Liquid Helium Enhanced Vitrification Efficiency of Human Bone-Derived Mesenchymal Stem Cells and Human Embryonic Stem Cells
Abstract
:1. Introduction
2. Materials and Methods
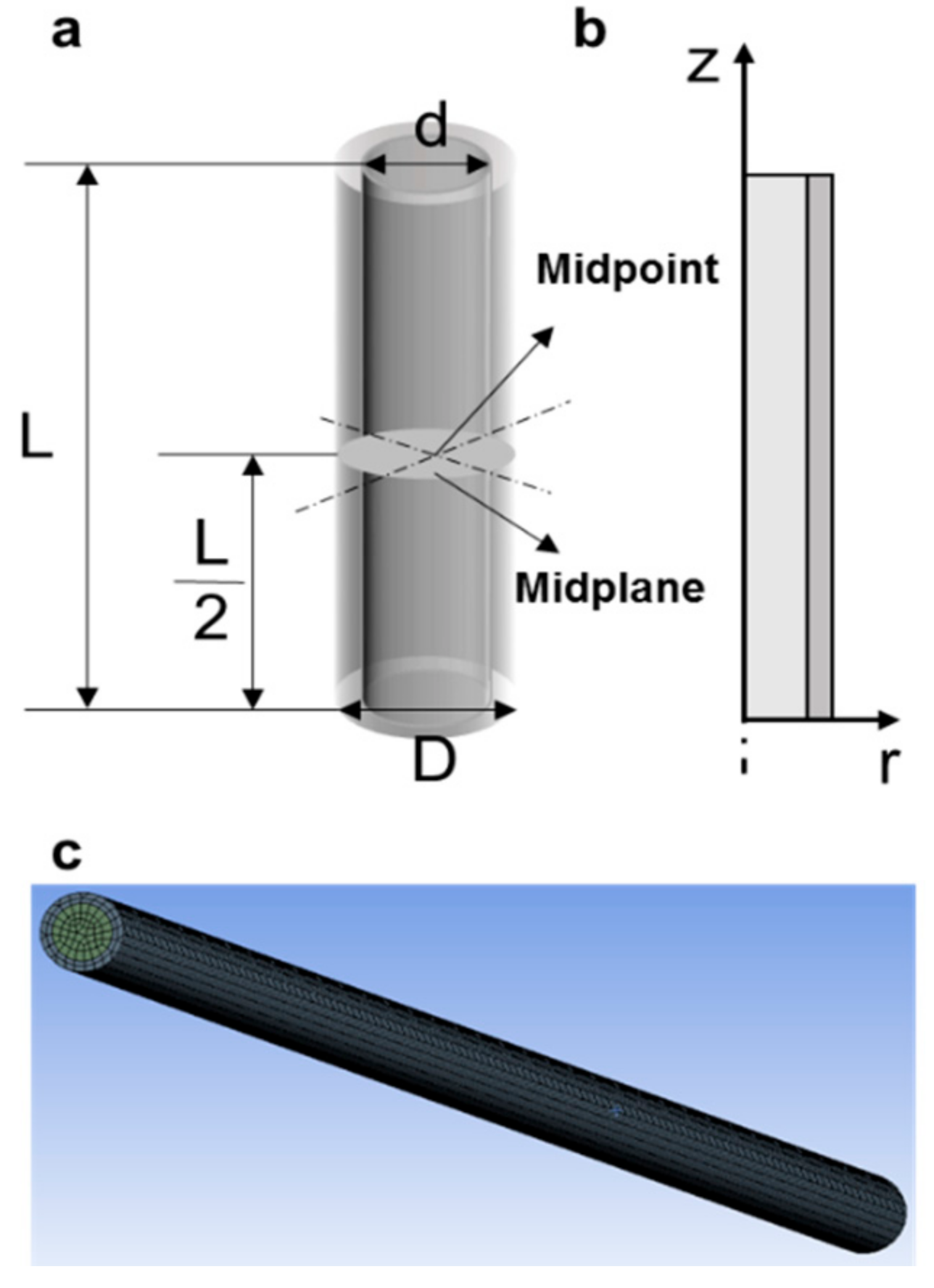
2.1. Physical Model
2.2. Cell Culture
2.3. Vitrification
2.4. Immediate Cell Viability
2.5. Attachment Efficiency
2.6. Morphology and Proliferation
2.7. Flow Cytometry Analysis
2.8. Immunofluorescence Staining
2.9. Reverse Transcriptase Quantitative Polymerase Chain Reaction (RT-qPCR) Analysis
2.10. Differentiation Capacity of hBMSCs
2.11. Karyotype Analysis of hESCs
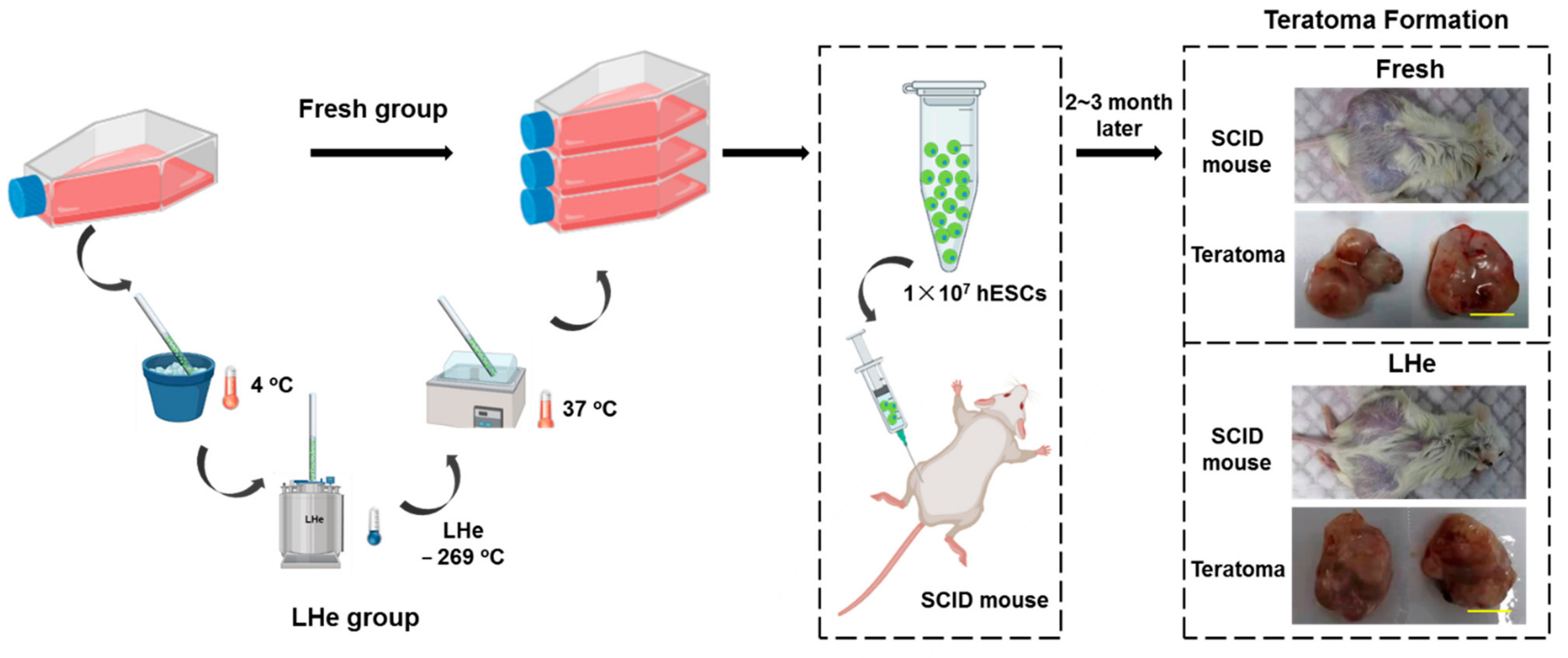
2.12. Teratoma Formation
2.13. Statistical Analysis
3. Results and Discussion
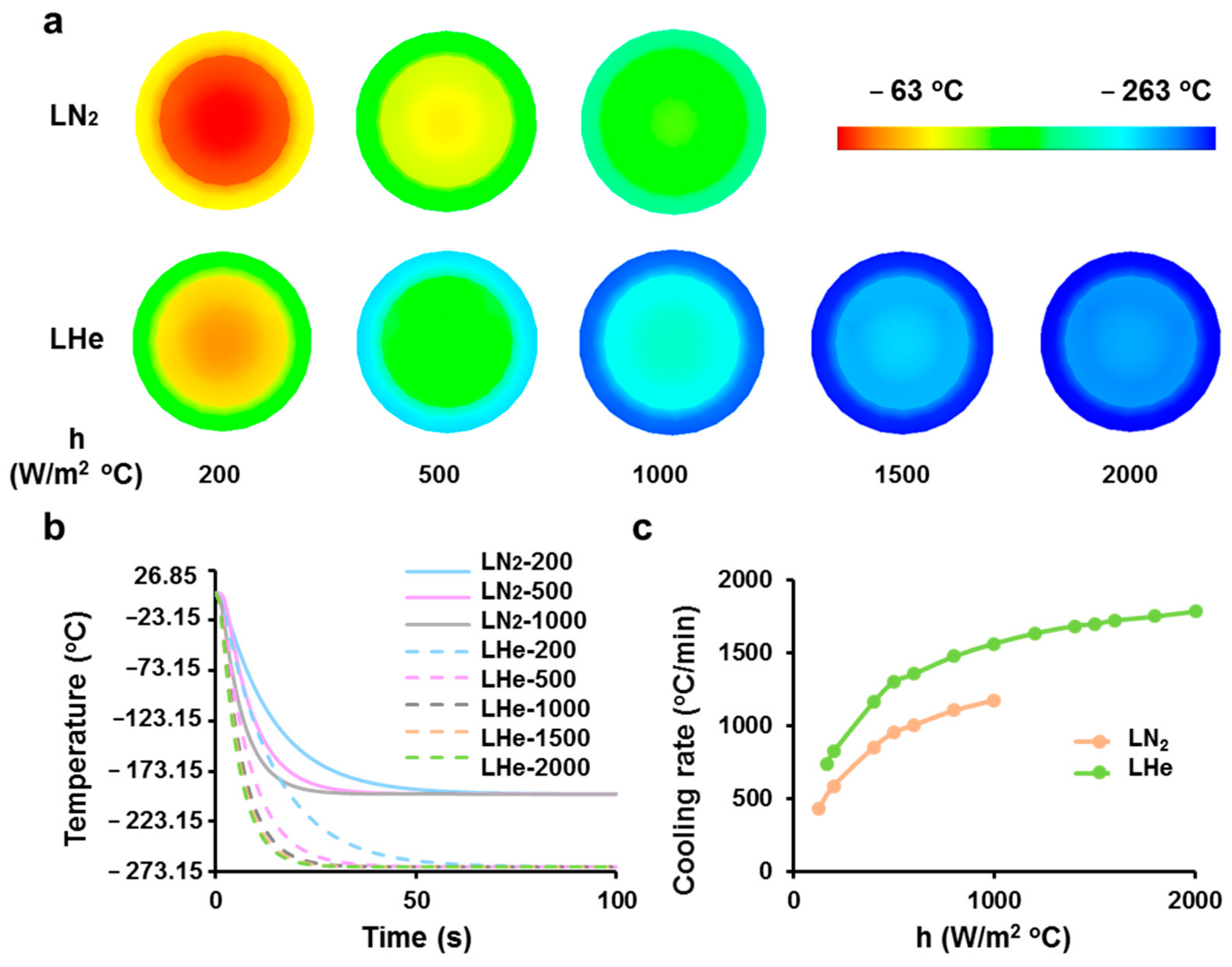
3.1. Theoretical Prediction of Cooling Rates of Vitrification Straws Plunged into Liquid Nitrogen or Helium
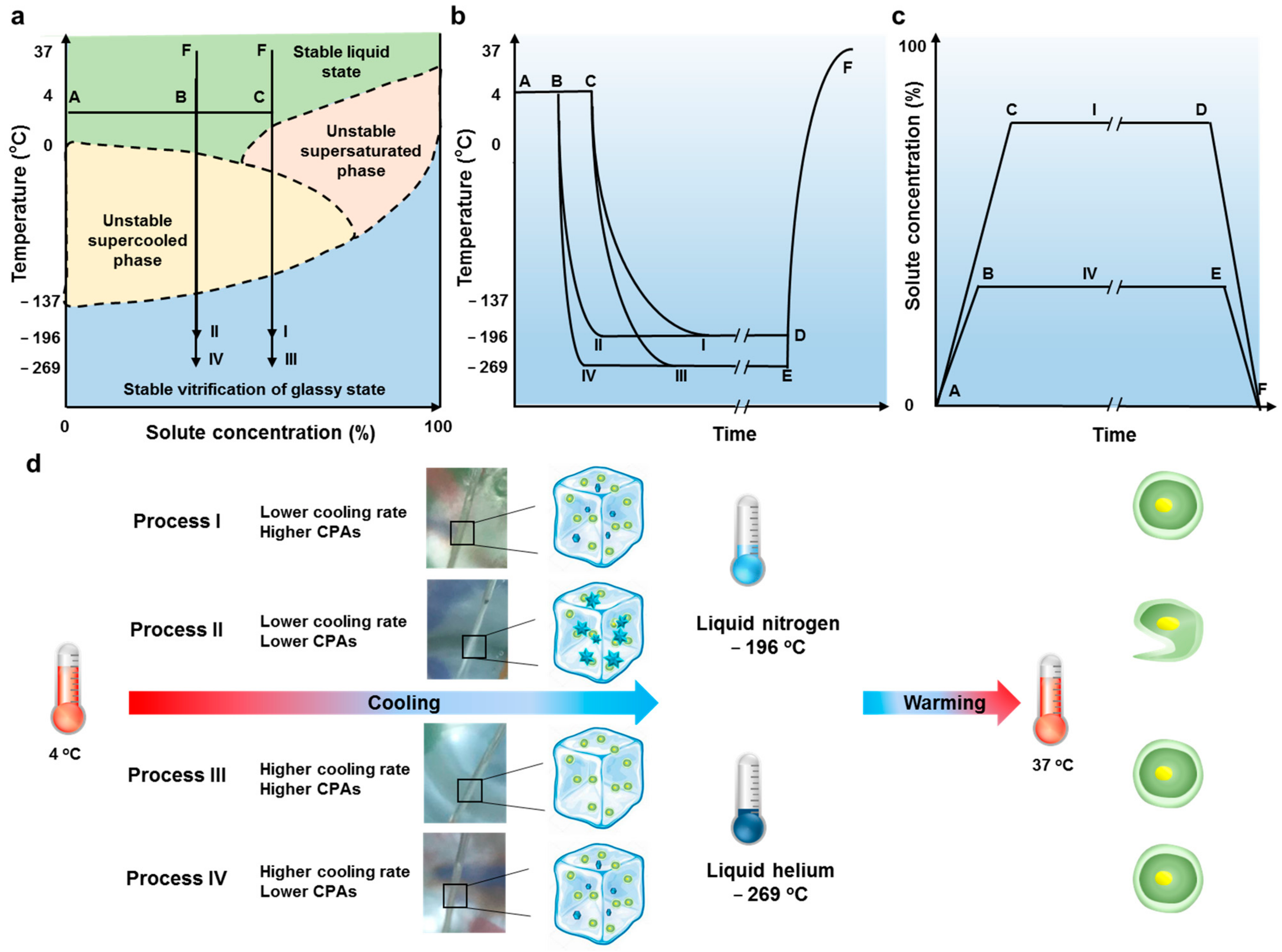
3.2. Vitrification in Liquid Helium Assisting Survival of Cells
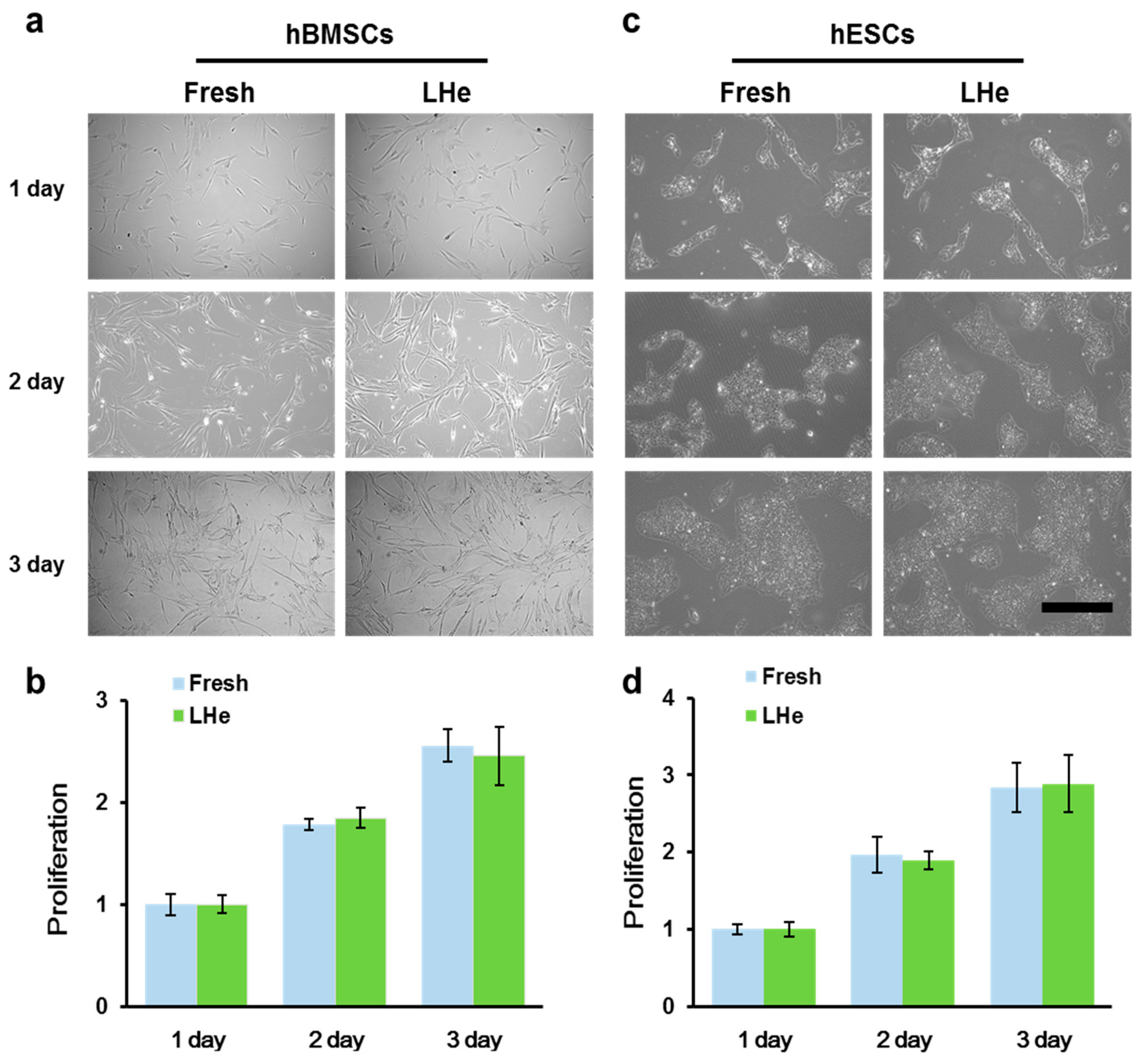
3.3. Maintenance of Cell Morphology and Proliferation after Vitrification in Liquid Helium
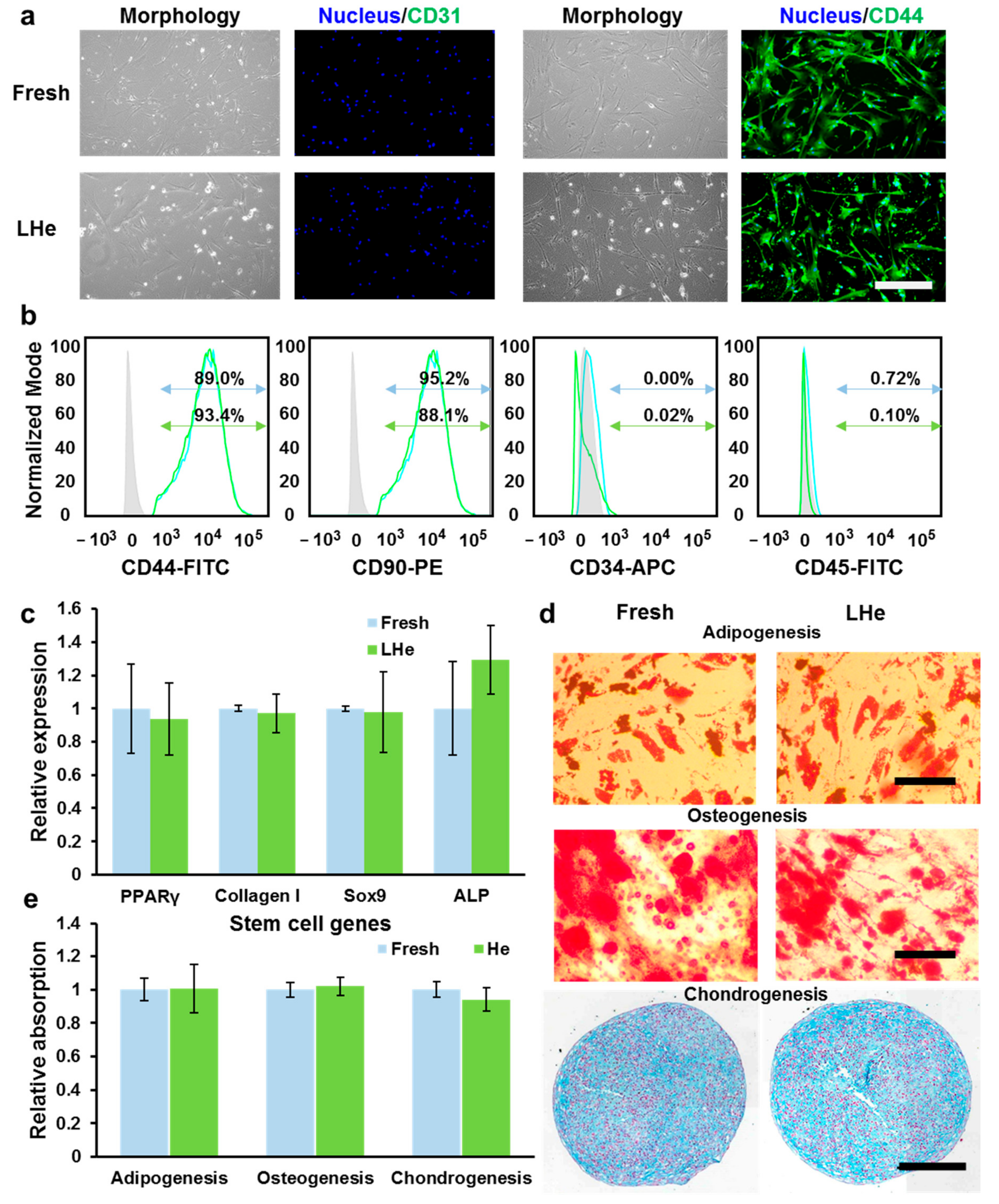
3.4. Stemness and Multilineage Differentiation of hBMSCs Vitrified in Liquid Helium
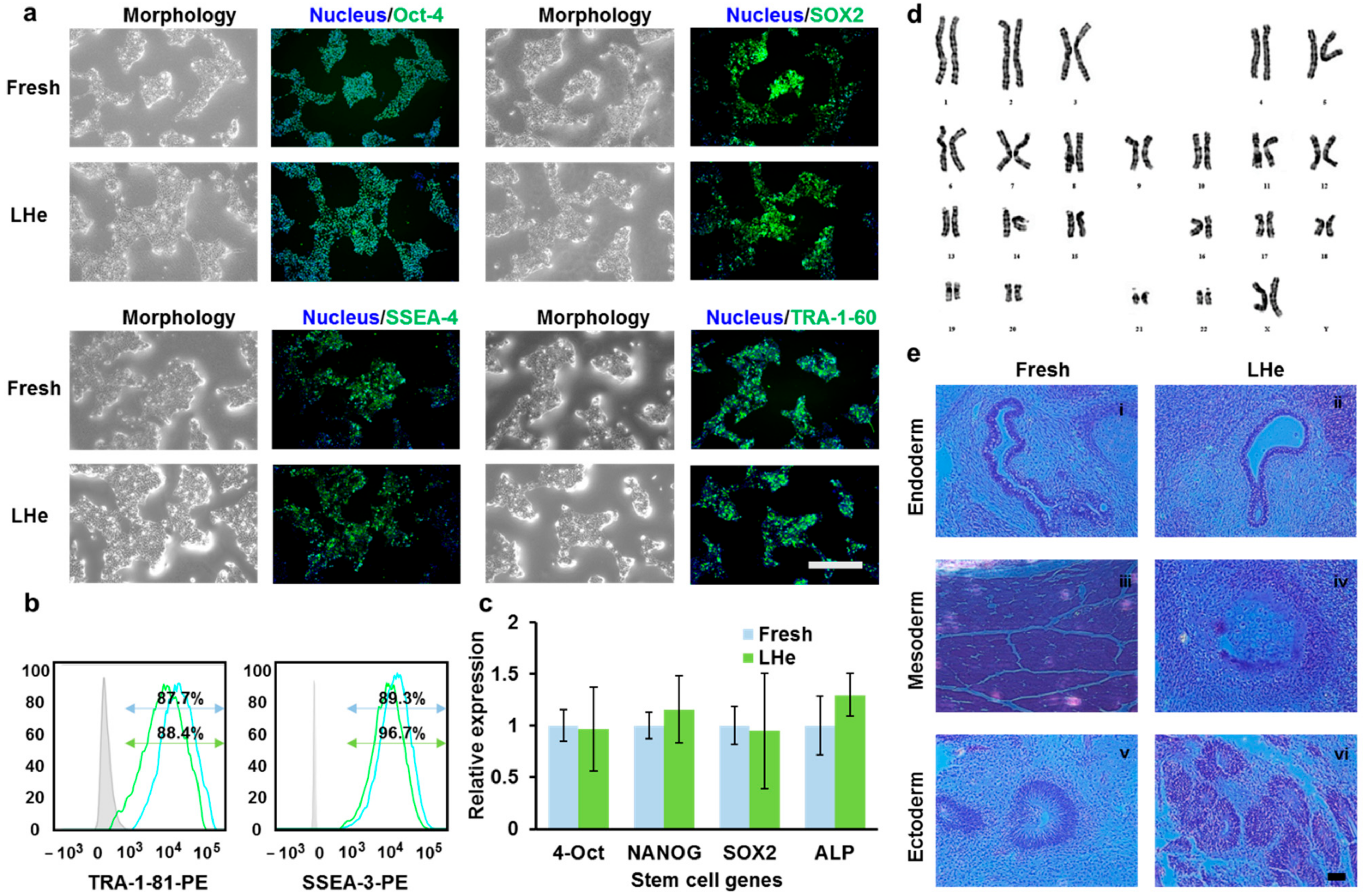
3.5. Pluripotency of Vitrified hESCs
4. Conclusions
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Data Availability Statement
Acknowledgments
Conflicts of Interest
References
- Baraniak, P.R.; Mcdevitt, T.C. Stem cell paracrine actions and tissue regeneration. Regen. Med. 2010, 5, 121–143. [Google Scholar] [CrossRef] [Green Version]
- De Miguel, M.P.; Fuentes-Julián, S.; Blázquez-Martínez, A.; Pascual, C.Y.; Aller, M.A.; Arias, J.; Arnalich-Montiel, F. Immunosuppressive properties of mesenchymal stem cells: Advances and applications. Curr. Mol. Med. 2012, 12, 574–591. [Google Scholar] [CrossRef] [PubMed]
- Ha, S.Y.; Jee, B.C.; Suh, C.S.; Kim, H.S.; Oh, S.K.; Kim, S.H.; Moon, S.Y. Cryopreservation of human embryonic stem cells without the use of a programmable freezer. Hum. Reprod. 2005, 20, 1779. [Google Scholar] [CrossRef]
- Dou, M.; Lu, C.; Rao, W. Bioinspired materials and technology for advanced cryopreservation. Trends Biotechnol. 2021. [Google Scholar] [CrossRef] [PubMed]
- Fan, Q.; Dou, M.; Mao, J.; Hou, Y.; Liu, S.; Zhao, L.; Lv, J.; Liu, Z.; Wang, Y.; Rao, W.; et al. Strong Hydration Ability of Silk Fibroin Suppresses Formation and Recrystallization of Ice Crystals During Cryopreservation. Biomacromolecules 2021. [Google Scholar] [CrossRef]
- Coopman, K. Large-scale compatible methods for the preservation of human embryonic stem cells: Current perspectives. Biotechnol. Prog. 2011, 27, 1511–1521. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Hunt, C.J. Cryopreservation: Vitrification and Controlled Rate Cooling. Methods Mol. Biol. 2017, 1590, 41–77. [Google Scholar] [PubMed]
- Best, B.P. Cryoprotectant Toxicity: Facts, Issues, and Questions. Rejuvenation Res. 2015, 18, 422–436. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Shu, Z.; Heimfeld, S.; Gao, D. Hematopoietic SCT with cryopreserved grafts: Adverse reactions after transplantation and cryoprotectant removal before infusion. Bone Marrow Transplant. 2014, 49, 469–476. [Google Scholar] [CrossRef] [PubMed]
- Dou, M.; Lu, C.; Sun, Z.; Rao, W. Natural cryoprotectants combinations of l-proline and trehalose for red blood cells cryopreservation. Cryobiology 2019, 91, 23–29. [Google Scholar] [CrossRef]
- Dou, M.; Li, Y.; Sun, Z.; Li, L.; Rao, W. L-proline feeding for augmented freeze tolerance of Camponotus japonicus Mayr. Sci. Bull. 2019, 64, 1795–1804. [Google Scholar] [CrossRef] [Green Version]
- Deller, R.C.; Vatish, M.; Mitchell, D.A.; Gibson, M.I. Synthetic polymers enable non-vitreous cellular cryopreservation by reducing ice crystal growth during thawing. Nat. Commun. 2014, 5, 3244. [Google Scholar] [CrossRef] [Green Version]
- Hou, Y.; Lu, C.; Dou, M.; Zhang, C.; Chang, H.; Liu, J.; Rao, W. Soft liquid metal nanoparticles achieve reduced crystal nucleation and ultrarapid rewarming for human bone marrow stromal cell and blood vessel cryopreservation. Acta Biomater. 2020, 102, 403–415. [Google Scholar] [CrossRef]
- Manuchehrabadi, N.; Gao, Z.; Zhang, J.; Ring, H.L.; Shao, Q.; Liu, F.; McDermott, M.; Fok, A.; Rabin, Y.; Brockbank, K.G.; et al. Improved tissue cryopreservation using inductive heating of magnetic nanoparticles. Sci. Transl. Med. 2017, 9. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Khosla, K.; Wang, Y.; Hagedorn, M.; Qin, Z.; Bischof, J. Gold Nanorod Induced Warming of Embryos from the Cryogenic State Enhances Viability. ACS Nano 2017, 11, 7869–7878. [Google Scholar] [CrossRef] [PubMed]
- Chen, J.-Y.; Li, X.-X.; Xu, Y.-K.; Wu, H.; Zheng, J.-J.; Yu, X.-L. Developmental competence and gene expression of immature oocytes following liquid helium vitrification in bovine. Cryobiology 2014, 69, 428–433. [Google Scholar] [CrossRef]
- Saragusty, J.; Arav, A. Current progress in oocyte and embryo cryopreservation by slow freezing and vitrification. Reproduction 2011, 141, 1–19. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Suzuki, N.; Yoshioka, N.; Takae, S.; Sugishita, Y.; Tamura, M.; Hashimoto, S.; Morimoto, Y.; Kawamura, K. Successful fertility preservation following ovarian tissue vitrification in patients with primary ovarian insufficiency. Hum. Reprod. 2015, 30, 608–615. [Google Scholar] [CrossRef] [Green Version]
- Sansinena, M.; Santos, M.V.; Zaritzky, N.; Chirife, J. Comparison of heat transfer in liquid and slush nitrogen by numerical simulation of cooling rates for French straws used for sperm cryopreservation. Theriogenology 2012, 77, 1717–1721. [Google Scholar] [CrossRef] [Green Version]
- Ugraitskaya, S.V.; Shishova, N.V.; Gagarinskiy, E.L.; Shvirst, N.E.; Fesenko, E. The Effect of Helium on Cryopreservation of HeLa and L929 Cells. Biophysics 2018, 63, 387–392. [Google Scholar] [CrossRef]
- Li, J.; Meng, Q.; Ouyang, Z.; Shi, L.; Ai, X.; Chen, X. Iop, “Helium recovery and purification at CHMFL”. In Proceedings of the 26th International Cryogenic Engineering Conference/International Cryogenic Materials Conference, New Delhi, India, 7–11 March 2016. [Google Scholar]
- Guo, X.F.; Yu, X.L.; Zhang, F.; Wu, H.; Pei, X.Z.; Li, X.X.; Li, Y.H. Effect of liquid helium vitrification on cytoskeleton of immature cattle oocytes. Anim. Reprod. Sci. 2017, 187, 91–99. [Google Scholar] [CrossRef] [PubMed]
- Sansinena, M.; Santos, M.V.; Zaritzky, N.; Baeza, R.; Chirife, J. Theoretical prediction of the effect of heat transfer parameters on cooling rates of liquid-filled plastic straws used for cryopreservation of spermatozoa. CryoLetters 2010, 31, 120–129. [Google Scholar]
- Santos, M.V.; Sansinena, M.; Zaritzky, N.; Chirife, J. Assessment of external heat transfer coefficient during oocyte vitrification in liquid and slush nitrogen using numerical simulations to determine cooling rates. CryoLetters 2012, 33, 31–40. [Google Scholar] [PubMed]
- Deev, V.I.; Keilin, V.E.; Kovalev, I.A.; Kondratenko, A.K.; Petrovichev, V.I. Nucleate and film pool boiling heat transfer to saturated liquid helium. Cryogenics 1977, 17, 562–577. [Google Scholar] [CrossRef]
- Zhang, W.; Zhao, S.; Rao, W.; Snyder, J.; Choi, J.K.; Wang, J.; Khan, I.A.; Saleh, N.B.; Mohler, P.J.; Yu, J.; et al. A Novel Core-Shell Microcapsule for Encapsulation and 3D Culture of Embryonic Stem Cells. J. Mater. Chem. B 2013, 2013, 1002–1009. [Google Scholar] [CrossRef]
- Huang, H.; He, X.; Yarmush, M.L. Advanced technologies for the preservation of mammalian biospecimens. Nat. Biomed. Eng. 2021, 5, 793–804. [Google Scholar] [CrossRef]

| Cell Types | Equilibration Solution | Vitrification Solution | ||
|---|---|---|---|---|
| hBMSCs | EFS15 | 15%EG-DPBS containing 20% FBS | EFS30 | 30%EG + 18% Ficoll 70 + 0.3 sucrose-DPBS containing 20% FBS |
| EFS17.5 | 17.5%EG-DPBS containing 20% FBS | EFS35 | 35%EG + 18% Ficoll 70 + 0.3 sucrose-DPBS containing 20% FBS | |
| EFS20 | 20%EG-DPBS containing 20% FBS | EFS40 | 40%EG + 18% Ficoll 70 + 0.3 sucrose-DPBS containing 20% FBS | |
| hESCs | EDS15 | 7.5%EG + 7.5%DMSO-culture medium | EDS30 | 15%EG + 15%DMSO + 0.5M sucrose-culture medium |
| EDS17.5 | 8.75%EG + 8.75%DMSO-culture medium | EDS35 | 17.5%EG + 17.5%DMSO + 0.5M sucrose-culture medium | |
| EDS20 | 10%EG + 10%DMSO-culture medium | EDS40 | 20%EG + 20%DMSO + 0.5M sucrose-culture medium | |
| Cells | Antigens | Primary Antibody | Secondary Antibody |
|---|---|---|---|
| hBMSCs | Surface antigens | Rabbit anti-CD44 (Abcam) (ab189524) | AF 488 Goat anti-rabbit (Abcam) (ab150077) |
| Mouse anti-CD31 (Abcam) (ab24590) | AF 488 Goat anti-mouse (Abcam) (ab150113) | ||
| hESCs | Intranuclear antigens | Rabbit anti-Oct-4 (Signalway Antibody) (#49129) | Mouse anti-rabbit IgG FITC (Bioss Antibodies) (bs-0295M-FITC) |
| Mouse anti-SOX2 (Santa cruz Biotechnology) (sc-365823) | Anti-mouse IgGκ BP-FITC (Santa cruz Biotechnology) (sc-516140) | ||
| Surface antigens | Mouse anti-SSEA-4 (Santa cruz Biotechnology) (sc-21704) | ||
| Mouse anti-TRA-1-60 (Santa cruz Biotechnology) (sc-21705) |
| Cell Types | Gene | Primer | |
|---|---|---|---|
| hBMSCs | PPARγ | Sense primer | 5′-TCT CTC CGT AAT GGA AGA CC-3′ |
| Anti-sense primer | 5′-GCA TTA TGA GAC ATC CCC AC-3′ | ||
| Collagen type I | Sense primer | 5′-GGG CAA GAC AGT GAT TGA ATA CA-3′ | |
| Anti-sense primer | 5′-GGA TGG AGG GAG TTT ACA GGA A-3′ | ||
| Sox9 | Sense primer | 5′-GCA GAG ACT GAA GAC CCT ACA CAG A-3′ | |
| Anti-sense primer | 5′-GAG GCA ACT TCA CGC TGC AA-3′ | ||
| ALP | Sense primer | 5′-GCA AGA GCA CAA AGA GAA GAG-3′ | |
| Anti-sense primer | 5′-AAG GGG TCT ACA TGG CAA CT-3′ | ||
| GAPDH | Sense primer | 5′-GCA AGA GCA CAA AGA GAA GAG-3′ | |
| Anti-sense primer | 5′-AAG GGG TCT ACA TGG CAA CT-3′ | ||
| hESCs | Oct-4 | Sense primer | 5′-CTTGCTGCAGAAGTGGGTGGAGGAA-3′ |
| Anti-sense primer | 5′-CTGVCAGTGTGGGTTTCGGGCA-3′ | ||
| NANOG | Sense primer | 5′-CAACTGGCCGAAGAATAGCAATGGTAT-3′ | |
| Anti-sense primer | 5′-AAGGCAAGTCAGCAGCCATCTCAT-3′ | ||
| SOX2 | Sense primer | 5′-GCCTGGGCGCCAAGTAGA-3′ | |
| Anti-sense primer | 5′-GAACGAGCCGTTCATGTAGTCTG-3′ | ||
| GAPDH | Sense primer | 5′-AGCCACATCGCTCAGACACC-3′ | |
| Anti-sense primer | 5′-AAGCTCTGTGGGACCTCTTG-3′ | ||
Publisher’s Note: MDPI stays neutral with regard to jurisdictional claims in published maps and institutional affiliations. |
© 2021 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Dou, M.; Lu, C.; Liu, J.; Rao, W. Liquid Helium Enhanced Vitrification Efficiency of Human Bone-Derived Mesenchymal Stem Cells and Human Embryonic Stem Cells. Bioengineering 2021, 8, 162. https://doi.org/10.3390/bioengineering8110162
Dou M, Lu C, Liu J, Rao W. Liquid Helium Enhanced Vitrification Efficiency of Human Bone-Derived Mesenchymal Stem Cells and Human Embryonic Stem Cells. Bioengineering. 2021; 8(11):162. https://doi.org/10.3390/bioengineering8110162
Chicago/Turabian StyleDou, Mengjia, Chennan Lu, Jing Liu, and Wei Rao. 2021. "Liquid Helium Enhanced Vitrification Efficiency of Human Bone-Derived Mesenchymal Stem Cells and Human Embryonic Stem Cells" Bioengineering 8, no. 11: 162. https://doi.org/10.3390/bioengineering8110162
APA StyleDou, M., Lu, C., Liu, J., & Rao, W. (2021). Liquid Helium Enhanced Vitrification Efficiency of Human Bone-Derived Mesenchymal Stem Cells and Human Embryonic Stem Cells. Bioengineering, 8(11), 162. https://doi.org/10.3390/bioengineering8110162







