Engineering Breast Cancer Cells and hUMSCs Microenvironment in 2D and 3D Scaffolds: A Mechanical Study Approach of Stem Cells in Anticancer Therapy
Abstract
:1. Introduction
2. Materials and Methods
2.1. Cell Culture and Reagents
2.2. Preparation of 2D Surfaces
2.3. Fabrication of 3D Scaffolds
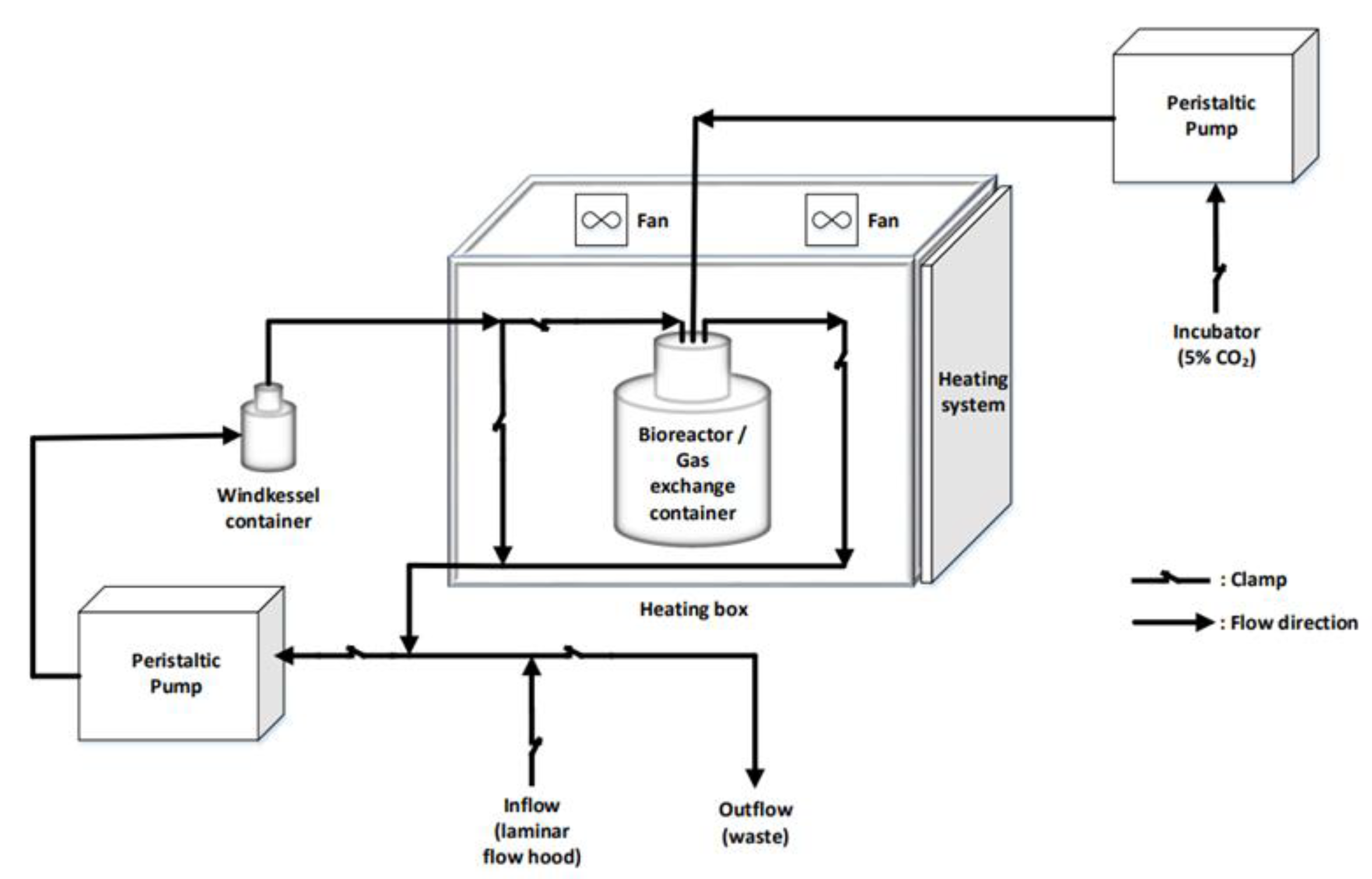
2.4. Dynamic Cell Culture
2.5. Treatment of Breast Cancer Cells with hUMSCSs-CM
2.6. Cell Viability and Cell Proliferation Assays
2.7. Migration Assay
2.8. Mechanical Properties of Breast Cancer Cells Prior to and Post Treatment
2.8.1. Tensile Strength of PCL Scaffolds
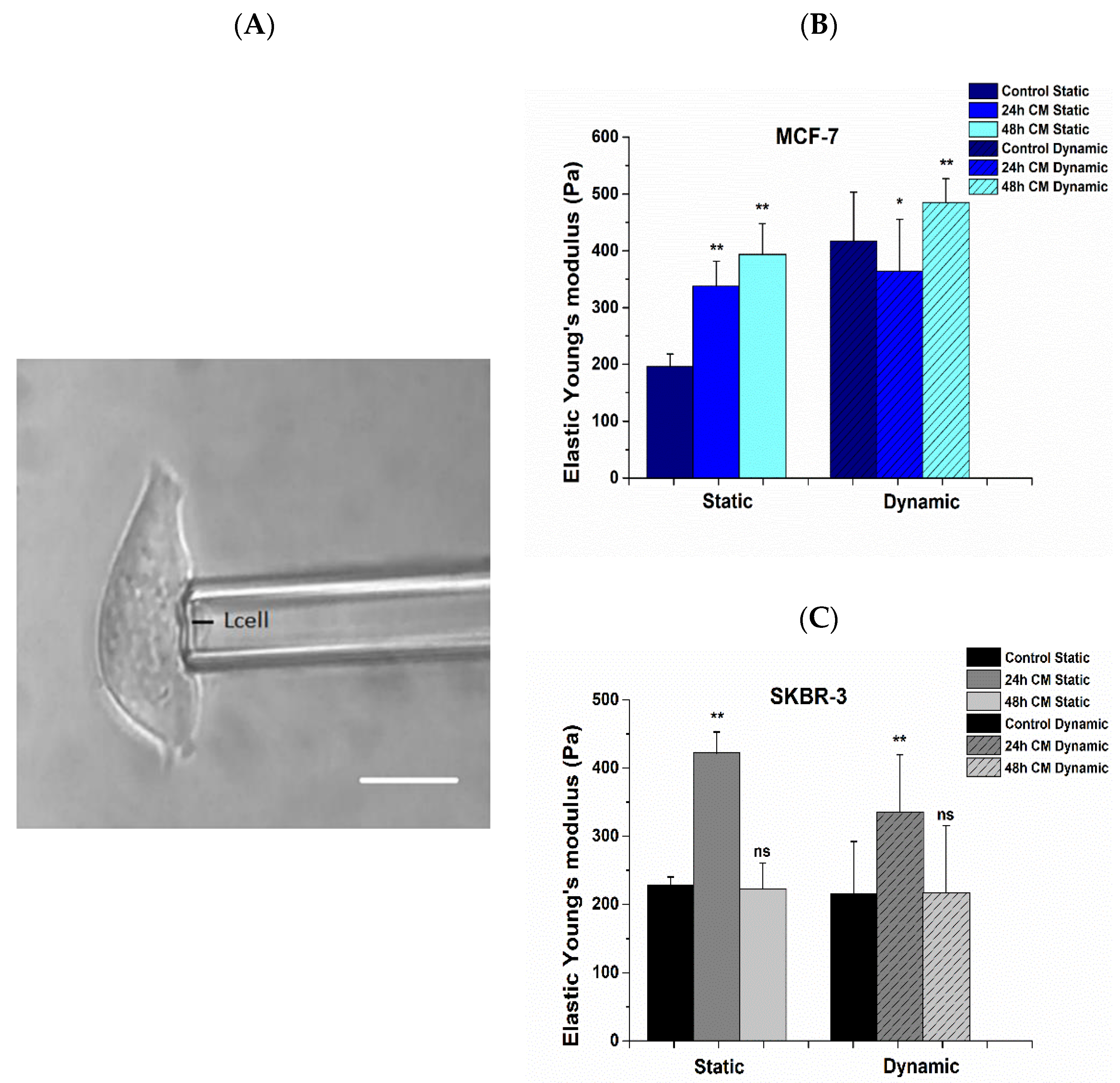
2.8.2. Cell Elasticity
2.8.3. Cell Membrane Roughness
2.9. Immunofluorescence Assay Prior to and Post Treatment
2.10. Cell Morphology Prior to and Post Treatment
2.11. Statistical Analysis
3. Results
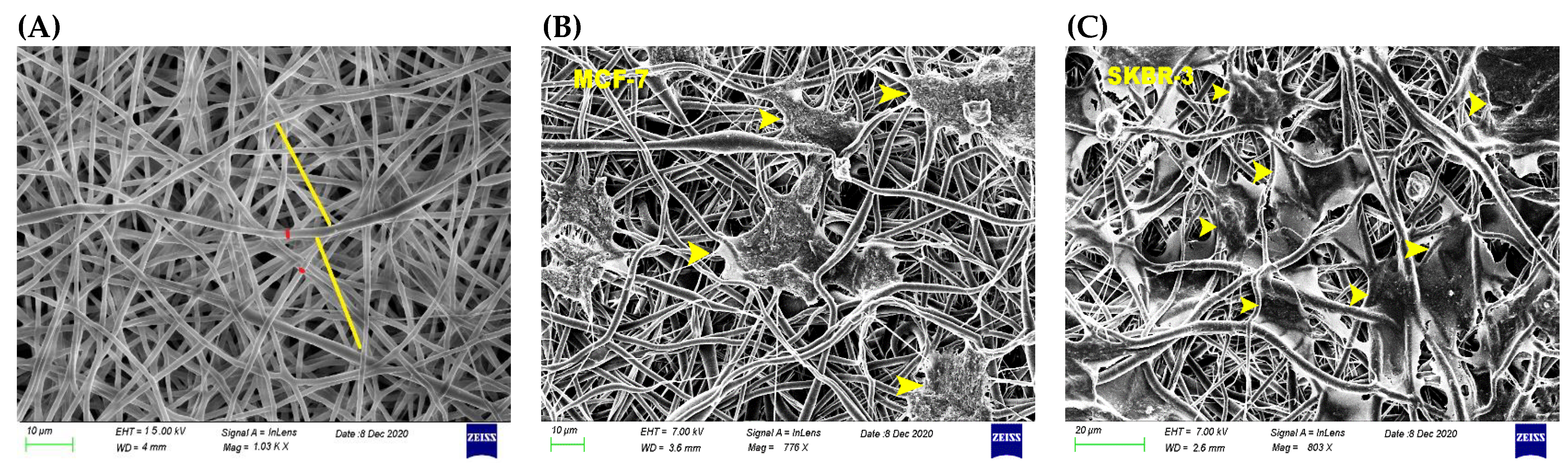
3.1. Physicomechanical Propertied of 3D Scaffold
3.2. Effect of hUMSCs-CM Therapy on Cell Viability after Dynamic Cell Culture
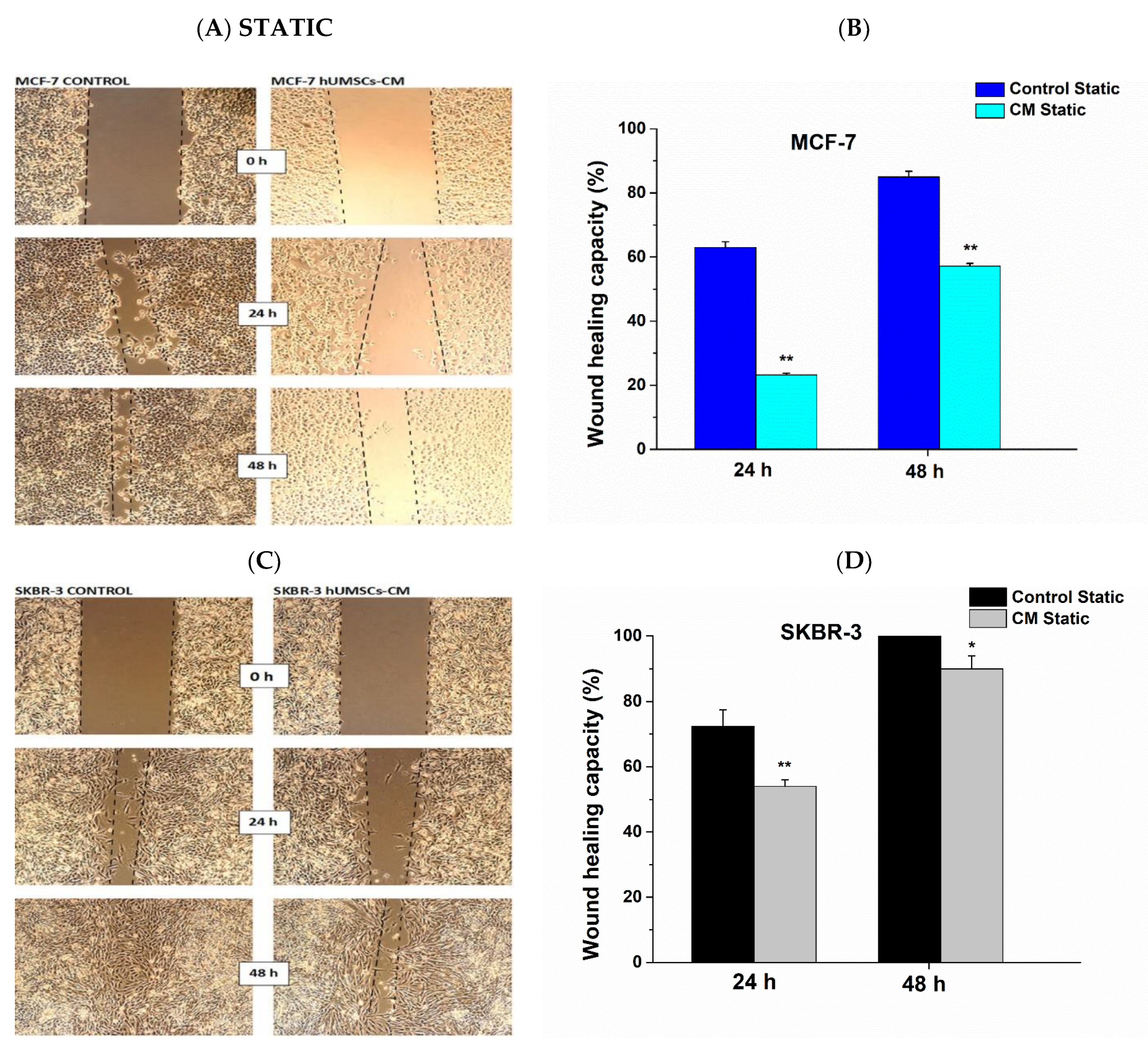
3.3. Attenuation of Cell Migration Post hUMSCs-CM Treatment
3.4. Alterations in Mechanical Properties Post Treatment with hUMSCs-CM
3.5. Effect of hUMSCs-CM on Cell Membrane Roughness
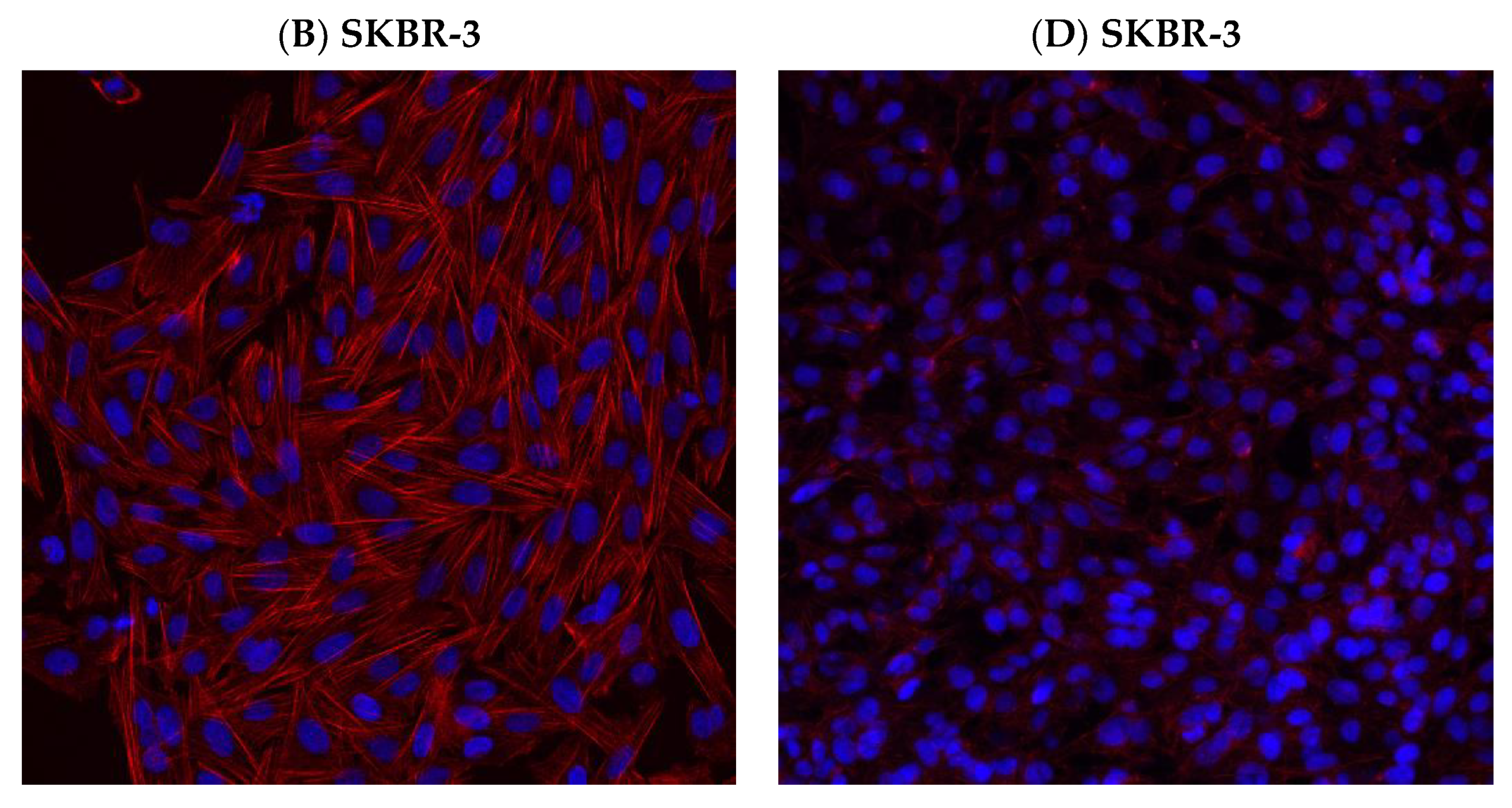
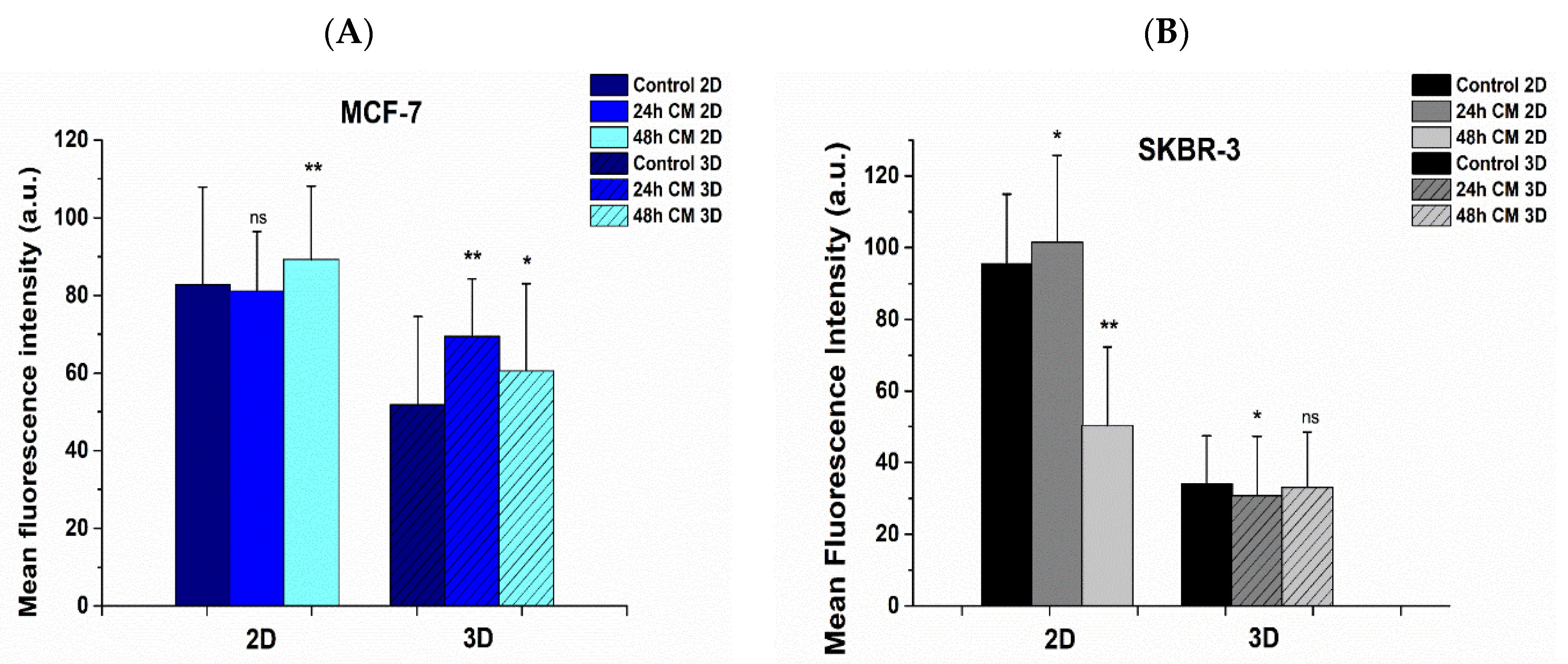
3.6. Effect of hUMSCs-CM Treatment on F-actin Morphology and Mean Intensity
4. Discussion
5. Conclusions
Supplementary Materials
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Data Availability Statement
Acknowledgments
Conflicts of Interest
References
- Korkmaz, U.; Ustun, F. Experimental Breast Cancer Models: Preclinical Imaging Perspective. Curr. Radiopharm. 2021, 14, 5–14. [Google Scholar] [CrossRef]
- Liang, Y.; Zhang, H.; Song, X.; Yang, Q. Metastatic heterogeneity of breast cancer: Molecular mechanism and potential therapeutic targets. Semin. Cancer Biol. 2020, 60, 14–27. [Google Scholar] [CrossRef] [PubMed]
- Kuchuk, I.; Bouganim, N.; Beusterien, K.; Grinspan, J.; Vandermeer, L.; Gertler, S.; Dent, S.F.; Song, X.; Segal, R.; Mazzarello, S.; et al. Preference weights for chemotherapy side effects from the perspective of women with breast cancer. Breast Cancer Res. Treat 2013, 142, 101–107. [Google Scholar] [CrossRef] [PubMed]
- Levy, O.; Zhao, W.; Mortensen, L.J.; Leblanc, S.; Tsang, K.; Fu, M.; Phillips, J.A.; Sagar, V.; Anandakumaran, P.; Ngai, J.; et al. mRNA-engineered mesenchymal stem cells for targeted delivery of interleukin-10 to sites of inflammation. Blood 2013, 122, e23–e32. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Rühle, A.; Huber, P.E.; Saffrich, R.; Lopez Perez, R.; Nicolay, N.H. The current understanding of mesenchymal stem cells as potential attenuators of chemotherapy-induced toxicity. Int. J. Cancer 2018, 143, 2628–2639. [Google Scholar] [CrossRef] [Green Version]
- Serakinci, N.; Tulay, P.; Kalkan, R. Role of Mesenchymal Stem Cells in Cancer Development and Their Use in Cancer Therapy. Adv. Exp. Med. Biol. 2018, 1083, 45–62. [Google Scholar] [CrossRef]
- Ayuzawa, R.; Doi, C.; Rachakatla, R.S.; Pyle, M.M.; Maurya, D.K.; Troyer, D.; Tamura, M. Naive human umbilical cord matrix derived stem cells significantly attenuate growth of human breast cancer cells in vitro and in vivo. Cancer Lett. 2009, 280, 31–37. [Google Scholar] [CrossRef] [Green Version]
- Matsuzuka, T.; Rachakatla, R.S.; Doi, C.; Maurya, D.K.; Ohta, N.; Kawabata, A.; Pyle, M.M.; Pickel, L.; Reischman, J.; Marini, F.; et al. Human umbilical cord matrix-derived stem cells expressing interferon-beta gene significantly attenuate bronchioloalveolar carcinoma xenografts in SCID mice. Lung Cancer 2010, 70, 28–36. [Google Scholar] [CrossRef] [Green Version]
- Marofi, F.; Vahedi, G.; Biglari, A.; Esmaeilzadeh, A.; Athari, S.S. Mesenchymal Stromal/Stem Cells: A New Era in the Cell-Based Targeted Gene Therapy of Cancer. Front. Immunol. 2017, 8, 1770. [Google Scholar] [CrossRef] [Green Version]
- Yuan, Y.; Zhou, C.; Chen, X.; Tao, C.; Cheng, H.; Lu, X. Suppression of tumor cell proliferation and migration by human umbilical cord mesenchymal stem cells: A possible role for apoptosis and Wnt signaling. Oncol. Lett. 2018, 15, 8536–8544. [Google Scholar] [CrossRef]
- Qiao, L.; Xu, Z.-l.; Zhao, T.-j.; Ye, L.-h.; Zhang, X.-d. Dkk-1 secreted by mesenchymal stem cells inhibits growth of breast cancer cells via depression of Wnt signalling. Cancer Lett. 2008, 269, 67–77. [Google Scholar] [CrossRef]
- Lage, H. Gene Therapeutic Approaches to Overcome ABCB1-Mediated Drug Resistance. In Current Strategies in Cancer Gene Therapy; Walther, W., Ed.; Springer International Publishing: Cham, Switzerland, 2016; pp. 87–94. [Google Scholar] [CrossRef]
- Dai, X.; Cheng, H.; Bai, Z.; Li, J. Breast Cancer Cell Line Classification and Its Relevance with Breast Tumor Subtyping. J. Cancer 2017, 8, 3131–3141. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Holliday, D.L.; Speirs, V. Choosing the right cell line for breast cancer research. Breast Cancer Res. 2011, 13, 215. [Google Scholar] [CrossRef] [Green Version]
- Chatzistamatiou, T.K.; Papassavas, A.C.; Michalopoulos, E.; Gamaloutsos, C.; Mallis, P.; Gontika, I.; Panagouli, E.; Koussoulakos, S.L.; Stavropoulos-Giokas, C. Optimizing isolation culture and freezing methods to preserve Wharton’s jelly’s mesenchymal stem cell (MSC) properties: An MSC banking protocol validation for the Hellenic Cord Blood Bank. Transfusion 2014, 54, 3108–3120. [Google Scholar] [CrossRef] [PubMed]
- Metsiou, D.N.; Siatis, K.E.; Giannopoulou, E.; Papachristou, D.J.; Kalofonos, H.P.; Koutras, A.; Athanassiou, G. The Impact of Anti-tumor Agents on ER-Positive MCF-7 and HER2-Positive SKBR-3 Breast Cancer Cells Biomechanics. Ann. Biomed. Eng. 2019, 47, 1711–1724. [Google Scholar] [CrossRef]
- Giannopoulou, E.; Siatis, K.E.; Metsiou, D.; Kritikou, I.; Papachristou, D.J.; Kalofonou, M.; Koutras, A.; Athanassiou, G.; Kalofonos, H.P. The inhibition of aromatase alters the mechanical and rheological properties of non-small-cell lung cancer cell lines affecting cell migration. Biochim. Biophys. Acta 2015, 1853, 328–337. [Google Scholar] [CrossRef] [Green Version]
- Page, J.M.; Merkel, A.R.; Ruppender, N.S.; Guo, R.; Dadwal, U.C.; Cannonier, S.; Basu, S.; Guelcher, S.A.; Sterling, J.A. Matrix rigidity regulates the transition of tumor cells to a bone-destructive phenotype through integrin β3 and TGF-β receptor type II. Biomaterials 2015, 64, 33–44. [Google Scholar] [CrossRef] [Green Version]
- Liu, Y.-L.; Chou, C.-K.; Kim, M.; Vasisht, R.; Kuo, Y.-A.; Ang, P.; Liu, C.; Perillo, E.P.; Chen, Y.-A.; Blocher, K.; et al. Assessing metastatic potential of breast cancer cells based on EGFR dynamics. Sci. Rep. 2019, 9, 3395. [Google Scholar] [CrossRef] [PubMed]
- Kraning-Rush, C.M.; Reinhart-King, C.A. Controlling matrix stiffness and topography for the study of tumor cell migration. Cell Adhes. Migr. 2012, 6, 274–279. [Google Scholar] [CrossRef] [Green Version]
- Keren, K.; Pincus, Z.; Allen, G.M.; Barnhart, E.L.; Marriott, G.; Mogilner, A.; Theriot, J.A. Mechanism of shape determination in motile cells. Nature 2008, 453, 475–480. [Google Scholar] [CrossRef] [Green Version]
- D Antonio, P.; Lasalvia, M.; Perna, G.; Capozzi, V. Scale-independent roughness value of cell membranes studied by means of AFM technique. Biochim. Biophys. Acta (BBA)-Biomembr. 2012, 1818, 3141–3148. [Google Scholar] [CrossRef] [Green Version]
- Fraczkowska, K.; Bacia, M.; Przybyło, M.; Drabik, D.; Kaczorowska, A.; Rybka, J.; Stefanko, E.; Drobczynski, S.; Masajada, J.; Podbielska, H.; et al. Alterations of biomechanics in cancer and normal cells induced by doxorubicin. Biomed. Pharmacother. 2018, 97, 1195–1203. [Google Scholar] [CrossRef]
- Huang, J.; Zhang, L.; Wan, D.; Zhou, L.; Zheng, S.; Lin, S.; Qiao, Y. Extracellular matrix and its therapeutic potential for cancer treatment. Signal Transduct. Target. Ther. 2021, 6, 153. [Google Scholar] [CrossRef] [PubMed]
- Knight, E.; Przyborski, S. Advances in 3D cell culture technologies enabling tissue-like structures to be created in vitro. J. Anat. 2015, 227, 746–756. [Google Scholar] [CrossRef] [Green Version]
- Park, Y.; Huh, K.M.; Kang, S.-W. Applications of Biomaterials in 3D Cell Culture and Contributions of 3D Cell Culture to Drug Development and Basic Biomedical Research. Int. J. Mol. Sci. 2021, 22, 2491. [Google Scholar] [CrossRef] [PubMed]
- Wang, J.; Wan, Z.; Liu, W.; Li, L.; Ren, L.; Wang, X.; Sun, P.; Ren, L.; Zhao, H.; Tu, Q.; et al. Atomic force microscope study of tumor cell membranes following treatment with anti-cancer drugs. Biosens. Bioelectron. 2009, 25, 721–727. [Google Scholar] [CrossRef] [PubMed]
- Alibert, C.; Goud, B.; Manneville, J.B. Are cancer cells really softer than normal cells? Biol. Cell 2017, 109, 167–189. [Google Scholar] [CrossRef] [Green Version]
- Gensbittel, V.; Krater, M.; Harlepp, S.; Busnelli, I.; Guck, J.; Goetz, J.G. Mechanical Adaptability of Tumor Cells in Metastasis. Dev. Cell 2021, 56, 164–179. [Google Scholar] [CrossRef]
- Paul, R.; Heil, P.; Spatz, J.P.; Schwarz, U.S. Propagation of mechanical stress through the actin cytoskeleton toward focal adhesions: Model and experiment. Biophys. J. 2008, 94, 1470–1482. [Google Scholar] [CrossRef] [Green Version]
- Rückerl, F.; Lenz, M.; Betz, T.; Manzi, J.; Martiel, J.-L.; Safouane, M.; Paterski-Boujemaa, R.; Blanchoin, L.; Sykes, C. Adaptive Response of Actin Bundles under Mechanical Stress. Biophys. J. 2017, 113, 1072–1079. [Google Scholar] [CrossRef] [Green Version]
- Chang, C.-H.; Lee, H.-H.; Lee, C.-H. Substrate properties modulate cell membrane roughness by way of actin filaments. Sci. Rep. 2017, 7, 9068. [Google Scholar] [CrossRef]
- Alhalhooly, L.; Mamnoon, B.; Kim, J.; Mallik, S.; Choi, Y. Dynamic cellular biomechanics in responses to chemotherapeutic drug in hypoxia probed by atomic force spectroscopy. Oncotarget 2021, 12, 1165. [Google Scholar] [CrossRef] [PubMed]
- Flamini, M.I.; Gauna, G.V.; Sottile, M.L.; Nadin, B.S.; Sanchez, A.M.; Vargas-Roig, L.M. Retinoic acid reduces migration of human breast cancer cells: Role of retinoic acid receptor beta. J. Cell. Mol. Med. 2014, 18, 1113–1123. [Google Scholar] [CrossRef]
- Bartel, C.A.; Jackson, M.W. HER2-positive breast cancer cells expressing elevated FAM83A are sensitive to FAM83A loss. PLoS ONE 2017, 12, e0176778. [Google Scholar] [CrossRef]
- Hart, V.; Gautrey, H.; Kirby, J.; Tyson-Capper, A. HER2 splice variants in breast cancer: Investigating their impact on diagnosis and treatment outcomes. Oncotarget 2020, 11, 4338. [Google Scholar] [CrossRef]
- Cai, K.; Jiang, L.; Wang, J.; Zhang, H.; Wang, X.; Cheng, D.; Dou, J. Downregulation of β-catenin decreases the tumorigenicity, but promotes epithelial-mesenchymal transition in breast cancer cells. J. Cancer Res. 2014, 10, 1063–1070. [Google Scholar] [CrossRef]
- Cusack, S.; Miller, A. Determination of the elastic constants of collagen by Brillouin light scattering. J. Mol. Biol. 1979, 135, 39–51. [Google Scholar] [CrossRef]
- Wenger, M.P.E.; Bozec, L.; Horton, M.A.; Mesquida, P. Mechanical properties of collagen fibrils. Biophys. J. 2007, 93, 1255–1263. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Shukla, V.C.; Higuita-Castro, N.; Nana-Sinkam, P.; Ghadiali, S.N. Substrate stiffness modulates lung cancer cell migration but not epithelial to mesenchymal transition. J. Biomed. Mater. Res. Part A 2016, 104, 1182–1193. [Google Scholar] [CrossRef] [PubMed]
- Gradilone, A.; Naso, G.; Raimondi, C.; Cortesi, E.; Gandini, O.; Vincenzi, B.; Saltarelli, R.; Chiapparino, E.; Spremberg, F.; Cristofanilli, M.; et al. Circulating tumor cells (CTCs) in metastatic breast cancer (MBC): Prognosis, drug resistance and phenotypic characterization. Ann. Oncol. 2011, 22, 86–92. [Google Scholar] [CrossRef]
- Hughes, A.D.; Marshall, J.R.; Keller, E.; Powderly, J.D.; Greene, B.T.; King, M.R. Differential drug responses of circulating tumor cells within patient blood. Cancer Lett. 2014, 352, 28–35. [Google Scholar] [CrossRef] [PubMed] [Green Version]




Publisher’s Note: MDPI stays neutral with regard to jurisdictional claims in published maps and institutional affiliations. |
© 2021 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Metsiou, D.N.; Kozaniti, F.K.; Deligianni, D.D. Engineering Breast Cancer Cells and hUMSCs Microenvironment in 2D and 3D Scaffolds: A Mechanical Study Approach of Stem Cells in Anticancer Therapy. Bioengineering 2021, 8, 189. https://doi.org/10.3390/bioengineering8110189
Metsiou DN, Kozaniti FK, Deligianni DD. Engineering Breast Cancer Cells and hUMSCs Microenvironment in 2D and 3D Scaffolds: A Mechanical Study Approach of Stem Cells in Anticancer Therapy. Bioengineering. 2021; 8(11):189. https://doi.org/10.3390/bioengineering8110189
Chicago/Turabian StyleMetsiou, Despoina Nektaria, Foteini K. Kozaniti, and Despina D. Deligianni. 2021. "Engineering Breast Cancer Cells and hUMSCs Microenvironment in 2D and 3D Scaffolds: A Mechanical Study Approach of Stem Cells in Anticancer Therapy" Bioengineering 8, no. 11: 189. https://doi.org/10.3390/bioengineering8110189
APA StyleMetsiou, D. N., Kozaniti, F. K., & Deligianni, D. D. (2021). Engineering Breast Cancer Cells and hUMSCs Microenvironment in 2D and 3D Scaffolds: A Mechanical Study Approach of Stem Cells in Anticancer Therapy. Bioengineering, 8(11), 189. https://doi.org/10.3390/bioengineering8110189




