Preclinical Efficacy of Pro- and Anti-Angiogenic Peptide Hydrogels to Treat Age-Related Macular Degeneration
Abstract
:1. Introduction
2. Materials and Methods
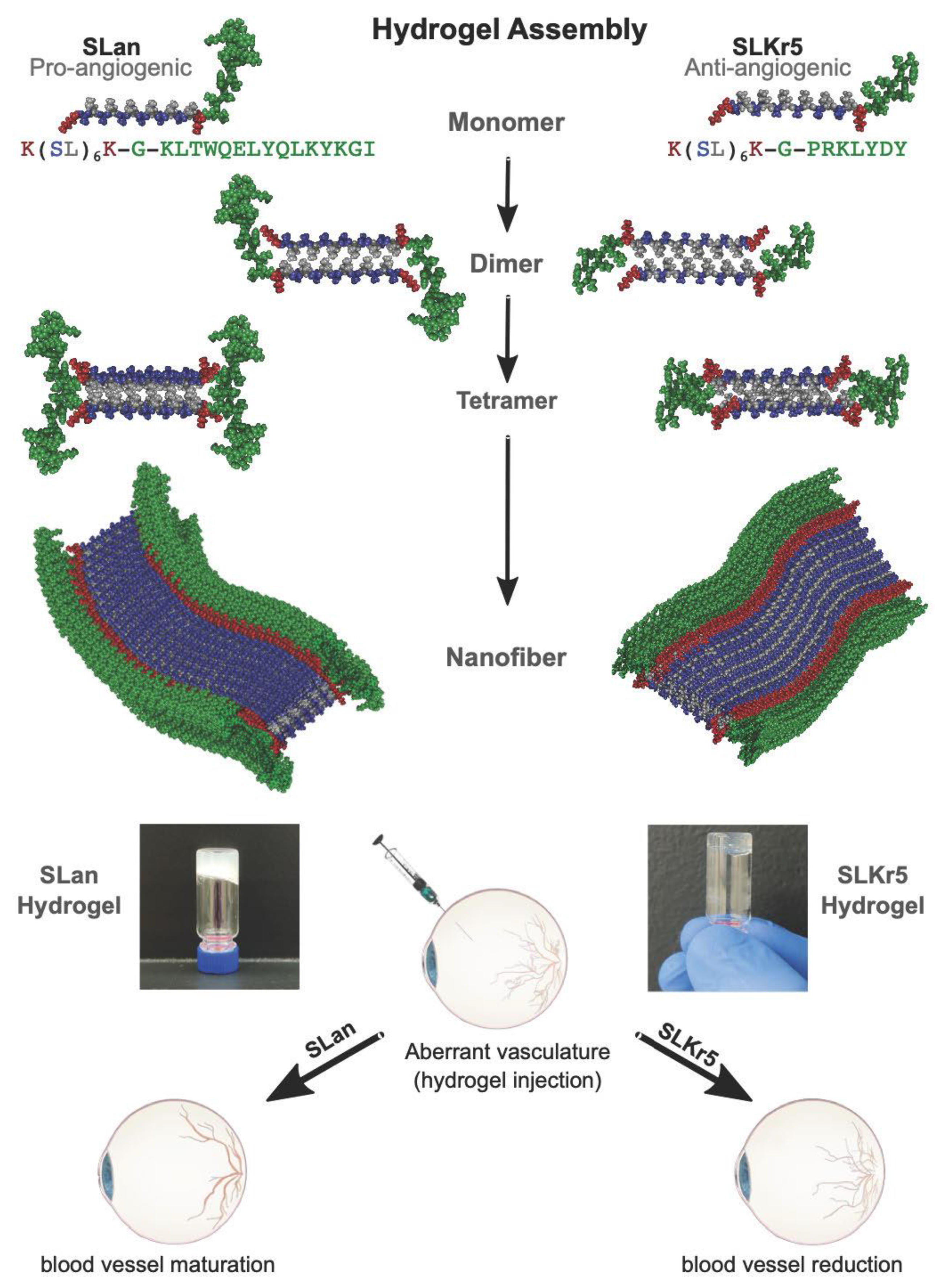
2.1. Peptide Preparation and Characterization
2.2. Rat Laser-Induced Choroidal Neovascularization Model
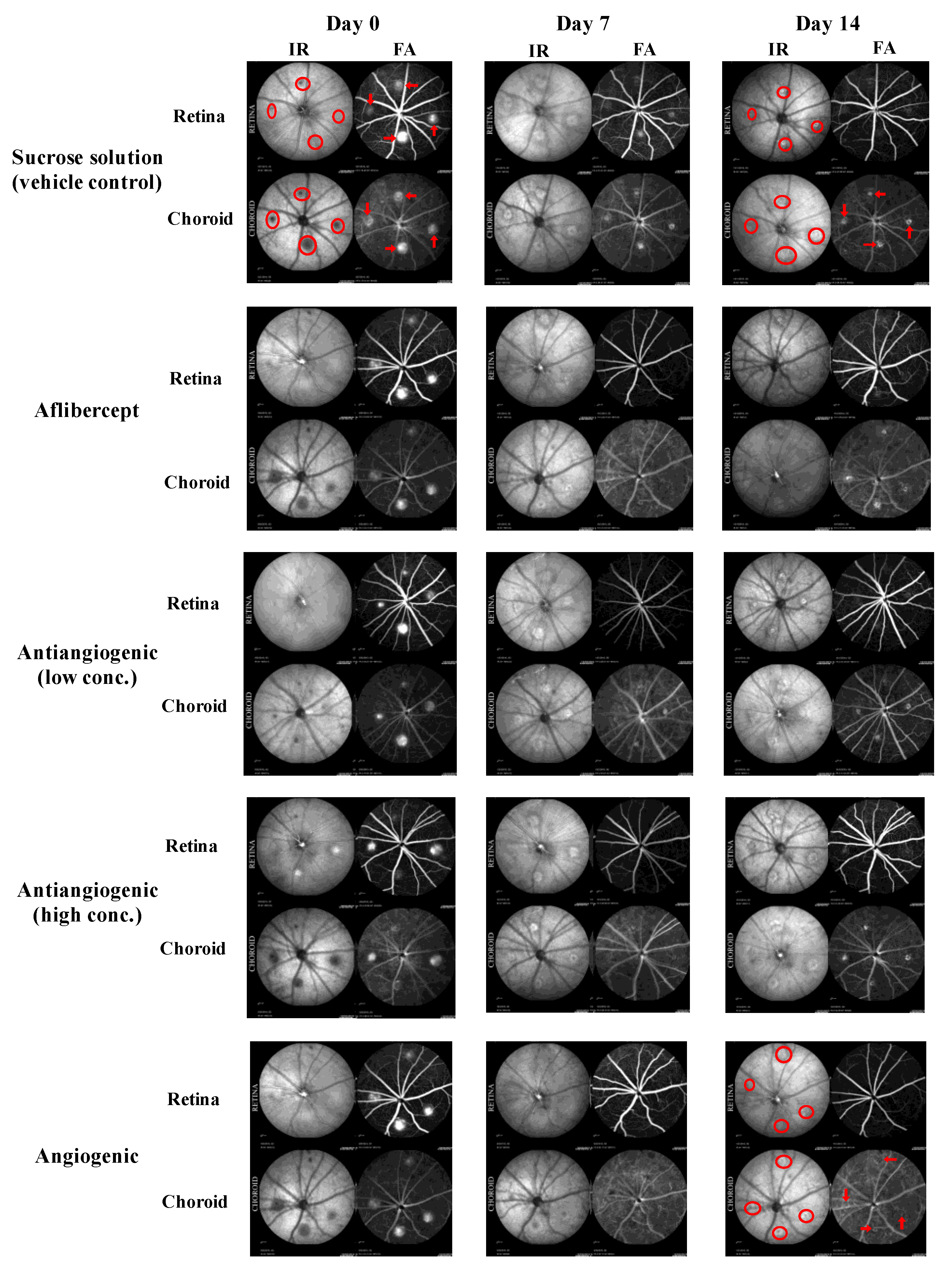
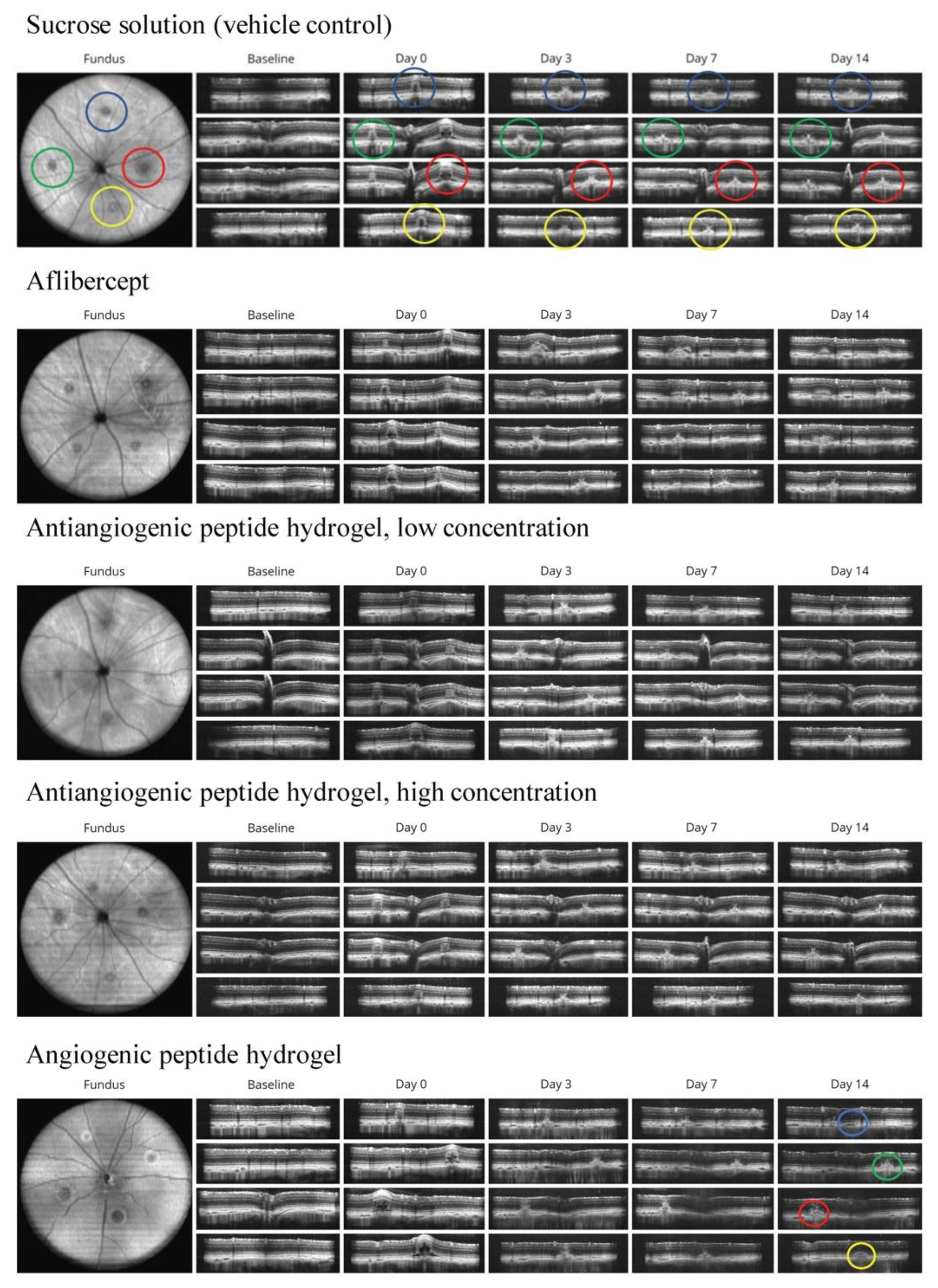
2.3. Analysis of Choroidal Neovascularization Lesions with In Vivo Imaging
2.4. Data Analysis
3. Results
3.1. Tolerability of Peptide Hydrogels
3.2. Qualitative Analysis of CNV Lesions
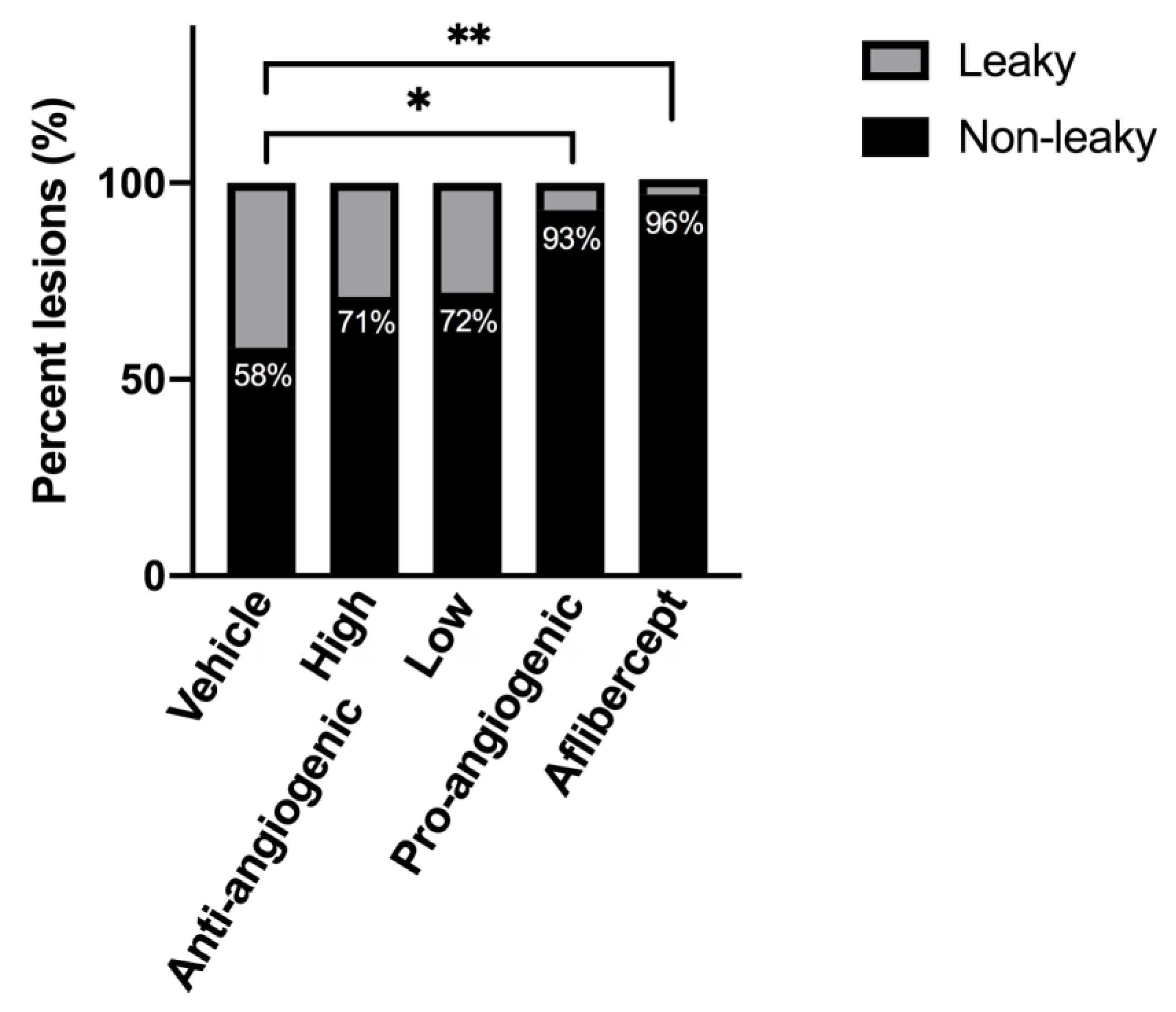
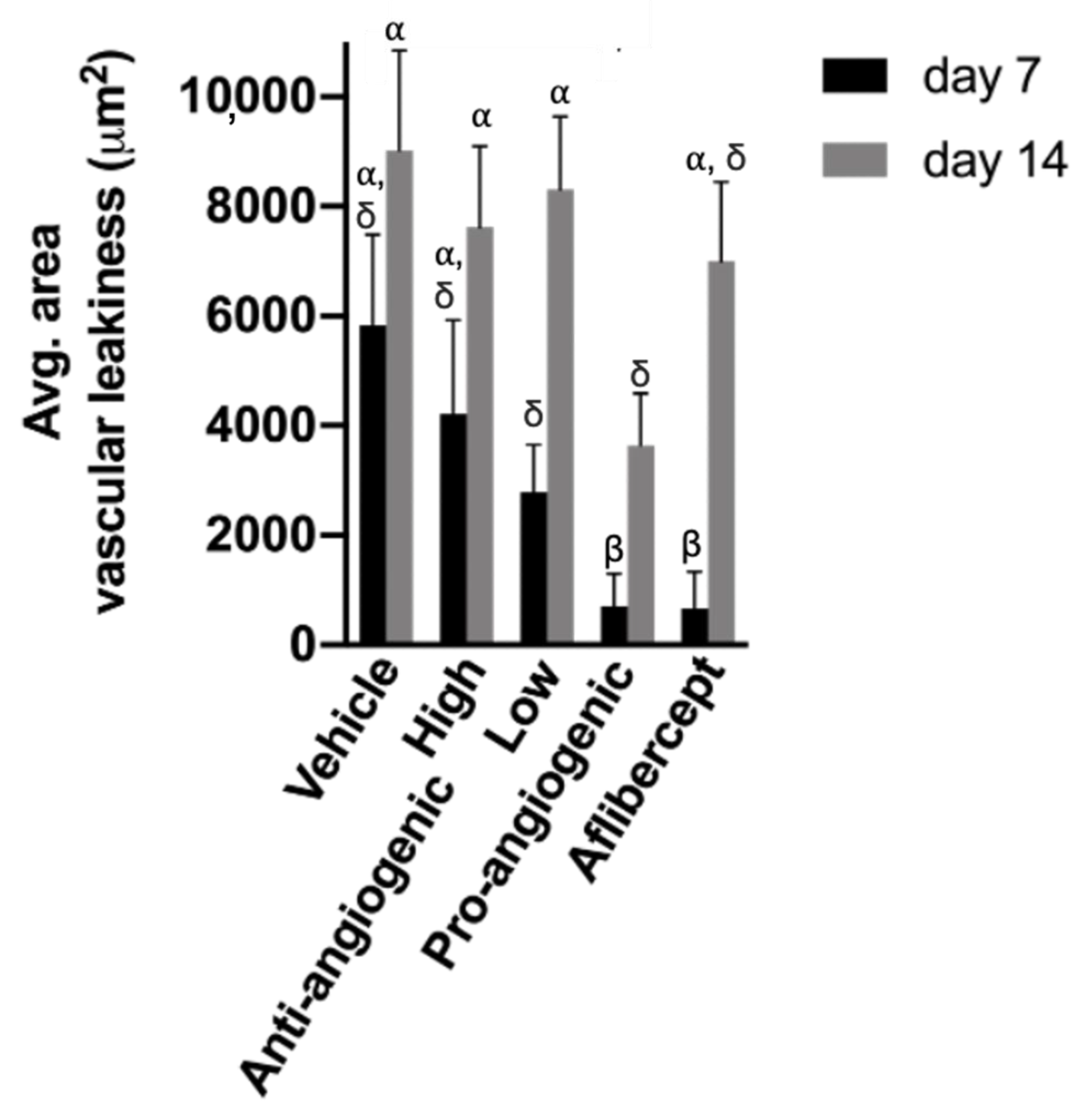
3.3. Quantitative Analysis of CNV Lesions
4. Discussion
5. Conclusions
Supplementary Materials
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Data Availability Statement
Conflicts of Interest
References
- Pennington, K.L.; DeAngelis, M.M. Epidemiology of age-related macular degeneration (AMD): Associations with cardiovascular disease phenotypes and lipid factors. Eye Vis. 2016, 3, 34–54. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Ambati, J.; Fowler, B.J. Mechanisms of age-related macular degeneration. Neuron 2012, 75, 26–39. [Google Scholar] [CrossRef] [Green Version]
- Gass, J.D.M.; Agarwal, A.; Lavina, A.M.; Tawansy, K.A. Focal inner retinal hemorrhages in patients with drusen: An early sign of occult choroidal neovascularization and chorioretinal anastomosis. Retina 2003, 23, 741–751. [Google Scholar] [CrossRef]
- Amadio, M.; Govoni, S.; Pascale, A. Targeting VEGF in eye neovascularization: What’s new?: A comprehensive review on current therapies and oligonucleotide-based interventions under development. Pharmacol. Res. 2016, 103, 253–269. [Google Scholar] [CrossRef] [Green Version]
- Daien, V.; Nguyen, V.; Essex, R.W.; Morlet, N.; Barthelmes, D.; Gillies, M.C. Incidence and outcomes of infectious and noninfectious endophthalmitis after intravitreal injections for age-related macular degeneration. Ophthalmology 2018, 125, 66–74. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Seah, I.; Zhao, X.; Lin, Q.; Liu, Z.; Su, S.Z.Z.; Yuen, Y.S.; Hunziker, W.; Lingam, G.; Loh, X.J.; Su, X. Use of biomaterials for sustained delivery of anti-VEGF to treat retinal diseases. Eye 2020, 34, 1341–1356. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Li, J.; Xing, R.; Bai, S.; Yan, X. Recent advances of self-assembling peptide-based hydrogels for biomedical applications. Soft Matter 2019, 15, 1704–1715. [Google Scholar] [CrossRef]
- Radvar, E.; Azevedo, H.S. Supramolecular Peptide/Polymer Hybrid Hydrogels for Biomedical Applications. Macromol. Biosci. 2019, 19, e1800221. [Google Scholar] [CrossRef] [Green Version]
- Hu, C.C.; Chiu, Y.C.; Chaw, J.R.; Chen, C.F.; Liu, H.W. Thermo-responsive hydrogel as an anti-VEGF drug delivery system to inhibit retinal angiogenesis in Rex rabbits. Technol. Health Care 2019, 27, 153–163. [Google Scholar] [CrossRef] [Green Version]
- Tang, J.D.; Mura, C.; Lampe, K.J. Stimuli-Responsive, Pentapeptide, Nanofiber Hydrogel for Tissue Engineering. J. Am. Chem. Soc. 2019, 141, 4886–4899. [Google Scholar] [CrossRef]
- Van Hove, A.H.; Burke, K.; Antonienko, E.; Brown, E.; Benoit, D.S. Enzymatically-responsive pro-angiogenic peptide-releasing poly(ethylene glycol) hydrogels promote vascularization in vivo. J. Control Release 2015, 217, 191–201. [Google Scholar] [CrossRef] [Green Version]
- Hammer, J.A.; Ruta, A.; West, J.L. Using Tools from Optogenetics to Create Light-Responsive Biomaterials: LOVTRAP-PEG Hydrogels for Dynamic Peptide Immobilization. Ann. Biomed. Eng. 2020, 48, 1885–1894. [Google Scholar] [CrossRef] [PubMed]
- Wang, K.; Mitra, R.N.; Zheng, M.; Han, Z. Nanoceria-loaded injectable hydrogels for potential age-related macular degeneration treatment. J. Biomed. Mater. Res. A 2018, 106, 2795–2804. [Google Scholar] [CrossRef] [PubMed]
- Yu, Y.; Lin, X.; Wang, Q.; He, M.; Chau, Y. Long-term therapeutic effect in nonhuman primate eye from a single injection of anti-VEGF controlled release hydrogel. Bioeng. Transl. Med. 2019, 4, e10128. [Google Scholar] [CrossRef] [Green Version]
- Bakota, E.L.A.; Galler, K.M.; Hartgerink, J.D. Enzymatic Cross-Linking of a Nanofibrous Peptide Hydrogel. Biomacromolecules 2011, 12, 82–87. [Google Scholar] [CrossRef] [PubMed]
- Drury, J.L.; Boontheekul, T.; Mooney, D.J. Cellular cross-linking of peptide modified hydrogels. J. Biomech. Eng. 2005, 127, 220–228. [Google Scholar] [CrossRef] [PubMed]
- Pugliese, R.; Gelain, F. Cross-Linked Self-Assembling Peptides and Their Post-Assembly Functionalization via One-Pot and In Situ Gelation System. Int. J. Mol. Sci. 2020, 21, 4261. [Google Scholar] [CrossRef]
- Pugliese, R.; Maleki, M.; Zuckermann, R.N.; Gelain, F. Self-assembling peptides cross-linked with genipin: Resilient hydrogels and self-standing electrospun scaffolds for tissue engineering applications. Biomater. Sci. 2018, 7, 76–91. [Google Scholar] [CrossRef]
- Pugliese, R.; Marchini, A.; Saracino, G.A.A.; Zuckermann, R.N.; Gelain, F. Cross-linked self-assembling peptide scaffolds. Nano Res. 2017, 11, 586–602. [Google Scholar] [CrossRef] [Green Version]
- Li, I.C.; Hartgerink, J.D. Covalent Capture of Aligned Self-Assembling Nanofibers. J. Am. Chem. Soc. 2017, 139, 8044–8050. [Google Scholar] [CrossRef] [Green Version]
- Zeng, L.; Song, M.; Gu, J.; Xu, Z.; Xue, B.; Li, Y.; Cao, Y. A Highly Stretchable, Tough, Fast Self-Healing Hydrogel Based on Peptide(-)Metal Ion Coordination. Biomimetics 2019, 4, 36. [Google Scholar] [CrossRef] [Green Version]
- Kumar, V.A.; Wang, B.K.; Kanahara, S.M. Rational design of fiber forming supramolecular structures. Exp. Biol. Med. 2016, 241, 899–908. [Google Scholar] [CrossRef]
- Bakota, E.L.; Sensoy, O.; Ozgur, B.; Sayar, M.; Hartgerink, J.D. Self-assembling multidomain peptide fibers with aromatic cores. Biomacromolecules 2013, 14, 1370–1378. [Google Scholar] [CrossRef]
- Lopez-Silva, T.L.; Leach, D.G.; Azares, A.; Li, I.C.; Woodside, D.G.; Hartgerink, J.D. Chemical functionality of multidomain peptide hydrogels governs early host immune response. Biomaterials 2020, 231, 119667. [Google Scholar] [CrossRef] [PubMed]
- Roberts, S.; Harmon, T.S.; Schaal, J.L.; Miao, V.; Li, K.J.; Hunt, A.; Wen, Y.; Oas, T.G.; Collier, J.H.; Pappu, R.V.; et al. Injectable tissue integrating networks from recombinant polypeptides with tunable order. Nat. Mater. 2018, 17, 1154–1163. [Google Scholar] [CrossRef]
- Carrejo, N.C.; Moore, A.N.; Lopez Silva, T.L.; Leach, D.G.; Li, I.C.; Walker, D.R.; Hartgerink, J.D. Multidomain Peptide Hydrogel Accelerates Healing of Full-Thickness Wounds in Diabetic Mice. ACS Biomater. Sci. Eng. 2018, 4, 1386–1396. [Google Scholar] [CrossRef] [PubMed]
- Moore, A.N.; Hartgerink, J.D. Self-Assembling Multidomain Peptide Nanofibers for Delivery of Bioactive Molecules and Tissue Regeneration. Acc. Chem. Res. 2017, 50, 714–722. [Google Scholar] [CrossRef] [PubMed]
- Moore, A.N.; Lopez Silva, T.L.; Carrejo, N.C.; Origel Marmolejo, C.A.; Li, I.C.; Hartgerink, J.D. Nanofibrous peptide hydrogel elicits angiogenesis and neurogenesis without drugs, proteins, or cells. Biomaterials 2018, 161, 154–163. [Google Scholar] [CrossRef]
- Aulisa, L.D.; Hartgerink, J.D. Self-Assembly of Multidomain Peptides: Sequence Variation Allows Control over Cross-Linking and Viscoelasticity. Biomacromolecules 2009, 10, 2694–2698. [Google Scholar] [CrossRef]
- Dong, H.P.; Aulisa, L.; Bakota, E.L.; Hartgerink, J.D. Self-Assembly of Multidomain Peptides: Balancing Molecular Frustration Controls Conformation and Nanostructure. J. Am. Chem. Soc. 2007, 129, 12468–12472. [Google Scholar] [CrossRef] [PubMed]
- Lopez-Silva, T.L.; Leach, D.G.; Li, I.C.; Wang, X.; Hartgerink, J.D. Self-Assembling Multidomain Peptides: Design and Characterization of Neutral Peptide-Based Materials with pH and Ionic Strength Independent Self-Assembly. ACS Biomater. Sci. Eng. 2019, 5, 977–985. [Google Scholar] [CrossRef] [PubMed]
- Leach, D.G.; Dharmaraj, N.; Piotrowski, S.L.; Lopez-Silva, T.L.; Lei, Y.L.; Sikora, A.G.; Young, S.; Hartgerink, J.D. STINGel: Controlled release of a cyclic dinucleotide for enhanced cancer immunotherapy. Biomaterials 2018, 163, 67–75. [Google Scholar] [CrossRef]
- Kim, K.K.; Siddiqui, Z.; Patel, M.; Sarkar, B.; Kumar, V.A. A self-assembled peptide hydrogel for cytokine sequestration. J. Mater. Chem. B 2020, 8, 945–950. [Google Scholar] [CrossRef]
- Kumar, V.A.; Wickremasinghe, N.C.; Shi, S.; Hartgerink, J.D. Nanofibrous Snake Venom Hemostat. ACS Biomater. Sci. Eng. 2015, 12, 1300–1305. [Google Scholar] [CrossRef] [Green Version]
- Kumar, V.A.; Taylor, N.L.; Shi, S.; Wickremasinghe, N.C.; D’Souza, R.N.; Hartgerink, J.D. Self-assembling multidomain peptides tailor biological responses through biphasic release. Biomaterials 2015, 52, 71–78. [Google Scholar] [CrossRef] [Green Version]
- Wickremasinghe, N.C.; Kumar, V.A.; Hartgerink, J.D. Two-step self-assembly of liposome-multidomain peptide nanofiber hydrogel for time-controlled release. Biomacromolecules 2014, 15, 3587–3595. [Google Scholar] [CrossRef] [PubMed]
- Leach, D.G.; Newton, J.M.; Florez, M.A.; Lopez-Silva, T.L.; Jones, A.A.; Young, S.; Sikora, A.G.; Hartgerink, J.D. Drug-Mimicking Nanofibrous Peptide Hydrogel for Inhibition of Inducible Nitric Oxide Synthase. ACS Biomater. Sci. Eng. 2019, 12, 6755–6765. [Google Scholar] [CrossRef] [PubMed]
- Lopez-Silva, T.L.; Cristobal, C.D.; Edwin Lai, C.S.; Leyva-Aranda, V.; Lee, H.K.; Hartgerink, J.D. Self-assembling multidomain peptide hydrogels accelerate peripheral nerve regeneration after crush injury. Biomaterials 2021, 265, 120401. [Google Scholar] [CrossRef] [PubMed]
- Harbour, V.; Casillas, C.; Siddiqui, Z.; Sarkar, B.; Sanyal, S.; Nguyen, P.; Kim, K.K.; Roy, A.; Iglesias-Montoro, P.; Patel, S.; et al. Regulation of Lipoprotein Homeostasis by Self-Assembling Peptides. ACS Appl. Bio Mater. 2020, 12, 8978–8988. [Google Scholar] [CrossRef]
- Ma, X.; Agas, A.; Siddiqui, Z.; Kim, K.; Iglesias-Montoro, P.; Kalluru, J.; Kumar, V.; Haorah, J. Angiogenic peptide hydrogels for treatment of traumatic brain injury. Bioact. Mater. 2020, 5, 124–132. [Google Scholar] [CrossRef]
- Nguyen, P.K.; Gao, W.; Patel, S.D.; Siddiqui, Z.; Weiner, S.; Shimizu, E.; Sarkar, B.; Kumar, V.A. Self-Assembly of a Dentinogenic Peptide Hydrogel. ACS Omega 2018, 6, 5980–5987. [Google Scholar] [CrossRef]
- Sarkar, B.; Siddiqui, Z.; Nguyen, P.K.; Dube, N.; Fu, W.; Park, S.; Jaisinghani, S.; Paul, R.; Kozuch, S.D.; Deng, D.; et al. Membrane-Disrupting Nanofibrous Peptide Hydrogels. ACS Biomater. Sci. Eng. 2019, 9, 4657–4670. [Google Scholar] [CrossRef] [PubMed]
- Siddiqui, Z.; Sarkar, B.; Kim, K.K.; Kadincesme, N.; Paul, R.; Kumar, A.; Kobayashi, Y.; Roy, A.; Choudhury, M.; Yang, J.; et al. Angiogenic hydrogels for dental pulp revascularization. Acta Biomater. 2021, 126, 109–118. [Google Scholar] [CrossRef]
- Sarkar, B.; Ma, X.; Agas, A.; Siddiqui, Z.; Iglesias-Montoro, P.; Nguyen, P.K.; Kim, K.K.; Haorah, J.; Kumar, V.A. In vivo neuroprotective effect of a self-assembled peptide hydrogel. Chem. Eng. J. 2021, 408, 127295. [Google Scholar] [CrossRef]
- Kumar, P.; Kumar, S.; Udupa, E.P.; Kumar, U.; Rao, P.; Honnegowda, T. Role of angiogenesis and angiogenic factors in acute and chronic wound healing. Plast. Aesthetic Res. 2015, 5, 243–249. [Google Scholar] [CrossRef] [Green Version]
- Kumar, V.A.; Liu, Q.; Wickremasinghe, N.C.; Shi, S.; Cornwright, T.T.; Deng, Y.; Azares, A.; Moore, A.N.; Acevedo-Jake, A.M.; Agudo, N.R.; et al. Treatment of hind limb ischemia using angiogenic peptide nanofibers. Biomaterials 2016, 98, 113–119. [Google Scholar] [CrossRef] [Green Version]
- Kumar, V.A.; Taylor, N.L.; Shi, S.; Wang, B.K.; Jalan, A.A.; Kang, M.K.; Wickremasinghe, N.C.; Hartgerink, J.D. Highly angiogenic peptide nanofibers. ACS Nano 2015, 9, 860–868. [Google Scholar] [CrossRef] [Green Version]
- Nguyen, P.K.; Sarkar, B.; Siddiqui, Z.; McGowan, M.; Iglesias-Montoro, P.; Rachapudi, S.; Kim, S.; Gao, W.; Lee, E.J.; Kumar, V.A. Self-Assembly of an Antiangiogenic Nanofibrous Peptide Hydrogel. ACS Appl. Bio Mater. 2018, 3, 865–870. [Google Scholar] [CrossRef]
- Sarkar, B.; Nguyen, P.K.; Gao, W.; Dondapati, A.; Siddiqui, Z.; Kumar, V.A. Angiogenic Self-Assembling Peptide Scaffolds for Functional Tissue Regeneration. Biomacromolecules 2018, 19, 3597–3611. [Google Scholar] [CrossRef] [PubMed]
- Wickremasinghe, N.C.; Kumar, V.A.; Shi, S.; Hartgerink, J.D. Controlled Angiogenesis in Peptide Nanofiber Composite Hydrogels. ACS Biomater. Sci. Eng. 2015, 1, 845–854. [Google Scholar] [CrossRef] [Green Version]
- Ragauskas, S.; Kielczewski, E.; Vance, J.; Kaja, S.; Kalesnykas, G. In Vivo Multimodal Imaging and Analysis of Mouse Laser-Induced Choroidal Neovascularization Model. J. Vis. Exp. 2018, 131, e56173. [Google Scholar] [CrossRef]
- Ebneter, A.; Agca, C.; Dysli, C.; Zinkernagel, M.S. Investigation of retinal morphology alterations using spectral domain optical coherence tomography in a mouse model of retinal branch and central retinal vein occlusion. PLoS ONE 2015, 3, e0119046. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Chen, X.; Kezic, J.M.; Forrester, J.V.; Goldberg, G.L.; Wicks, I.P.; Bernard, C.C.; McMenamin, P.G. In vivo multi-modal imaging of experimental autoimmune uveoretinitis in transgenic reporter mice reveals the dynamic nature of inflammatory changes during disease progression. J. Neuroinflammation 2015, 12, 17. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Grossniklaus, H.E.; Kang, S.J.; Berglin, L. Animal Models of Choroidal and Retinal Neovascularization. Prog. Retin. Eye Res. 2010, 29, 500–519. [Google Scholar] [CrossRef] [Green Version]
- Garweg, J.G.; Zirpel, J.J.; Gerhardt, C.; Pfister, I.B. The fate of eyes with wet AMD beyond four years of anti-VEGF therapy. Graefes Arch. Clin. Exp. Ophthalmol. 2018, 256, 823–831. [Google Scholar] [CrossRef]
- Grzybowski, A.; Told, R.; Sacu, S.; Bandello, F.; Moisseiev, E.; Loewenstein, A.; Schmidt-Erfurth, U.; on behalf of the Euretina Board. Update on Intravitreal Injections: Euretina Expert Consensus Recommendations. OPH 2018, 239, 181–193. [Google Scholar]
- Blasiak, J.; Petrovski, G.; Veréb, Z.; Facskó, A.; Kaarniranta, K. Oxidative Stress, Hypoxia, and Autophagy in the Neovascular Processes of Age-Related Macular Degeneration. BioMed Res. Inter. 2014, e768026. [Google Scholar] [CrossRef]
- Ourradi, K.; Blythe, T.; Jarrett, C.; Barratt, S.L.; Welsh, G.I.; Millar, A.B. VEGF isoforms have differential effects on permeability of human pulmonary microvascular endothelial cells. Respirat. Res. 2017, 18, 116. [Google Scholar] [CrossRef] [PubMed]
- Chen, L.; Cui, Y.; Li, B.; Weng, J.; Wang, W.; Zhang, S.; Huang, X.; Guo, X.; Huang, Q. Advanced glycation end products induce immature angiogenesis in in vivo and ex vivo mouse models. Am. J. Physiol.-Heart Circulat. Physiol. 2020, 318, H519–H533. [Google Scholar] [CrossRef]
Publisher’s Note: MDPI stays neutral with regard to jurisdictional claims in published maps and institutional affiliations. |
© 2021 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Acevedo-Jake, A.; Shi, S.; Siddiqui, Z.; Sanyal, S.; Schur, R.; Kaja, S.; Yuan, A.; Kumar, V.A. Preclinical Efficacy of Pro- and Anti-Angiogenic Peptide Hydrogels to Treat Age-Related Macular Degeneration. Bioengineering 2021, 8, 190. https://doi.org/10.3390/bioengineering8120190
Acevedo-Jake A, Shi S, Siddiqui Z, Sanyal S, Schur R, Kaja S, Yuan A, Kumar VA. Preclinical Efficacy of Pro- and Anti-Angiogenic Peptide Hydrogels to Treat Age-Related Macular Degeneration. Bioengineering. 2021; 8(12):190. https://doi.org/10.3390/bioengineering8120190
Chicago/Turabian StyleAcevedo-Jake, Amanda, Siyu Shi, Zain Siddiqui, Sreya Sanyal, Rebecca Schur, Simon Kaja, Alex Yuan, and Vivek A. Kumar. 2021. "Preclinical Efficacy of Pro- and Anti-Angiogenic Peptide Hydrogels to Treat Age-Related Macular Degeneration" Bioengineering 8, no. 12: 190. https://doi.org/10.3390/bioengineering8120190
APA StyleAcevedo-Jake, A., Shi, S., Siddiqui, Z., Sanyal, S., Schur, R., Kaja, S., Yuan, A., & Kumar, V. A. (2021). Preclinical Efficacy of Pro- and Anti-Angiogenic Peptide Hydrogels to Treat Age-Related Macular Degeneration. Bioengineering, 8(12), 190. https://doi.org/10.3390/bioengineering8120190





