Bioactive Scaffolds Integrated with Liposomal or Extracellular Vesicles for Bone Regeneration
Abstract
:1. Introduction
2. Liposomal Vesicle-Integrated Scaffolds for Bone Regeneration
2.1. Functionalization of Liposomes
2.1.1. Thermosensitive Liposomes
2.1.2. Adhesive Liposomes
2.1.3. Bone-Targeting Liposomes
2.1.4. Osteoinductive Liposomes
2.2. Cargo Loading into Liposomes
2.2.1. Proteins and Peptides
2.2.2. Hydrophobic Small Molecule Drugs
2.2.3. Hydrophilic Small Molecule Drugs
2.2.4. Genes
2.3. Integration of Liposomes into Scaffolds
2.4. Signaling Pathways Explored in Liposome-Integrated Scaffolds
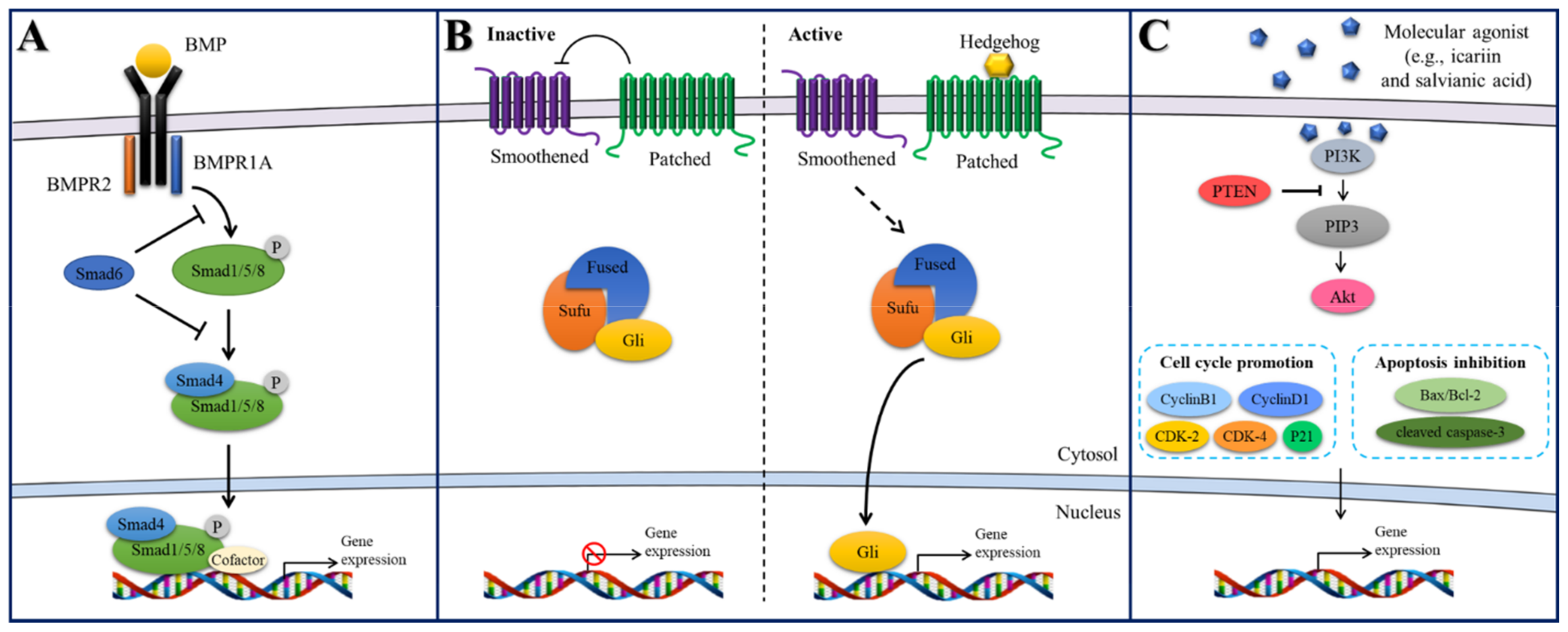
2.4.1. BMP/Smad Signaling
2.4.2. Hedgehog Signaling
2.4.3. PI3K/Akt Signaling
3. Extracellular Vesicle-Integrated Scaffolds for Bone Regeneration
3.1. Source and Function of Extracellular Vesicles
3.1.1. Bone Cells
3.1.2. Other Stem Cells
| Source of Exosomes | Pre-conditioning | Scaffold | In Vivo Model | Function of Exosome-Integrated Scaffolds | Reference |
|---|---|---|---|---|---|
| rBMSCs | OM | Alginate-PCL | Nude mouse subcutaneous bone formation model | Pro-angiogenic and pro-bone regeneration activities in vitro and in vivo. | [68] |
| rBMSCs | OM | Decalcified bone matrix (DBM) | Nude mouse subcutaneous bone formation model | Pro-angiogenic and pro-bone regeneration activities in vitro and in vivo. | [69] |
| hBMSCs | - | Gelatin methacrylate (GelMA) | Rabbit osteochondral defect model | Enhanced mitochondrial biogenesis in vitro and facilitated cartilage regeneration in vivo. | [70] |
| hBMSCs | Hypoxia | Commercial HA scaffold | Rat calvarial defect model | Pro-angiogenic activities via AKT/mTOR pathway. | [71] |
| hBMSCs | OM, Noggin Suppression | Injectable chitosan hydrogel | Mouse calvarial defect model | Elevated osteogenesis via inhibition of miR-29a in BMP/Smad signaling. | [72] |
| hBMSCs | - | DBM | Rat subcutaneous implantation and Rabbit femoral condyle bone defect model | Human BMP2 plasmids were coated onto the vesicles. Promoted bone formation and angiogenesis in animal model. | [73] |
| hBMSCs | - | Calcium sulfate/ nanohydroxyapatite based nanocement | Femur neck canal defect model in osteoporotic rats | Enhanced bone formation in the absence of BMP. | [74] |
| hBMSCs | OM | Titanium alloy | Rat radial bone defect model | Promoted osteogenic differentiation via PI3K/Akt and MAPK signaling pathways. | [75] |
| rBMSCs | OM | Mesoporous bioactive glass (MBG) | Rat calvarial defect model | Osteoinductivity attributed to exosomal miRNAs (let-7a-5p, let-7c-5p, miR-328a-5p, and miR-31a-5p). | [66] |
| hBMSCs | - | Titanium alloy | Osteoporotic bone defect model | miR-20a in hBMSC-EVs was shown to play a key role in promoting osteogenesis. | [76] |
| hBMSCs | BMP2 overexpression | Alginate-RGD hydrogel | Rat calvarial defect model | EVs were tethered to biomaterial scaffolds with ECM proteins, which promoted bone repair and prolonged delivery in vivo. | [77] |
| Preosteoblasts MC3T3 | - | Alginate hydrogel | - | Verified the osteogenic potential of MC3T3-derived EVs in vitro. | [78] |
| Murine-derived macrophage | BMP2 stimulation | Titanium dioxide nanotubes | - | BMP2/macrophage-derived exosomes enhanced the osteogenic differentiation of MSCs in vitro. | [60] |
| Osteoclast from osteoporotic rats | - | Magnetic nanoparticle-infiltrated hydroxyapatite | - | The presence of magnetic nanoparticles altered osteoclast-derived exosomal cargo and decreased the uptake efficiency of osteoclast exosomes in osteoblasts. | [61] |
| Osteoclast from mice | - | DBM | Rat calvarial defect model | Elevated osteogenesis via miR-324 in ARHGAP1/RhoA/ROCK signaling. | [62] |
| Source of Exosomes | Pre-Conditioning | Scaffold | In Vivo Model | Function of Exosome-Integrated Scaffolds | Reference |
|---|---|---|---|---|---|
| hASCs | OM | Polydopamine-coated PLGA | Rat calvarial defect model | Promoted proliferation, migration, and osteogenic differentiation of hBMSCs in vitro. Enhanced migration and homing of hBMSCs in vivo. | [63] |
| hASCs | - | Biotin-doped polypyrrole titanium | Nude mouse subcutaneous bone formation model | Osteoinductive ability of hASC-EVs was evaluated via analysis of its content miRNAs. | [65] |
| hASCs | - | CaSi-coated PLA | - | Enhanced osteogenic properties in vitro. | [79] |
| hASCs | - | Silk fibroin | Rat calvarial defect model | Improved osteogenic differentiation of hBMSCs in vitro. Promoted the production of collagenous tissues and bone-like tissue in vivo. | [64] |
| Chondrogenic progenitor cells | - | Core-shell nanofiber film of CS/PLA | Rat calvarial defect model | VEGF plasmid DNA was sustainably delivered, resulting in elevated vascularized osteogenesis in vivo. | [80] |
| Chondrogenic progenitor cells | - | 3D printed PCL scaffolds | Rat radial bone defect model | Osteogenic differentiation of hBMSCs in vitro. Vascularized bone regeneration in vivo. | [81] |
| hiPSCs | - | Commercial β-TCP | Rat ovariectomized model | Enhanced angiogenesis and osteogenesis under osteoporotic conditions. | [82] |
| hiPSCs | - | Commercial β-TCP | Rat calvarial defect model | Osteogenic differentiation of hBMSCs via PI3K/Akt signaling. | [83] |
| hUCMSCs | - | Commercial hyaluronan-heparin hydrogel | Rat model of femoral fracture | HIF-1-mediated promotion of angiogenesis. | [67] |
| hUCMSCs | CHA/SF/GCS/DF-PEG hydrogel | Rat femoral condyle bone defect model | Sustained delivery of exosomes at the bone defect sites. | [84] | |
| hUCMSCs | - | Hyaluronic acid-alginate hydrogel with HAP | Rat calvarial defect model | Controlled delivery of exosomes at the bone defect sites. | [85] |
| hUCMSCs | - | Hyaluronic acid hydrogel combined with customized HAP/poly-ε-caprolactone | Rat calvarial defect model | miR-21 is a potential intercellular messenger that promoted angiogenesis by upregulating the NOTCH1/DLL4 pathway. | [86] |
| hGMSCs | - | PLLA scaffold | Rat calvarial defect model | Improved vascular network and osteogenic regeneration. | [87] |
| hDPSCs | OM | PLLA scaffold | Nude mouse subcutaneous bone formation model | PLGA-PEG-PLGA triblock copolymer microsphere Controlled release of Exos from scaffolds was characterized. | [88] |
| rDPSCs | - | Commercial collagen, β-TCP, or HA scaffold | Rat calvarial defect model | DPSC-EVs-loaded scaffold showed a comparable bone regeneration effect to the DSPC-loaded scaffolds. | [89] |
| hSHED | - | β-TCP | Rat periodontal defect model | Periodontal bone regeneration through AMPK signaling. | [90] |
3.2. Endogenous Engineering of Extracellular Vesicles
3.2.1. Pre-Conditioning Parent Cells to Enhance Angiogenic Potential
3.2.2. Pre-Conditioning Parent Cells to Enhance Osteogenic Potential
3.3. Exogenous Cargo Loading into Extracellular Vesicles
3.4. Integration of Extracellular Vesicles into Scaffolds
3.4.1. Post-Solution Adsorption
3.4.2. Encapsulation during In Situ Gelation
3.4.3. Surface Modification Strategies
4. Conclusions and Future Perspectives
Author Contributions
Funding
Conflicts of Interest
References
- Office of the Surgeon General (US). Bone Health and Osteoporosis: A Report of the Surgeon General; Reports of the Surgeon General; Office of the Surgeon General (US): Rockville, MD, USA, 2004. [Google Scholar]
- Baldwin, P.; Li, D.J.; Auston, D.A.; Mir, H.S.; Yoon, R.S.; Koval, K.J. Autograft, allograft, and bone graft substitutes: Clinical evidence and indications for use in the setting of orthopaedic trauma surgery. J. Orthop. Trauma 2019, 33, 203–213. [Google Scholar] [CrossRef]
- Betz, R.R. Limitations of autograft and allograft: New synthetic solutions. Orthopedics 2002, 25, S561–S570. [Google Scholar] [CrossRef] [PubMed]
- Henkel, J.; Woodruff, M.A.; Epari, D.R.; Steck, R.; Glatt, V.; Dickinson, I.C.; Choong, P.F.M.; Schuetz, M.A.; Hutmacher, D.W. Bone regeneration based on tissue engineering conceptions—A 21st century perspective. Bone Res. 2013, 1, 216–248. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Kim, S.; Lee, M. Rational design of hydrogels to enhance osteogenic potential. Chem. Mater. 2020, 32, 9508–9530. [Google Scholar] [CrossRef]
- De Witte, T.-M.; Fratila-Apachitei, L.E.; Zadpoor, A.A.; Peppas, N.A. Bone tissue engineering via growth factor delivery: From scaffolds to complex matrices. Regen. Biomater. 2018, 5, 197–211. [Google Scholar] [CrossRef] [Green Version]
- Sarigol-Calamak, E.; Hascicek, C. Tissue scaffolds as a local drug delivery system for bone regeneration. In Cutting-Edge Enabling Technologies for Regenerative Medicine; Advances in Experimental Medicine and Biology; Chun, H.J., Park, C.H., Kwon, I.K., Khang, G., Eds.; Springer: Singapore, 2018; Volume 1078, pp. 475–493. ISBN 9789811309496. [Google Scholar]
- Xie, C.; Ye, J.; Liang, R.; Yao, X.; Wu, X.; Koh, Y.; Wei, W.; Zhang, X.; Ouyang, H. Advanced strategies of biomimetic tissue-engineered grafts for bone regeneration. Adv. Healthc. Mater. 2021, 10, 2100408. [Google Scholar] [CrossRef] [PubMed]
- Cheng, R.; Liu, L.; Xiang, Y.; Lu, Y.; Deng, L.; Zhang, H.; Santos, H.A.; Cui, W. Advanced liposome-loaded scaffolds for therapeutic and tissue engineering applications. Biomaterials 2020, 232, 119706. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Al-Sowayan, B.; Alammari, F.; Alshareeda, A. Preparing the bone tissue regeneration ground by exosomes: From diagnosis to therapy. Molecules 2020, 25, 4205. [Google Scholar] [CrossRef]
- Zhou, Y.; Xiao, Y. The development of extracellular vesicle-integrated biomaterials for bone regeneration. In Biomimicked Biomaterials; Advances in Experimental Medicine and Biology; Chun, H.J., Reis, R.L., Motta, A., Khang, G., Eds.; Springer: Singapore, 2020; Volume 1250, pp. 97–108. ISBN 9789811532610. [Google Scholar]
- Yan, H.-C.; Yu, T.-T.; Li, J.; Qiao, Y.-Q.; Wang, L.-C.; Zhang, T.; Li, Q.; Zhou, Y.-H.; Liu, D.-W. The delivery of extracellular vesicles loaded in biomaterial scaffolds for bone regeneration. Front. Bioeng. Biotechnol. 2020, 8, 1015. [Google Scholar] [CrossRef]
- Bozzuto, G.; Molinari, A. Liposomes as nanomedical devices. Int. J. Nanomed. 2015, 10, 975. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- van Niel, G.; D’Angelo, G.; Raposo, G. Shedding light on the cell biology of extracellular vesicles. Nat. Rev. Mol. Cell. Biol. 2018, 19, 213–228. [Google Scholar] [CrossRef]
- Koog, L.; Gandek, T.B.; Nagelkerke, A. Liposomes and extracellular vesicles as drug delivery systems: A comparison of composition, pharmacokinetics, and functionalization. Adv. Healthc. Mater. 2021, 2100639. [Google Scholar] [CrossRef]
- Phinney, D.G.; Pittenger, M.F. Concise review: MSC-derived exosomes for cell-free therapy: MSC-derived exosomes. Stem. Cells. 2017, 35, 851–858. [Google Scholar] [CrossRef] [Green Version]
- Bueno, E.M.; Glowacki, J. Cell-free and Cell-based approaches for bone regeneration. Nat. Rev. Rheumatol. 2009, 5, 685–697. [Google Scholar] [CrossRef] [PubMed]
- López-Noriega, A.; Ruiz-Hernández, E.; Quinlan, E.; Storm, G.; Hennink, W.E.; O’Brien, F.J. Thermally triggered release of a pro-osteogenic peptide from a functionalized collagen-based scaffold using thermosensitive liposomes. J. Control. Release 2014, 187, 158–166. [Google Scholar] [CrossRef]
- Liu, P.; Guo, B.; Wang, S.; Ding, J.; Zhou, W. A thermo-responsive and self-healing liposome-in-hydrogel system as an antitubercular drug carrier for localized bone tuberculosis therapy. Int. J. Pharm. 2019, 558, 101–109. [Google Scholar] [CrossRef]
- Liu, L.; Xiang, Y.; Wang, Z.; Yang, X.; Yu, X.; Lu, Y.; Deng, L.; Cui, W. Adhesive liposomes loaded onto an injectable, self-healing and antibacterial hydrogel for promoting bone reconstruction. NPG Asia. Mater. 2019, 11, 81. [Google Scholar] [CrossRef]
- Wang, G.; Babadağli, M.E.; Uludağ, H. Bisphosphonate-derivatized liposomes to control drug release from collagen/hydroxyapatite scaffolds. Mol. Pharm. 2011, 8, 1025–1034. [Google Scholar] [CrossRef] [PubMed]
- Zhang, X.; Fan, J.; Lee, C.; Kim, S.; Chen, C.; Aghaloo, T.; Lee, M. Apatite-binding nanoparticulate agonist of hedgehog signaling for bone repair. Adv. Funct. Mater. 2020, 30, 1909218. [Google Scholar] [CrossRef] [PubMed]
- Liu, Y.; Jia, Z.; Akhter, M.P.; Gao, X.; Wang, X.; Wang, X.; Zhao, G.; Wei, X.; Zhou, Y.; Wang, X.; et al. Bone-targeting liposome formulation of salvianic acid a accelerates the healing of delayed fracture union in mice. Nanomed. Nanotechnol. Biol. Med. 2018, 14, 2271–2282. [Google Scholar] [CrossRef] [PubMed]
- Zhou, L.; Wu, H.; Gao, X.; Zheng, X.; Chen, H.; Li, H.; Peng, J.; Liang, W.; Wang, W.; Qiu, Z.; et al. Bone-targeting liposome-encapsulated salvianic acid a improves nonunion healing through the regulation of HDAC3-mediated endochondral ossification. Drug Des. Dev. Ther. 2020, 14, 3519–3533. [Google Scholar] [CrossRef]
- Xu, G.; Hu, X.; Han, L.; Zhao, Y.; Li, Z. The construction of a novel xenograft bovine bone scaffold, (DSS)6-liposome/CKIP-1 SiRNA/Calcine bone and its osteogenesis evaluation on skull defect in Rats. J. Orthop. Transl. 2021, 28, 74–82. [Google Scholar] [CrossRef]
- Vhora, I.; Lalani, R.; Bhatt, P.; Patil, S.; Misra, A. Lipid-nucleic acid nanoparticles of novel ionizable lipids for systemic BMP-9 gene delivery to bone-marrow mesenchymal stem cells for osteoinduction. Int. J. Pharm. 2019, 563, 324–336. [Google Scholar] [CrossRef]
- Cui, Z.-K.; Kim, S.; Baljon, J.J.; Doroudgar, M.; Lafleur, M.; Wu, B.M.; Aghaloo, T.; Lee, M. Design and characterization of a therapeutic non-phospholipid liposomal nanocarrier with osteoinductive characteristics to promote bone formation. ACS Nano 2017, 11, 8055–8063. [Google Scholar] [CrossRef]
- Cottrill, E.; Lazzari, J.; Pennington, Z.; Ehresman, J.; Schilling, A.; Dirckx, N.; Theodore, N.; Sciubba, D.; Witham, T. Oxysterols as promising small molecules for bone tissue engineering: Systematic review. World J. Orthop. 2020, 11, 328–344. [Google Scholar] [CrossRef]
- Lee, C.-S.; Kim, S.; Fan, J.; Hwang, H.S.; Aghaloo, T.; Lee, M. Smoothened agonist sterosome immobilized hybrid scaffold for bone regeneration. Sci. Adv. 2020, 6, eaaz7822. [Google Scholar] [CrossRef] [Green Version]
- Lee, C.-S.; Hsu, G.C.-Y.; Sono, T.; Lee, M.; James, A.W. Development of a biomaterial scaffold integrated with osteoinductive oxysterol liposomes to enhance hedgehog signaling and bone repair. Mol. Pharm. 2021, 18, 1677–1689. [Google Scholar] [CrossRef]
- Crasto, G.J.; Kartner, N.; Reznik, N.; Spatafora, M.V.; Chen, H.; Williams, R.; Burns, P.N.; Clokie, C.; Manolson, M.F.; Peel, S.A.F. Controlled bone formation using ultrasound-triggered release of BMP-2 from liposomes. J. Control. Release 2016, 243, 99–108. [Google Scholar] [CrossRef] [PubMed]
- Matsuo, T.; Sugita, T.; Kubo, T.; Yasunaga, Y.; Ochi, M.; Murakami, T. injectable magnetic liposomes as a novel carrier of recombinant human BMP-2 for bone formation in a rat bone-defect model. J. Biomed. Mater. Res. 2003, 66A, 747–754. [Google Scholar] [CrossRef] [PubMed]
- Mohammadi, M.; Alibolandi, M.; Abnous, K.; Salmasi, Z.; Jaafari, M.R.; Ramezani, M. fabrication of hybrid scaffold based on hydroxyapatite-biodegradable nanofibers incorporated with liposomal formulation of BMP-2 peptide for bone tissue engineering. Nanomed. Nanotechnol. Biol. Med. 2018, 14, 1987–1997. [Google Scholar] [CrossRef] [PubMed]
- Sarkar, N.; Bose, S. liposome-encapsulated curcumin-loaded 3D printed scaffold for bone tissue engineering. ACS Appl. Mater. Interfaces 2019, 11, 17184–17192. [Google Scholar] [CrossRef]
- Li, Y.; Bai, Y.; Pan, J.; Wang, H.; Li, H.; Xu, X.; Fu, X.; Shi, R.; Luo, Z.; Li, Y.; et al. A hybrid 3D-printed aspirin-laden liposome composite scaffold for bone tissue engineering. J. Mater. Chem. B 2019, 7, 619–629. [Google Scholar] [CrossRef]
- Li, Y.; Li, Q.; Li, H.; Xu, X.; Fu, X.; Pan, J.; Wang, H.; Fuh, J.Y.H.; Bai, Y.; Wei, S. An effective dual-factor modified 3D-printed PCL scaffold for bone defect repair. J. Biomed. Mater. Res. 2020, 108, 2167–2179. [Google Scholar] [CrossRef]
- Yang, J.; Zhu, Y.; Wang, F.; Deng, L.; Xu, X.; Cui, W. Microfluidic liposomes-anchored microgels as extended delivery platform for treatment of osteoarthritis. Chem. Eng. J. 2020, 400, 126004. [Google Scholar] [CrossRef]
- Cheng, R.; Yan, Y.; Liu, H.; Chen, H.; Pan, G.; Deng, L.; Cui, W. Mechanically enhanced lipo-hydrogel with controlled release of multi-type drugs for bone regeneration. Appl. Mater. Today 2018, 12, 294–308. [Google Scholar] [CrossRef]
- Han, X.; Sun, M.; Chen, B.; Saiding, Q.; Zhang, J.; Song, H.; Deng, L.; Wang, P.; Gong, W.; Cui, W. Lotus seedpod-inspired internal vascularized 3D printed scaffold for bone tissue repair. Bioact. Mater. 2021, 6, 1639–1652. [Google Scholar] [CrossRef]
- Monteiro, N.; Ribeiro, D.; Martins, A.; Faria, S.; Fonseca, N.A.; Moreira, J.N.; Reis, R.L.; Neves, N.M. Instructive nanofibrous scaffold comprising runt-related transcription factor 2 gene delivery for bone tissue engineering. ACS Nano 2014, 8, 8082–8094. [Google Scholar] [CrossRef]
- Cui, Z.-K.; Fan, J.; Kim, S.; Bezouglaia, O.; Fartash, A.; Wu, B.M.; Aghaloo, T.; Lee, M. Delivery of SiRNA via cationic sterosomes to enhance osteogenic differentiation of mesenchymal stem cells. J. Control. Release 2015, 217, 42–52. [Google Scholar] [CrossRef] [Green Version]
- Cui, Z.-K.; Sun, J.A.; Baljon, J.J.; Fan, J.; Kim, S.; Wu, B.M.; Aghaloo, T.; Lee, M. Simultaneous delivery of hydrophobic small molecules and sirna using sterosomes to direct mesenchymal stem cell differentiation for bone repair. Acta Biomater. 2017, 58, 214–224. [Google Scholar] [CrossRef] [PubMed]
- Brito Barrera, Y.A.; Hause, G.; Menzel, M.; Schmelzer, C.E.H.; Lehner, E.; Mäder, K.; Wölk, C.; Groth, T. Engineering osteogenic microenvironments by combination of multilayers from collagen type i and chondroitin sulfate with novel cationic liposomes. Mater. Today Bio 2020, 7, 100071. [Google Scholar] [CrossRef] [PubMed]
- Monteiro, N.; Martins, A.; Pires, R.; Faria, S.; Fonseca, N.A.; Moreira, J.N.; Reis, R.L.; Neves, N.M. Immobilization of bioactive factor-loaded liposomes on the surface of electrospun nanofibers targeting tissue engineering. Biomater. Sci. 2014, 2, 1195–1209. [Google Scholar] [CrossRef] [PubMed]
- Ding, Y.H.; Floren, M.; Tan, W. Mussel-inspired polydopamine for bio-surface functionalization. Biosurface Biotribol. 2016, 2, 121–136. [Google Scholar] [CrossRef]
- Wu, X.; Walker, J.; Zhang, J.; Ding, S.; Schultz, P.G. Purmorphamine induces osteogenesis by activation of the hedgehog signaling pathway. Chem. Biol. 2004, 11, 1229–1238. [Google Scholar] [CrossRef] [Green Version]
- Ten Dijke, P.; Korchynskyi, O.; Valdimarsdottir, G.; Goumans, M.-J. Controlling cell fate by bone morphogenetic protein receptors. Mol. Cell. Endocrinol. 2003, 211, 105–113. [Google Scholar] [CrossRef] [PubMed]
- Valcourt, U.; Moustakas, A. BMP signaling in osteogenesis, bone remodeling and repair. Eur. J. Trauma 2005, 31, 464–479. [Google Scholar] [CrossRef]
- Isomoto, S.; Hattori, K.; Ohgushi, H.; Nakajima, H.; Tanaka, Y.; Takakura, Y. Rapamycin as an inhibitor of osteogenic differentiation in bone marrow-derived mesenchymal stem cells. J. Orthop. Sci. 2007, 12, 83–88. [Google Scholar] [CrossRef]
- Harada, S.; Rodan, G.A. Control of osteoblast function and regulation of bone mass. Nature 2003, 423, 349–355. [Google Scholar] [CrossRef]
- Jin, X.; Sun, J.; Yu, B.; Wang, Y.; Sun, W.J.; Yang, J.; Huang, S.H.; Xie, W.L. Daidzein stimulates osteogenesis facilitating proliferation, differentiation, and antiapoptosis in human osteoblast-like MG-63 cells via estrogen receptor–dependent MEK/ERK and PI3K/Akt activation. Nutr. Res. 2017, 42, 20–30. [Google Scholar] [CrossRef] [PubMed]
- Hu, J.; Mao, Z.; He, S.; Zhan, Y.; Ning, R.; Liu, W.; Yan, B.; Yang, J. Icariin protects against glucocorticoid induced osteoporosis, increases the expression of the bone enhancer DEC1 and modulates the PI3K/Akt/GSK3β/β-catenin integrated signaling pathway. Biochem. Pharmacol. 2017, 136, 109–121. [Google Scholar] [CrossRef]
- Kalluri, R.; LeBleu, V.S. The biology, function, and biomedical applications of exosomes. Science 2020, 367, eaau6977. [Google Scholar] [CrossRef] [PubMed]
- Théry, C.; Witwer, K.W.; Aikawa, E.; Alcaraz, M.J.; Anderson, J.D.; Andriantsitohaina, R.; Antoniou, A.; Arab, T.; Archer, F.; Atkin-Smith, G.K.; et al. Minimal information for studies of extracellular vesicles 2018 (MISEV2018): A position statement of the international society for extracellular vesicles and update of the MISEV2014 guidelines. J. Extracell. Vesicles 2018, 7, 1535750. [Google Scholar] [CrossRef] [Green Version]
- Luz, I.; Cooks, T. Extracellular vesicles: What secrets do they hold inside? Cell. Death. Dis. 2019, 10, 406. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Mathieu, M.; Martin-Jaular, L.; Lavieu, G.; Théry, C. Specificities of secretion and uptake of exosomes and other extracellular vesicles for cell-to-cell communication. Nat. Cell. Biol. 2019, 21, 9–17. [Google Scholar] [CrossRef]
- Zhang, J.; Li, S.; Li, L.; Li, M.; Guo, C.; Yao, J.; Mi, S. Exosome and exosomal microRNA: Trafficking, sorting, and function. Genom. Proteom. Bioinform. 2015, 13, 17–24. [Google Scholar] [CrossRef] [Green Version]
- Xu, J.-F.; Yang, G.; Pan, X.-H.; Zhang, S.-J.; Zhao, C.; Qiu, B.-S.; Gu, H.-F.; Hong, J.-F.; Cao, L.; Chen, Y.; et al. Altered microRNA expression profile in exosomes during osteogenic differentiation of human bone marrow-derived mesenchymal stem cells. PLoS ONE 2014, 9, e114627. [Google Scholar] [CrossRef]
- Yao, Y.; Cai, X.; Ren, F.; Ye, Y.; Wang, F.; Zheng, C.; Qian, Y.; Zhang, M. The macrophage-osteoclast axis in osteoimmunity and osteo-related diseases. Front. Immunol. 2021, 12, 664871. [Google Scholar] [CrossRef]
- Wei, F.; Li, M.; Crawford, R.; Zhou, Y.; Xiao, Y. Exosome-integrated titanium oxide nanotubes for targeted bone regeneration. Acta Biomater. 2019, 86, 480–492. [Google Scholar] [CrossRef] [PubMed]
- Zhu, Y.; Li, Z.; Zhang, Y.; Lan, F.; He, J.; Wu, Y. The essential role of osteoclast-derived exosomes in magnetic nanoparticle-infiltrated hydroxyapatite scaffold modulated osteoblast proliferation in an osteoporosis model. Nanoscale 2020, 12, 8720–8726. [Google Scholar] [CrossRef]
- Liang, M.; Yin, X.; Zhang, S.; Ai, H.; Luo, F.; Xu, J.; Dou, C.; Dong, S.; Ma, Q. Osteoclast-derived small extracellular vesicles induce osteogenic differentiation via inhibiting ARHGAP1. Mol. Ther. Nucleic Acids 2021, 23, 1191–1203. [Google Scholar] [CrossRef]
- Li, W.; Liu, Y.; Zhang, P.; Tang, Y.; Zhou, M.; Jiang, W.; Zhang, X.; Wu, G.; Zhou, Y. Tissue-engineered bone immobilized with human adipose stem cells-derived exosomes promotes bone regeneration. ACS Appl. Mater. Interfaces 2018, 10, 5240–5254. [Google Scholar] [CrossRef] [PubMed]
- Kim, D.K.; Lee, S.; Kim, M.; Jeong, Y.; Lee, S. Exosome-coated silk fibroin 3D-scaffold for inducing osteogenic differentiation of bone marrow derived mesenchymal stem cells. Chem. Eng. J. 2021, 406, 127080. [Google Scholar] [CrossRef]
- Chen, L.; Mou, S.; Li, F.; Zeng, Y.; Sun, Y.; Horch, R.E.; Wei, W.; Wang, Z.; Sun, J. Self-assembled human adipose-derived stem cell-derived extracellular vesicle-functionalized biotin-doped polypyrrole titanium with long-term stability and potential osteoinductive ability. ACS Appl. Mater. Interfaces 2019, 11, 46183–46196. [Google Scholar] [CrossRef]
- Liu, A.; Lin, D.; Zhao, H.; Chen, L.; Cai, B.; Lin, K.; Shen, S.G.F. Optimized BMSC-derived osteoinductive exosomes immobilized in hierarchical scaffold via lyophilization for bone repair through Bmpr2/Acvr2b competitive receptor-activated smad pathway. Biomaterials 2021, 272, 120718. [Google Scholar] [CrossRef]
- Zhang, Y.; Hao, Z.; Wang, P.; Xia, Y.; Wu, J.; Xia, D.; Fang, S.; Xu, S. Exosomes from human umbilical cord mesenchymal stem cells enhance fracture healing through HIF-1α-mediated promotion of angiogenesis in a rat model of stabilized fracture. Cell. Prolif. 2019, 52, e12570. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Xie, H.; Wang, Z.; Zhang, L.; Lei, Q.; Zhao, A.; Wang, H.; Li, Q.; Chen, Z.; Zhang, W. Development of an angiogenesis-promoting microvesicle-alginate-polycaprolactone composite graft for bone tissue engineering applications. PeerJ 2016, 4, e2040. [Google Scholar] [CrossRef] [PubMed]
- Xie, H.; Wang, Z.; Zhang, L.; Lei, Q.; Zhao, A.; Wang, H.; Li, Q.; Cao, Y.; Jie Zhang, W.; Chen, Z. Extracellular vesicle-functionalized decalcified bone matrix scaffolds with enhanced pro-angiogenic and pro-bone regeneration activities. Sci. Rep. 2017, 7, 45622. [Google Scholar] [CrossRef] [PubMed]
- Chen, P.; Zheng, L.; Wang, Y.; Tao, M.; Xie, Z.; Xia, C.; Gu, C.; Chen, J.; Qiu, P.; Mei, S.; et al. Desktop-stereolithography 3D printing of a radially oriented extracellular matrix/mesenchymal stem cell exosome bioink for osteochondral defect regeneration. Theranostics 2019, 9, 2439–2459. [Google Scholar] [CrossRef] [PubMed]
- Liang, B.; Liang, J.-M.; Ding, J.-N.; Xu, J.; Xu, J.-G.; Chai, Y.-M. Dimethyloxaloylglycine-stimulated human bone marrow mesenchymal stem cell-derived exosomes enhance bone regeneration through angiogenesis by targeting the AKT/MTOR pathway. Stem. Cell. Res. 2019, 10, 335. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Fan, J.; Lee, C.-S.; Kim, S.; Chen, C.; Aghaloo, T.; Lee, M. Generation of small RNA-modulated exosome mimetics for bone regeneration. ACS Nano 2020, 14, 11973–11984. [Google Scholar] [CrossRef]
- Liang, Z.; Luo, Y.; Lv, Y. Mesenchymal stem cell-derived microvesicles mediate BMP2 gene delivery and enhance bone regeneration. J. Mater. Chem. B 2020, 8, 6378–6389. [Google Scholar] [CrossRef]
- Qayoom, I.; Teotia, A.K.; Kumar, A. Nanohydroxyapatite based ceramic carrier promotes bone formation in a femoral neck canal defect in osteoporotic rats. Biomacromolecules 2020, 21, 328–337. [Google Scholar] [CrossRef] [PubMed]
- Zhai, M.; Zhu, Y.; Yang, M.; Mao, C. Human mesenchymal stem cell derived exosomes enhance cell-free bone regeneration by altering their MiRNAs profiles. Adv. Sci. 2020, 7, 2001334. [Google Scholar] [CrossRef]
- Liu, W.; Huang, J.; Chen, F.; Xie, D.; Wang, L.; Ye, C.; Zhu, Q.; Li, X.; Li, X.; Yang, L. MSC-derived small extracellular vesicles overexpressing MiR-20a promoted the osteointegration of porous titanium alloy by enhancing osteogenesis via targeting BAMBI. Stem. Cell. Res. 2021, 12, 348. [Google Scholar] [CrossRef]
- Huang, C.-C.; Kang, M.; Shirazi, S.; Lu, Y.; Cooper, L.F.; Gajendrareddy, P.; Ravindran, S. 3D encapsulation and tethering of functionally engineered extracellular vesicles to hydrogels. Acta Biomater. 2021, 126, 199–210. [Google Scholar] [CrossRef] [PubMed]
- Holkar, K.; Kale, V.; Ingavle, G. Hydrogel-assisted 3D model to investigate the osteoinductive potential of MC3T3-derived extracellular vesicles. ACS Biomater. Sci. Eng. 2021, 7, 2687–2700. [Google Scholar] [CrossRef]
- Gandolfi, M.G.; Gardin, C.; Zamparini, F.; Ferroni, L.; Esposti, M.D.; Parchi, G.; Ercan, B.; Manzoli, L.; Fava, F.; Fabbri, P.; et al. Mineral-doped poly(L-Lactide) acid scaffolds enriched with exosomes improve osteogenic commitment of human adipose-derived mesenchymal stem cells. Nanomaterials 2020, 10, 432. [Google Scholar] [CrossRef] [Green Version]
- Zha, Y.; Lin, T.; Li, Y.; Zhang, X.; Wang, Z.; Li, Z.; Ye, Y.; Wang, B.; Zhang, S.; Wang, J. Exosome-mimetics as an engineered gene-activated matrix induces in-situ vascularized osteogenesis. Biomaterials 2020, 247, 119985. [Google Scholar] [CrossRef]
- Zha, Y.; Li, Y.; Lin, T.; Chen, J.; Zhang, S.; Wang, J. Progenitor cell-derived exosomes endowed with VEGF plasmids enhance osteogenic induction and vascular remodeling in large segmental bone defects. Theranostics 2021, 11, 397–409. [Google Scholar] [CrossRef]
- Qi, X.; Zhang, J.; Yuan, H.; Xu, Z.; Li, Q.; Niu, X.; Hu, B.; Wang, Y.; Li, X. Exosomes secreted by human-induced pluripotent stem cell-derived mesenchymal stem cells repair critical-sized bone defects through enhanced angiogenesis and osteogenesis in osteoporotic rats. Int. J. Biol. Sci. 2016, 12, 836–849. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Zhang, J.; Liu, X.; Li, H.; Chen, C.; Hu, B.; Niu, X.; Li, Q.; Zhao, B.; Xie, Z.; Wang, Y. Exosomes/tricalcium phosphate combination scaffolds can enhance bone regeneration by activating the PI3K/Akt signaling pathway. Stem. Cell. Res. 2016, 7, 136. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Wang, L.; Wang, J.; Zhou, X.; Sun, J.; Zhu, B.; Duan, C.; Chen, P.; Guo, X.; Zhang, T.; Guo, H. A new self-healing hydrogel containing HucMSC-derived exosomes promotes bone regeneration. Front. Bioeng. Biotechnol. 2020, 8, 564731. [Google Scholar] [CrossRef] [PubMed]
- Yang, S.; Zhu, B.; Yin, P.; Zhao, L.; Wang, Y.; Fu, Z.; Dang, R.; Xu, J.; Zhang, J.; Wen, N. Integration of human umbilical cord mesenchymal stem cells-derived exosomes with hydroxyapatite-embedded hyaluronic acid-alginate hydrogel for bone regeneration. ACS Biomater. Sci. Eng. 2020, 6, 1590–1602. [Google Scholar] [CrossRef] [PubMed]
- Zhang, Y.; Xie, Y.; Hao, Z.; Zhou, P.; Wang, P.; Fang, S.; Li, L.; Xu, S.; Xia, Y. Umbilical mesenchymal stem cell-derived exosome-encapsulated hydrogels accelerate bone repair by enhancing angiogenesis. ACS Appl. Mater. Interfaces 2021, 13, 18472–18487. [Google Scholar] [CrossRef] [PubMed]
- Diomede, F.; Gugliandolo, A.; Cardelli, P.; Merciaro, I.; Ettorre, V.; Traini, T.; Bedini, R.; Scionti, D.; Bramanti, A.; Nanci, A.; et al. Three-dimensional printed PLA scaffold and human gingival stem cell-derived extracellular vesicles: A new tool for bone defect repair. Stem. Cell. Res. Ther. 2018, 9, 104. [Google Scholar] [CrossRef] [Green Version]
- Swanson, W.B.; Zhang, Z.; Xiu, K.; Gong, T.; Eberle, M.; Wang, Z.; Ma, P.X. Scaffolds with controlled release of pro-mineralization exosomes to promote craniofacial bone healing without cell transplantation. Acta Biomater. 2020, 118, 215–232. [Google Scholar] [CrossRef]
- Imanishi, Y.; Hata, M.; Matsukawa, R.; Aoyagi, A.; Omi, M.; Mizutani, M.; Naruse, K.; Ozawa, S.; Honda, M.; Matsubara, T.; et al. Efficacy of extracellular vesicles from dental pulp stem cells for bone regeneration in rat calvarial bone defects. Inflamm. Regen. 2021, 41, 12. [Google Scholar] [CrossRef] [PubMed]
- Wu, J.; Chen, L.; Wang, R.; Song, Z.; Shen, Z.; Zhao, Y.; Huang, S.; Lin, Z. Exosomes secreted by stem cells from human exfoliated deciduous teeth promote alveolar bone defect repair through the regulation of angiogenesis and osteogenesis. ACS Biomater. Sci. Eng. 2019, 5, 3561–3571. [Google Scholar] [CrossRef]
- Jafari, D.; Shajari, S.; Jafari, R.; Mardi, N.; Gomari, H.; Ganji, F.; Forouzandeh Moghadam, M.; Samadikuchaksaraei, A. Designer exosomes: A new platform for biotechnology therapeutics. BioDrugs 2020, 34, 567–586. [Google Scholar] [CrossRef]
- Razban, V.; Lotfi, A.S.; Soleimani, M.; Ahmadi, H.; Massumi, M.; Khajeh, S.; Ghaedi, M.; Arjmand, S.; Najavand, S.; Khoshdel, A. HIF-1α overexpression induces angiogenesis in mesenchymal stem cells. BioRes. Open Access 2012, 1, 174–183. [Google Scholar] [CrossRef]
- James, A.W.; LaChaud, G.; Shen, J.; Asatrian, G.; Nguyen, V.; Zhang, X.; Ting, K.; Soo, C. A review of the clinical side effects of bone morphogenetic protein-2. Tissue Eng. Part. B Rev. 2016, 22, 284–297. [Google Scholar] [CrossRef]
- Wei, F.; Zhou, Y.; Wang, J.; Liu, C.; Xiao, Y. The immunomodulatory role of BMP-2 on macrophages to accelerate osteogenesis. Tissue Eng. Part. A 2018, 24, 584–594. [Google Scholar] [CrossRef]
- Huang, C.-C.; Kang, M.; Lu, Y.; Shirazi, S.; Diaz, J.I.; Cooper, L.F.; Gajendrareddy, P.; Ravindran, S. Functionally engineered extracellular vesicles improve bone regeneration. Acta Biomater. 2020, 109, 182–194. [Google Scholar] [CrossRef]
- Wu, X.-B.; Li, Y.; Schneider, A.; Yu, W.; Rajendren, G.; Iqbal, J.; Yamamoto, M.; Alam, M.; Brunet, L.J.; Blair, H.C.; et al. Impaired osteoblastic differentiation, reduced bone formation, and severe osteoporosis in noggin-overexpressing mice. J. Clin. Invest. 2003, 112, 924–934. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Wan, D.C.; Pomerantz, J.H.; Brunet, L.J.; Kim, J.-B.; Chou, Y.-F.; Wu, B.M.; Harland, R.; Blau, H.M.; Longaker, M.T. Noggin suppression enhances in vitro osteogenesis and accelerates in vivo bone formation. J. Biol. Chem. 2007, 282, 26450–26459. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Fan, J.; Im, C.S.; Guo, M.; Cui, Z.-K.; Fartash, A.; Kim, S.; Patel, N.; Bezouglaia, O.; Wu, B.M.; Wang, C.-Y.; et al. Enhanced osteogenesis of adipose-derived stem cells by regulating bone morphogenetic protein signaling antagonists and agonists: Enhanced osteogenesis of ASCs by BMP signaling. Stem Cells Transl. Med. 2016, 5, 539–551. [Google Scholar] [CrossRef] [PubMed]
- Kibria, G.; Ramos, E.K.; Wan, Y.; Gius, D.R.; Liu, H. Exosomes as a drug delivery system in cancer therapy: Potential and challenges. Mol. Pharm. 2018, 15, 3625–3633. [Google Scholar] [CrossRef]
- Johnsen, K.B.; Gudbergsson, J.M.; Skov, M.N.; Pilgaard, L.; Moos, T.; Duroux, M. A comprehensive overview of exosomes as drug delivery vehicles—endogenous nanocarriers for targeted cancer therapy. Biochim. Biophys. Acta BBA Rev. Cancer 2014, 1846, 75–87. [Google Scholar] [CrossRef]
- Li, X.; Corbett, A.L.; Taatizadeh, E.; Tasnim, N.; Little, J.P.; Garnis, C.; Daugaard, M.; Guns, E.; Hoorfar, M.; Li, I.T.S. Challenges and opportunities in exosome research—perspectives from biology, engineering, and cancer therapy. APL Bioeng. 2019, 3, 011503. [Google Scholar] [CrossRef] [Green Version]
- Bunggulawa, E.J.; Wang, W.; Yin, T.; Wang, N.; Durkan, C.; Wang, Y.; Wang, G. Recent advancements in the use of exosomes as drug delivery systems. J. Nanobiotechnol. 2018, 16, 81. [Google Scholar] [CrossRef] [Green Version]
- Grosso, A.; Burger, M.G.; Lunger, A.; Schaefer, D.J.; Banfi, A.; Di Maggio, N. It takes two to tango: Coupling of angiogenesis and osteogenesis for bone regeneration. Front. Bioeng. Biotechnol. 2017, 5, 68. [Google Scholar] [CrossRef]
- Hu, K.; Olsen, B.R. The roles of vascular endothelial growth factor in bone repair and regeneration. Bone 2016, 91, 30–38. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Qin, Y.; Wang, L.; Gao, Z.; Chen, G.; Zhang, C. Bone marrow stromal/stem cell-derived extracellular vesicles regulate osteoblast activity and differentiation in vitro and promote bone regeneration in vivo. Sci. Rep. 2016, 6, 21961. [Google Scholar] [CrossRef] [PubMed]


| Drug | Liposome Composition | Scaffold | Cross-Linking Method | Notes | Reference |
|---|---|---|---|---|---|
| Plasmid DNA encoding RUNX2 | DODAP, HSPC, Cholesterol, and DSPE-PEG or DSPE-PEG-Mal | Polycaprolactone nanofiber meshes | Thioether linkage of maleimide and thiol group | Osteogenic activities in human bone-marrow mesenchymal stem cells in vitro | [40] |
| Dexamethasone | DODAP, HSPC, Cholesterol, and DSPE-PEG or DSPE-PEG-Mal | Polycaprolactone nanofiber meshes | Thioether linkage of maleimide and thiol group | Osteogenic activities in human bone-marrow mesenchymal stem cells in vitro | [44] |
| Noggin siRNA | Stearylamine and cholesterol | Methacrylated glycol chitosan hydrogel | Encapsulation | Osteogenic and bone regeneration activities in vitro and in vivo | [41] |
| Phenamil and Noggin siRNA | Stearylamine and cholesterol | Methacrylated glycol chitosan hydrogel | Encapsulation | Osteogenic and bone regeneration activities in vitro and in vivo | [42] |
| 20(s)-hydroxycholesterol and Plasmid DNA encoding sonic hedgehog | Palmitic acid and 20(s)-hydroxycholesterol | Porous hydroxyapatite-coated PLGA scaffold | Electrostatic interaction of alendronate and apatite | Osteogenic and bone regeneration activities in vitro and in vivo | [22] |
| Kartogenin | Lecithin and cholesterol | Gelatin methacryloyl hydrogel | Encapsulation via the physical network hindrance and non-covalent interaction | Extended joint retention, in vitro chondrogenic activities, and therapeutic effects in osteoarthritis model in vivo | [37] |
| Dexamethasone | (N-{6-amino-1-[N-(9Z) -octadec9-enylamino] -1-oxohexan-(2S) -2- yl} –N’- {2- [N, N-bis(2-aminoethyl) amino] ethyl} -2-hexadecylpropandiamide) (OO4) and DOPE | Glass coverslips, gold sensors, and silicon substrates | Layer-by-Layer coating with polyethyleneimine, collagen type I, chondroitin sulfate, and liposome | Enhanced adhesion and osteogenic differentiation of C2C12 myoblasts in vitro | [43] |
| Salvianic acid A | Lecithin, cholesterol, and cholesterol-pyrophosphate | Collagen sponge | Absorption | Improved bone healing via the regulation of HDAC3-mediated endochondral ossification in rabbit segmental defect model | [23] |
| Deferoxamine and BMP-2 | Phosphatidylcholin and Chol | Gelatin methacryloyl hydrogel | Hydrogen bond and hydrogel network micro-cross-linking | Enhanced mechanical property by liposome encapsulation, controlled phase release of various type of drugs, osteogenesis, angiogenesis, mature lamella bone formation in vivo | [38] |
| Deferoxamine | Lecithin and cholesterol | Gelatin methacryloyl hydrogel and 3D printed bioceramic scaffold | Encapsulation | Designed biomimetic ‘lotus’ biological structure, increased the expression of vascularization, and pro-osteogenic effects in vitro/in vivo | [39] |
| Curcumin | 1,2-dimyristoylsn-glycero-3-phosphocholine (DMPC) and 1,2-dimyristoyl-sn-glycero-3-phospho-(1′-rac-glycerol) (sodium salt) (DMPG) | 3D printed calcium phosphate scaffolds | Absorption and ionic interaction | Cytotoxic against in vitro osteosarcoma (bone cancer) cells and promoted osteoblast (healthy bone cell) cell growth | [34] |
| 20(s)-hydroxycholesterol | Stearylamine and 20(s)-hydroxycholesterol | Methacrylated glycol chitosan hydrogel | Encapsulation | Designed non-phospholipid liposome, named sterosome, which has intrinsic osteoinductivity, and enhanced osteogenic activities in vitro and bone formation in vivo via hedgehog signaling | [27] |
| 20(s)-hydroxycholesterol and purmorphamine | Stearylamine and 20(s)-hydroxycholesterol | Porous PLGA scaffold | Polydopamine-mediated layer-by-layer coating (Schiff base formation and Michael-type addition) | Osteogenic activities in vitro and bone formation in vivo via hedgehog signaling | [29] |
| 20(s)-hydroxycholesterol and smoothened agonist (SAG) | Stearylamine and 20(s)-hydroxycholesterol | Porous hydroxyapatite-coated PLGA scaffold | Polydopamine-mediated layer-by-layer coating (Schiff base formation and Michael-type addition) | Osteogenic activities in vitro and bone formation in vivo via hedgehog signaling | [30] |
| Aspirin and bone forming peptide-1 | DSPE-PEG-NH2, 1,2- dipalmitoyl-sn-glycero-3-phosphocholine (DPPC), and cholesterol | 3D printed polycaprolactone scaffold | Polydopamine-mediated coating | Osteogenic activities in vitro and bone formation in vivo via PI3K/AKT signaling | [36] |
| Aspirin | DSPE-PEG-NH2, DPPC, and cholesterol | 3D printed polycaprolactone scaffold | Polydopamine-mediated coating | Osteogenic activities in vitro and ectopic bone formation in vivo | [35] |
| BMP-2 peptide | HSPC or DPPC, Cholesterol, and mPEG-DSPE-maleimide | Electrospun poly L-lactic acid nanofibers | Thioether linkage of maleimide and thiol group | Sustained release of BMP-2 peptide up to 21 days, enhanced in vitro osteogenic activities, and initiated ectopic bone formation | [33] |
| 107–111 pentapeptide of the parathyroid hormone-related protein (PTHrP 107-111) | MSPC, DSPE-PEG-maleimide, and DPPC | Collagen-hydroxyapatite scaffolds | Thioether linkage of maleimide and thiol group | Triggered release of PTHrP 107-111 by thermal stimulation and enhanced pro-osteogenic activities in vitro | [18] |
| N′-Dodecanoylisonicotinohydrazide | Phospholipid | PLGA-PEG-PLGA hydrogel | Encapsulation | Developed a liposome-in-hydrogel and sustained drug release | [19] |
| Carboxyfluorescein, doxorubicin, and lysozyme; not for bone tissue engineering | DSPC, cholesterol, and DSPE-PEG or DSPE-PEG-thiol bisphosphonate | Collagen-hydroxyapatite scaffolds | Electrostatic interaction of bisphosphonate and apatite | Increased affinity to the scaffold and sustained drug release | [21] |
| BMP-2 | Lecithin, cholesterol, and octadecylamine | PEG and Ag ion hydrogel | Encapsulation | Promoted osteogenic differentiation in vitro and local bone remodeling of osteoporotic fracture in vivo because of increased localization efficacy at injected site | [20] |
| CKIP-1 siRNA | DOTAP, DOPE, cholesterol, DSPE-mPEG2000, and DSPE-PEG2000-maleimide | Bovine bone scaffold | Electrostatic interaction | Osteogenic activities in vitro and bone repair in vivo via CKIP-1 knock down | [25] |
Publisher’s Note: MDPI stays neutral with regard to jurisdictional claims in published maps and institutional affiliations. |
© 2021 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Kang, M.; Lee, C.-S.; Lee, M. Bioactive Scaffolds Integrated with Liposomal or Extracellular Vesicles for Bone Regeneration. Bioengineering 2021, 8, 137. https://doi.org/10.3390/bioengineering8100137
Kang M, Lee C-S, Lee M. Bioactive Scaffolds Integrated with Liposomal or Extracellular Vesicles for Bone Regeneration. Bioengineering. 2021; 8(10):137. https://doi.org/10.3390/bioengineering8100137
Chicago/Turabian StyleKang, Minjee, Chung-Sung Lee, and Min Lee. 2021. "Bioactive Scaffolds Integrated with Liposomal or Extracellular Vesicles for Bone Regeneration" Bioengineering 8, no. 10: 137. https://doi.org/10.3390/bioengineering8100137
APA StyleKang, M., Lee, C.-S., & Lee, M. (2021). Bioactive Scaffolds Integrated with Liposomal or Extracellular Vesicles for Bone Regeneration. Bioengineering, 8(10), 137. https://doi.org/10.3390/bioengineering8100137






