Synergistic Effect of Magnetite and Bioelectrochemical Systems on Anaerobic Digestion
Abstract
:1. Introduction
2. Materials and Methods
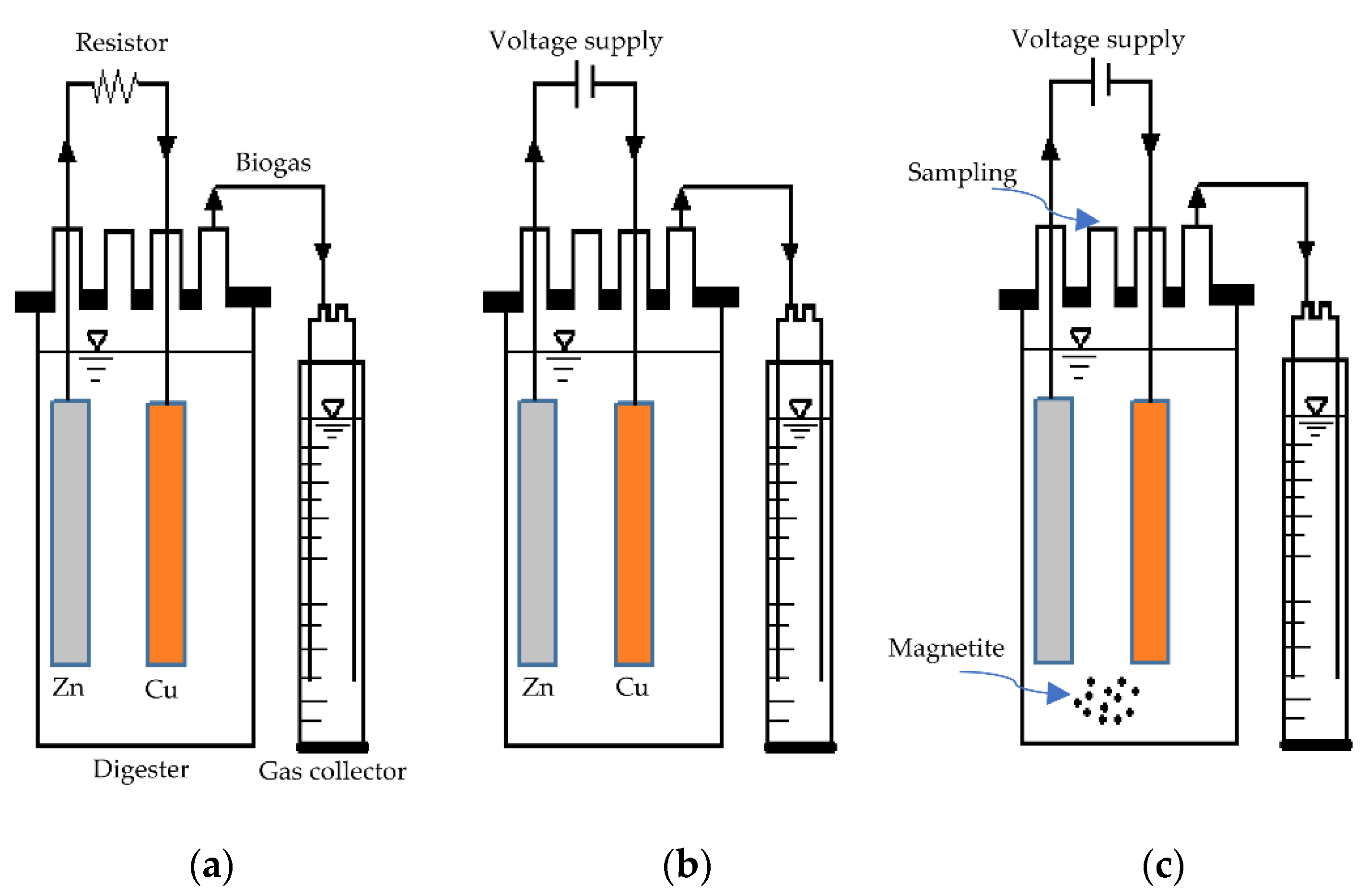
2.1. Anaerobic Digester and Operation
2.2. Synthesis of Magnetite (Fe3O4) Nanoparticles and Substrate/Chemical Reagents
2.3. Analyses and Calculations
3. Results and Discussion
3.1. Biogas Accumulation and Methane Content in Biogas
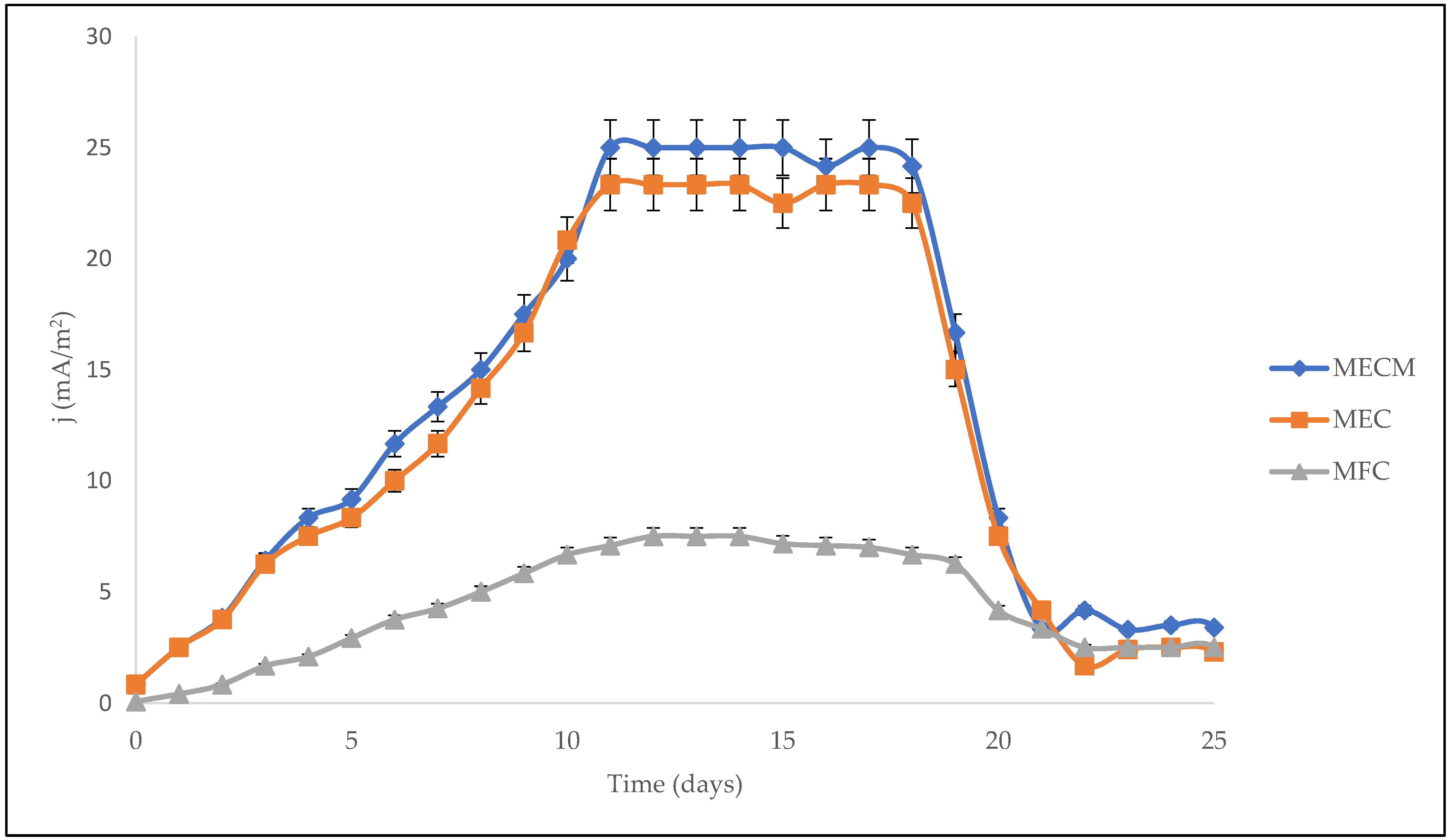
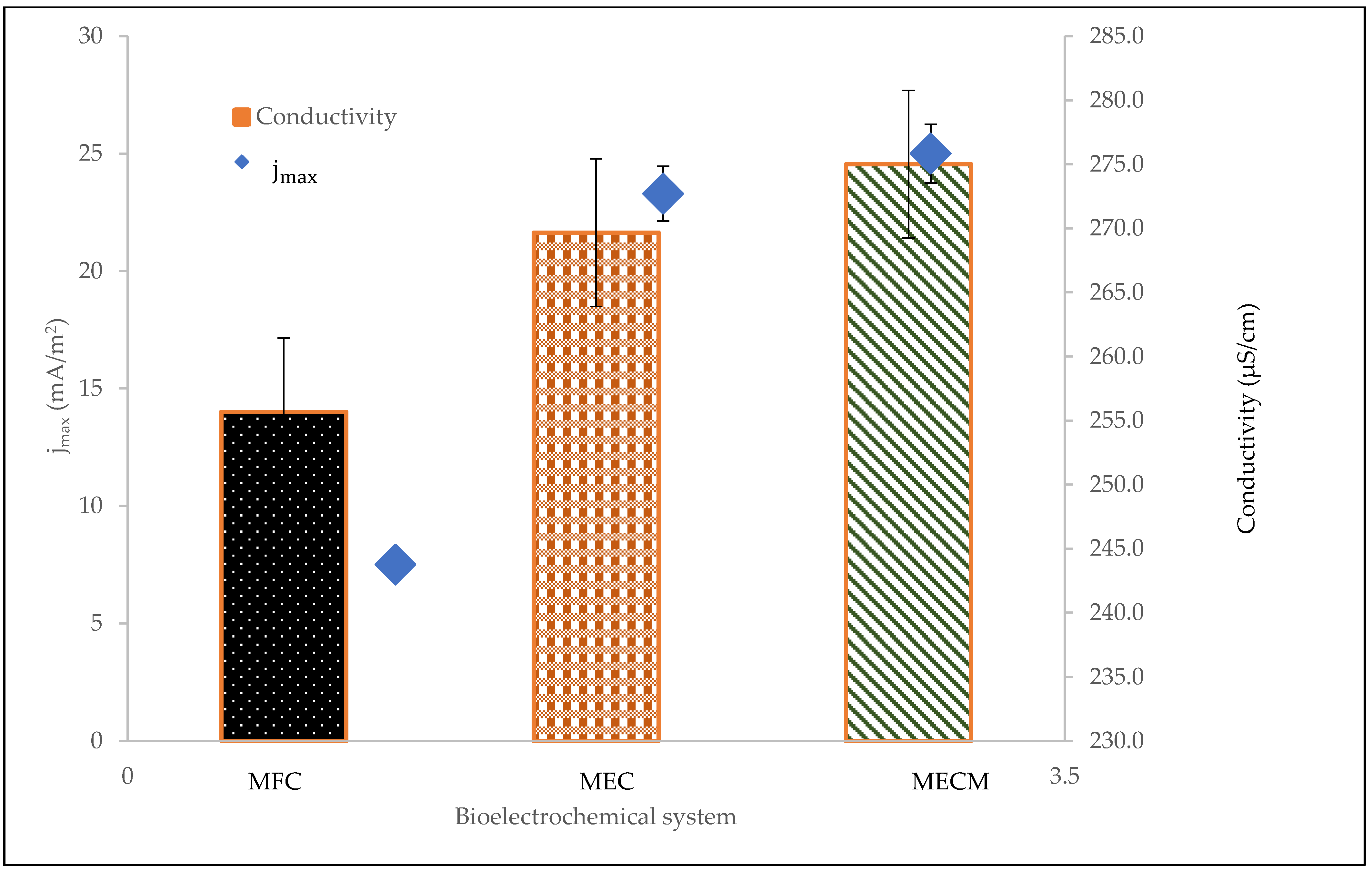
3.2. Electrochemical Characterisation
3.3. Stability Indicator
3.4. Effect of Anaerobic Bioelectrochemical Process on Contaminant Removal
4. Conclusions
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Data Availability Statement
Acknowledgments
Conflicts of Interest
References
- Whiting, A.; Azapagic, A. Life cycle environmental impacts of generating electricity and heat from biogas produced by anaerobic digestion. Energy 2014, 70, 181–193. [Google Scholar] [CrossRef]
- Halim, A.; Rahman, O.; Ibrahim, M.; Kundu, R. Effect of Anolyte pH on the Performance of a Dual-Chambered Microbial Fuel Cell Operated with Different Biomass Feed. J. Chem. 2021, 2021, 5465680. [Google Scholar] [CrossRef]
- Nwokolo, N.; Mukumba, P.; Obileke, K.; Enebe, M. Waste to Energy: A Focus on the Impact of Substrate Type in Biogas Production. Processes 2020, 8, 1224. [Google Scholar] [CrossRef]
- Suryawanshi, P.C.; Chaudhari, A.B.; Kothari, R.M. Thermophilic anaerobic digestion: The best option for waste treatment. Crit. Rev. Biotechnol. 2010, 30, 259–282. [Google Scholar] [CrossRef] [PubMed]
- Gebreeyessus, G.; Jenicek, P. Thermophilic versus Mesophilic Anaerobic Digestion of Sewage Sludge: A Comparative Review. Bioengineering 2016, 3, 15. [Google Scholar] [CrossRef] [PubMed]
- Lin, Q.; He, G.; Rui, J.; Fang, X.; Tao, Y.; Li, J.; Li, X. Microorganism-regulated mechanisms of temperature effects on the performance of anaerobic digestion. Microb. Cell Fact. 2016, 15, 1–18. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Matos, J.C.; Rozensky, L.; Vrba, X.; Hansen, J.M.; Hajek, M.; Lipa, J.; Rodrigues, C.V.; Castro, F.G.F.; Maintinguer, S.I. Application of Electromagnetic Field in Anaerobic Biodigestion in Batch Reactors. BioResources 2020, 15, 4972–4981. [Google Scholar] [CrossRef]
- Zielinski, M.; Debowski, M.; Kazimierowicz, J. The Effect of Static Magnetic Field on Methanogenesis in the Anaerobic Digestion of Municipal Sewage Sludge. Energies 2021, 14, 590. [Google Scholar] [CrossRef]
- Hamelers, H.M.; Heijne, A.; Sleutels, T.J.A.; Jeremiasse, A.; Strik, D.B.T.B.; Buisman, C.N. New applications and performance of bioelectrochemical systems. Appl. Microbiol. Biotechnol. 2010, 85, 1673–1685. [Google Scholar] [CrossRef]
- Moreno, M.C. Anaerobic Digestion and Bioelectrochemical Systems Combination for Energy and Nitrogen Recovery Optimization. Ph.D. Thesis, The Polytechnic University of Catalonia, Barcelona, Spain, 2016. [Google Scholar]
- Tsuchiya, T.; Imura, M.; Koide, Y.; Terabe, K. Magnetic Control of Magneto-Electrochemical Cell and Electric Double Layer Transistor. Sci. Rep. 2017, 7, 10534. [Google Scholar] [CrossRef] [Green Version]
- Bhagchandanii, D.D.; Babu, R.P.; Sonawane, J.M.; Khanna, N.; Pandit, S.; Jadhav, D.A.; Khilari, S.; Prasad, R. A Comprehensive Understanding of Electro-Fermentation. Fermentation 2020, 6, 92. [Google Scholar] [CrossRef]
- Shah, F.A.; Mahmood, Q.; Shah, M.M.; Pervez, A.; Asad, S.A. Microbial Ecology of Anaerobic Digesters: The Key Players of Anaerobiosis. Sci. World J. 2014, 2014, 1–21. [Google Scholar]
- Feng, Q.; Song, Y.S.; Bae, B.U. Influence of applied voltage on the performance of bioelectrochemical anaerobic digestion of sewage sludge and planktonic microbial communities at ambient temperature. Bioresour. Technol. 2016, 220, 500–508. [Google Scholar] [CrossRef]
- Paritosh, K.; Yadav, M.; Chawade, A.; Sahoo, D.; Kesharwani, N.; Pareek, N.; Vivekanand, V. Additives as a Support Structure for Specific Biochemical Activity Boosts in Anaerobic Digestion: A Review. Front. Energy Res. 2020, 8, 88. [Google Scholar] [CrossRef]
- Baek, G.; Kim, J.; Kim, S.; Lee, C. Role and Potential of Direct Interspecies Electron Transfer in Anaerobic Digestion. Energies 2018, 11, 107. [Google Scholar] [CrossRef] [Green Version]
- Cheng, S.; Xing, D.; Call, D.F.; Logan, B.E. Direct biological conversion of electrical current into methane by electromethanogenesis. Environ. Sci. Technol. 2009, 43, 3953–3958. [Google Scholar] [CrossRef]
- Villano, M.; Aulenta, F.; Ciucci, C.; Ferri, T.; Giuliano, A.; Majone, M. Bioelectrochemical reduction of CO2 to CH4 via direct and indirect extracellular electron transfer by a hydrogenophilic methanogenic culture. Bioresour. Technol. 2010, 101, 3085–3090. [Google Scholar] [CrossRef]
- Wu, S.; Sun, A.; Zhai, F.; Wang, J.; Xu, W.; Zhang, Q.; Volinsky, A.A. Fe3O4 magnetic nanoparticles synthesis from tailings by ultrasonic chemical co-precipitation. Mater. Lett. 2011, 65, 1882–1884. [Google Scholar] [CrossRef]
- Nelabhotla, A.B.; Dinamarca, C. Bioelectrochemical CO2 Reduction to Methane: MES Integration in Biogas Production Processes. Appl. Sci. 2019, 9, 1056. [Google Scholar] [CrossRef] [Green Version]
- Rolfe, M.D.; Rice, C.J.; Lucchini, S.; Pin, C.; Thompson, A.; Cameron, A.D.S.; Alston, M.; Stringer, M.F.; Betts, R.P.; Baranyi, J.; et al. Lag Phase Is a Distinct Phase That Prepares Bacteria for Exponential Growth and Involves Transient Metal Accumulation. J. Bacteriol. 2012, 194, 686–701. [Google Scholar] [CrossRef] [Green Version]
- An, Z.; Feng, Q.; Zhao, R.; Wang, X. Bioelectrochemical Methane Production from Food Waste in Anaerobic Digestion Using a Carbon-Modified Copper Foam Electrode. Processes 2020, 8, 416. [Google Scholar] [CrossRef] [Green Version]
- Anukam, A.; Mohammadi, A.; Naqvi, M.; Granstrom, K. A review of the Chemistry of Anaerobic Digestion: Methods of Accelerating and Optimizing Process Efficiency. Processes 2019, 7, 504. [Google Scholar] [CrossRef] [Green Version]
- Paulo, L.; Stams, A.; Sousa, D. Methanogens, sulphate and heavy metals: A complex system. Rev. Environ. Sci. Biotechnol. 2015, 14, 537–553. [Google Scholar] [CrossRef] [Green Version]
- Zhang, J.; Lu, T.; Wang, Z.; Wang, Y.; Zhong, H.; Shen, P.; Wei, Y. Effects of magnetite on anaerobic digestion of swine manure: Attention to methane production and fate of antibiotic resistance genes. Bioresour. Technol. 2019, 291, 121847. [Google Scholar] [CrossRef]
- Song, Y.C.; Feng, Q.; Ahn, Y. Performance of the bioelectrochemical anaerobic digestion of sewage sludge at different HRTs. Energy Fuels 2015, 30, 352–359. [Google Scholar] [CrossRef]
- Feng, Q.; Song, Y.C.; Kim, D.H.; Kim, M.S.; Kim, D.H. Influence of the temperature and hydraulic retention time in bioelectrochemical anaerobic digestion of sewage sludge. Int. J. Hydrogen Energy 2019, 44, 2170–2179. [Google Scholar] [CrossRef]
- Hamed, M.S.; Majdi, H.S.; Hasan, B.O. Effect of Electrode Material and Hydrodynamics on Produced Current in Double Chamber Microbial Fuel Cells. ACS Omega 2020, 5, 10339–10348. [Google Scholar] [CrossRef] [PubMed]
- Ghaly, A.E. Effect of reseeding and pH control on the performance of a two-stage mesophilic anaerobic digester operating on acid cheese whey. Can. Agric. Eng. 2000, 42, 173–183. [Google Scholar]
- Lim, S.S.; Fontmorin, J.M.; Izadi, P.; Daud, W.R.W.; Scott, K.; Yu, E.H. Impact of applied cell voltage on the performance of a microbial electrolysis cell fully catalysed by microorganisms. Int. J. Hydrogen Energy 2020, 45, 2557–2568. [Google Scholar] [CrossRef]
- Sun, J.; Cao, H. Progress in Nitrogen Removal in Bioelectrochemical Systems. Processes 2020, 8, 831. [Google Scholar] [CrossRef]





| Parameters | Unit | Amount |
|---|---|---|
| pH | - | 6.7 ± 0.5 |
| NH3-N | mg/L | 41.4 ± 2.5 |
| TOC | mg/L | 3633 ± 47 |
| Phosphate | mg/L | 9.9 ± 0.1 |
| TSS | mg/L | 37.3 ± 1.3 |
| COD | mg/L | 2300 ± 216 |
| Colour | Pt.Co | 234 ± 5.3 |
| Turbidity | NTU | 519 ± 8.0 |
| Electrical conductivity | µS/cm | 604 ± 61 |
Publisher’s Note: MDPI stays neutral with regard to jurisdictional claims in published maps and institutional affiliations. |
© 2021 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Madondo, N.I.; Tetteh, E.K.; Rathilal, S.; Bakare, B.F. Synergistic Effect of Magnetite and Bioelectrochemical Systems on Anaerobic Digestion. Bioengineering 2021, 8, 198. https://doi.org/10.3390/bioengineering8120198
Madondo NI, Tetteh EK, Rathilal S, Bakare BF. Synergistic Effect of Magnetite and Bioelectrochemical Systems on Anaerobic Digestion. Bioengineering. 2021; 8(12):198. https://doi.org/10.3390/bioengineering8120198
Chicago/Turabian StyleMadondo, Nhlanganiso Ivan, Emmanuel Kweinor Tetteh, Sudesh Rathilal, and Babatunde Femi Bakare. 2021. "Synergistic Effect of Magnetite and Bioelectrochemical Systems on Anaerobic Digestion" Bioengineering 8, no. 12: 198. https://doi.org/10.3390/bioengineering8120198
APA StyleMadondo, N. I., Tetteh, E. K., Rathilal, S., & Bakare, B. F. (2021). Synergistic Effect of Magnetite and Bioelectrochemical Systems on Anaerobic Digestion. Bioengineering, 8(12), 198. https://doi.org/10.3390/bioengineering8120198









