Facile Fabrication of Three-Dimensional Hydrogel Film with Complex Tissue Morphology
Abstract
1. Introduction
2. Materials and Methods
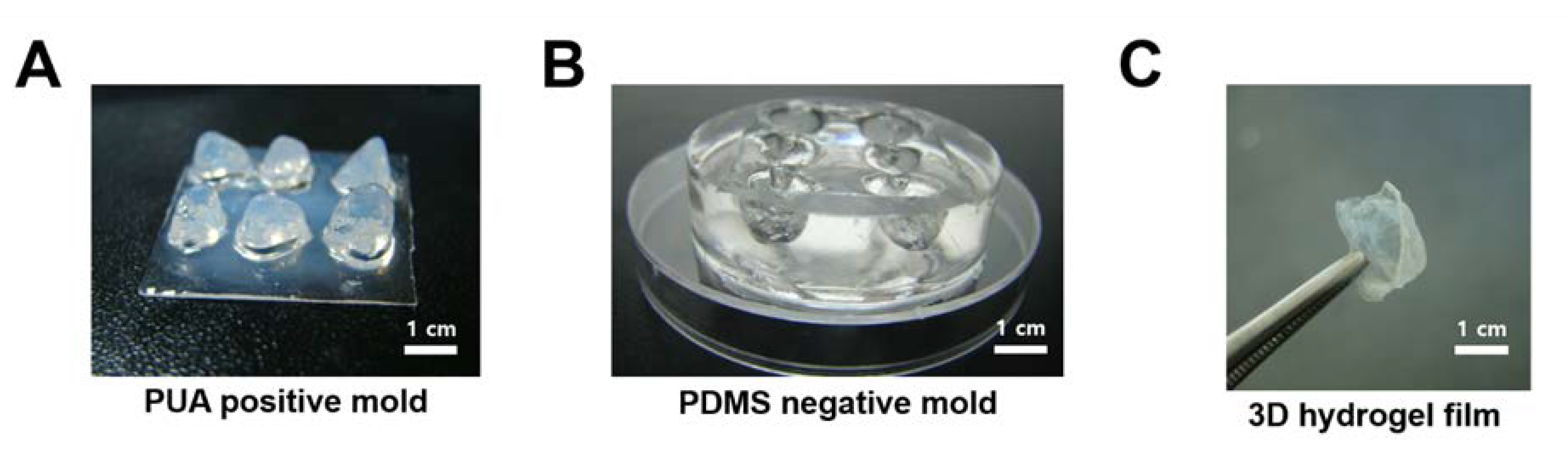
2.1. Fabrication of Cardiac Tissue-Shaped 3D Replica Structure
2.2. Fabrication of 3D Hydrogel Film Structure
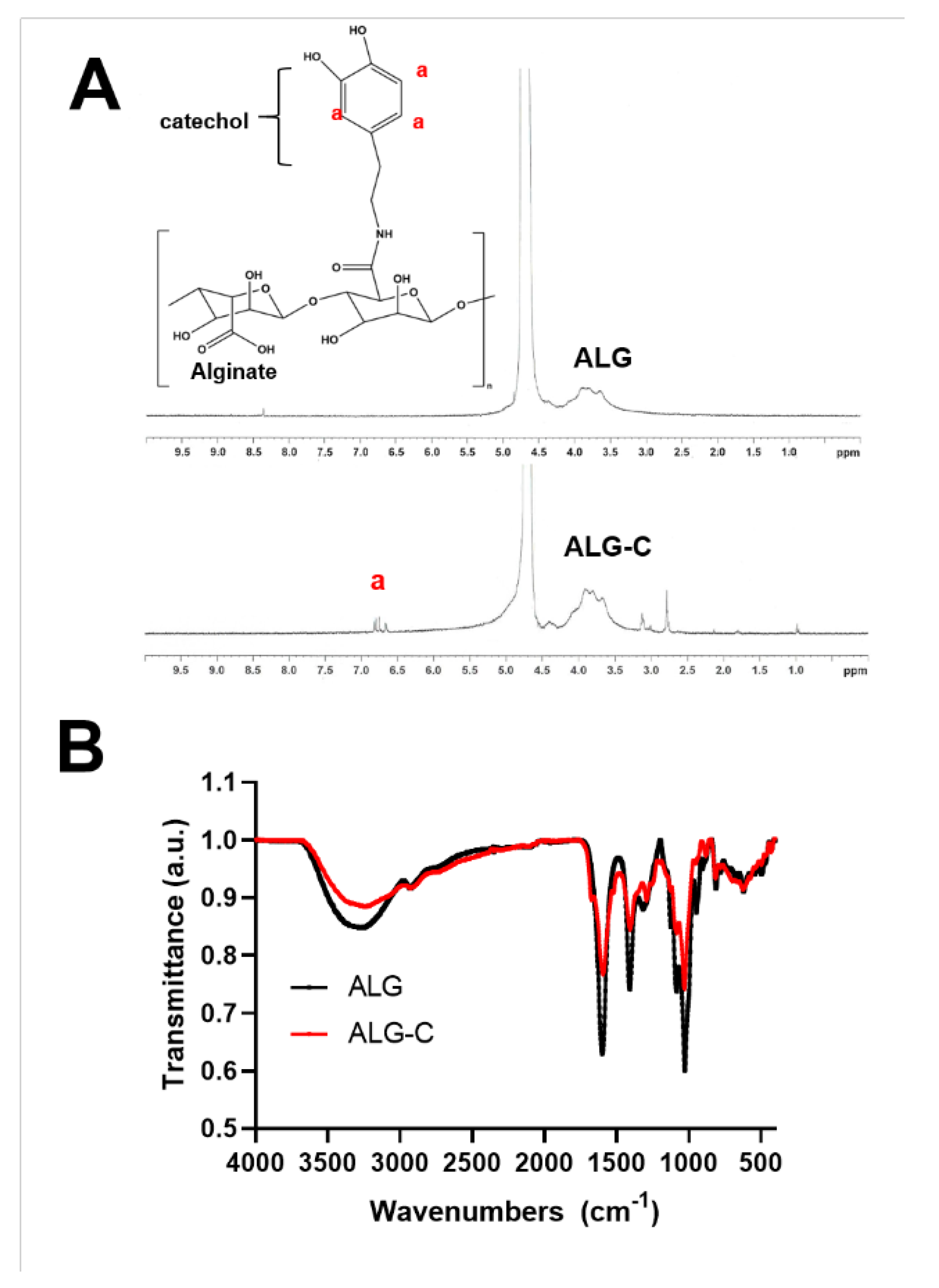
2.3. Synthesis of Catechol-Conjugated Alginate (ALG-C)
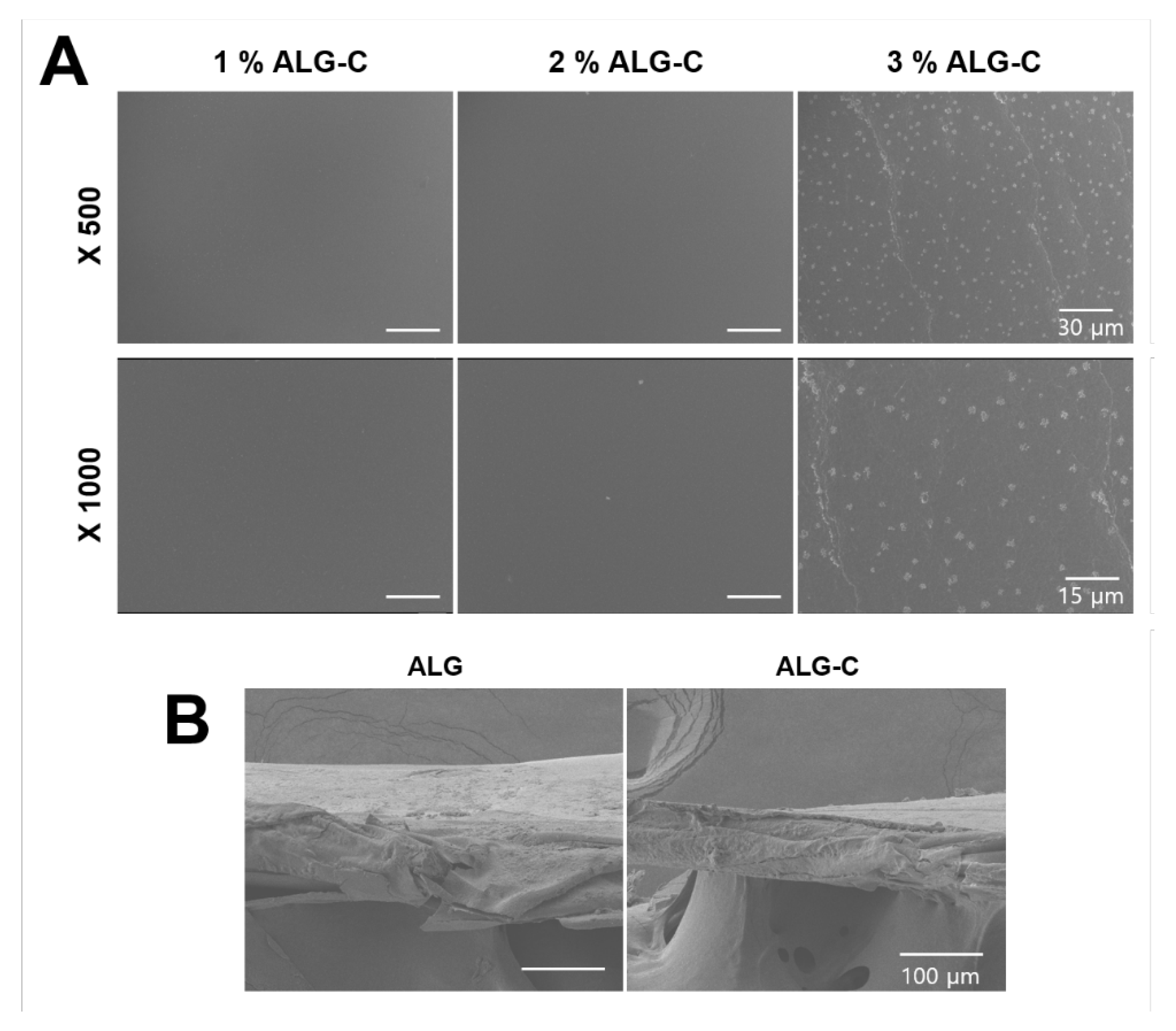
2.4. Scanning Electron Microscopy (SEM) Analysis
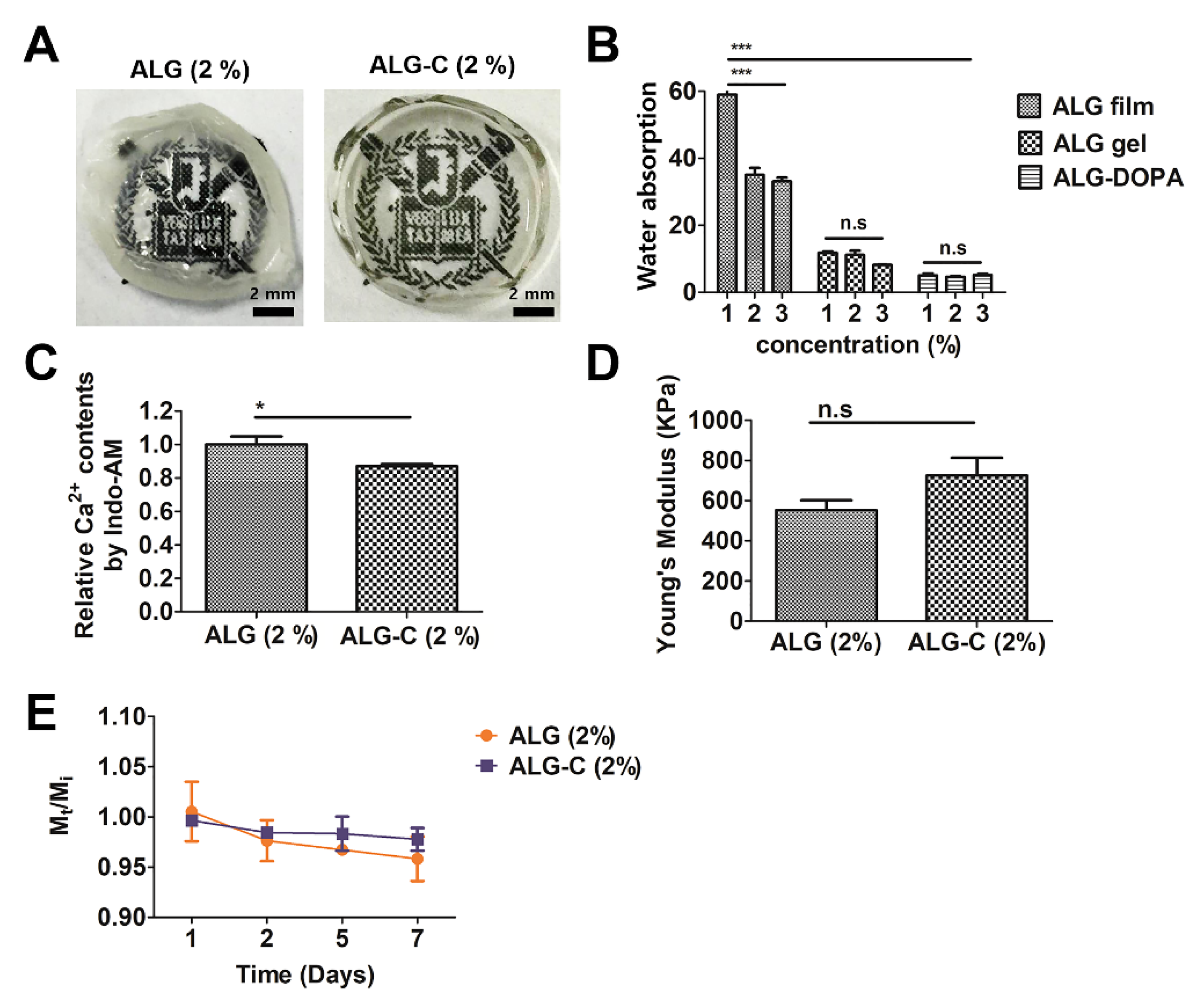
2.5. Measurement of Water Absorption
2.6. Measurement of Calcium Content on Alginate Hydrogel and Alginate Film
2.7. Quantification of Young’s Modulus
2.8. Determination of Degradation Kinetics
2.9. Live/Dead Assay
2.10. Synthesis of Lentiviral Vectors
2.11. Determination of Drug Coating Efficiency
2.12. Statistical Analysis
3. Results
3.1. Fabrication of 3D Hydrogel Film for Cardiac Tissue Engineering
3.2. Physicochemical Properties of the 3D Hydrogel Film
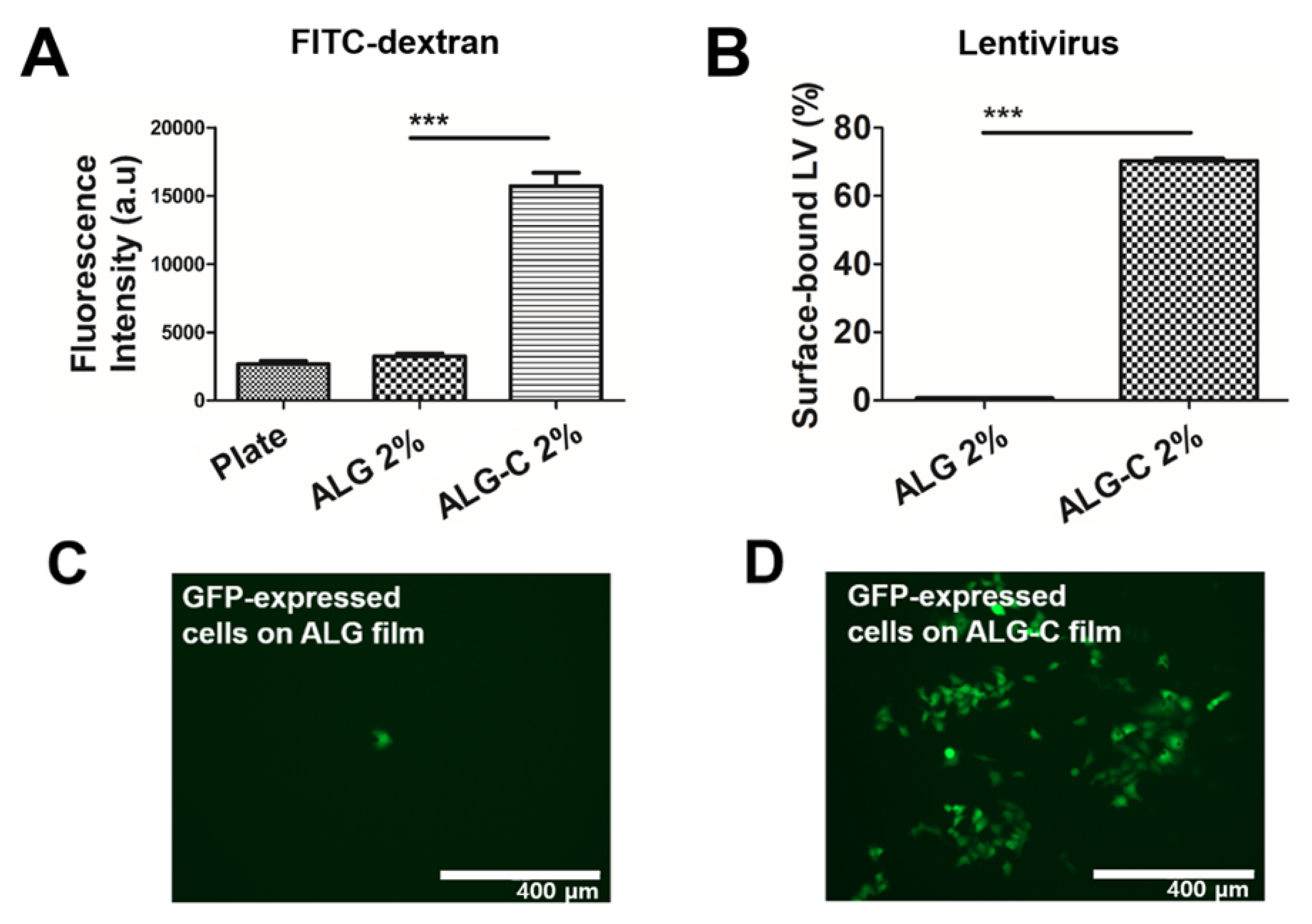
3.3. Determination of Drug Loading Efficacy
4. Conclusions
Supplementary Materials
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Conflicts of Interest
References
- Kim, S.H.; Yu, S.J.; Kim, I.; Choi, J.; Choi, Y.H.; Im, S.G.; Hwang, N.S. A biofunctionalized viral delivery patch for spatially defined transfection. Chem. Commun. 2019, 55, 2317–2320. [Google Scholar] [CrossRef]
- Tavana, H.; Jovic, A.; Mosadegh, B.; Lee, Q.Y.; Liu, X.; Luker, K.E.; Luker, G.D.; Weiss, S.J.; Takayama, S. Nanolitre liquid patterning in aqueous environments for spatially defined reagent delivery to mammalian cells. Nat. Mater. 2009, 8, 736–741. [Google Scholar] [CrossRef]
- Kim, E.; Lee, S.; Hong, S.; Jin, G.; Kim, M.; Park, K.I.; Lee, H.; Jang, J.-H. Sticky “delivering-from” strategies using viral vectors for efficient human neural stem cell infection by bioinspired catecholamines. ACS Appl. Mater. Interfaces 2014, 6, 8288–8294. [Google Scholar] [CrossRef] [PubMed]
- Lee, S.; Yun, S.; Park, K.I.; Jang, J.-H. Sliding fibers: Slidable, injectable, and gel-like electrospun nanofibers as versatile cell carriers. ACS Nano 2016, 10, 3282–3294. [Google Scholar] [CrossRef] [PubMed]
- Bidez, P.R.; Li, S.; MacDiarmid, A.G.; Venancio, E.C.; Wei, Y.; Lelkes, P.I. Polyaniline, an electroactive polymer, supports adhesion and proliferation of cardiac myoblasts. J. Biomater. Sci. Polym. Ed. 2006, 17, 199–212. [Google Scholar] [CrossRef]
- Castellano, D.; Blanes, M.; Marco, B.; Cerrada, I.; Ruiz-Saurí, A.; Pelacho, B.; Arana, M.; Montero, J.A.; Cambra, V.; Prosper, F. A comparison of electrospun polymers reveals poly (3-hydroxybutyrate) fiber as a superior scaffold for cardiac repair. Stem Cells Dev. 2014, 23, 1479–1490. [Google Scholar] [CrossRef]
- Vunjak-Novakovic, G.; Tandon, N.; Godier, A.; Maidhof, R.; Marsano, A.; Martens, T.P.; Radisic, M. Challenges in cardiac tissue engineering. Tissue Eng. Part B Rev. 2010, 16, 169–187. [Google Scholar] [CrossRef]
- Golob, M.; Moss, R.L.; Chesler, N.C. Cardiac tissue structure, properties, and performance: A materials science perspective. Ann. Biomed. Eng. 2014, 42, 2003–2013. [Google Scholar] [CrossRef]
- Feinberg, A.W.; Alford, P.W.; Jin, H.W.; Ripplinger, C.M.; Werdich, A.A.; Sheehy, S.P.; Grosberg, A.; Parker, K.K. Controlling the contractile strength of engineered cardiac muscle by hierarchal tissue architecture. Biomaterials 2012, 33, 5732–5741. [Google Scholar] [CrossRef]
- Lapierre-Landry, M.; Kolesova, H.; Liu, Y.; Watanabe, M.; Jenkins, M.W. Three-dimensional alignment of microvasculature and cardiomyocytes in the developing ventricle. Sci. Rep. 2020, 10, 14955. [Google Scholar] [CrossRef] [PubMed]
- Kumar, N.; Sridharan, D.; Palaniappan, A.; Dougherty, J.A.; Czirok, A.; Isai, D.G.; Mergaye, M.; Angelos, M.G.; Powell, H.M.; Khan, M. Scalable Biomimetic Coaxial Aligned Nanofiber Cardiac Patch: A Potential Model for “Clinical Trials in a Dish”. Front Bioeng. Biotech. 2020, 8. [Google Scholar] [CrossRef]
- Yildirim, Y.; Naito, H.; Didié, M.; Karikkineth, B.C.; Biermann, D.; Eschenhagen, T.; Zimmermann, W.-H. Development of a biological ventricular assist device: Preliminary data from a small animal model. Circulation 2007, 116, I-16–I-23. [Google Scholar] [CrossRef]
- MacQueen, L.A.; Sheehy, S.P.; Chantre, C.O.; Zimmerman, J.F.; Pasqualini, F.S.; Liu, X.; Goss, J.A.; Campbell, P.H.; Gonzalez, G.M.; Park, S.-J. A tissue-engineered scale model of the heart ventricle. Nat. Biomed. Eng. 2018, 2, 930–941. [Google Scholar] [CrossRef] [PubMed]
- Kupfer, M.E.; Lin, W.-H.; Ravikumar, V.; Qiu, K.; Wang, L.; Gao, L.; Bhuiyan, D.B.; Lenz, M.; Ai, J.; Mahutga, R.R. In situ expansion, differentiation, and electromechanical coupling of human cardiac muscle in a 3D bioprinted, chambered organoid. Circ. Res. 2020, 127, 207–224. [Google Scholar] [CrossRef] [PubMed]
- Lee, H.; Dellatore, S.M.; Miller, W.M.; Messersmith, P.B. Mussel-inspired surface chemistry for multifunctional coatings. Science 2007, 318, 426–430. [Google Scholar] [CrossRef]
- Dalsin, J.L.; Hu, B.-H.; Lee, B.P.; Messersmith, P.B. Mussel adhesive protein mimetic polymers for the preparation of nonfouling surfaces. J. Am. Chem. Soc. 2003, 125, 4253–4258. [Google Scholar] [CrossRef] [PubMed]
- Kord Forooshani, P.; Lee, B.P. Recent approaches in designing bioadhesive materials inspired by mussel adhesive protein. J. Polym. Sci. Part A Polym. Chem. 2017, 55, 9–33. [Google Scholar] [CrossRef]
- Lee, H.; Rho, J.; Messersmith, P.B. Facile conjugation of biomolecules onto surfaces via mussel adhesive protein inspired coatings. Adv. Mater. 2009, 21, 431–434. [Google Scholar] [CrossRef]
- Madhurakkat Perikamana, S.K.; Lee, J.; Lee, Y.B.; Shin, Y.M.; Lee, E.J.; Mikos, A.G.; Shin, H. Materials from mussel-inspired chemistry for cell and tissue engineering applications. Biomacromolecules 2015, 16, 2541–2555. [Google Scholar] [CrossRef]
- Kim, S.H.; Lee, S.H.; Lee, J.E.; Park, S.J.; Kim, K.; Kim, I.S.; Lee, Y.S.; Hwang, N.S.; Kim, B.G. Tissue adhesive, rapid forming, and sprayable ECM hydrogel via recombinant tyrosinase crosslinking. Biomaterials 2018, 178, 401–412. [Google Scholar] [CrossRef]
- Xu, J.; Strandman, S.; Zhu, J.X.; Barralet, J.; Cerruti, M. Genipin-crosslinked catechol-chitosan mucoadhesive hydrogels for buccal drug delivery. Biomaterials 2015, 37, 395–404. [Google Scholar] [CrossRef]
- Zhang, W.; Wang, R.; Sun, Z.; Zhu, X.; Zhao, Q.; Zhang, T.; Cholewinski, A.; Yang, F.K.; Zhao, B.; Pinnaratip, R. Catechol-functionalized hydrogels: Biomimetic design, adhesion mechanism, and biomedical applications. Chem. Soc. Rev. 2020, 49, 433–464. [Google Scholar] [CrossRef]
- Jin, Y.J.; Koh, R.H.; Kim, S.H.; Kim, K.M.; Park, G.K.; Hwang, N.S. Injectable anti-inflammatory hyaluronic acid hydrogel for osteoarthritic cartilage repair. Mat. Sci. Eng. C Mater. 2020, 115. [Google Scholar] [CrossRef] [PubMed]
- Kim, B.S.; Kim, S.H.; Kim, K.; An, Y.H.; So, K.H.; Kim, B.G.; Hwang, N.S. Enzyme-mediated one-pot synthesis of hydrogel with the polyphenol cross-linker for skin regeneration. Mater. Today Bio 2020, 8. [Google Scholar] [CrossRef] [PubMed]
- Anene-Nzelu, C.G.; Choudhury, D.; Li, H.P.; Fraiszudeen, A.; Peh, K.Y.; Toh, Y.C.; Ng, S.H.; Leo, H.L.; Yu, H. Scalable cell alignment on optical media substrates. Biomaterials 2013, 34, 5078–5087. [Google Scholar] [CrossRef] [PubMed]
- Sumi, D.; Abe, K.; Himeno, S. Arsenite retards the cardiac differentiation of rat cardiac myoblast H9c2 cells. Biochem. Biophs. Res. Co 2013, 436, 175–179. [Google Scholar] [CrossRef]
- Comelli, M.; Domenis, R.; Bisetto, E.; Contin, M.; Marchini, M.; Ortolani, F.; Tomasetig, L.; Mavelli, I. Cardiac differentiation promotes mitochondria development and ameliorates oxidative capacity in H9c2 cardiomyoblasts. Mitochondrion 2011, 11, 315–326. [Google Scholar] [CrossRef]
- Bonyár, A.; Sántha, H.; Varga, M.; Ring, B.; Vitéz, A.; Harsányi, G. Characterization of rapid PDMS casting technique utilizing molding forms fabricated by 3D rapid prototyping technology (RPT). Int. J. Mater. Form. 2014, 7, 189–196. [Google Scholar] [CrossRef]
- Ye, X.; Liu, H.; Ding, Y.; Li, H.; Lu, B. Research on the cast molding process for high quality PDMS molds. Microelectron. Eng. 2009, 86, 310–313. [Google Scholar] [CrossRef]
- Hong, S.; Sycks, D.; Chan, H.F.; Lin, S.; Lopez, G.P.; Guilak, F.; Leong, K.W.; Zhao, X. 3D printing of highly stretchable and tough hydrogels into complex, cellularized structures. Adv. Mater. 2015, 27, 4035–4040. [Google Scholar] [CrossRef]
- Kirchmajer, D.M.; Gorkin Iii, R. An overview of the suitability of hydrogel-forming polymers for extrusion-based 3D-printing. J. Mater. Chem. B 2015, 3, 4105–4117. [Google Scholar] [CrossRef] [PubMed]
- Highley, C.B.; Rodell, C.B.; Burdick, J.A. Direct 3D printing of shear-thinning hydrogels into self-healing hydrogels. Adv. Mater. 2015, 27, 5075–5079. [Google Scholar] [CrossRef]
- Hinton, T.J.; Jallerat, Q.; Palchesko, R.N.; Park, J.H.; Grodzicki, M.S.; Shue, H.-J.; Ramadan, M.H.; Hudson, A.R.; Feinberg, A.W. Three-dimensional printing of complex biological structures by freeform reversible embedding of suspended hydrogels. Sci. Adv. 2015, 1, e1500758. [Google Scholar] [CrossRef]
- Noor, N.; Shapira, A.; Edri, R.; Gal, I.; Wertheim, L.; Dvir, T. 3D Printing of Personalized Thick and Perfusable Cardiac Patches and Hearts. Adv. Sci. 2019, 6. [Google Scholar] [CrossRef]
- Lee, A.; Hudson, A.R.; Shiwarski, D.J.; Tashman, J.W.; Hinton, T.J.; Yerneni, S.; Bliley, J.M.; Campbell, P.G.; Feinberg, A.W. 3D bioprinting of collagen to rebuild components of the human heart. Science 2019, 365, 482–487. [Google Scholar] [CrossRef] [PubMed]
- Lee, E.J.; Kim, D.E.; Azeloglu, E.U.; Costa, K.D. Engineered cardiac organoid chambers: Toward a functional biological model ventricle. Tissue Eng. Pt A 2008, 14, 215–225. [Google Scholar] [CrossRef] [PubMed]
- Li, R.A.; Keung, W.; Cashman, T.J.; Backeris, P.C.; Johnson, B.V.; Bardot, E.S.; Wong, A.O.T.; Chan, P.K.W.; Chan, C.W.Y.; Costa, K.D. Bioengineering an electro-mechanically functional miniature ventricular heart chamber from human pluripotent stem cells. Biomaterials 2018, 163, 116–127. [Google Scholar] [CrossRef]
- Badiger, H.; Shukla, S.; Kalyani, S.; Sridhar, S. Thin film composite sodium alginate membranes for dehydration of acetic acid and isobutanol. J. Appl. Polym. Sci. 2014, 131. [Google Scholar] [CrossRef]
- Senturk Parreidt, T.; Müller, K.; Schmid, M. Alginate-based edible films and coatings for food packaging applications. Foods 2018, 7, 170. [Google Scholar] [CrossRef]
- Soazo, M.; Baez, G.; Barboza, A.; Busti, P.A.; Rubiolo, A.; Verdini, R.; Delorenzi, N.J. Heat treatment of calcium alginate films obtained by ultrasonic atomizing: Physicochemical characterization. Food Hydrocolloid 2015, 51, 193–199. [Google Scholar] [CrossRef]
- Shin, J.; Lee, J.S.; Lee, C.; Park, H.J.; Yang, K.; Jin, Y.; Ryu, J.H.; Hong, K.S.; Moon, S.H.; Chung, H.M. Tissue adhesive catechol-modified hyaluronic acid hydrogel for effective, minimally invasive cell therapy. Adv. Funct. Mater. 2015, 25, 3814–3824. [Google Scholar] [CrossRef]
- Ryu, J.H.; Lee, Y.; Kong, W.H.; Kim, T.G.; Park, T.G.; Lee, H. Catechol-functionalized chitosan/pluronic hydrogels for tissue adhesives and hemostatic materials. Biomacromolecules 2011, 12, 2653–2659. [Google Scholar] [CrossRef]
- Prausnitz, M.R.; Langer, R. Transdermal drug delivery. Nat. Biotechnol. 2008, 26, 1261–1268. [Google Scholar] [CrossRef] [PubMed]
- Tibbitt, M.W.; Dahlman, J.E.; Langer, R. Emerging frontiers in drug delivery. J. Am. Chem. Soc. 2016, 138, 704–717. [Google Scholar] [CrossRef]
- Magliaro, C.; Mattei, G.; Iacoangeli, F.; Corti, A.; Piemonte, V.; Ahluwalia, A. Oxygen Consumption Characteristics in 3D Constructs Depend on Cell Density. Front Bioeng. Biotech. 2019, 7. [Google Scholar] [CrossRef] [PubMed]
- Kim, S.H.; Kim, K.; Kim, B.S.; An, Y.H.; Lee, U.J.; Lee, S.H.; Kim, S.L.; Kim, B.G.; Hwang, N.S. Fabrication of polyphenol-incorporated anti-inflammatory hydrogel via high-affinity enzymatic crosslinking for wet tissue adhesion. Biomaterials 2020, 242. [Google Scholar] [CrossRef]
- Kang, B.-J.; Kim, H.; Lee, S.K.; Kim, J.; Shen, Y.; Jung, S.; Kang, K.-S.; Im, S.G.; Lee, S.Y.; Choi, M. Umbilical-cord-blood-derived mesenchymal stem cells seeded onto fibronectin-immobilized polycaprolactone nanofiber improve cardiac function. Acta Biomater. 2014, 10, 3007–3017. [Google Scholar] [CrossRef]
- Wu, Y.; Wang, L.; Guo, B.; Ma, P.X. Interwoven aligned conductive nanofiber yarn/hydrogel composite scaffolds for engineered 3D cardiac anisotropy. Acs Nano 2017, 11, 5646–5659. [Google Scholar] [CrossRef]
- Gao, C.M.; Liu, M.Z.; Chen, J.; Zhang, X. Preparation and controlled degradation of oxidized sodium alginate hydrogel. Polym Degrad Stabil. 2009, 94, 1405–1410. [Google Scholar] [CrossRef]
- Rodell, C.B.; Wade, R.J.; Purcell, B.P.; Dusaj, N.N.; Burdick, J.A. Selective proteolytic degradation of guest–host assembled, injectable hyaluronic acid hydrogels. ACS Biomater. Sci. Eng. 2015, 1, 277–286. [Google Scholar] [CrossRef]
- Wade, R.J.; Bassin, E.J.; Rodell, C.B.; Burdick, J.A. Protease-degradable electrospun fibrous hydrogels. Nat. Commun. 2015, 6, 1–10. [Google Scholar] [CrossRef]
- Kim, E.; Song, I.T.; Lee, S.; Kim, J.S.; Lee, H.; Jang, J.H. Drawing Sticky Adeno-Associated Viruses on Surfaces for Spatially Patterned Gene Expression. Angew. Chem. Int. Ed. 2012, 51, 5598–5601. [Google Scholar] [CrossRef]
- Kim, J.S.; Chu, H.S.; Park, K.I.; Won, J.I.; Jang, J.H. Elastin-like polypeptide matrices for enhancing adeno-associated virus-mediated gene delivery to human neural stem cells. Gene 2012, 19, 329–337. [Google Scholar] [CrossRef]
- Campostrini, G.; Windt, L.M.; van Meer, B.J.; Bellin, M.; Mummery, C.L. Cardiac tissues from stem cells: New routes to maturation and cardiac regeneration. Circ. Res. 2021, 128, 775–801. [Google Scholar] [CrossRef]
- Barile, L.; Messina, E.; Giacomello, A.; Marbán, E. Endogenous cardiac stem cells. Prog. Cardiovasc. Dis. 2007, 50, 31–48. [Google Scholar] [CrossRef]
- Feyen, D.A.; McKeithan, W.L.; Bruyneel, A.A.; Spiering, S.; Hörmann, L.; Ulmer, B.; Zhang, H.; Briganti, F.; Schweizer, M.; Hegyi, B. Metabolic maturation media improve physiological function of human iPSC-derived cardiomyocytes. Cell Rep. 2020, 32, 107925. [Google Scholar] [CrossRef] [PubMed]
- Senyo, S.E.; Steinhauser, M.L.; Pizzimenti, C.L.; Yang, V.K.; Cai, L.; Wang, M.; Wu, T.-D.; Guerquin-Kern, J.-L.; Lechene, C.P.; Lee, R.T. Mammalian heart renewal by pre-existing cardiomyocytes. Nature 2013, 493, 433–436. [Google Scholar] [CrossRef]
- Burridge, P.W.; Matsa, E.; Shukla, P.; Lin, Z.C.; Churko, J.M.; Ebert, A.D.; Lan, F.; Diecke, S.; Huber, B.; Mordwinkin, N.M. Chemically defined generation of human cardiomyocytes. Nat. Methods 2014, 11, 855–860. [Google Scholar] [CrossRef] [PubMed]
- Sasaki, H.; Yamamura, K.; Tei, C.; Nishida, K.; Nakamura, J. Ocular permeability of FITC-dextran with absorption promoter for ocular delivery of peptide drug. J. Drug Target 1995, 3, 129–135. [Google Scholar] [CrossRef]
- Saiz-Poseu, J.; Mancebo-Aracil, J.; Nador, F.; Busqué, F.; Ruiz-Molina, D. The chemistry behind catechol-based adhesion. Angew. Chem. Int. Ed. 2019, 58, 696–714. [Google Scholar] [CrossRef] [PubMed]
- Zeng, X.-H.; Du, H.; Zhao, H.-M.; Xiang, L.; Feng, N.-X.; Li, H.; Li, Y.-W.; Cai, Q.-Y.; Mo, C.-H.; Wong, M.-H. Insights into the binding interaction of substrate with catechol 2, 3-dioxygenase from biophysics point of view. J. Hazard. Mater. 2020, 391, 122211. [Google Scholar] [CrossRef] [PubMed]


Publisher’s Note: MDPI stays neutral with regard to jurisdictional claims in published maps and institutional affiliations. |
© 2021 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
An, Y.-H.; Kim, S.-H. Facile Fabrication of Three-Dimensional Hydrogel Film with Complex Tissue Morphology. Bioengineering 2021, 8, 164. https://doi.org/10.3390/bioengineering8110164
An Y-H, Kim S-H. Facile Fabrication of Three-Dimensional Hydrogel Film with Complex Tissue Morphology. Bioengineering. 2021; 8(11):164. https://doi.org/10.3390/bioengineering8110164
Chicago/Turabian StyleAn, Young-Hyeon, and Su-Hwan Kim. 2021. "Facile Fabrication of Three-Dimensional Hydrogel Film with Complex Tissue Morphology" Bioengineering 8, no. 11: 164. https://doi.org/10.3390/bioengineering8110164
APA StyleAn, Y.-H., & Kim, S.-H. (2021). Facile Fabrication of Three-Dimensional Hydrogel Film with Complex Tissue Morphology. Bioengineering, 8(11), 164. https://doi.org/10.3390/bioengineering8110164





