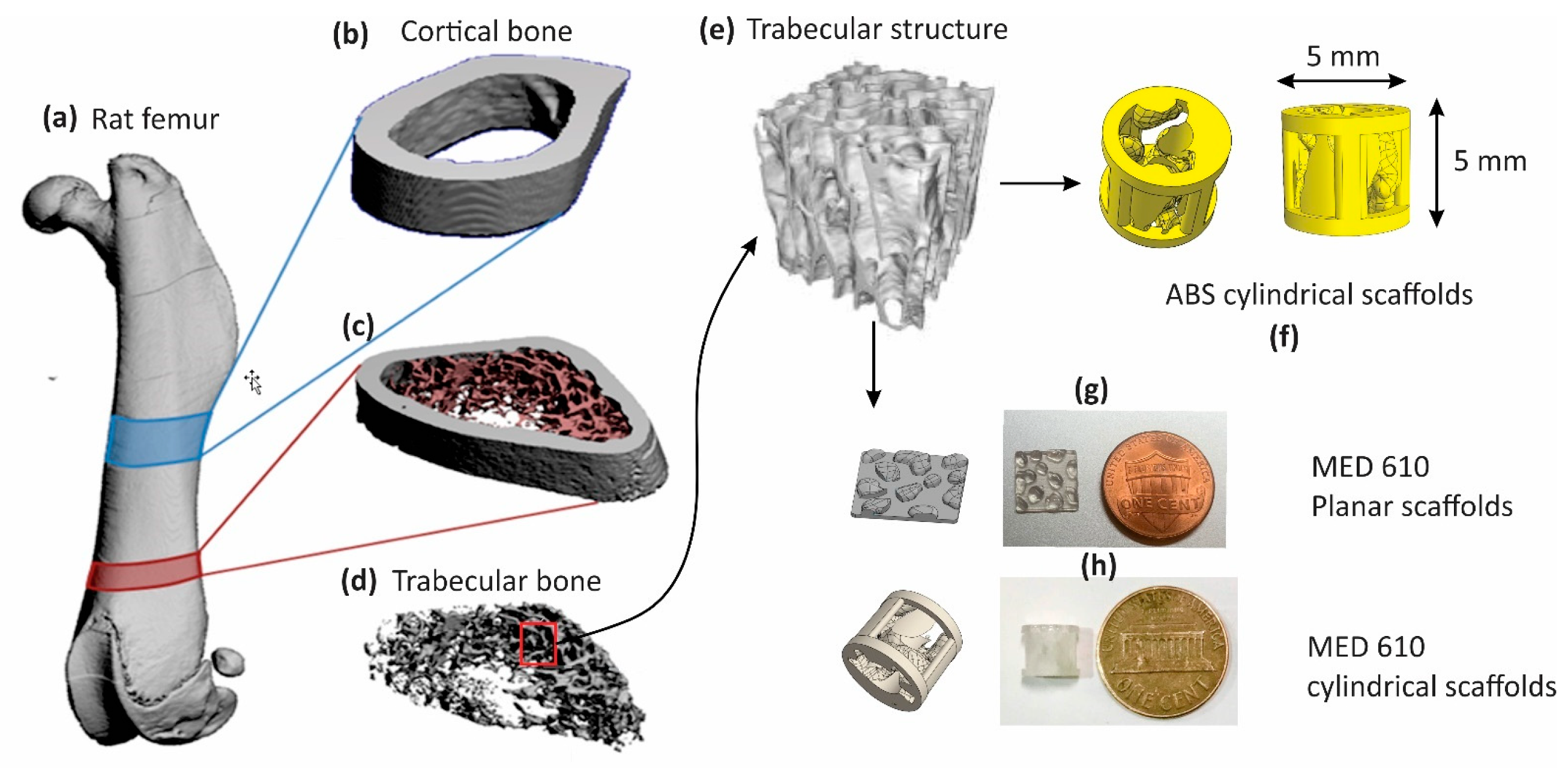
2.1. Design and Fabrication of Cylindrical Scaffolds
2.2. Preparation of the Scaffolds
2.3. Seeding MC3T3 Cells
2.4. Bone Matrix Deposition on the 3D-Printed Scaffold
2.5. Osteoconductivity of MED610 Scaffolds
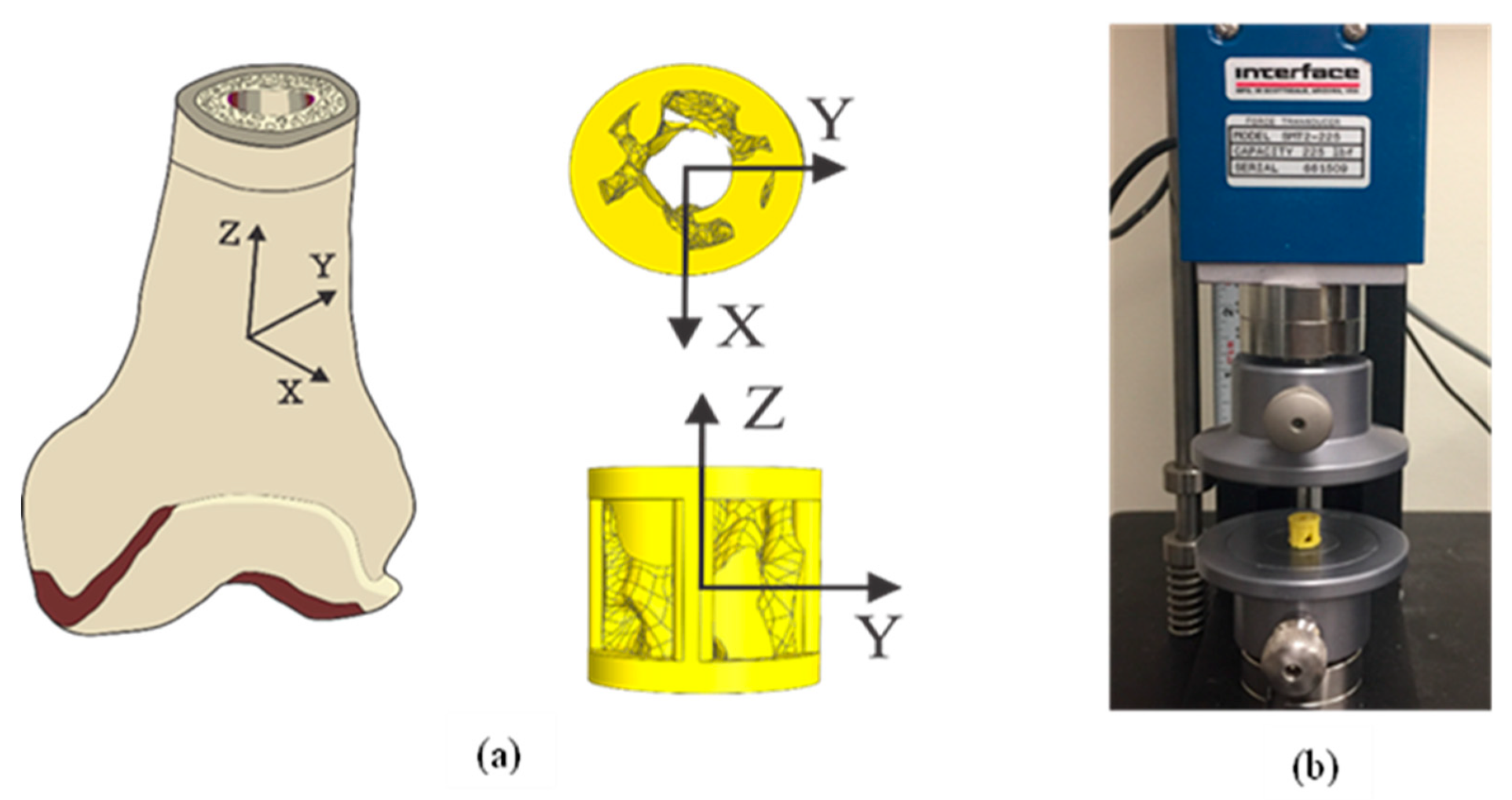
2.6. Mechanical Properties of Scaffolds
2.7. In Vitro Study and Strength Analysis of Cylindrical Scaffolds Treated with CRFP
3. Results
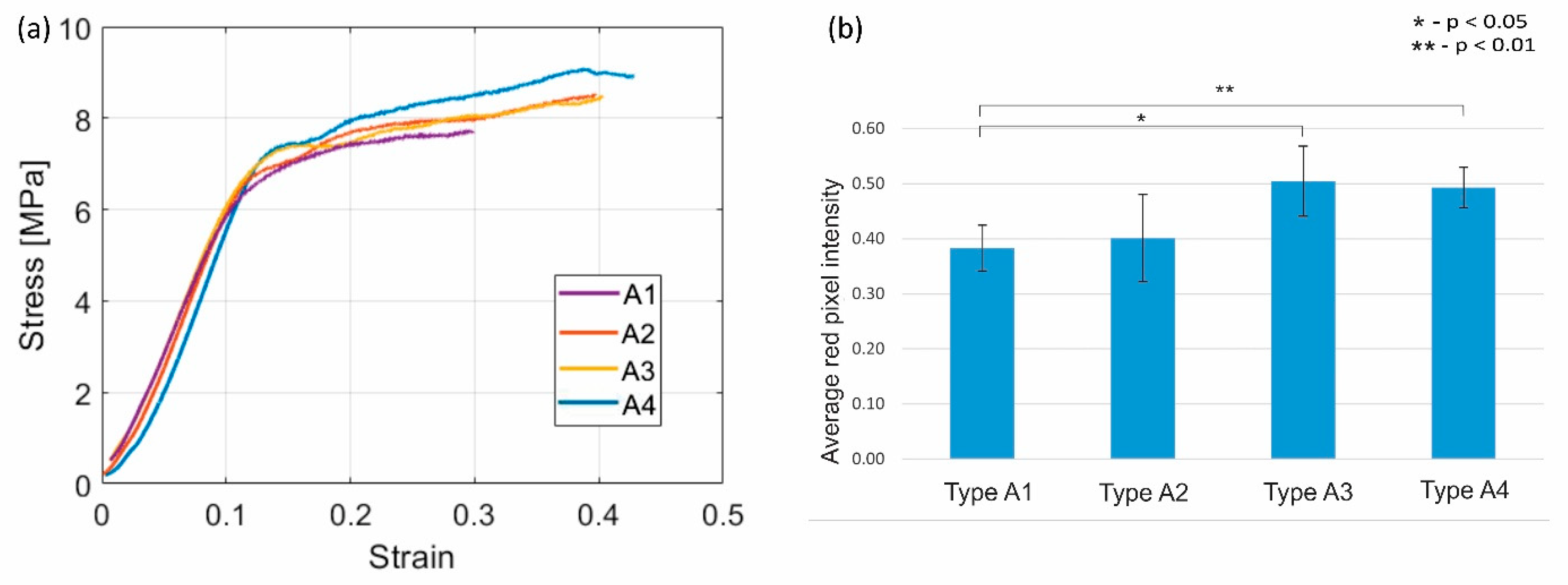
3.1. Bone Matrix Deposition on MED610 Planar Scaffolds
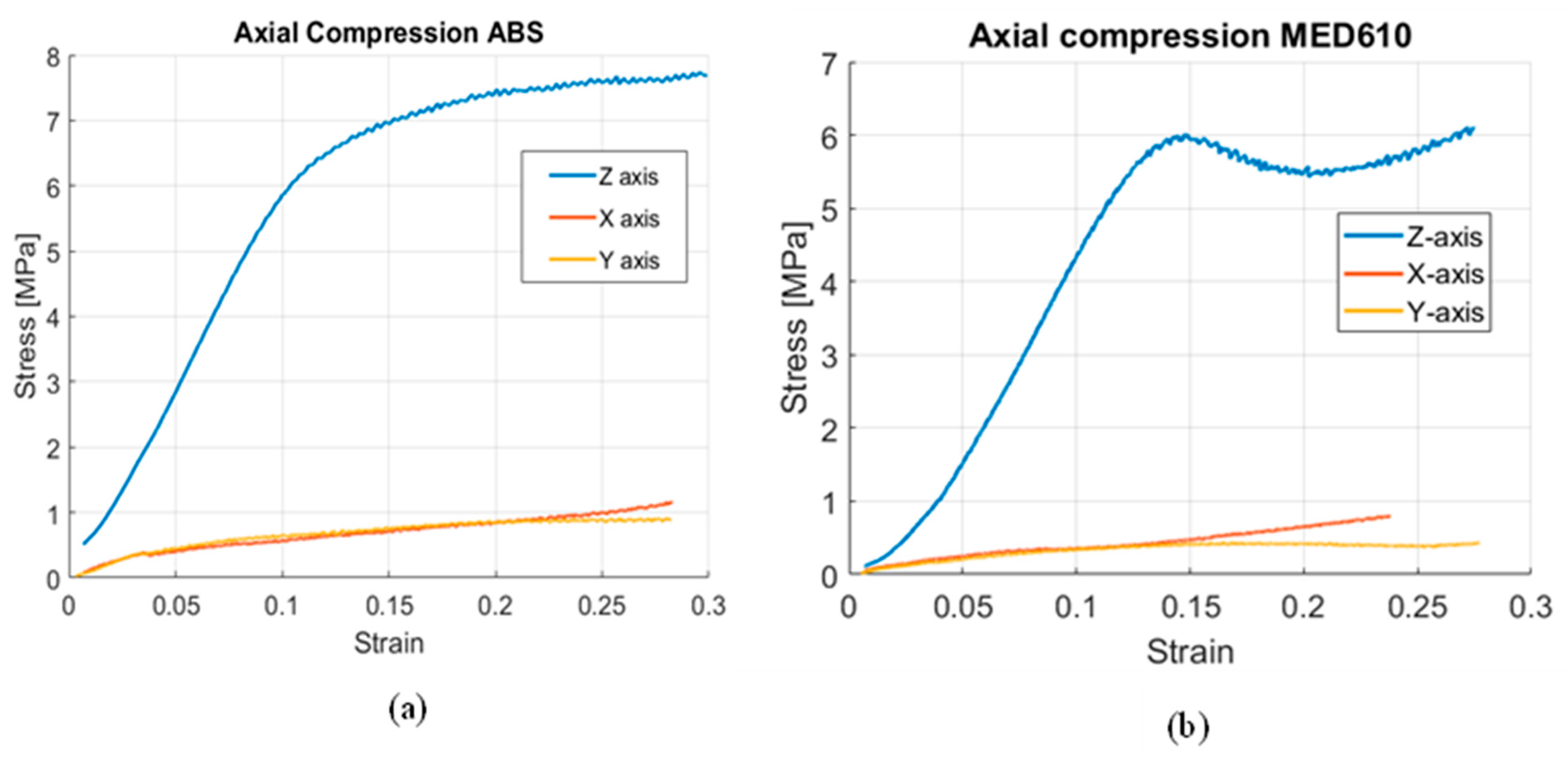
3.2. Mechanical Properties of the Cylindrical Scaffolds
4. Discussion
5. Conclusions
Author Contributions
Funding
Conflicts of Interest
References
- de Grado, G.F.; Keller, L.; Idoux-Gillet, Y.; Wagner, Q.; Musset, A.-M.; Benkirane-Jessel, N.; Bornert, F.; Offner, D. Bone substitutes: A review of their characteristics, clinical use, and perspectives for large bone defects management. J. Tissue Eng. 2018, 9, 2041731418776819. [Google Scholar]
- Sohn, H.S.; Oh, J.K. Review of bone graft and bone substitutes with an emphasis on fracture surgeries. Biomater. Res. 2019, 23, 9. [Google Scholar] [CrossRef] [PubMed]
- Fillingham, Y.; Jacobs, J. Bone grafts and their substitutes. Bone Jt. J. 2016, 98, 6–9. [Google Scholar] [CrossRef] [PubMed]
- Allison, D.C.; Lindberg, A.W.; Samimi, B.; Mirzayan, R.; Menendez, L.R. A Comparison of Mineral Bone Graft Substitutes for Bone Defects. Oncol. Hematol. Rev. 2011, 7, 38–49. [Google Scholar] [CrossRef][Green Version]
- Gazdag, A.R.; Lane, J.M.; Glaser, D.; Forster, R.A. Alternatives to autogenous bone graft: Efficacy and indications. JAAOS-J. Am. Acad. Orthop. Surg. 1995, 3, 1–8. [Google Scholar] [CrossRef] [PubMed]
- De Long, W.G., Jr.; Einhorn, T.A.; Koval, K.; McKee, M.; Smith, W.; Sanders, R.; Watson, T. Bone grafts and bone graft substitutes in orthopaedic trauma surgery. A critical analysis. J. Bone Jt. Surg. Am. Vol. 2007, 89, 649–658. [Google Scholar] [CrossRef]
- Finkemeier, C.G. Bone-grafting and bone-graft substitutes. J. Bone Jt. Surg. Am. Vol. 2002, 84, 454–464. [Google Scholar] [CrossRef] [PubMed]
- Tomford, W.W. Transmission of disease through transplantation of musculoskeletal allografts. JBJS 1995, 77, 1742–1754. [Google Scholar] [CrossRef] [PubMed]
- Boyce, T.; Edwards, J.; Scarborough, N. Allograft bone: The influence of processing on safety and performance. Orthop. Clin. 1999, 30, 571–581. [Google Scholar] [CrossRef]
- Buck, B.E.; Malinin, T.I.; Brown, M.D. Bone transplantation and human immunodeficiency virus. An estimate of risk of acquired immunodeficiency syndrome (AIDS). Clin. Orthop. Relat. Res. 1989, 240, 129–136. [Google Scholar] [CrossRef]
- Mroz, T.E.; Joyce, M.J.; Steinmetz, M.P.; Lieberman, I.H.; Wang, J.C. Musculoskeletal allograft risks and recalls in the United States. J. Am. Acad. Orthop. Surg. 2008, 16, 559–565. [Google Scholar] [CrossRef]
- Khan, S.N.; Cammisa, F.P., Jr.; Sandhu, H.S.; Diwan, A.D.; Girardi, F.P.; Lane, J.M. The biology of bone grafting. J. Am. Acad. Orthop. Surg. 2005, 13, 77–86. [Google Scholar] [CrossRef]
- Ullmark, G.; Obrant, K.J. Histology of impacted bone-graft incorporation. J. Arthroplast. 2002, 17, 150–157. [Google Scholar] [CrossRef]
- Saikia, K.; Bhattacharya, T.; Bhuyan, S.; Talukdar, D.; Saikia, S.; Jitesh, P. Calcium phosphate ceramics as bone graft substitutes in filling bone tumor defects. Indian J. Orthop. 2008, 42, 169. [Google Scholar] [CrossRef]
- Gruskin, E.; Doll, B.A.; Futrell, F.W.; Schmitz, J.P.; Hollinger, J.O. Demineralized bone matrix in bone repair: History and use. Adv. Drug Deliv. Rev. 2012, 64, 1063–1077. [Google Scholar] [CrossRef] [PubMed]
- Wang, J.C.; Alanay, A.; Mark, D.; Kanim, L.E.; Campbell, P.A.; Dawson, E.G.; Lieberman, J.R. A comparison of commercially available demineralized bone matrix for spinal fusion. Eur. Spine J. 2007, 16, 1233–1240. [Google Scholar] [CrossRef] [PubMed]
- Chung, H.-J.; Hur, J.-W.; Ryu, K.-S.; Kim, J.-S.; Seong, J.-H. Surgical outcomes of anterior cervical fusion using deminaralized bone matrix as stand-alone graft material: Single arm, pilot study. Korean J. Spine 2016, 13, 114. [Google Scholar] [CrossRef] [PubMed]
- Campana, V.; Milano, G.; Pagano, E.; Barba, M.; Cicione, C.; Salonna, G.; Lattanzi, W.; Logroscino, G. Bone substitutes in orthopaedic surgery: From basic science to clinical practice. J. Mater. Sci. Mater. Med. 2014, 25, 2445–2461. [Google Scholar] [CrossRef] [PubMed]
- Russell, N.; Walsh, W.R.; Lovric, V.; Kim, P.; Chen, J.H.; Larson, M.J.; Vizesi, F. In-vivo Performance of Seven Commercially Available Demineralized Bone Matrix Fiber and Putty Products in a Rat Posterolateral Fusion Model. Front. Surg. 2020, 7, 10. [Google Scholar] [CrossRef] [PubMed]
- Flatley, T.J.; Lynch, K.L.; Benson, M. Tissue response to implants of calcium phosphate ceramic in the rabbit spine. Clin. Orthop. Relat. Res. 1983, 179, 246–252. [Google Scholar] [CrossRef]
- Parikh, S.N. Bone graft substitutes in modern orthopedics. Orthopedics 2002, 25, 1301–1309. [Google Scholar] [CrossRef]
- Hing, K.A.; Wilson, L.F.; Buckland, T. Comparative performance of three ceramic bone graft substitutes. Spine J. 2007, 7, 475–490. [Google Scholar] [CrossRef]
- Koshino, T.; Murase, T.; Takagi, T.; Saito, T. New bone formation around porous hydroxyapatite wedge implanted in opening wedge high tibial osteotomy in patients with osteoarthritis. Biomaterials 2001, 22, 1579–1582. [Google Scholar] [CrossRef]
- Myers, G.J.C.; Abudu, A.T.; Carter, S.R.; Tillman, R.M.; Grimer, R.J. Endoprosthetic replacement of the distal femur for bone tumours: Long-term results. Bone Jt. J. 2007, 89, 521–526. [Google Scholar] [CrossRef]
- Shin, D.S.; Choong, P.F.; Chao, E.Y.; Sim, F.H. Large tumor endoprostheses and extracortical bone-bridging: 28 patients followed 10–20 years. Acta Orthop. Scand. 2000, 71, 305–311. [Google Scholar] [CrossRef] [PubMed]
- Plotz, W.; Rechl, H.; Burgkart, R.; Messmer, C.; Schelter, R.; Hipp, E.; Gradinger, R. Limb salvage with tumor endoprostheses for malignant tumors of the knee. Clin. Orthop. Relat. Res. 2002, 405, 207–215. [Google Scholar] [CrossRef] [PubMed]
- Engh, C.A.; Bobyn, J.D.; Glassman, A.H. Porous-coated hip replacement. The factors governing bone ingrowth, stress shielding, and clinical results. J. Bone Jt. Surg. Br. Vol. 1987, 69, 45–55. [Google Scholar] [CrossRef] [PubMed]
- Epari, D.R.; Taylor, W.R.; Heller, M.O.; Duda, G.N. Mechanical conditions in the initial phase of bone healing. Clin. Biomech. 2006, 21, 646–655. [Google Scholar] [CrossRef] [PubMed]
- Bose, S.; Vahabzadeh, S.; Bandyopadhyay, A. Bone tissue engineering using 3D printing. Mater. Today 2013, 16, 496–504. [Google Scholar] [CrossRef]
- Wieding, J.; Fritsche, A.; Heinl, P.; Korner, C.; Cornelsen, M.; Seitz, H.; Mittelmeier, W.; Bader, R. Biomechanical behavior of bone scaffolds made of additive manufactured tricalciumphosphate and titanium alloy under different loading conditions. J. Appl. Biomater. Funct. Mater. 2013, 11, e159–e166. [Google Scholar] [CrossRef] [PubMed]
- Helguero, C.G.; Amaya, J.L.; Ramirez, E.A.; Komatsu, D.E.; Kao, I.; Pentyala, S. A manufacturing approach to functional biomimetic three-dimensional-printed bone implants. Proc. Inst. Mech. Eng. Part L J. Mater. 2019, 233, 383–392. [Google Scholar] [CrossRef]
- Wong, K.C. 3D-printed patient-specific applications in orthopedics. Orthop. Res. Rev. 2016, 8, 57–66. [Google Scholar] [CrossRef] [PubMed]
- Jariwala, S.H.; Lewis, G.S.; Bushman, Z.J.; Adair, J.H.; Donahue, H.J. 3D Printing of Personalized Artificial Bone Scaffolds. 3D Print. Addit. Manuf. 2015, 2, 56–64. [Google Scholar] [CrossRef]
- Konopnicki, S.; Sharaf, B.; Resnick, C.; Patenaude, A.; Pogal-Sussman, T.; Hwang, K.G.; Abukawa, H.; Troulis, M.J. Tissue-engineered bone with 3-dimensionally printed beta-tricalcium phosphate and polycaprolactone scaffolds and early implantation: An in vivo pilot study in a porcine mandible model. J. Oral Maxillofac. Surg. 2015, 73, 1016.e1–1016.e11. [Google Scholar] [CrossRef] [PubMed]
- Yoshimoto, H.; Shin, Y.M.; Terai, H.; Vacanti, J.P. A biodegradable nanofiber scaffold by electrospinning and its potential for bone tissue engineering. Biomaterials 2003, 24, 2077–2082. [Google Scholar] [CrossRef]
- Chacón, J.; Caminero, M.A.; García-Plaza, E.; Núnez, P.J. Additive manufacturing of PLA structures using fused deposition modelling: Effect of process parameters on mechanical properties and their optimal selection. Mater. Des. 2017, 124, 143–157. [Google Scholar] [CrossRef]
- Hutmacher, D.W.; Schantz, T.; Zein, I.; Ng, K.W.; Teoh, S.H.; Tan, K.C. Mechanical properties and cell cultural response of polycaprolactone scaffolds designed and fabricated via fused deposition modeling. J. Biomed. Mater. Res. 2001, 55, 203–216. [Google Scholar] [CrossRef]
- Kim, J.; McBride, S.; Tellis, B.; Alvarez-Urena, P.; Song, Y.H.; Dean, D.D.; Sylvia, V.L.; Elgendy, H.; Ong, J.; Hollinger, J.O. Rapid-prototyped PLGA/beta-TCP/hydroxyapatite nanocomposite scaffolds in a rabbit femoral defect model. Biofabrication 2012, 4, 025003. [Google Scholar] [CrossRef] [PubMed]
- Zhang, B.; Wang, L.; Song, P.; Pei, X.; Sun, H.; Wu, L.; Zhou, C.; Wang, K.; Fan, Y.; Zhang, X. 3D printed bone tissue regenerative PLA/HA scaffolds with comprehensive performance optimizations. Mater. Des. 2021, 201, 109490. [Google Scholar] [CrossRef]
- Gunatillake, P.A.; Adhikari, R. Biodegradable synthetic polymers for tissue engineering. Eur. Cell Mater. 2003, 5, 1–16, discussion 16. [Google Scholar] [CrossRef]
- Gregor, A.; Filova, E.; Novak, M.; Kronek, J.; Chlup, H.; Buzgo, M.; Blahnova, V.; Lukasova, V.; Bartos, M.; Necas, A.; et al. Designing of PLA scaffolds for bone tissue replacement fabricated by ordinary commercial 3D printer. J. Biol. Eng. 2017, 11, 31. [Google Scholar] [CrossRef]
- Helguero, C.G.; Mustahsan, V.M.; Parmar, S.; Pentyala, S.; Pfail, J.L.; Kao, I.; Komatsu, D.E.; Pentyala, S. Biomechanical properties of 3D-printed bone scaffolds are improved by treatment with CRFP. J. Orthop. Surg. Res. 2017, 12, 195. [Google Scholar] [CrossRef] [PubMed]
- Rosenzweig, D.H.; Carelli, E.; Steffen, T.; Jarzem, P.; Haglund, L. 3D-Printed ABS and PLA Scaffolds for Cartilage and Nucleus Pulposus Tissue Regeneration. Int. J. Mol. Sci. 2015, 16, 15118–15135. [Google Scholar] [CrossRef]
- Komatsu, D.E.; Hadjiargyrou, M.; Udin, S.M.; Trasolini, N.A.; Pentyala, S. Identification and Characterization of a Synthetic Osteogenic Peptide. Calcif. Tissue Int. 2015, 97, 611–623. [Google Scholar] [CrossRef] [PubMed][Green Version]
- Orcel, P.; Tajima, H.; Murayama, Y.; Fujita, T.; Krane, S.M.; Ogata, E.; Goldring, S.R.; Nishimoto, I. Multiple domains interacting with Gs in the porcine calcitonin receptor. Mol. Endocrinol. 2000, 14, 170–182. [Google Scholar] [CrossRef] [PubMed]
- Stratasys Ltd. Biocompatible Clear MED610 Material Data Sheet. Available online: https://www.stratasys.com/materials/search/biocompatible (accessed on 19 October 2021).
- Gulcan, O.; Gunaydin, K.; Tamer, A. The State of the Art of Material Jetting-A Critical Review. Polymers 2021, 13, 2829. [Google Scholar] [CrossRef]
- Udroiu, R.; Braga, I.C. Polyjet technology applications for rapid tooling. In Proceedings of the MATEC Web of Conferences, Iasi, Romania, 24–27 May 2017; Volume 112, p. 03011. [Google Scholar]
- Wang, T.; Yang, X.; Qi, X.; Jiang, C. Osteoinduction and proliferation of bone-marrow stromal cells in three-dimensional poly (epsilon-caprolactone)/ hydroxyapatite/collagen scaffolds. J. Transl. Med. 2015, 13, 152. [Google Scholar] [CrossRef] [PubMed]
- Gunn, N.M.; Bachman, M.; Li, G.P.; Nelson, E.L. Fabrication and biological evaluation of uniform extracellular matrix coatings on discontinuous photolithography generated micropallet arrays. J. Biomed. Mater. Res. A 2010, 95, 401–412. [Google Scholar] [CrossRef] [PubMed]
- Wang, D.; Christensen, K.; Chawla, K.; Xiao, G.; Krebsbach, P.H.; Franceschi, R.T. Isolation and characterization of MC3T3-E1 preosteoblast subclones with distinct in vitro and in vivo differentiation/mineralization potential. J. Bone Miner. Res. Off. J. Am. Soc. Bone Miner. Res. 1999, 14, 893–903. [Google Scholar] [CrossRef] [PubMed]
- Tenenbaum, H.C.; Torontali, M.; Sukhu, B. Effects of bisphosphonates and inorganic pyrophosphate on osteogenesis in vitro. Bone 1992, 13, 249–255. [Google Scholar] [CrossRef]
- Franceschi, R.T.; Iyer, B.S.; Cui, Y. Effects of ascorbic acid on collagen matrix formation and osteoblast differentiation in murine MC3T3-E1 cells. J. Bone Miner. Res. Off. J. Am. Soc. Bone Miner. Res. 1994, 9, 843–854. [Google Scholar] [CrossRef] [PubMed]
- USP. United States Pharmacopeia and National Formulary. Available online: https://www.usp.org/reference-standards (accessed on 29 September 2020).
- ISO 604: 2002. Plastics-Determination of Compressive Properties, 3rd ed.; International Organization for Standardization: Geneva, Switzerland, 2002; pp. 1–18. [Google Scholar]
- Zhao, S.; Arnold, M.; Ma, S.; Abel, R.L.; Cobb, J.P.; Hansen, U.; Boughton, O. Standardizing compression testing for measuring the stiffness of human bone. Bone Jt. Res. 2018, 7, 524–538. [Google Scholar] [CrossRef] [PubMed]
- Novitskaya, E.; Chen, P.Y.; Lee, S.; Castro-Cesena, A.; Hirata, G.; Lubarda, V.A.; McKittrick, J. Anisotropy in the compressive mechanical properties of bovine cortical bone and the mineral and protein constituents. Acta Biomater. 2011, 7, 3170–3177. [Google Scholar] [CrossRef] [PubMed]
- Hasegawa, T.; Miwa, M.; Sakai, Y.; Niikura, T.; Lee, S.; Oe, K.; Iwakura, T.; Kurosaka, M.; Komori, T. Efficient cell-seeding into scaffolds improves bone formation. J. Dent. Res. 2010, 89, 854–859. [Google Scholar] [CrossRef] [PubMed]
- Mi, Z.R.; Shuib, S.; Hassan, A.; Shorki, A.; Ibrahim, M. Problem of stress shielding and improvement to the hip Implat designs: A review. J. Med. Sci. 2007, 7, 460–467. [Google Scholar]
- Polo-Corrales, L.; Latorre-Esteves, M.; Ramirez-Vick, J.E. Scaffold design for bone regeneration. J. Nanosci. Nanotechnol. 2014, 14, 15–56. [Google Scholar] [CrossRef] [PubMed]
- DeLustro, F.; Dasch, J.; Keefe, J.; Ellingsworth, L. Immune responses to allogeneic and xenogeneic implants of collagen and collagen derivatives. Clin. Orthop. Relat. Res. 1990, 260, 263–279. [Google Scholar] [CrossRef]
- Chen, Y.W.; Fang, H.Y.; Shie, M.Y.; Shen, Y.F. The mussel-inspired assisted apatite mineralized on PolyJet material for artificial bone scaffold. Int. J. Bioprint. 2019, 5, 197. [Google Scholar] [CrossRef] [PubMed]


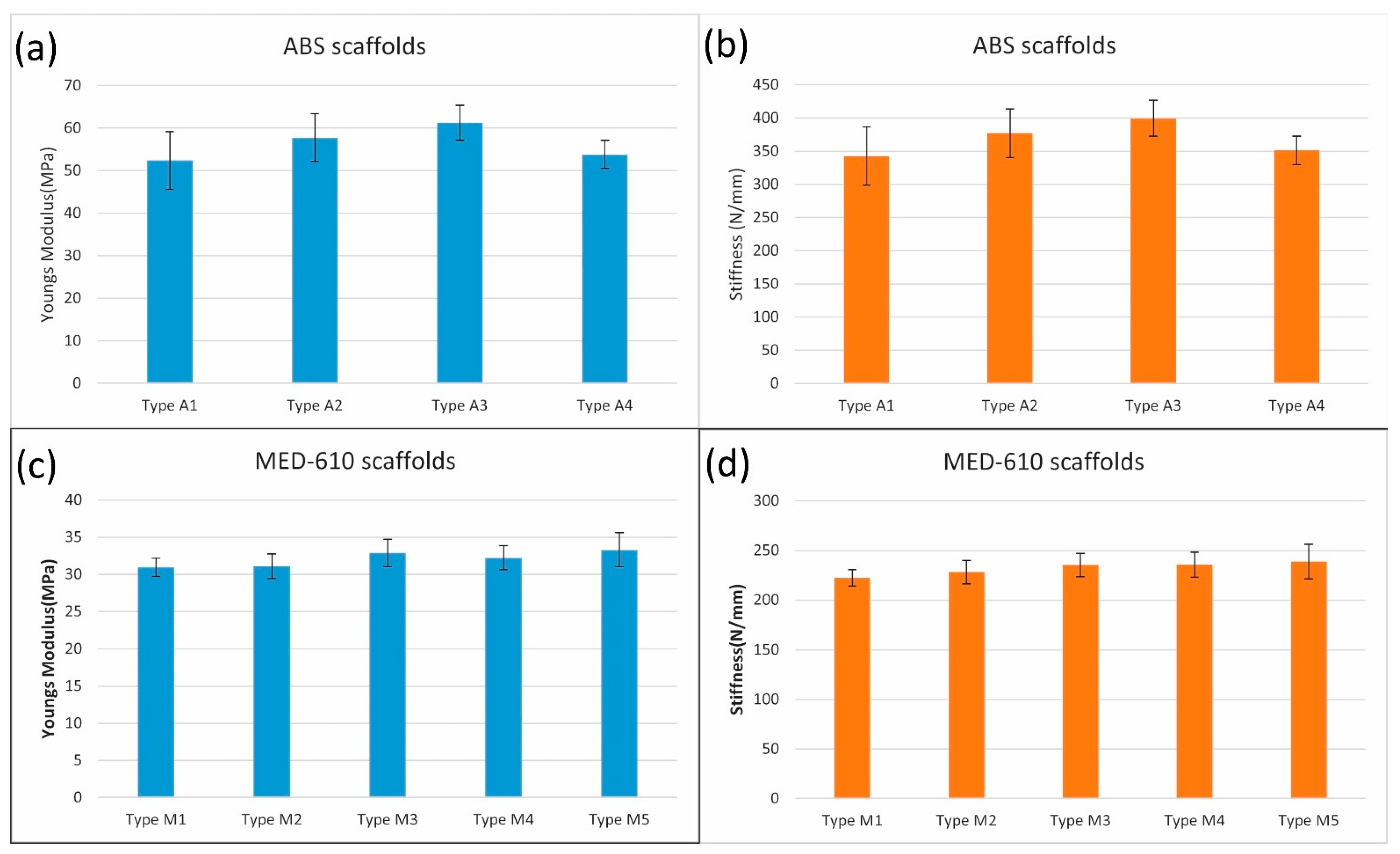

| Type | Coating | Osteogenic Reagent |
|---|---|---|
| A1 | Only scaffold | No reagent |
| A2 | Polylysine coating | G6P + AA |
| A3 | Polylysine coating | G6P + AA + CRFP |
| A4 | No coating | G6P + AA + CRFP |
| Type | Coating | Osteogenic Reagent |
|---|---|---|
| M1 | Only scaffold | No reagent |
| M2 | Polylysine coating | G6P + AA |
| M3 | Polylysine coating | G6P + AA + CRFP |
| M4 | No coating | G6P + AA |
| M5 | No coating | G6P + AA + CRFP |
Publisher’s Note: MDPI stays neutral with regard to jurisdictional claims in published maps and institutional affiliations. |
© 2021 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Mustahsan, V.M.; Anugu, A.; Komatsu, D.E.; Kao, I.; Pentyala, S. Biocompatible Customized 3D Bone Scaffolds Treated with CRFP, an Osteogenic Peptide. Bioengineering 2021, 8, 199. https://doi.org/10.3390/bioengineering8120199
Mustahsan VM, Anugu A, Komatsu DE, Kao I, Pentyala S. Biocompatible Customized 3D Bone Scaffolds Treated with CRFP, an Osteogenic Peptide. Bioengineering. 2021; 8(12):199. https://doi.org/10.3390/bioengineering8120199
Chicago/Turabian StyleMustahsan, Vamiq M., Amith Anugu, David E. Komatsu, Imin Kao, and Srinivas Pentyala. 2021. "Biocompatible Customized 3D Bone Scaffolds Treated with CRFP, an Osteogenic Peptide" Bioengineering 8, no. 12: 199. https://doi.org/10.3390/bioengineering8120199
APA StyleMustahsan, V. M., Anugu, A., Komatsu, D. E., Kao, I., & Pentyala, S. (2021). Biocompatible Customized 3D Bone Scaffolds Treated with CRFP, an Osteogenic Peptide. Bioengineering, 8(12), 199. https://doi.org/10.3390/bioengineering8120199






