Bioengineering of Extracellular Vesicles: Exosome-Based Next-Generation Therapeutic Strategy in Cancer
Abstract
1. Introduction
2. Biogenesis, Structure, and Composition of Exosomes
3. Exosomes in Cancer Regulation
3.1. The Protumorigenic Activity of Exosomes
3.2. The Antitumorigenic Activity of Exosomes
4. Exosomes—A Tool in Cancer Management
4.1. Exosomal Isolation Methods
4.1.1. Ultracentrifugation
4.1.2. Ultrafiltration
4.1.3. Size Exclusion Chromatography
4.1.4. Immuno-Affinity Capture
4.1.5. Polymer-Based Precipitation
| Method | Principle | Advantage | Disadvantage | Reference |
|---|---|---|---|---|
| Ultracentrifugation | The constituents are separated based on their density and size | High yielding capacity, cost effective, low risk of contamination | Damage prone due to high speed, requires special equipment, time consuming | [58] |
| Ultrafiltration | Different exosomes are separated based on their size | Fast, cost effective, no need for special equipment, reduced labor | Low purity | [59] |
| Size exclusion chromatography | Different exosomes are separated based on their size | High purity, biological activity is preserved | Moderate cost, requires special equipment | [60] |
| Immunoaffinity capture | Exosomes are separated based on their membrane-bound protein and receptors. | High purity, isolation of ligand specific exosome | Specific ligands need to be established, yield and capacity are low, receptor may be blocked | [61] |
| Polymer-based precipitation | Exosomes are precipitated using a water excluding polymer | Possibility for kit-based isolation, user friendly, no requirement of special equipment | Risk of contamination is high, similar to proteins. | [62] |
4.2. Exosomal Incorporation Methods
4.2.1. Simple Incubation
4.2.2. Electroporation
4.2.3. Saponin Permeabilization
4.2.4. Sonication
4.2.5. Extrusion
4.2.6. Freeze–Thaw Cycles
4.2.7. Incubation of Donor Cells
4.2.8. Transfection
4.2.9. Chemical Conjugation
| Type of Strategies | Functional Utility | Advantage | Disadvantage | Reference |
|---|---|---|---|---|
| Incubation | Incorporate drugs, nucleic acids, proteins, peptides, nanomaterials | Easiest way of cargo loading | Loading efficiency is low, inserted cargoes are difficult to manipulate | [68] |
| Surfactant treatment | Incorporate proteins, peptides, nanomaterials | High loading efficiency | Damage exosome integrity, inactivate loaded cargo | [68] |
| Electroporation | Helps to incorporate drugs, nucleic acids, proteins, peptides, nanomaterials | High loading efficiency | Cargo aggregation | [69] |
| Sonication | Incorporate drugs, proteins, peptides, nanomaterials | High loading efficiency | Damages exosome integrity | [71] |
| Extrusion | Incorporate drugs | High loading efficiency | Alters the immune status of exosome | [72] |
| Freeze–thaw | Incorporate proteins, peptides | High loading efficiency | Cargo aggregation, protein inactivation | [73] |
| Transfection | Incorporate nucleic acids, proteins, peptides | High loading efficiency for nucleic acids, proteins, and peptides; stable | Cargo sorting is difficult to manipulate | [75] |
5. Bioengineering of Exosomes
5.1. ncRNAs
5.1.1. lncRNAs
5.1.2. miRNAs
5.1.3. siRNAs
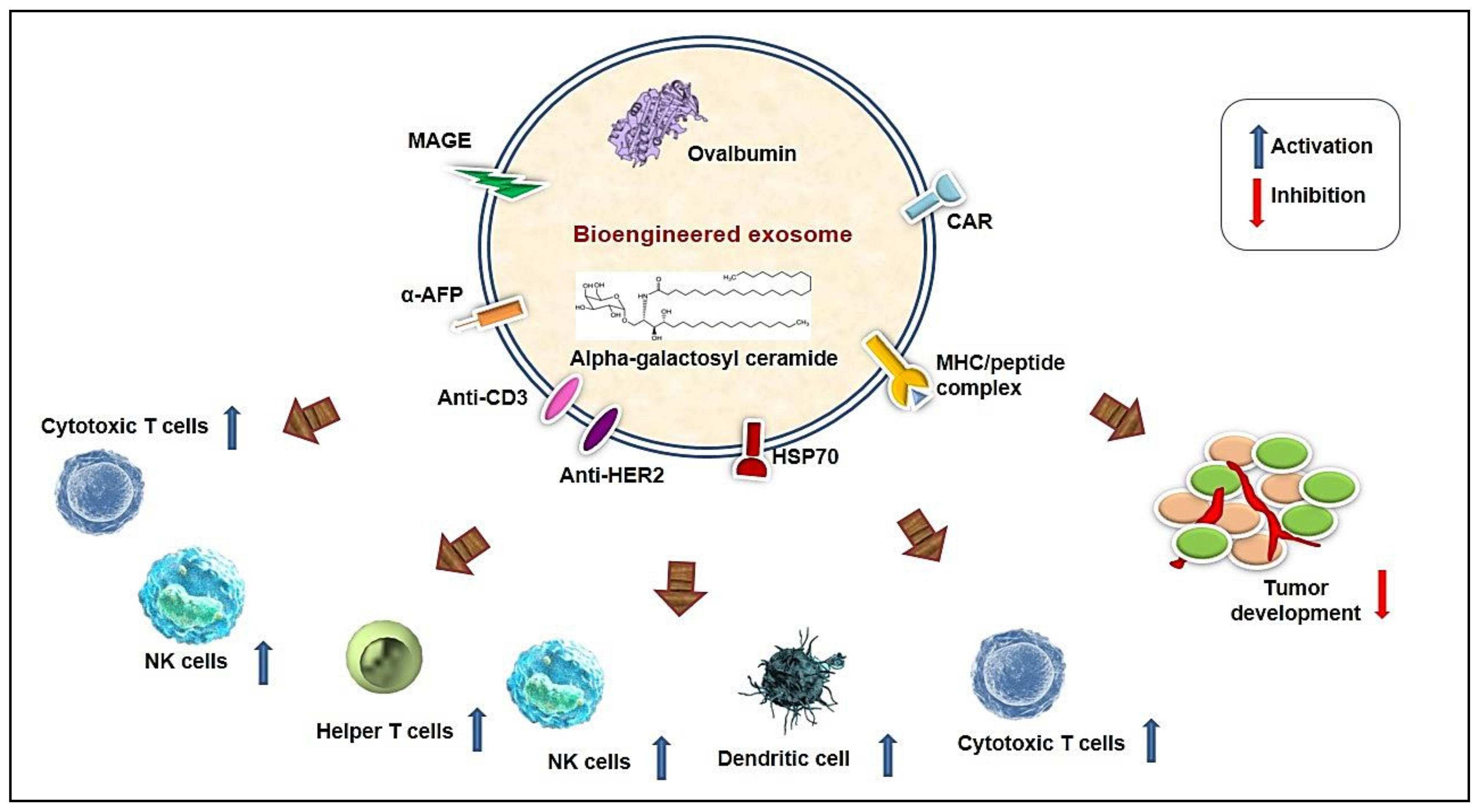
5.2. Bioengineered Exosome-Based Immune Modulation
5.2.1. Lymphocytes
5.2.2. Dendritic Cells (DC)
5.2.3. Macrophages
5.2.4. Indirect Bioengineering of Exosomes for Immune Modulation
5.3. Chemotherapy
5.4. Exosomal Delivery of Small Molecules
5.4.1. Natural Phytochemicals
5.4.2. Other Small Molecules
5.5. Recombinant Protein
5.6. Fusogenic Exosome
5.7. Vexosomes (Vector Exosomes)
6. Future Prospects and Conclusions
Author Contributions
Funding
Acknowledgments
Conflicts of Interest
References
- Zhang, Y.; Liu, Y.; Liu, H.; Tang, W.H. Exosomes: Biogenesis, biologic function and clinical potential. Cell Biosci. 2019, 9, 19. [Google Scholar] [CrossRef]
- Meng, W.; He, C.; Hao, Y.; Wang, L.; Li, L.; Zhu, G. Prospects and challenges of extracellular vesicle-based drug delivery system: Considering cell source. Drug Deliv. 2020, 27, 585–598. [Google Scholar] [CrossRef]
- Mishra, A.; Singh, P.; Qayoom, I.; Prasad, A.; Kumar, A. Current strategies in tailoring methods for engineered exosomes and future avenues in biomedical applications. J. Mater. Chem. B 2021, 9, 6281–6309. [Google Scholar] [CrossRef] [PubMed]
- Han, C.; Sun, X.; Liu, L.; Jiang, H.; Shen, Y.; Xu, X.; Li, J.; Zhang, G.; Huang, J.; Lin, Z.; et al. Exosomes and their therapeutic potentials of stem cells. Stem Cells Int. 2016, 2016, 7653489. [Google Scholar] [CrossRef] [PubMed]
- Sinha, D.; Roy, S.; Saha, P.; Chatterjee, N.; Bishayee, A. Trends in Research on Exosomes in Cancer Progression and Anticancer Therapy. Cancers 2021, 13, 326. [Google Scholar] [CrossRef] [PubMed]
- Kalluri, R.; LeBleu, V.S. The biology, function, and biomedical applications of exosomes. Science 2020, 367, eaau6977. [Google Scholar] [CrossRef] [PubMed]
- Dai, J.; Su, Y.; Zhong, S.; Cong, L.; Liu, B.; Yang, J.; Tao, Y.; He, Z.; Chen, C.; Jiang, Y. Exosomes: Key players in cancer and potential therapeutic strategy. Signal Transduct. Target. Ther. 2020, 5, 1–10. [Google Scholar] [CrossRef] [PubMed]
- Chen, H.; Chengalvala, V.; Hu, H.; Sun, D. Tumor-derived exosomes: Nanovesicles made by cancer cells to promote cancer metastasis. Acta Pharm. Sin. B 2021, 11, 2136–2149. [Google Scholar] [CrossRef]
- De la Torre Gomez, C.; Goreham, R.V.; Bech Serra, J.J.; Nann, T.; Kussmann, M. “Exosomics”-A review of biophysics, biology and biochemistry of exosomes with a focus on human breast milk. Front. Genet. 2018, 9, 92. [Google Scholar] [CrossRef]
- Bondhopadhyay, B.; Sisodiya, S.; Alzahrani, F.A.; Bakhrebah, M.A.; Chikara, A.; Kasherwal, V.; Khan, A.; Rani, J.; Dar, S.A.; Akhter, N.; et al. Exosomes: A Forthcoming Era of Breast Cancer Therapeutics. Cancers 2021, 13, 4672. [Google Scholar] [CrossRef]
- Sun, Y.; Zheng, W.; Guo, Z.; Ju, Q.; Zhu, L.; Gao, J.; Zhou, L.; Liu, F.; Xu, Y.; Zhan, Q.; et al. A novel TP53 pathway influences the HGS-mediated exosome formation in colorectal cancer. Sci. Rep. 2016, 6, 28083. [Google Scholar] [CrossRef] [PubMed]
- Tamkovich, S.N.; Tutanov, O.S.; Laktionov, P.P. Exosomes: Generation, structure, transport, biological activity, and diagnostic application. Biochem. Suppl. Ser. A Membr. Cell Biol. 2016, 10, 163–173. [Google Scholar] [CrossRef]
- Simons, M.; Raposo, G. Exosomes - vesicular carriers for intercellular communication. Curr. Opin. Cell Biol. 2009, 21, 575–581. [Google Scholar] [CrossRef] [PubMed]
- Hessvik, N.P.; Llorente, A. Current knowledge on exosome biogenesis and release. Cell. Mol. Life Sci. 2018, 75, 193. [Google Scholar] [CrossRef] [PubMed]
- Kowal, J.; Tkach, M.; Théry, C. Biogenesis and secretion of exosomes. Curr. Opin. Cell Biol. 2014, 29, 116–125. [Google Scholar] [CrossRef] [PubMed]
- Mathivanan, S.; Fahner, C.J.; Reid, G.E.; Simpson, R.J. ExoCarta 2012: Database of exosomal proteins, RNA and lipids. Nucleic Acids Res. 2012, 40, D1241–D1244. [Google Scholar] [CrossRef] [PubMed]
- Beach, A.; Zhang, H.-G.; Ratajczak, M.Z.; Kakar, S.S. Exosomes: An overview of biogenesis, composition and role in ovarian cancer. J. Ovarian Res. 2014, 7, 1–10. [Google Scholar] [CrossRef]
- Hsu, C.; Morohashi, Y.; Yoshimura, S.; Manrique-Hoyos, N.; Jung, S.; Lauterbach, M.A.; Bakhti, M.; Grønborg, M.; Möbius, W.; Rhee, J.; et al. Regulation of exosome secretion by Rab35 and its GTPase-activating proteins TBC1D10A–C. J. Cell Biol. 2010, 189, 223. [Google Scholar] [CrossRef]
- Ostrowski, M.; Carmo, N.B.; Krumeich, S.; Fanget, I.; Raposo, G.; Savina, A.; Moita, C.F.; Schauer, K.; Hume, A.N.; Freitas, R.P.; et al. Rab27a and Rab27b control different steps of the exosome secretion pathway. Nat. Cell Biol. 2010, 12, 19–30. [Google Scholar] [CrossRef]
- Wilcke, M.; Johannes, L.; Galli, T.; Mayau, V.; Goud, B.; Salamero, J. Rab11 regulates the compartmentalization of early endosomes required for efficient transport from early endosomes to the trans-Golgi network. J. Cell Biol. 2000, 151, 1207–1220. [Google Scholar] [CrossRef]
- Nazarenko, I.; Rana, S.; Baumann, A.; McAlear, J.; Hellwig, A.; Trendelenburg, M.; Lochnit, G.; Preissner, K.T.; Zöller, M. Cell surface tetraspanin Tspan8 contributes to molecular pathways of exosome-induced endothelial cell activation. Cancer Res. 2010, 70, 1668–1678. [Google Scholar] [CrossRef]
- Van Niel, G.; Charrin, S.; Simoes, S.; Romao, M.; Rochin, L.; Saftig, P.; Marks, M.S.; Rubinstein, E.; Raposo, G. The Tetraspanin CD63 Regulates ESCRT-Independent and -Dependent Endosomal Sorting during Melanogenesis. Dev. Cell 2011, 21, 708–721. [Google Scholar] [CrossRef]
- Laulagnier, K.; Motta, C.; Hamdi, S.; Roy, S.; Fauvelle, F.; Pageaux, J.F.; Kobayashi, T.; Salles, J.P.; Perret, B.; Bonnerot, C.; et al. Mast cell- and dendritic cell-derived display a specific lipid composition and an unusual membrane organization. Biochem. J. 2004, 380, 161–171. [Google Scholar] [CrossRef]
- Vlassov, A.V.; Magdaleno, S.; Setterquist, R.; Conrad, R. Exosomes: Current knowledge of their composition, biological functions, and diagnostic and therapeutic potentials. Biochim. Biophys. Acta Gen. Subj. 2012, 1820, 940–948. [Google Scholar] [CrossRef]
- Osaki, M.; Okada, F. Exosomes and their role in cancer progression. Yonago Acta Med. 2019, 62, 182–190. [Google Scholar] [CrossRef] [PubMed]
- Svensson, K.J.; Kucharzewska, P.; Christianson, H.C.; Sköld, S.; Löfstedt, T.; Johansson, M.C.; Mörgelin, M.; Bengzon, J.; Ruf, W.; Belting, M. Hypoxia triggers a proangiogenic pathway involving cancer cell microvesicles and PAR-2–mediated heparin-binding EGF signaling in endothelial cells. Proc. Natl. Acad. Sci. USA 2011, 108, 13147–13152. [Google Scholar] [CrossRef] [PubMed]
- Zhou, M.; Chen, J.; Zhou, L.; Chen, W.; Ding, G.; Cao, L. Pancreatic cancer derived exosomes regulate the expression of TLR4 in dendritic cells via miR-203. Cell. Immunol. 2014, 292, 65–69. [Google Scholar] [CrossRef] [PubMed]
- Luga, V.; Zhang, L.; Viloria-Petit, A.M.; Ogunjimi, A.A.; Inanlou, M.R.; Chiu, E.; Buchanan, M.; Hosein, A.N.; Basik, M.; Wrana, J.L. Exosomes mediate stromal mobilization of autocrine Wnt-PCP signaling in breast cancer cell migration. Cell 2012, 151, 1542–1556. [Google Scholar] [CrossRef] [PubMed]
- Costa-Silva, B.; Aiello, N.M.; Ocean, A.J.; Singh, S.; Zhang, H.; Thakur, B.K.; Becker, A.; Hoshino, A.; Mark, M.T.; Molina, H.; et al. Pancreatic cancer exosomes initiate pre-metastatic niche formation in the liver. Nat. Cell Biol. 2015, 17, 816–826. [Google Scholar] [CrossRef] [PubMed]
- Lu, H.; Bowler, N.; Harshyne, L.A.; Craig Hooper, D.; Krishn, S.R.; Kurtoglu, S.; Fedele, C.; Liu, Q.; Tang, H.Y.; Kossenkov, A.V.; et al. Exosomal αvβ6 integrin is required for monocyte M2 polarization in prostate cancer. Matrix Biol. 2018, 70, 20–35. [Google Scholar] [CrossRef]
- Li, X.; Wang, S.; Zhu, R.; Li, H.; Han, Q.; Zhao, R.C. Lung tumor exosomes induce a pro-inflammatory phenotype in mesenchymal stem cells via NFκB-TLR signaling pathway. J. Hematol. Oncol. 2016, 9, 42. [Google Scholar] [CrossRef] [PubMed]
- Huang, Z.; Feng, Y. Exosomes derived from hypoxic colorectal cancer cells promote angiogenesis through Wnt4-Induced β-catenin signaling in endothelial cells. Oncol. Res. 2017, 25, 651–661. [Google Scholar] [CrossRef]
- Zhang, H.; Deng, T.; Liu, R.; Bai, M.; Zhou, L.; Wang, X.; Li, S.; Wang, X.; Yang, H.; Li, J.; et al. Exosome-delivered EGFR regulates liver microenvironment to promote gastric cancer liver metastasis. Nat. Commun. 2017, 8, 15016. [Google Scholar] [CrossRef] [PubMed]
- Zhao, S.; Mi, Y.; Guan, B.; Zheng, B.; Wei, P.; Gu, Y.; Zhang, Z.; Cai, S.; Xu, Y.; Li, X.; et al. Tumor-derived exosomal miR-934 induces macrophage M2 polarization to promote liver metastasis of colorectal cancer. J. Hematol. Oncol. 2020, 13, 33. [Google Scholar] [CrossRef] [PubMed]
- Sakha, S.; Muramatsu, T.; Ueda, K.; Inazawa, J. Exosomal microRNA miR-1246 induces cell motility and invasion through the regulation of DENND2D in oral squamous cell carcinoma. Sci. Reports 2016, 6, 1–11. [Google Scholar] [CrossRef] [PubMed]
- Yin, X.; Zheng, X.; Liu, M.; Wang, D.; Sun, H.; Qiu, Y.; Chen, J.; Shi, B. Exosomal miR-663b targets Ets2-repressor factor to promote proliferation and the epithelial–mesenchymal transition of bladder cancer cells. Cell Biol. Int. 2020, 44, 958–965. [Google Scholar] [CrossRef]
- Lin, F.; Yin, H.-B.; Li, X.Y.; Zhu, G.M.; He, W.Y.; Gou, X. Bladder cancer cell-secreted exosomal miR-21 activates the PI3K/AKT pathway in macrophages to promote cancer progression. Int. J. Oncol. 2020, 56, 151–164. [Google Scholar] [CrossRef]
- Zhou, W.; Fong, M.Y.; Min, Y.; Somlo, G.; Liu, L.; Palomares, M.R.; Yu, Y.; Chow, A.; O’Connor, S.T.F.; Chin, A.R.; et al. Cancer-Secreted miR-105 destroys vascular endothelial barriers to promote metastasis. Cancer Cell 2014, 25, 501–515. [Google Scholar] [CrossRef]
- Tominaga, N.; Kosaka, N.; Ono, M.; Katsuda, T.; Yoshioka, Y.; Tamura, K.; Lötvall, J.; Nakagama, H.; Ochiya, T. Brain metastatic cancer cells release microRNA-181c-containing extracellular vesicles capable of destructing blood-brain barrier. Nat. Commun. 2015, 6, 6716. [Google Scholar] [CrossRef]
- Sun, S.; Chen, H.; Xu, C.; Zhang, Y.; Zhang, Q.; Chen, L.; Ding, Q.; Deng, Z. Exosomal miR-106b serves as a novel marker for lung cancer and promotes cancer metastasis via targeting PTEN. Life Sci. 2020, 244, 117297. [Google Scholar] [CrossRef]
- Li, Z.; Tao, Y.; Wang, X.; Jiang, P.; Li, J.; Peng, M.; Zhang, X.; Chen, K.; Liu, H.; Zhen, P.; et al. Tumor-secreted exosomal miR-222 promotes tumor progression via regulating P27 expression and re-localization in pancreatic cancer. Cell. Physiol. Biochem. 2018, 51, 610–629. [Google Scholar] [CrossRef] [PubMed]
- Li, Z.; Jiang, P.; Li, J.; Peng, M.; Zhao, X.; Zhang, X.; Chen, K.; Zhang, Y.; Liu, H.; Gan, L.; et al. Tumor-derived exosomal lnc-Sox2ot promotes EMT and stemness by acting as a ceRNA in pancreatic ductal adenocarcinoma. Oncogene 2018, 37, 3822–3838. [Google Scholar] [CrossRef] [PubMed]
- Pan, L.; Liang, W.; Fu, M.; Huang, Z.-h.; Li, X.; Zhang, W.; Zhang, P.; Qian, H.; Jiang, P.-c.; Xu, W.-r.; et al. Exosomes-mediated transfer of long noncoding RNA ZFAS1 promotes gastric cancer progression. J. Cancer Res. Clin. Oncol. 2017, 143, 991–1004. [Google Scholar] [CrossRef] [PubMed]
- Wang, J.; Lv, B.; Su, Y.; Wang, X.; Bu, J.; Yao, L. Exosome-mediated transfer of lncRNA HOTTIP promotes cisplatin resistance in gastric cancer cells by regulating HMGA1/miR-218 axis. Onco. Targets. Ther. 2019, 12, 11325–11338. [Google Scholar] [CrossRef]
- Li, R.; Wang, Y.; Zhang, X.; Feng, M.; Ma, J.; Li, J.; Yang, X.; Fang, F.; Xia, Q.; Zhang, Z.; et al. Exosome-mediated secretion of LOXL4 promotes hepatocellular carcinoma cell invasion and metastasis. Mol. Cancer 2019, 18, 18. [Google Scholar] [CrossRef]
- Wang, Y.; Qin, X.; Zhu, X.; Chen, W.; Zhang, J.; Chen, W. Oral cancer-derived exosomal NAP1 enhances cytotoxicity of natural killer cells via the IRF-3 pathway. Oral Oncol. 2018, 76, 34–41. [Google Scholar] [CrossRef]
- Shoae-Hassani, A.; Hamidieh, A.A.; Behfar, M.; Mohseni, R.; Mortazavi-Tabatabaei, S.A.; Asgharzadeh, S. NK Cell-derived Exosomes from NK Cells Previously Exposed to Neuroblastoma Cells Augment the Antitumor Activity of Cytokine-Activated NK Cells. J. Immunother. 2017, 40, 265–276. [Google Scholar] [CrossRef]
- Zheng, R.; Du, M.; Wang, X.; Xu, W.; Liang, J.; Wang, W.; Lv, Q.; Qin, C.; Chu, H.; Wang, M.; et al. Exosome-transmitted long non-coding RNA PTENP1 suppresses bladder cancer progression. Mol. Cancer 2018, 17, 143. [Google Scholar] [CrossRef]
- Liang, Y.; Zhang, D.; Li, L.; Xin, T.; Zhao, Y.; Ma, R.; Du, J. Exosomal microRNA-144 from bone marrow-derived mesenchymal stem cells inhibits the progression of non-small cell lung cancer by targeting CCNE1 and CCNE2. Stem Cell Res. Ther. 2020, 11, 87. [Google Scholar] [CrossRef]
- Shi, H.; Li, H.; Zhen, T.; Dong, Y.; Pei, X.; Zhang, X. The Potential Therapeutic Role of Exosomal MicroRNA-520b Derived from Normal Fibroblasts in Pancreatic Cancer. Mol. Ther. Nucleic Acids 2020, 20, 373–384. [Google Scholar] [CrossRef]
- Jeong, K.; Yu, Y.J.; You, J.Y.; Rhee, W.J.; Kim, J.A. Exosome-mediated microRNA-497 delivery for anti-cancer therapy in a microfluidic 3D lung cancer model. Lab Chip 2020, 20, 548–557. [Google Scholar] [CrossRef]
- Chen, W.; Quan, Y.; Fan, S.; Wang, H.; Liang, J.; Huang, L.; Chen, L.; Liu, Q.; He, P.; Ye, Y. Exosome-transmitted circular RNA hsa_circ_0051443 suppresses hepatocellular carcinoma progression. Cancer Lett. 2020, 475, 119–128. [Google Scholar] [CrossRef]
- Zaharie, F.; Muresan, M.S.; Petrushev, B.; Berce, C.; Gafencu, G.A.; Selicean, S.; Jurj, A.; Cojocneanu-Petric, R.; Lisencu, C.I.; Pop, L.A.; et al. Exosome-carried microRNA-375 inhibits cell progression and dissemination via Bcl-2 blocking in colon cancer. J. Gastrointest. Liver Dis. 2015, 24, 435–443. [Google Scholar] [CrossRef]
- Yoon, J.H.; Ashktorab, H.; Smoot, D.T.; Nam, S.W.; Hur, H.; Park, W.S. Uptake and tumor-suppressive pathways of exosome-associated GKN1 protein in gastric epithelial cells. Gastric Cancer 2020, 23, 848–862. [Google Scholar] [CrossRef] [PubMed]
- Xu, G.; Zhang, B.; Ye, J.; Cao, S.; Shi, J.; Zhao, Y.; Wang, Y.; Sang, J.; Yao, Y.; Guan, W.; et al. Exosomal miRNA-139 in cancer-associated fibroblasts inhibits gastric cancer progression by repressing mmp11 expression. Int. J. Biol. Sci. 2019, 15, 2320–2329. [Google Scholar] [CrossRef] [PubMed]
- Nam, G.H.; Choi, Y.; Kim, G.B.; Kim, S.; Kim, S.A.; Kim, I.S. Emerging Prospects of Exosomes for Cancer Treatment: From Conventional Therapy to Immunotherapy. Adv. Mater. 2020, 32, 1–37. [Google Scholar] [CrossRef] [PubMed]
- Muralikumar, M. Current understanding of the mesenchymal stem cell-derived exosomes in cancer and aging. Biotechnol. Reports 2021, 31, 1–13. [Google Scholar] [CrossRef] [PubMed]
- Li, P.; Kaslan, M.; Lee, S.H.; Yao, J.; Gao, Z. Progress in Exosome Isolation Techniques. Theranostics 2017, 7, 789–804. [Google Scholar] [CrossRef]
- Yu, L.L.; Zhu, J.; Liu, J.X.; Jiang, F.; Ni, W.K.; Qu, L.S.; Ni, R.Z.; Lu, C.H.; Xiao, M.B. A Comparison of Traditional and Novel Methods for the Separation of Exosomes from Human Samples. Biomed Res. Int. 2018, 2018, 1–10. [Google Scholar] [CrossRef] [PubMed]
- Sidhom, K.; Obi, P.O.; Saleem, A. A review of exosomal isolation methods: Is size exclusion chromatography the best option? Int. J. Mol. Sci. 2020, 21, 6466. [Google Scholar] [CrossRef]
- Greening, D.W.; Xu, R.; Ji, H.; Tauro, B.J.; Simpson, R.J. A Protocol for Exosome Isolation and Characterization: Evaluation of Ultracentrifugation, Density-Gradient Separation, and Immunoaffinity Capture Methods. Methods Mol. Biol. 2015, 1295, 179–209. [Google Scholar] [CrossRef] [PubMed]
- PN, B.; H, Y. Polymer-Based Purification of Extracellular Vesicles. Methods Mol. Biol. 2017, 1660, 91–103. [Google Scholar] [CrossRef]
- Peng, H.; Ji, W.; Zhao, R.; Yang, J.; Lu, Z.; Li, Y.; Zhang, X. Exosome: A significant nano-scale drug delivery carrier. J. Mater. Chem. B 2020, 8, 7591–7608. [Google Scholar] [CrossRef]
- Franzen, C.A.; Simms, P.E.; Van Huis, A.F.; Foreman, K.E.; Kuo, P.C.; Gupta, G.N. Characterization of uptake and internalization of exosomes by bladder cancer cells. Biomed Res. Int. 2014, 2014, 1–12. [Google Scholar] [CrossRef] [PubMed]
- Feng, D.; Zhao, W.L.; Ye, Y.Y.; Bai, X.C.; Liu, R.Q.; Chang, L.F.; Zhou, Q.; Sui, S.F. Cellular internalization of exosomes occurs through phagocytosis. Traffic 2010, 11, 675–687. [Google Scholar] [CrossRef] [PubMed]
- Gonda, A.; Moyron, R.; Kabagwira, J.; Vallejos, P.A.; Wall, N.R. Cellular-Defined Microenvironmental Internalization of Exosomes. In Extracellular Vesicles and Their Importance in Human Health; IntechOpen: London, UK, 2019. [Google Scholar] [CrossRef]
- Susa, F.; Limongi, T.; Dumontel, B.; Vighetto, V.; Cauda, V. Engineered extracellular vesicles as a reliable tool in cancer nanomedicine. Cancers 2019, 11, 1979. [Google Scholar] [CrossRef]
- Fu, S.; Wang, Y.; Xia, X.; Zheng, J.C. Exosome engineering: Current progress in cargo loading and targeted delivery. NanoImpact 2020, 20, 1–20. [Google Scholar] [CrossRef]
- Yang, Z.; Shi, J.; Xie, J.; Wang, Y.; Sun, J.; Liu, T.; Zhao, Y.; Zhao, X.; Wang, X.; Ma, Y.; et al. Large-scale generation of functional mRNA-encapsulating exosomes via cellular nanoporation. Nat. Biomed. Eng. 2019, 4, 69–83. [Google Scholar] [CrossRef]
- Xu, M.; Yang, Q.; Sun, X.; Wang, Y. Recent Advancements in the Loading and Modification of Therapeutic Exosomes. Front. Bioeng. Biotechnol. 2020, 8, 1–12. [Google Scholar] [CrossRef]
- Lamichhane, T.N.; Jeyaram, A.; Patel, D.B.; Parajuli, B.; Livingston, N.K.; Arumugasaamy, N.; Schardt, J.S.; Jay, S.M. Oncogene Knockdown via Active Loading of Small RNAs into Extracellular Vesicles by Sonication. Cell. Mol. Bioeng. 2016, 9, 315–324. [Google Scholar] [CrossRef]
- Van Deun, J.; Roux, Q.; Deville, S.; Van Acker, T.; Rappu, P.; Miinalainen, I.; Heino, J.; Vanhaecke, F.; De Geest, B.G.; De Wever, O.; et al. Feasibility of Mechanical Extrusion to Coat Nanoparticles with Extracellular Vesicle Membranes. Cells 2020, 9, 1979. [Google Scholar] [CrossRef]
- Jeyaram, A.; Jay, S.M. Preservation and Storage Stability of Extracellular Vesicles for Therapeutic Applications. AAPS J. 2017, 20, 1–13. [Google Scholar] [CrossRef]
- Wang, X.; Zhang, H.; Yang, H.; Bai, M.; Ning, T.; Li, S.; Li, J.; Deng, T.; Ying, G.; Ba, Y. Cell-derived Exosomes as Promising Carriers for Drug Delivery and Targeted Therapy. Curr. Cancer Drug Targets 2018, 18, 347–354. [Google Scholar] [CrossRef]
- Xia, S. Drug loading techniques for exosome-based drug delivery systems. Pharmazie 2021, 76, 61–67. [Google Scholar] [CrossRef]
- Srivastava, A.; Babu, A.; Filant, J.; Moxley, K.M.; Ruskin, R.; Dhanasekaran, D.; Sood, A.K.; McMeekin, S.; Ramesh, R. Exploitation of exosomes as nanocarriers for gene-, chemo-, and immune-therapy of cancer. J. Biomed. Nanotechnol. 2016, 12, 1159–1173. [Google Scholar] [CrossRef]
- Born, L.J.; Harmon, J.W.; Jay, S.M. Therapeutic potential of extracellular vesicle-associated long noncoding RNA. Bioeng. Transl. Med. 2020, 5, 1–13. [Google Scholar] [CrossRef]
- Wang, M.; Zhou, L.; Yu, F.; Zhang, Y.; Li, P.; Wang, K. The functional roles of exosomal long non-coding RNAs in cancer. Cell. Mol. Life Sci. 2019, 76, 2059–2076. [Google Scholar] [CrossRef] [PubMed]
- Sun, Z.; Yang, S.; Zhou, Q.; Wang, G.; Song, J.; Li, Z.; Zhang, Z.; Xu, J.; Xia, K.; Chang, Y.; et al. Emerging role of exosome-derived long non-coding RNAs in tumor microenvironment. Mol. Cancer 2018, 17, 1–9. [Google Scholar] [CrossRef] [PubMed]
- Silva, M.; Melo, S.A. Non-coding RNAs in Exosomes: New Players in Cancer Biology. Curr. Genomics 2015, 16, 295–303. [Google Scholar] [CrossRef] [PubMed]
- Zhou, H.; Chen, A.; Shen, J.; Zhang, X.; Hou, M.; Li, J.; Chen, J.; Zou, H.; Zhang, Y.; Deng, Q.; et al. Long non-coding RNA LOC285194 functions as a tumor suppressor by targeting p53 in non-small cell lung cancer. Oncol. Rep. 2019, 41, 15–26. [Google Scholar] [CrossRef]
- Zhang, M.Y.; Zhang, Z.L.; Cui, H.X.; Wang, R.K.; Fu, L. Long non-coding RNA FENDRR inhibits NSCLC cell growth and aggressiveness by sponging miR-761. Eur. Rev. Med. Pharmacol. Sci. 2018, 22, 8324–8332. [Google Scholar] [CrossRef]
- Xu, J.; Su, C.; Zhao, F.; Tao, J.; Hu, D.; Shi, A.; Pan, J.; Zhang, Y. Paclitaxel promotes lung cancer cell apoptosis via MEG3-P53 pathway activation. Biochem. Biophys. Res. Commun. 2018, 504, 123–128. [Google Scholar] [CrossRef]
- Wu, W.; Chen, F.; Cui, X.; Yang, L.; Chen, J.; Zhao, J.; Huang, D.; Liu, J.; Yang, L.; Zeng, J.; et al. LncRNA NKILA suppresses TGF-β-induced epithelial–mesenchymal transition by blocking NF-κB signaling in breast cancer. Int. J. Cancer 2018, 143, 2213–2224. [Google Scholar] [CrossRef]
- Qiu, S.; Chen, G.; Peng, J.; Liu, J.; Chen, J.; Wang, J.; Li, L.; Yang, K. LncRNA EGOT decreases breast cancer cell viability and migration via inactivation of the Hedgehog pathway. FEBS Open Bio 2020, 10, 817–826. [Google Scholar] [CrossRef] [PubMed]
- Guo, W.; Jiang, H.; Li, H.; Li, F.; Yu, Q.; Liu, Y.; Jiang, W.; Zhang, M. LncRNA-SRA1 Suppresses Osteosarcoma Cell Proliferation While Promoting Cell Apoptosis. Technol. Cancer Res. Treat. 2019, 18, 1–11. [Google Scholar] [CrossRef] [PubMed]
- Mei, X.L.; Zhong, S.; Lyu, P. Long noncoding RNA LINC00520 prevents the progression of cutaneous squamous cell carcinoma through the inactivation of the PI3K/Akt signaling pathway by downregulating EGFR. Chin. Med. J. 2019, 132, 454–465. [Google Scholar] [CrossRef] [PubMed]
- Gilligan, K.E.; Dwyer, R.M. Engineering exosomes for cancer therapy. Int. J. Mol. Sci. 2017, 18, 1122. [Google Scholar] [CrossRef] [PubMed]
- Katakowski, M.; Buller, B.; Zheng, X.; Lu, Y.; Rogers, T.; Osobamiro, O.; Shu, W.; Jiang, F.; Chopp, M. Exosomes from marrow stromal cells expressing miR-146b inhibit glioma growth. Cancer Lett. 2013, 335, 201–204. [Google Scholar] [CrossRef]
- Liang, G.; Kan, S.; Zhu, Y.; Feng, S.; Feng, W.; Gao, S. Engineered exosome-mediated delivery of functionally active miR-26a and its enhanced suppression effect in HepG2 cells. Int. J. Nanomedicine 2018, 13, 585–599. [Google Scholar] [CrossRef]
- Akao, Y.; Iio, A.; Itoh, T.; Noguchi, S.; Itoh, Y.; Ohtsuki, Y.; Naoe, T. Microvesicle-mediated RNA molecule delivery system using monocytes/macrophages. Mol. Ther. 2011, 19, 395–399. [Google Scholar] [CrossRef]
- O’Brien, K.; Lowry, M.C.; Corcoran, C.; Martinez, V.G.; Daly, M.; Rani, S.; Gallagher, W.M.; Radomski, M.W.; MacLeod, R.A.F.; O’Driscoll, L. miR-134 in extracellular vesicles reduces triple-negative breast cancer aggression and increases drug sensitivity. Oncotarget 2015, 6, 32774–32789. [Google Scholar] [CrossRef]
- Lou, G.; Song, X.; Yang, F.; Wu, S.; Wang, J.; Chen, Z.; Liu, Y. Exosomes derived from MIR-122-modified adipose tissue-derived MSCs increase chemosensitivity of hepatocellular carcinoma. J. Hematol. Oncol. 2015, 8, 122. [Google Scholar] [CrossRef]
- Liang, G.; Zhu, Y.; Ali, D.J.; Tian, T.; Xu, H.; Si, K.; Sun, B.; Chen, B.; Xiao, Z. Engineered exosomes for targeted co-delivery of miR-21 inhibitor and chemotherapeutics to reverse drug resistance in colon cancer. J. Nanobiotechnology 2020, 18, 1–15. [Google Scholar] [CrossRef]
- Limoni, S.K.; Moghadam, M.F.; Moazzeni, S.M.; Gomari, H.; Salimi, F. Engineered Exosomes for Targeted Transfer of siRNA to HER2 Positive Breast Cancer Cells. Appl. Biochem. Biotechnol. 2019, 187, 352–364. [Google Scholar] [CrossRef]
- Li, J.; Zhao, Y.; Wang, J. Extracellular vesicle-associated microRNA-221-3p secreted by drug-resistant lung cancer cells targets HMBOX1 to promote the progression of lung cancer. Cancer Gene Ther. 2021, 28, 679–692. [Google Scholar] [CrossRef] [PubMed]
- Krebs, M.; Solimando, A.G.; Kalogirou, C.; Marquardt, A.; Frank, T.; Sokolakis, I.; Hatzichristodoulou, G.; Kneitz, S.; Bargou, R.; Kübler, H.; et al. Mir-221-3p regulates vegfr2 expression in high- risk prostate cancer and represents an escape mechanism from sunitinib in vitro. J. Clin. Med. 2020, 9, 670. [Google Scholar] [CrossRef] [PubMed]
- Zhou, Y.; Yuan, Y.; Liu, M.; Hu, X.; Quan, Y.; Chen, X. Tumor-specific delivery of KRAS siRNA with iRGD-exosomes efficiently inhibits tumor growth. ExRNA 2019, 1, 1–7. [Google Scholar] [CrossRef]
- Bai, J.; Duan, J.; Liu, R.; Du, Y.; Luo, Q.; Cui, Y.; Su, Z.; Xu, J.; Xie, Y.; Lu, W. Engineered targeting tLyp-1 exosomes as gene therapy vectors for efficient delivery of siRNA into lung cancer cells. Asian J. Pharm. Sci. 2020, 15, 461–471. [Google Scholar] [CrossRef] [PubMed]
- Bellavia, D.; Raimondo, S.; Calabrese, G.; Forte, S.; Cristaldi, M.; Patinella, A.; Memeo, L.; Manno, M.; Raccosta, S.; Diana, P.; et al. Interleukin 3- receptor targeted exosomes inhibit in vitro and in vivo chronic myelogenous Leukemia cell growth. Theranostics 2017, 7, 1333–1345. [Google Scholar] [CrossRef]
- Pi, F.; Binzel, D.W.; Lee, T.J.; Li, Z.; Sun, M.; Rychahou, P.; Li, H.; Haque, F.; Wang, S.; Croce, C.M.; et al. Nanoparticle Orientation to Control RNA Loading and Ligand Display on Extracellular Vesicles for Cancer Regression. Nat. Nanotechnol. 2018, 13, 82–89. [Google Scholar] [CrossRef]
- Yang, Z.; Xie, J.; Zhu, J.; Kang, C.; Chiang, C.; Wang, X.; Wang, X.; Kuang, T.; Chen, F.; Chen, Z.; et al. Functional exosome-mimic for delivery of siRNA to cancer: In vitro and in vivo evaluation. J. Control. Release 2016, 243, 160–171. [Google Scholar] [CrossRef] [PubMed]
- Pi, Y.N.; Xia, B.R.; Jin, M.Z.; Jin, W.L.; Lou, G. Exosomes: Powerful weapon for cancer nano-immunoengineering. Biochem. Pharmacol. 2021, 186, 114487. [Google Scholar] [CrossRef] [PubMed]
- Zhao, Z.; McGill, J.; Gamero-Kubota, P.; He, M. Microfluidic on-demand engineering of exosomes towards cancer immunotherapy. Lab Chip 2019, 19, 1877–1886. [Google Scholar] [CrossRef] [PubMed]
- Wahlgren, J.; Karlson, T.D.L.; Brisslert, M.; Vaziri Sani, F.; Telemo, E.; Sunnerhagen, P.; Valadi, H. Plasma exosomes can deliver exogenous short interfering RNA to monocytes and lymphocytes. Nucleic Acids Res. 2012, 40, e130. [Google Scholar] [CrossRef]
- Zhu, L.; Kalimuthu, S.; Gangadaran, P.; Oh, J.M.; Lee, H.W.; Baek, S.H.; Jeong, S.Y.; Lee, S.-W.; Lee, J.; Ahn, B.-C. Exosomes Derived From Natural Killer Cells Exert Therapeutic Effect in Melanoma. Theranostics 2017, 7, 2732. [Google Scholar] [CrossRef]
- Gehrmann, U.; Hiltbrunner, S.; Georgoudaki, A.-M.; Karlsson, M.C.; Näslund, T.I.; Gabrielsson, S. Synergistic Induction of Adaptive Antitumor Immunity by Codelivery of Antigen with α-Galactosylceramide on Exosomes. Cancer Res. 2013, 73, 3865–3876. [Google Scholar] [CrossRef]
- Xie, Y.; Bai, O.; Zhang, H.; Yuan, J.; Zong, S.; Chibbar, R.; Slattery, K.; Qureshi, M.; Wei, Y.; Deng, Y.; et al. Membrane-bound HSP70-engineered myeloma cell-derived exosomes stimulate more efficient CD8+ CTL- and NK-mediated antitumour immunity than exosomes released from heat-shocked tumour cells expressing cytoplasmic HSP70. J. Cell. Mol. Med. 2010, 14, 2655–2666. [Google Scholar] [CrossRef]
- Fu, W.; Lei, C.; Liu, S.; Cui, Y.; Wang, C.; Qian, K.; Li, T.; Shen, Y.; Fan, X.; Lin, F.; et al. CAR exosomes derived from effector CAR-T cells have potent antitumour effects and low toxicity. Nat. Commun. 2019, 10, 1–12. [Google Scholar] [CrossRef]
- Shi, X.; Cheng, Q.; Hou, T.; Han, M.; Smbatyan, G.; Lang, J.E.; Epstein, A.L.; Lenz, H.J.; Zhang, Y. Genetically Engineered Cell-Derived Nanoparticles for Targeted Breast Cancer Immunotherapy. Mol. Ther. 2020, 28, 536–547. [Google Scholar] [CrossRef]
- Delcayre, A.; Shu, H.; Le Pecq, J.B. Dendritic cell-derived exosomes in cancer immunotherapy: Exploiting nature’s antigen delivery pathway. Expert Rev. Anticancer Ther. 2005, 5, 537–547. [Google Scholar] [CrossRef]
- Escudier, B.; Dorval, T.; Chaput, N.; André, F.; Caby, M.P.; Novault, S.; Flament, C.; Leboulaire, C.; Borg, C.; Amigorena, S.; et al. Vaccination of metastatic melanoma patients with autologous dendritic cell (DC) derived-exosomes: Results of the first phase 1 clinical trial. J. Transl. Med. 2005, 3, 10. [Google Scholar] [CrossRef] [PubMed]
- Morse, M.A.; Garst, J.; Osada, T.; Khan, S.; Hobeika, A.; Clay, T.M.; Valente, N.; Shreeniwas, R.; Sutton, M.A.; Delcayre, A.; et al. A phase I study of dexosome immunotherapy in patients with advanced non-small cell lung cancer. J. Transl. Med. 2005, 3, 1–8. [Google Scholar] [CrossRef]
- Besse, B.; Charrier, M.; Lapierre, V.; Dansin, E.; Lantz, O.; Planchard, D.; Le Chevalier, T.; Livartoski, A.; Barlesi, F.; Laplanche, A.; et al. Dendritic cell-derived exosomes as maintenance immunotherapy after first line chemotherapy in NSCLC. Oncoimmunology 2016, 5, 1071008. [Google Scholar] [CrossRef] [PubMed]
- NM, M.; ME, K.-L.; J, D.; JG, A.; JP, H. Tumour-derived exosomes as antigen delivery carriers in dendritic cell-based immunotherapy for malignant mesothelioma. J. Extracell. vesicles 2013, 2, 22492. [Google Scholar] [CrossRef]
- Lu, Z.; Zuo, B.; Jing, R.; Gao, X.; Rao, Q.; Liu, Z.; Qi, H.; Guo, H.; Yin, H.F. Dendritic cell-derived exosomes elicit tumor regression in autochthonous hepatocellular carcinoma mouse models. J. Hepatol. 2017, 67, 739–748. [Google Scholar] [CrossRef]
- Viaud, S.; Terme, M.; Flament, C.; Taieb, J.; André, F.; Novault, S.; Escudier, B.; Robert, C.; Caillat-Zucman, S.; Tursz, T.; et al. Dendritic cell-derived exosomes promote natural killer cell activation and proliferation: A role for NKG2D ligands and IL-15Rα. PLoS One 2009, 4, e4942. [Google Scholar] [CrossRef]
- Xiao, L.; Erb, U.; Zhao, K.; Hackert, T.; Zöller, M. Efficacy of vaccination with tumor-exosome loaded dendritic cells combined with cytotoxic drug treatment in pancreatic cancer. Oncoimmunology 2017, 6, e1319044. [Google Scholar] [CrossRef]
- Cheng, L.; Wang, Y.; Huang, L. Exosomes from M1-Polarized Macrophages Potentiate the Cancer Vaccine by Creating a Pro-inflammatory Microenvironment in the Lymph Node. Mol. Ther. 2017, 25, 1665–1675. [Google Scholar] [CrossRef]
- Morishita, M.; Takahashi, Y.; Matsumoto, A.; Nishikawa, M.; Takakura, Y. Exosome-based tumor antigens–adjuvant co-delivery utilizing genetically engineered tumor cell-derived exosomes with immunostimulatory CpG DNA. Biomaterials 2016, 111, 55–65. [Google Scholar] [CrossRef]
- Xiao, W.; Dong, W.; Zhang, C.; Saren, G.; Geng, P.; Zhao, H.; Li, Q.; Zhu, J.; Li, G.; Zhang, S.; et al. Effects of the epigenetic drug MS-275 on the release and function of exosome-related immune molecules in hepatocellular carcinoma cells. Eur. J. Med. Res. 2013, 18, 61. [Google Scholar] [CrossRef]
- Wang, J.; Wang, L.; Lin, Z.; Tao, L.; Chen, M. More efficient induction of antitumor T cell immunity by exosomes from CD40L gene-modified lung tumor cells. Mol. Med. Rep. 2014, 9, 125–131. [Google Scholar] [CrossRef]
- Vulpis, E.; Cecere, F.; Molfetta, R.; Soriani, A.; Fionda, C.; Peruzzi, G.; Caracciolo, G.; Palchetti, S.; Masuelli, L.; Simonelli, L.; et al. Genotoxic stress modulates the release of exosomes from multiple myeloma cells capable of activating NK cell cytokine production: Role of HSP70/TLR2/NF-kB axis. Oncoimmunology 2017, 6, e1279372. [Google Scholar] [CrossRef]
- Gebeyehu, A.; Kommineni, N.; Meckes, D.G.; Sachdeva, M.S. Role of Exosomes for Delivery of Chemotherapeutic Drugs. Crit. Rev. Ther. Drug Carrier Syst. 2021, 38, 53–97. [Google Scholar] [CrossRef]
- Ha, D.; Yang, N.; Nadithe, V. Exosomes as therapeutic drug carriers and delivery vehicles across biological membranes: Current perspectives and future challenges. Acta Pharm. Sin. B 2016, 6, 287–296. [Google Scholar] [CrossRef] [PubMed]
- Kim, M.S.; Haney, M.J.; Zhao, Y.; Mahajan, V.; Deygen, I.; Klyachko, N.L.; Inskoe, E.; Piroyan, A.; Sokolsky, M.; Okolie, O.; et al. Development of exosome-encapsulated paclitaxel to overcome MDR in cancer cells. Nanomedicine Nanotechnology Biol. Med. 2016, 12, 655–664. [Google Scholar] [CrossRef] [PubMed]
- Munagala, R.; Aqil, F.; Jeyabalan, J.; Gupta, R.C. Bovine milk-derived exosomes for drug delivery. Cancer Lett. 2016, 371, 48–61. [Google Scholar] [CrossRef] [PubMed]
- Yong, T.; Zhang, X.; Bie, N.; Zhang, H.; Zhang, X.; Li, F.; Hakeem, A.; Hu, J.; Gan, L.; Santos, H.A.; et al. Tumor exosome-based nanoparticles are efficient drug carriers for chemotherapy. Nat. Commun. 2019, 10, 1–16. [Google Scholar] [CrossRef]
- Chul Jang, S.; Youn Kim, O.; Min Yoon, C.; Choi, D.-S.; Roh, T.-Y.; Park, J.; Nilsson, J.; Lö tvall, J.; Kim, Y.-K.; Song Gho, Y. Bioinspired Exosome-Mimetic Nanovesicles for Targeted Delivery of Chemotherapeutics to Malignant Tumors. ACS Nano 2013, 7, 7698–7710. [Google Scholar] [CrossRef]
- Li, Y.J.; Wu, J.Y.; Wang, J.M.; Hu, X.-B.; Cai, J.X.; Xiang, D.X. Gemcitabine loaded autologous exosomes for effective and safe chemotherapy of pancreatic cancer. Acta Biomater. 2020, 101, 519–530. [Google Scholar] [CrossRef]
- Tang, K.; Zhang, Y.; Zhang, H.; Xu, P.; Liu, J.; Ma, J.; Lv, M.; Li, D.; Katirai, F.; Shen, G.X.; et al. Delivery of chemotherapeutic drugs in tumour cell-derived microparticles. Nat. Commun. 2012, 3, 1–11. [Google Scholar] [CrossRef]
- Donoso-Quezada, J.; Guajardo-Flores, D.; González-Valdez, J. Enhanced exosome-mediated delivery of black bean phytochemicals (Phaseolus vulgaris L.) for cancer treatment applications. Biomed. Pharmacother. 2020, 131, 1–9. [Google Scholar] [CrossRef]
- Munagala, R.; Aqil, F.; Jeyabalan, J.; Agrawal, A.K.; Mudd, A.M.; Kyakulaga, A.H.; Singh, I.P.; Vadhanam, M.V.; Gupta, R.C. Exosomal formulation of anthocyanidins against multiple cancer types. Cancer Lett. 2017, 393, 94–102. [Google Scholar] [CrossRef]
- Aqil, F.; Jeyabalan, J.; Agrawal, A.K.; Kyakulaga, A.H.; Munagala, R.; Parker, L.; Gupta, R.C. Exosomal delivery of berry anthocyanidins for the management of ovarian cancer. Food Funct. 2017, 8, 4100–4107. [Google Scholar] [CrossRef]
- Kanchanapally, R.; Khan, M.A.; Deshmukh, S.K.; Srivastava, S.K.; Khushman, M.; Singh, S.; Singh, A.P. Exosomal Formulation Escalates Cellular Uptake of Honokiol Leading to the Enhancement of Its Antitumor Efficacy. ACS Omega 2020, 5, 23299–23307. [Google Scholar] [CrossRef] [PubMed]
- Liu, Y.; Bai, L.; Guo, K.; Jia, Y.; Zhang, K.; Liu, Q.; Wang, P.; Wang, X. Focused ultrasound-augmented targeting delivery of nanosonosensitizers from homogenous exosomes for enhanced sonodynamic cancer therapy. Theranostics 2019, 9, 5261–5281. [Google Scholar] [CrossRef]
- Tran, P.H.L.; Wang, T.; Yin, W.; Tran, T.T.D.; Nguyen, T.N.G.; Lee, B.J.; Duan, W. Aspirin-loaded nanoexosomes as cancer therapeutics. Int. J. Pharm. 2019, 572, 1–12. [Google Scholar] [CrossRef] [PubMed]
- Altanerova, U.; Babincova, M.; Babinec, P.; Benejova, K.; Jakubechova, J.; Altanerova, V.; Zduriencikova, M.; Altaner, C. human mesenchymal stem cell-derived iron oxide exosomes allow targeted ablation of tumor cells via magnetic hyperthermia. Int. J. Nanomedicine 2017, 12, 7923–7936. [Google Scholar] [CrossRef]
- Fuhrmann, G.; Serio, A.; Mazo, M.; Nair, R.; Stevens, M.M. Active loading into extracellular vesicles significantly improves the cellular uptake and photodynamic effect of porphyrins. J. Control. Release 2015, 205, 35–44. [Google Scholar] [CrossRef] [PubMed]
- Aqil, F.; Kausar, H.; Agrawal, A.K.; Jeyabalan, J.; Kyakulaga, A.H.; Munagala, R.; Gupta, R. Exosomal formulation enhances therapeutic response of celastrol against lung cancer. Exp. Mol. Pathol. 2016, 101, 12–21. [Google Scholar] [CrossRef]
- Zhuang, X.; Xiang, X.; Grizzle, W.; Sun, D.; Zhang, S.; Axtell, R.C.; Ju, S.; Mu, J.; Zhang, L.; Steinman, L.; et al. Treatment of brain inflammatory diseases by delivering exosome encapsulated anti-inflammatory drugs from the nasal region to the brain. Mol. Ther. 2011, 19, 1769–1779. [Google Scholar] [CrossRef] [PubMed]
- Hong, Y.; Nam, G.-H.; Koh, E.; Jeon, S.; Kim, G.B.; Jeong, C.; Kim, D.-H.; Yang, Y.; Kim, I.-S. Exosome as a Vehicle for Delivery of Membrane Protein Therapeutics, PH20, for Enhanced Tumor Penetration and Antitumor Efficacy. Adv. Funct. Mater. 2018, 28, 1703074. [Google Scholar] [CrossRef]
- Hong, Y.; Kim, Y.K.; Kim, G.B.; Nam, G.H.; Kim, S.A.; Park, Y.; Yang, Y.; Kim, I.S. Degradation of tumour stromal hyaluronan by small extracellular vesicle-PH20 stimulates CD103+ dendritic cells and in combination with PD-L1 blockade boosts anti-tumour immunity. J. Extracell. Vesicles 2019, 8, 1670893. [Google Scholar] [CrossRef]
- Zhuang, M.; Chen, X.; Du, D.; Shi, J.; Deng, M.; Long, Q.; Yin, X.; Wang, Y.; Rao, L. SPION decorated exosome delivery of TNF-α to cancer cell membranes through magnetism. Nanoscale 2020, 12, 173–188. [Google Scholar] [CrossRef] [PubMed]
- Koh, E.; Lee, E.J.; Nam, G.H.; Hong, Y.; Cho, E.; Yang, Y.; Kim, I.S. Exosome-SIRPα, a CD47 blockade increases cancer cell phagocytosis. Biomaterials 2017, 121, 121–129. [Google Scholar] [CrossRef] [PubMed]
- Kalimuthu, S.; Gangadaran, P.; Rajendran, R.L.; Zhu, L.; Oh, J.M.; Lee, H.W.; Gopal, A.; Baek, S.H.; Jeong, S.Y.; Lee, S.W.; et al. A new approach for loading anticancer drugs into mesenchymal stem cell-derived exosome mimetics for cancer therapy. Front. Pharmacol. 2018, 9, 1116. [Google Scholar] [CrossRef] [PubMed]
- Choudhari, A.S.; Mandave, P.C.; Deshpande, M.; Ranjekar, P.; Prakash, O. Phytochemicals in Cancer Treatment: From Preclinical Studies to Clinical Practice. Front. Pharmacol. 2020, 10, 1–17. [Google Scholar] [CrossRef] [PubMed]
- Mandal, S. Curcumin, a Promising Anti-Cancer Therapeutic: It’S Bioactivity and Development of Drug Delivery Vehicles. Int. J. Drug Res. Tech. Int. J. Drug Res. Technol. 2016, 6, 43–57. [Google Scholar] [CrossRef]
- Luo, C.; Hu, X.; Peng, R.; Huang, H.; Liu, Q.; Tan, W. Biomimetic Carriers Based on Giant Membrane Vesicles for Targeted Drug Delivery and Photodynamic/Photothermal Synergistic Therapy. ACS Appl. Mater. Interfaces 2019, 11, 43811–43819. [Google Scholar] [CrossRef]
- Lai, C.P.; Mardini, O.; Ericsson, M.; Prabhakar, S.; Maguire, C.A.; Chen, J.W.; Tannous, B.A.; Breakefield, X.O. Dynamic biodistribution of extracellular vesicles in vivo using a multimodal imaging reporter. ACS Nano 2014, 8, 483–494. [Google Scholar] [CrossRef]
- Wang, J.; Guo, R.; Yang, Y.; Jacobs, B.; Chen, S.; Iwuchukwu, I.; Gaines, K.J.; Chen, Y.; Simman, R.; Lv, G.; et al. The Novel Methods for Analysis of Exosomes Released from Endothelial Cells and Endothelial Progenitor Cells. Stem Cells Int. 2016, 2016, 1–12. [Google Scholar] [CrossRef]
- Qi, H.; Liu, C.; Long, L.; Ren, Y.; Zhang, S.; Chang, X.; Qian, X.; Jia, H.; Zhao, J.; Sun, J.; et al. Blood Exosomes Endowed with Magnetic and Targeting Properties for Cancer Therapy. ACS Nano 2016, 10, 3323–3333. [Google Scholar] [CrossRef] [PubMed]
- Hartman, Z.C.; Wei, J.; Glass, O.K.; Guo, H.; Lei, G.; Yang, X.Y.; Osada, T.; Hobeika, A.; Delcayre, A.; Le Pecq, J.B.; et al. Increasing vaccine potency through exosome antigen targeting. Vaccine 2011, 29, 9361–9367. [Google Scholar] [CrossRef] [PubMed]
- Kooijmans, S.A.A.; Aleza, C.G.; Roffler, S.R.; van Solinge, W.W.; Vader, P.; Schiffelers, R.M. Display of GPI-anchored anti-EGFR nanobodies on extracellular vesicles promotes tumour cell targeting. J. Extracell. Vesicles 2016, 5, 31053. [Google Scholar] [CrossRef] [PubMed]
- Rajendran, R.L.; Paudel, S.; Gangadaran, P.; Oh, J.M.; Oh, E.J.; Hong, C.M.; Lee, S.; Chung, H.Y.; Lee, J.; Ahn, B.-C. Extracellular Vesicles Act as Nano-Transporters of Tyrosine Kinase Inhibitors to Revert Iodine Avidity in Thyroid Cancer. Pharmaceutics 2021, 13, 248. [Google Scholar] [CrossRef] [PubMed]
- Fonsato, V.; De Lena, M.; Tritta, S.; Brossa, A.; Calvetti, R.; Tetta, C.; Camussi, G.; Bussolati, B. Human liver stem cell-derived extracellular vesicles enhance cancer stem cell sensitivity to tyrosine kinase inhibitors through Akt/mTOR/PTEN combined modulation. Oncotarget 2018, 9, 36151. [Google Scholar] [CrossRef][Green Version]
- Son, S.H.; Gangadaran, P.; Ahn, B.-C. A novel strategy of transferring NIS protein to cells using extracellular vesicles leads to increase in iodine uptake and cytotoxicity. Int. J. Nanomedicine 2019, 14, 1779. [Google Scholar] [CrossRef] [PubMed]
- Yang, Y.; Hong, Y.; Nam, G.-H.; Chung, J.H.; Koh, E.; Kim, I.-S. Virus-Mimetic Fusogenic Exosomes for Direct Delivery of Integral Membrane Proteins to Target Cell Membranes. Adv. Mater. 2017, 29, 1605604. [Google Scholar] [CrossRef]
- Kim, G.B.; Nam, G.-H.; Hong, Y.; Woo, J.; Cho, Y.; Kwon, I.C.; Yang, Y.; Kim, I.-S. Xenogenization of tumor cells by fusogenic exosomes in tumor microenvironment ignites and propagates antitumor immunity. Sci. Adv. 2020, 6, 2083. [Google Scholar] [CrossRef]
- Maguire, C.A.; Balaj, L.; Sivaraman, S.; Crommentuijn, M.H.W.; Ericsson, M.; Mincheva-Nilsson, L.; Baranov, V.; Gianni, D.; Tannous, B.A.; Sena-Esteves, M.; et al. Microvesicle-associated AAV Vector as a Novel Gene Delivery System. Mol. Ther. 2012, 20, 960–971. [Google Scholar] [CrossRef]
- Khan, N.; Maurya, S.; Bammidi, S.; Jayandharan, G.R. AAV6 Vexosomes Mediate Robust Suicide Gene Delivery in a Murine Model of Hepatocellular Carcinoma. Mol. Ther. Methods Clin. Dev. 2020, 17, 497–504. [Google Scholar] [CrossRef]
- Jafari, D.; Shajari, S.; Jafari, R.; Mardi, N.; Gomari, H.; Ganji, F.; Forouzandeh Moghadam, M.; Samadikuchaksaraei, A. Designer Exosomes: A New Platform for Biotechnology Therapeutics. BioDrugs 2020, 34, 567–586. [Google Scholar] [CrossRef] [PubMed]

| Nature of Cargo | Encapsulated Cargo | Nature of Study | Model | Target Tissue Type | Function | Mechanism | Reference |
|---|---|---|---|---|---|---|---|
| lncRNA | LOC285194 | In vitro | A549 and H1299 | NSCLC | ↓Tumor growth | ↑p53 | [81] |
| FENDRR | H1650, HCC827, H1975, and A549 | NSCLC | ↓Tumor growth, ↓ Invasion, ↓migration, and ↑apoptosis | ↓miR-761 | [82] | ||
| MEG3 | A549 | Advanced NSCLC | ↑Sensitivity to paclitaxel and ↓proliferation | ↑p53 | [83] | ||
| MEG3 and NKILA | MCF-7 and BT474 | Breast cancer | ↑Tumor suppression | ↑p53 and ↑NF-κB signaling pathways | [84] | ||
| EGOT | BT549 | Breast cancer | ↑Sensitivity to paclitaxel | ↑ITPR1, ↓GLI1, ↓smoothened protein, ↓protein patched homolog 1, and ↓ HHIP | [85] | ||
| SRA1 | SAOS-2, MG63, U2OS, SJSA1, and human osteoblast hFOB | Osteosarcoma | ↓Proliferation, ↓migration, and ↓invasion | Sponging of miR-208 | [86] | ||
| LINC00520 | A431 cSCC (cutaneous squamous cell carcinoma) | Cutaneous squamous cell carcinoma | ↓Tumor growth, ↓proliferation, and ↓migration | ↓PI3K/Akt and ↑EGFR | [87] | ||
| miRNA | miR-497 | In vitro | A549 | Lung cancer | ↓Tumor growth | ↓YAP1, ↓HDGF, ↓CCNE1, and ↓VEGF-A | [51] |
| Coculture of A549 and HUVECs | Human umbilical vein endothelial cells (HUVECs) | ↓Angiogenesis | ↓ VEGF-A | [51] | |||
| miRNA-26a | Class B type 1-expressing liver cancer cells | Hepatocellular carcinoma (HepG2) | ↓Tumor cell proliferation and ↓migration | ----- | [90] | ||
| miR-143 | THP-1 macrophages | Metastasis-associated in colon cancer-1 (MACC1) | ↓Cell growth, ↓migration, and ↓invasion | ↓EGFR and ↓NF-κB | [91] | ||
| Let-7a | Hs578Ts(i)8 cells | Triple negative breast cancer (Hs578T cells) | ↓Cell proliferation and ↑therapeutic efficacy of anti-Hsp90 | ↓ Hsp90 expression, ↓STAT5B, and ↓Bcl-2 levels | [92] | ||
| 5-fluorouracil anti miRNA-21 | HCT-1165FR | Colorectal cancer (HCT-119) | ↓Chemoresistance and ↑treatment efficiency | ↑PTEN | [94] | ||
| miRNA-Let7a | MDA-MA-231, leukemic cells | Nucleolin-positive cancer cells | Anti-cancer effect | ↑Delivery of small RNAs to the targeted tumor sites and ↓EGFR | [95] | ||
| miR-134 | In vivo | RAG2–/– mice | EGFR-expressing xenograft breast cancer tissue | Anti-tumor effect | ↓K-RAS, ↓H-RAS, and ↓N-RAS | [80] | |
| miR-146b | Male Fischer rats | Marrow stromal cells | ↓Glioma | ----- | [89] | ||
| miR-122 | BALB/c nude mice | Hepatocellular carcinomas (HCCs) | ↑Sensitivity towards sorafenib and ↓tumor size | ↓ CCNG1, ↓IGF1R, ↓ADAM10,↑ Caspase 3, and ↑Bax | [93] | ||
| siRNA | Tpd52 siRNA | In vitro | HER-2 positive cells (SKBR3 cells) | Breast cancer | ↑RNAi therapy | ↓TDP52 expression by about 70% | [95] |
| VEGF siRNA | MDA-MA-231 | Nucleolin-positive leukemic cells | Anti-cancer effect | ↑Delivery small RNAs to the targeted tumor sites | [95] | ||
| KRAS siRNAs | A549 | Lung cancer | ↓Tumor suppression | ↓ KRAS expression | [98] | ||
| SOX2 siRNA | NSCLC | Lung cancer | ↓Proliferation and growth | ----- | [99] | ||
| BCR-ABL siRNA | LAMA84, K562, and K562R | Chronic myelogenous leukemia | ↓Cancer cell growth and ↓size of tumors | ↓BCR-ABL expression by 17% to 45% | [100] | ||
| Survivin siRNA | MDA-MB-468 | Breast cancer cells | ↑RNAi therapy | ----- | [101] | ||
| siRNA | MCF-7 | Breast cancer | Anti-tumor effect | ↓CDK4 and cell cycle arrest in G1 phase | [102] |
| Source of Exosomes | Encapsulated Cargo | Target Cancer Model | Loading Method | Tumorigenic Effect | Mechanism | Reference |
|---|---|---|---|---|---|---|
| Chemotherapeutic Drugs | ||||||
| In vitro | ||||||
| RAW 264.7 macrophage | Paclitaxel | Renal carcinoma (MDCK) cells | Incubation, electroporation, and sonication | ↑Cytotoxicity, ↓drug-efflux pump, and resistance reversal | ↓Pgp | [126] |
| Milk from pasture-fed Holstein and Jersey cows | Paclitaxel and docetaxel | A549, H1299, MB-231, and T47D | Incubation and centrifugation | Anti-tumor effect and ↑anti-inflammatory effect | ___ | [127] |
| H22, Bel7402, or B16-F10 cells | Doxorubicin | H22 and B16-F10 cells | Electroporation | ↑Cytotoxicity,↑tissue-enrichment, ↓spheroid size, and ↓nonspecific adversities | ___ | [128] |
| U937 or Raw264.7 macrophages | Doxorubicin, 5-fluorouracil, gemcitabine, and carboplatin | HUVEC | Incubation and sonication | ↑Anti-inflammatory, ↓nonspecific adversities, and ↓tumor growth | ___ | [129] |
| PANC-1 cells | Gemcitabine | PANC-1 cells | Incubation or sonication | ↓Nonspecific adversities and ↓tumor growth | ___ | [130] |
| H22 and A2780 cells | Cisplatin | H22 and A2780 cells | Incubation and UV-irradiation | ↑Cytotoxicity and↓ drug efflux | ___ | [131] |
| In vivo | ||||||
| H22 and A2780 cells | Cisplatin | H22 and A2780 cell xenografted BALB/c mice | Incubation and UV-irradiation | ↑Tumor growth inhibition and ↑survivability of tumor-challenged mice | ___ | [131] |
| Small Molecules | ||||||
| In vitro | ||||||
| Milk from pasture-fed Holstein and Jersey cows | Withaferin A, bilberry-derived anthocyanidins, and curcumin | Human lung (A549 and H1299), breast (MDA-MB-231 and T47D) cancer cells, and normal bronchial epithelial (BEAS-2B) cells | Mixing | ↓Inflammatory stress | ↓ NF-κB | [127] |
| Human mammary (MCF7), prostate (PC3), colon (Caco2), and liver (HepG2) cells | Black bean-derived (myricetin, quercetin, kaempferol, and soyasaponins) | MCF7, Caco2, PC3, and HepG2 cells | Electroporation | ↑Apoptosis and ↑cell cycle arrest | ___ | [132] |
| Raw milk from dozens of mid-lactation, pasteurized Jersey cows | Berry-derived anthocyanidin | A549 and H1299; MDA-MB-231 and MCF7; and pancreatic (PANC1 and Mia PaCa2), prostate (PC3 and DU145), colon (HCT116), and ovarian (OVCA432) cancer cells | Simple mixing | ↑anti-proliferative and ↑anti-inflammatory | ___ | [133] |
| Mature bovine milk | Anthocyanidins | Cisplatin-sensitive (A2780) and cisplatin-resistant (A2780/CP70, OVCA432, and OVCA433) | Mixing | ↑anti-proliferative | ___ | [134] |
| Mesenchymal stem cells | Honokiol (extracted from Magnolia plant) | Pancreatic (MiaPaCa and Colo357); MDA-MB-231; and colon (HT-29), prostate (LNCaP), and ovarian (SKOV-3) cancer cells | Sonication | ↑Cell-cycle arrest, ↑apoptosis, and ↓survival- associated factors | ___ | [135] |
| 4T1 cells | Sinoporphyrin sodium D | 4T1 cells | Incubation | ↑Cell death, ↓metastasis, and ↑membrane permeability | ↑ROS | [136] |
| MDA-MB-231 and HT29 cells | Aspirin | MDA-MB-231 and HT29 cells | Freeze–thaw incubation and sonication | ↑Cytotoxicity, ↑apoptosis, and ↑autophagy | ___ | [137] |
| Mesenchymal stem cells | Venofer | PC3 cells | Incubation | ↓Proliferation | ___ | [138] |
| MDA-MB-231 cells | Porphyrine | MDA-MB-231 cells | Electroporation/saponin-assisted incubation/extrusion/dialysis | ↑Cytotoxicity | ___ | [139] |
| In vivo | ||||||
| Mature bovine milk | Anthocyanidins | Ovarian cancer xenografts | Mixing | ↓Tumor growth | ___ | [134] |
| Milk from pasture-fed Holstein and Jersey cows | Celastrol (a plant-derived triterpenoid) | A549 and H1299 NSCLC xenograft C57BL6 mice | Mixing | ↓Tumor growth | ___ | [140] |
| EL-4 cells | Curcumin | Lipopolysaccharide -induced brain inflammation in C57BL/6J mice | Mixing, incubation, and centrifugation | ↓LPS-induced brain inflammation and ↑apoptosis | ___ | [141] |
| EL-4 cells | JSI124 | Mouse (H-2b) glioblastoma (GL26) cell-xenografted C57BL/6J mice with brain tumor model | Mixing, incubation, and centrifugation | ↓Tumor growth and ↑microglial apoptosis | ↓Stat3 | [141] |
| Recombinant Proteins | ||||||
| In vitro | ||||||
| Human embryonic kidney HEK293T cells | PH20 | PC3 cells | Transfection | Anti-tumor effect | ↓HA | [142] |
| HEK293T cells | PH20-Oligo-HA | Murine breast cancer (4T1) cells | Transfection | ↑Immune response | ↓HA, ↑DC cells, ↑IFN-γ, and ↑cytotoxicity of CD8+ T cells | [143] |
| Human melanoma (A375), breast adenocarcinoma (MCF-7), lung carcinoma (A549), and colon adenocarcinoma (Colo201) cells | CTNF-α exosome SPIONs | A375, MCF-7, A549, and Colo201 cells | Transfection | Anti-cancer effect and ↑apoptosis | ↑TNF-α and ↑Cleaved caspase-2,3,8 | [144] |
| HEK293T cells | SIRPα | Human colon adenocarcinoma (HT29) cells, Raji Burkitt’s lymphoma (Raji), and mouse CT26.CL25 colon cancer cells | Transfection | Anti-tumor effect and ↓tumor growth | ↓CD47 | [145] |
Publisher’s Note: MDPI stays neutral with regard to jurisdictional claims in published maps and institutional affiliations. |
© 2021 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Saha, P.; Datta, S.; Ghosh, S.; Samanta, A.; Ghosh, P.; Sinha, D. Bioengineering of Extracellular Vesicles: Exosome-Based Next-Generation Therapeutic Strategy in Cancer. Bioengineering 2021, 8, 139. https://doi.org/10.3390/bioengineering8100139
Saha P, Datta S, Ghosh S, Samanta A, Ghosh P, Sinha D. Bioengineering of Extracellular Vesicles: Exosome-Based Next-Generation Therapeutic Strategy in Cancer. Bioengineering. 2021; 8(10):139. https://doi.org/10.3390/bioengineering8100139
Chicago/Turabian StyleSaha, Priyanka, Suchisnigdha Datta, Sukanya Ghosh, Anurima Samanta, Paramita Ghosh, and Dona Sinha. 2021. "Bioengineering of Extracellular Vesicles: Exosome-Based Next-Generation Therapeutic Strategy in Cancer" Bioengineering 8, no. 10: 139. https://doi.org/10.3390/bioengineering8100139
APA StyleSaha, P., Datta, S., Ghosh, S., Samanta, A., Ghosh, P., & Sinha, D. (2021). Bioengineering of Extracellular Vesicles: Exosome-Based Next-Generation Therapeutic Strategy in Cancer. Bioengineering, 8(10), 139. https://doi.org/10.3390/bioengineering8100139





