Additive Biomanufacturing with Collagen Inks
Abstract
1. Introduction
2. Processing Parameters
2.1. Sources of Collagen
2.2. Collagen Extraction
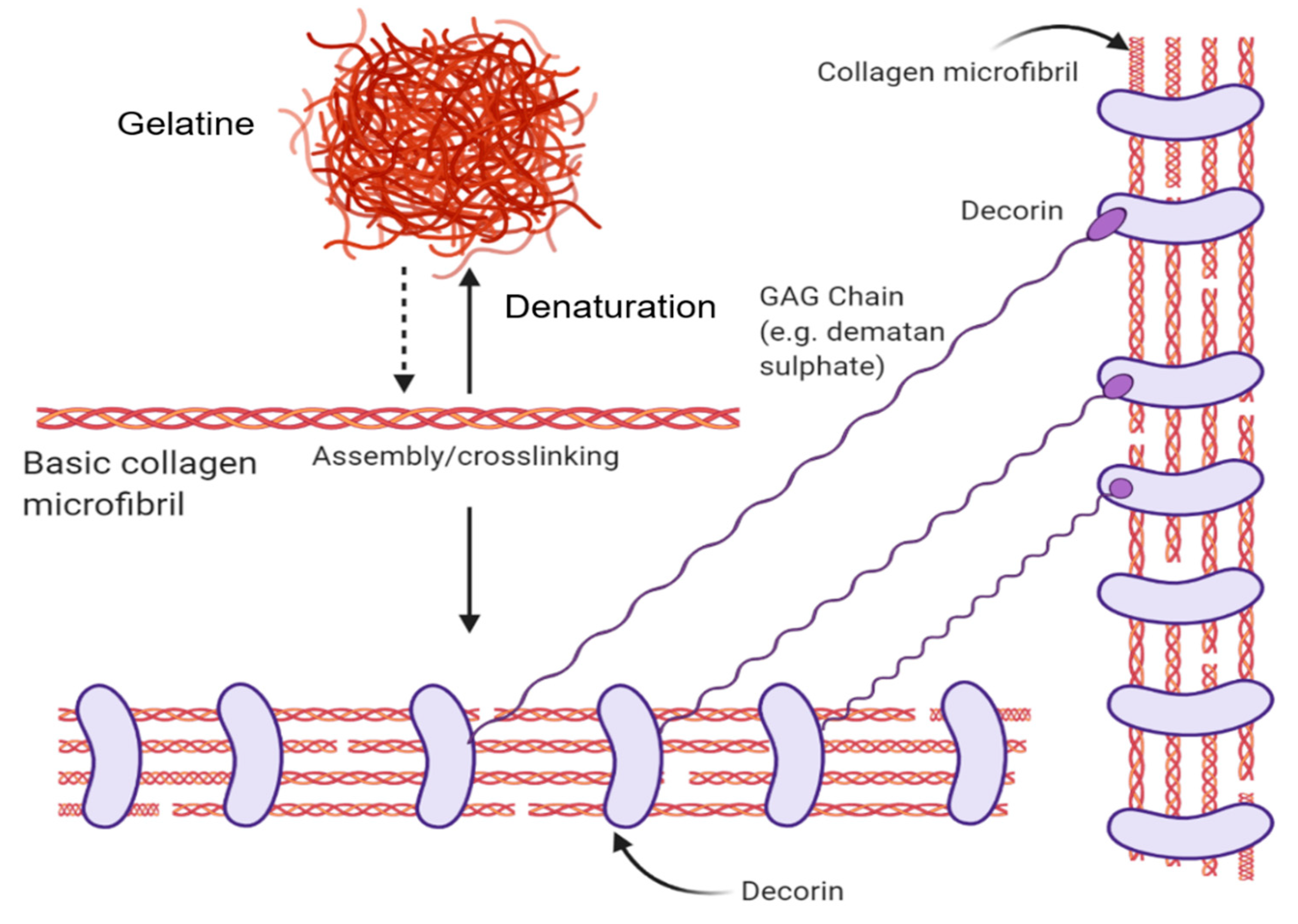
2.2.1. Various Forms–Native, Gelatin (Disordered), Collagen Peptides
2.2.2. Collagen Biocomposites
2.3. Methods of Collagen Crosslinking
2.3.1. Chemical Crosslinking
2.3.2. Physical Crosslinking
2.4. Collagen Analytical Methods
2.4.1. Structural Analysis
2.4.2. Morphological Analysis
2.4.3. Chemical Assays
3. Collagen-Based Ink Printing Applications
3.1. Non-Additive Manufacturing
Electrospinning
3.2. Additive Biomanufacturing
3.2.1. Extrusion
3.2.2. Inkjet Printing
3.2.3. Laser-Assisted Printing
3.2.4. Stereolithography Printing
4. Regulatory Considerations and Challenges for Collagen Biomanufacturing
5. Concluding Remarks
Author Contributions
Funding
Conflicts of Interest
References
- Frantz, C.; Stewart, K.M.; Weaver, V.M. The extracellular matrix at a glance. J. Cell Sci. 2010, 123, 4195–4200. [Google Scholar] [CrossRef] [PubMed]
- Sorushanova, A.; Delgado, L.M.; Wu, Z.; Shologu, N.; Kshirsagar, A.; Raghunath, R.; Mullen, A.M.; Bayon, Y.; Pandit, A.; Raghunath, M.; et al. The Collagen Suprafamily: From biosynthesis to advanced biomaterial development. Adv. Mater. 2019, 31, 1801651. [Google Scholar] [CrossRef] [PubMed]
- Sorushanova, A.; Coentro, J.Q.; Pandit, A.; Zeugolis, D.I.; Raghunath, M. Collagen: Materials Analysis and Implant Uses. In Comprehensive Biomaterials II; Elsevier Ltd.: Amsterdam, The Netherlands, 2017; pp. 332–350. ISBN 9780081006924. [Google Scholar]
- Lodish, H.; Berk, A.; Zipursky, S.L.; Matsudaira, P.; Baltimore, D.; Darnell, J.E. Collagen: The Fibrous Proteins of the Matrix. In Molecular Cell Biology, 4th ed.; W. H. Freeman and Company: New York, NY, USA, 2000. [Google Scholar]
- Cen, L.; Liu, W.; Cui, L.; Zhang, W.; Cao, Y. Collagen tissue engineering—Development of novel biomaterials and applications. Pediatr. Res. 2008, 63, 492–496. [Google Scholar] [CrossRef] [PubMed]
- Shoulders, M.D.; Raines, R.T. Collagen structure and stability. Annu. Rev. Biochem. 2009, 78, 929–958. [Google Scholar] [CrossRef]
- Hortensius, R.A.; Harley, B.A. The use of bioinspired alterations in the glycosaminoglycan content of collagen-GAG scaffolds to regulate cell activity. Biomaterials 2013, 34, 7645–7652. [Google Scholar] [CrossRef]
- Caliari, S.R.; Ramirez, M.A.; Harley, B.A. The development of collagen-GAG scaffold-membrane composites for tendon tissue engineering. Biomaterials 2011, 32, 8990–8998. [Google Scholar] [CrossRef]
- Fournet, M.; Bonté, F.; Desmoulière, A. Glycation Damage: A Possible hub for major pathophysiological disorders and aging. Aging Dis. 2018, 9, 880–900. [Google Scholar] [CrossRef]
- Justice, B.A.; Badr, N.A.; Felder, R.A. 3D cell culture opens new dimensions in cell-based assays. Drug Discov. Today 2009, 14, 102–107. [Google Scholar] [CrossRef]
- Grobstein, C. Morphogenetic interaction between embryonic mouse tissues separated by a membrane filter. Nature 1953, 172, 869–871. [Google Scholar] [CrossRef]
- Steele, R.E.; Preston, A.S.; Johnson, J.P.; Handler, J.S. Porous-bottom dishes for culture of polarized cells. Am. J. Physiol. Physiol. 1986, 251, C136–C139. [Google Scholar] [CrossRef]
- Copes, F.; Pien, N.; Van Vlierberghe, S.; Boccafoschi, F.; Mantovani, D. Collagen-based tissue engineering strategies for vascular medicine. Front. Bioeng. Biotechnol. 2019, 7, 166. [Google Scholar] [CrossRef] [PubMed]
- Hinderer, S.; Layland, S.L.; Schenke-Layland, K. ECM and ECM-like materials—Biomaterials for applications in regenerative medicine and cancer therapy. Adv. Drug Deliv. Rev. 2016, 97, 260–269. [Google Scholar] [CrossRef]
- Chattopadhyay, S.; Raines, R.T. Collagen-based biomaterials for wound healing. Biopolymers 2014, 101, 821–833. [Google Scholar] [CrossRef]
- Stephens, N.; Di Silvio, L.; Dunsford, I.; Ellis, M.; Glencross, A.; Sexton, A. Bringing cultured meat to market: Technical, socio-political, and regulatory challenges in cellular agriculture. Trends Food Sci.Technol. 2018, 78, 155–166. [Google Scholar] [CrossRef] [PubMed]
- Singh, S.; Choudhury, D.; Yu, F.; Mironov, V.; Naing, M.W. In situ bioprinting—Bioprinting from benchside to bedside? Acta Biomater. 2020, 101, 14–25. [Google Scholar] [CrossRef] [PubMed]
- Choudhury, D.; Anand, S.; Naing, M.W. The arrival of commercial bioprinters—Towards 3d bioprinting revolution! Int. J. Bioprinting. 2018, 4, 139. [Google Scholar] [CrossRef]
- Murphy, S.V.; Atala, A. 3D bioprinting of tissues and organs. Nat. Biotechnol. 2014, 32, 773–785. [Google Scholar] [CrossRef]
- Leberfinger, A.N.; Ravnic, D.J.; Dhawan, A.; Ozbolat, I.T. Concise review: Bioprinting of stem cells for transplantable tissue fabrication. Stem Cells Transl. Med. 2017, 6, 1940–1948. [Google Scholar] [CrossRef]
- Lee, A.; Hudson, A.R.; Shiwarski, D.J.; Tashman, J.W.; Hinton, T.J.; Yerneni, S.; Bliley, J.M.; Campbell, P.G.; Feinberg, A.W. 3D bioprinting of collagen to rebuild components of the human heart. Science 2019, 365, 482–487. [Google Scholar] [CrossRef]
- Hinton, T.J.; Jallerat, Q.; Palchesko, R.N.; Park, J.H.; Grodzicki, M.S.; Shue, H.-J.; Ramadan, M.H.; Hudson, A.R.; Feinberg, A.W. Three-dimensional printing of complex biological structures by freeform reversible embedding of suspended hydrogels. Sci. Adv. 2015, 1. [Google Scholar] [CrossRef]
- Choudhury, D.; Tun, H.W.; Wang, T.; Naing, M.W. Organ-derived decellularized extracellular matrix: A Game Changer for Bioink Manufacturing? Trends Biotechnol. 2018, 36, 787–805. [Google Scholar] [CrossRef] [PubMed]
- Marques, C.F.; Diogo, G.S.; Pina, S.; Oliveira, J.M.; Silva, T.H.; Reis, R.L. Collagen-based bioinks for hard tissue engineering applications: A comprehensive review. J. Mater. Sci Mater. Med. 2019, 30, 32. [Google Scholar] [CrossRef] [PubMed]
- Holmes, D.F.; Gilpin, C.J.; Baldock, C.; Ziese, U.; Koster, A.J.; Kadler, K.E. Corneal collagen fibril structure in three dimensions: Structural insights into fibril assembly, mechanical properties, and tissue organization. Proc. Natl. Acad. Sci. USA 2001, 98, 7307–7312. [Google Scholar] [CrossRef]
- Schmidt, M.M.; Dornelles, R.C.P.; Mello, R.O.; Kubota, E.H.; Mazutti, M.A.; Kempka, A.P.; Demiate, I.M. Collagen extraction process. Int. Food Res. J. 2015, 23, 913–922. [Google Scholar]
- Sawicki, L.A.; Choe, L.H.; Wiley, K.L.; Lee, K.H.; Kloxin, A.M. Isolation and Identification of Proteins Secreted by Cells Cultured within Synthetic Hydrogel-Based Matrices. ACS Biomater. Sci. Eng. 2018, 4, 836–845. [Google Scholar] [CrossRef] [PubMed]
- Kleinman, H.K. Isolation of laminin-1 and type IV collagen from the EHS sarcoma. J. Tissue Cult. Methods 1994, 16, 231–233. [Google Scholar] [CrossRef]
- Wang, T.; Lew, J.; Premkumar, J.; Poh, C.L.; Win Naing, M. Production of recombinant collagen: State of the art and challenges. Eng. Biol. 2017, 1, 18–23. [Google Scholar] [CrossRef]
- Tomita, M.; Munetsuna, H.; Sato, T.; Adachi, T.; Hino, R.; Hayashi, M.; Shimizu, K.; Nakamura, N.; Tamura, T.; Yoshizato, K. Transgenic silkworms produce recombinant human type III procollagen in cocoons. Nat. Biotechnol. 2003, 21, 52–56. [Google Scholar] [CrossRef]
- Rele, S.; Song, Y.; Apkarian, R.P.; Qu, Z.; Conticello, V.P.; Chaikof, E.L. D-periodic collagen-mimetic microfibers. J. Am. Chem. Soc. 2007, 129, 14780–14787. [Google Scholar] [CrossRef]
- O’Leary, L.E.R.; Fallas, J.A.; Bakota, E.L.; Kang, M.K.; Hartgerink, J.D. Multi-hierarchical self-assembly of a collagen mimetic peptide from triple helix to nanofibre and hydrogel. Nat. Chem. 2011, 3, 821–828. [Google Scholar] [CrossRef]
- Li, Z.-R.; Wang, B.; Chi, C.; Zhang, Q.-H.; Gong, Y.; Tang, J.-J.; Luo, H.; Ding, G. Isolation and characterization of acid soluble collagens and pepsin soluble collagens from the skin and bone of Spanish mackerel (Scomberomorous niphonius). Food Hydrocoll. 2013, 31, 103–113. [Google Scholar] [CrossRef]
- Meyer, M. Processing of collagen based biomaterials and the resulting materials properties. Biomed. Eng. Online 2019, 18, 24. [Google Scholar] [CrossRef] [PubMed]
- Davison, P.F.; Cannon, D.J.; Andersson, L.P. The effects of acetic acid on collagen cross-links. Connect. Tissue Res. 1972, 1, 205–216. [Google Scholar] [CrossRef]
- Lynn, A.K.; Yannas, I.V.; Bonfield, W. Antigenicity and immunogenicity of collagen. J. Biomed. Mater. Res. Part B Appl. Biomater. 2004, 71B, 343–354. [Google Scholar] [CrossRef]
- Parenteau-Bareil, R.; Gauvin, R.; Berthod, F. Collagen-based biomaterials for tissue engineering applications. Materials 2010, 3, 1863–1887. [Google Scholar] [CrossRef]
- Leon-Lopez, A.; Morales-Penaloza, A.; Martinez-Juarez, V.M.; Vargas-Torres, A.; Zeugolis, D.I.; Aguirre-Alvarez, G. Hydrolyzed collagen-sources and applications. Molecules 2019, 24, 4031. [Google Scholar] [CrossRef]
- Law, J.X.; Liau, L.L.; Saim, A.; Yang, Y.; Idrus, R. Electrospun collagen nanofibers and their applications in skin tissue engineering. Tissue Eng. Regen. Med. 2017, 14, 699–718. [Google Scholar] [CrossRef]
- Hiraoka, Y.; Kimura, Y.; Ueda, H.; Tabata, Y. Fabrication and biocompatibility of collagen sponge reinforced with poly (glycolic acid) fiber. Tissue Eng. 2004, 9, 1101–1112. [Google Scholar] [CrossRef]
- Mazzocchi, A.; Devarasetty, M.; Huntwork, R.; Soker, S.; Skardal, A. Optimization of collagen type I-hyaluronan hybrid bioink for 3D bioprinted liver microenvironments. Biofabrication 2018, 11, 15003. [Google Scholar] [CrossRef]
- Yang, X.; Lu, Z.; Wu, H.; Li, W.; Zheng, L.; Zhao, J. Collagen-alginate as bioink for three-dimensional (3D) cell printing based cartilage tissue engineering. Mater. Sci. Eng. C 2018, 83, 195–201. [Google Scholar] [CrossRef]
- Hortensius, R.A.; Harley, B.A.C. Collagen-GAG Materials. In Comprehensive Biomaterials II; Elsevier: Amsterdam, The Netherlands, 2017; pp. 351–380. ISBN 9780081006924. [Google Scholar]
- Ueda, H.; Hong, L.; Yamamoto, M.; Shigeno, K.; Inoue, M.; Toba, T.; Yoshitani, M.; Nakamura, T.; Tabata, Y.; Shimizu, Y. Use of collagen sponge incorporating transforming growth factor-beta1 to promote bone repair in skull defects in rabbits. Biomaterials 2002, 23, 1003–1010. [Google Scholar] [CrossRef]
- Wahl, D.A.; Czernuszka, J.T. Collagen-hydroxyapatite composites for hard tissue repair. Eur. Cell Mater. 2006, 11, 43–56. [Google Scholar] [CrossRef] [PubMed]
- Kim, W.; Jang, C.H.; Kim, G. Optimally designed collagen/polycaprolactone biocomposites supplemented with controlled release of HA/TCP/rhBMP-2 and HA/TCP/PRP for hard tissue regeneration. Mater. Sci. Eng. C 2017, 78, 763–772. [Google Scholar] [CrossRef] [PubMed]
- Osidak, E.O.; Karalkin, P.A.; Osidak, M.S.; Parfenov, V.A.; Sivogrivov, D.E.; Pereira, F.D.A.S.; Gryadunova, A.A.; Koudan, E.V.; Khesuani, Y.D.; Kasyanov, V.A.; et al. Viscoll collagen solution as a novel bioink for direct 3D bioprinting. J. Mater. Sci. Mater. Med. 2019, 30, 31. [Google Scholar] [CrossRef]
- Olde Damink, L.H.H.; Dijkstra, P.J.; Van Luyn, M.J.A.; Van Wachem, P.B.; Nieuwenhuis, P.; Feijen, J. Glutaraldehyde as a crosslinking agent for collagen-based biomaterials. J. Mater. Sci. Mater. Med. 1995, 6, 460–472. [Google Scholar] [CrossRef]
- Ahmed, R.M.; Venkateshwarlu, U.; Jayakumar, R. Multilayered peptide incorporated collagen tubules for peripheral nerve repair. Biomaterials 2004, 25, 2585–2594. [Google Scholar] [CrossRef]
- Yannas, I.V. Tissue regeneration by Use of Glycosaminoglycan Copolymer. Clin. Mater. 1992, 9, 179–187. [Google Scholar] [CrossRef]
- Haugh, M.G.; Murphy, C.M.; McKiernan, R.C.; Altenbuchner, C.; O’Brien, F.J. Crosslinking and mechanical properties significantly influence cell attachment, proliferation, and migration within collagen glycosaminoglycan scaffolds. Tissue Eng. Part A 2010, 17, 1201–1208. [Google Scholar] [CrossRef]
- Cote, M.F.; Sirois, E.; Doillon, C.J. In vitro contraction rate of collagen in sponge-shape matrices. J. Biomater. Sci. Polym. Ed. 1992, 3, 301–313. [Google Scholar] [CrossRef]
- White, M.J.; Kohno, I.; Rubin, A.L.; Stenzel, K.H.; Miyata, T. Collagen films—Effect of cross-linking on physical and biological properties. Biomater. Med. Devices. Artif. Organs 1973, 1, 703–715. [Google Scholar] [CrossRef]
- Ber, S.; Torun Kose, G.; Hasirci, V. Bone tissue engineering on patterned collagen films: An in vitro study. Biomaterials 2005, 26, 1977–1986. [Google Scholar] [CrossRef] [PubMed]
- Vasudev, S.C.; Chandy, T.; Sharma, C.P.; Mohanty, M.; Umasankar, P.R. Effects of double cross-linking technique on the enzymatic degradation and calcification of bovine pericardia. J. Biomater. Appl. 2000, 14, 273–295. [Google Scholar] [CrossRef] [PubMed]
- Damink, L.H.O.; Dijkstra, P.J.; van Luyn, M.J.A.; van Wachem, P.B.; Nieuwenhuis, P.; Feijen, J. Cross-linking of dermal sheep collagen using a water-soluble carbodiimide. Biomaterials 1996, 17, 765–773. [Google Scholar] [CrossRef]
- Sehgal, D.; Vijay, I.K. A Method for the High Efficiency of water-soluble carbodiimide-mediated amidation. Anal. Biochem. 1994, 218, 87–91. [Google Scholar] [CrossRef] [PubMed]
- Shin, K.-H.; Kim, J.-W.; Koh, Y.-H.; Kim, H.-E. Novel self-assembly-induced 3D plotting for macro/nano-porous collagen scaffolds comprised of nanofibrous collagen filaments. Mater. Lett. 2015, 143, 265–268. [Google Scholar] [CrossRef]
- Lee, H.; Yang, G.H.; Kim, M.; Lee, J.; Huh, J.; Kim, G. Fabrication of micro/nanoporous collagen/dECM/silk-fibroin biocomposite scaffolds using a low temperature 3D printing process for bone tissue regeneration. Mater. Sci. Eng. C 2018, 84, 140–147. [Google Scholar] [CrossRef]
- Wu, J.M.; Xu, Y.Y.; Li, Z.H.; Yuan, X.Y.; Wang, P.F.; Zhang, X.Z.; Liu, Y.Q.; Guan, J.; Guo, Y.; Li, R.X.; et al. Heparin-functionalized collagen matrices with controlled release of basic fibroblast growth factor. J. Mater. Sci. Mater. Med. 2011, 22, 107–114. [Google Scholar] [CrossRef]
- Tanaka, Y.; Baba, K.; Duncan, T.J.; Kubota, A.; Asahi, T.; Quantock, A.J.; Yamato, M.; Okano, T.; Nishida, K. Transparent, tough collagen laminates prepared by oriented flow casting, multi-cyclic vitrification and chemical cross-linking. Biomaterials 2011, 32, 3358–3366. [Google Scholar] [CrossRef]
- Liu, Y.; Ren, L.; Yao, H.; Wang, Y. Collagen films with suitable physical properties and biocompatibility for corneal tissue engineering prepared by ion leaching technique. Mater. Lett. 2012, 87, 1–4. [Google Scholar] [CrossRef]
- Damink, L.H.O.; Dijkstra, P.J.; van Luyn, M.J.A.; van Wachem, P.B.; Nieuwenhuis, P.; Feijen, J. Crosslinking of dermal sheep collagen using hexamethylene diisocyanate. J. Mater. Sci. Mater. Med. 1995, 6, 429–434. [Google Scholar] [CrossRef]
- Paul, R.G.; Bailey, A.J. Chemical stabilisation of collagen as a biomimetic. Sci. World J. 2003, 3, 138–155. [Google Scholar] [CrossRef] [PubMed]
- Zeugolis, D.I.; Paul, G.R.; Attenburrow, G. Cross-linking of extruded collagen fibers—A biomimetic three-dimensional scaffold for tissue engineering applications. J. Biomed. Mater. Res. Part A 2009, 89A, 895–908. [Google Scholar] [CrossRef]
- Heijmen, F.H.; du Pont, J.S.; Middelkoop, E.; Kreis, R.W.; Hoekstra, M.J. Cross-linking of dermal sheep collagen with tannic acid. Biomaterials 1997, 18, 749–754. [Google Scholar] [CrossRef]
- Yeo, M.G.; Kim, G.H. A cell-printing approach for obtaining {hASC}-laden scaffolds by using a collagen/polyphenol bioink. Biofabrication 2017, 9, 25004. [Google Scholar] [CrossRef] [PubMed]
- Lee, J.; Yeo, M.; Kim, W.; Koo, Y.; Kim, G.H. Development of a tannic acid cross-linking process for obtaining 3D porous cell-laden collagen structure. Int. J. Biol. Macromol. 2018, 110, 497–503. [Google Scholar] [CrossRef]
- Macaya, D.; Ng, K.K.; Spector, M. Injectable. Adv. Funct. Mater. 2011, 21, 4788–4797. [Google Scholar] [CrossRef]
- Kim, Y.B.; Lee, H.; Kim, G.H. Strategy to achieve highly porous/biocompatible macroscale cell blocks, using a collagen/genipin-bioink and an optimal 3d printing process. ACS Appl. Mater. Interfaces 2016, 8, 32230–32240. [Google Scholar] [CrossRef] [PubMed]
- Kumar, V.A.; Caves, J.M.; Haller, C.A.; Dai, E.; Li, L.; Grainger, S.; Chaikof, E.L. Collagen-based substrates with tunable strength for soft tissue engineering. Biomater. Sci. 2013, 1, 1193–1202. [Google Scholar] [CrossRef]
- HW, S.; RN, H.; LL, H.; CC, T.; CT, C. Feasibility study of a natural crosslinking reagent for biological tissue fixation. J. Biomed Mater Res. 1998, 42, 560–567. [Google Scholar] [CrossRef]
- Haugh, M.G.; Jaasma, M.J.; O’Brien, F.J. The effect of dehydrothermal treatment on the mechanical and structural properties of collagen-GAG scaffolds. J. Biomed. Mater. Res. Part A 2009, 89A, 363–369. [Google Scholar] [CrossRef]
- Weadock, K.S.; Miller, E.J.; Bellincampi, L.D.; Zawadsky, J.P.; Dunn, M.G. Physical crosslinking of collagen fibers—Comparison of ultraviolet irradiation and dehydrothermal treatment. J. Biomed. Mater. Res. 1995, 29, 1373–1379. [Google Scholar] [CrossRef] [PubMed]
- Davidenko, N.; Bax, D.V.; Schuster, C.F.; Farndale, R.W.; Hamaia, S.W.; Best, S.M.; Cameron, R.E. Optimisation of UV irradiation as a binding site conserving method for crosslinking collagen-based scaffolds. J. Mater. Sci. Mater. Med. 2016, 27, 14. [Google Scholar] [CrossRef]
- Zhang, X.; Xu, L.; Huang, X.; Wei, S.; Zhai, M. Structural study and preliminary biological evaluation on the collagen hydrogel crosslinked by γ-irradiation. J. Biomed. Mater. Res. A 2012, 100, 2960–2969. [Google Scholar] [CrossRef]
- Abraham, L.C.; Zuena, E.; Perez-Ramirez, B.; Kaplan, D.L. Guide to collagen characterization for biomaterial studies. J. Biomed Mater Res B Appl Biomater 2008, 87, 264–285. [Google Scholar] [CrossRef] [PubMed]
- Schroepfer, M.; Meyer, M. DSC investigation of bovine hide collagen at varying degrees of crosslinking and humidities. Int. J. Biol. Macromol. 2017, 103, 120–128. [Google Scholar] [CrossRef] [PubMed]
- Nomura, Y.; Sakai, H.; Ishii, Y.; Shirai, K. Preparation and some properties of type i collagen from fish scales. Biosci. Biotechnol. Biochem. 1996, 60, 2092–2094. [Google Scholar] [CrossRef] [PubMed]
- Wu, J.; Li, Z.; Yuan, X.; Wang, P.; Liu, Y.; Wang, H. Extraction and isolation of type I, III and V collagens and their SDS-PAGE analyses. Trans. Tianjin Univ. 2011, 17, 111. [Google Scholar] [CrossRef]
- Drzewiecki, K.E.; Grisham, D.R.; Parmar, A.S.; Nanda, V.; Shreiber, D.I. Circular dichroism spectroscopy of collagen fibrillogenesis: A new use for an old technique. Biophys. J. 2016, 111, 2377–2386. [Google Scholar] [CrossRef]
- Greenfield, N.J. Using circular dichroism spectra to estimate protein secondary structure. Nat. Protoc. 2006, 1, 2876–2890. [Google Scholar] [CrossRef]
- Martinez, M.G.; Bullock, A.J.; MacNeil, S.; Rehman, I.U. Characterisation of structural changes in collagen with Raman spectroscopy. Appl. Spectrosc. Rev. 2019, 54, 509–542. [Google Scholar] [CrossRef]
- Riaz, T.; Zeeshan, R.; Zarif, F.; Ilyas, K.; Muhammad, N.; Safi, S.Z.; Rahim, A.; Rizvi, S.A.A.; Rehman, I.U. FTIR analysis of natural and synthetic collagen. Appl. Spectrosc. Rev. 2018, 53, 703–746. [Google Scholar] [CrossRef]
- Beier, J.P.; Klumpp, D.; Rudisile, M.; Dersch, R.; Wendorff, J.H.; Bleiziffer, O.; Arkudas, A.; Polykandriotis, E.; Horch, R.E.; Kneser, U. Collagen matrices from sponge to nano: New perspectives for tissue engineering of skeletal muscle. BMC Biotechnol. 2009, 9, 34. [Google Scholar] [CrossRef] [PubMed]
- Lucchese, A.; Pilolli, G.P.; Petruzzi, M.; Crincoli, V.; Scivetti, M.; Favia, G. Analysis of collagen distribution in human crown dentin by confocal laser scanning microscopy. Ultrastruct. Pathol. 2008, 32, 107–111. [Google Scholar] [CrossRef] [PubMed]
- Dewavrin, J.-Y.; Hamzavi, N.; Shim, V.P.W.; Raghunath, M. Tuning the architecture of three-dimensional collagen hydrogels by physiological macromolecular crowding. Acta Biomater. 2014, 10, 4351–4359. [Google Scholar] [CrossRef]
- Quan, B.D.; Sone, E.D. Cryo-TEM analysis of collagen fibrillar structure. Methods Enzym. 2013, 532, 189–205. [Google Scholar] [CrossRef]
- Chernoff, E.A.G.; Chernoff, D.A. Atomic force microscope images of collagen fibers. J. Vac. Sci. Technol. A 1992, 10, 596–599. [Google Scholar] [CrossRef]
- Cissell, D.D.; Link, J.M.; Hu, J.C.; Athanasiou, K.A. A Modified hydroxyproline assay based on hydrochloric acid in ehrlich’s solution accurately measures tissue collagen content. Tissue Eng. Part C. Methods 2017, 23, 243–250. [Google Scholar] [CrossRef]
- Lareu, R.R.; Zeugolis, D.I.; Abu-Rub, M.; Pandit, A.; Raghunath, M. Essential modification of the Sircol Collagen Assay for the accurate quantification of collagen content in complex protein solutions. Acta Biomater. 2010, 6, 3146–3151. [Google Scholar] [CrossRef]
- Liang, H.; Russell, S.J.; Wood, D.J.; Tronci, G. A hydroxamic acid–methacrylated collagen conjugate for the modulation of inflammation-related MMP upregulation. J. Mater. Chem. B 2018, 6, 3703–3715. [Google Scholar] [CrossRef]
- Lai, J.-Y.; Wang, P.-R.; Luo, L.-J.; Chen, S.-T. Stabilization of collagen nanofibers with L-lysine improves the ability of carbodiimide cross-linked amniotic membranes to preserve limbal epithelial progenitor cells. Int. J. Nanomed. 2014, 9, 5117–5130. [Google Scholar] [CrossRef]
- Castagnaro, S.; Chrisam, M.; Cescon, M.; Braghetta, P.; Grumati, P.; Bonaldo, P. Extracellular collagen vi has prosurvival and autophagy instructive properties in mouse fibroblasts. Front. Physiol. 2018, 9, 1129. [Google Scholar] [CrossRef] [PubMed]
- Van Huizen, N.A.; Ijzermans, J.N.M.; Burgers, P.C.; Luider, T.M. Collagen analysis with mass spectrometry. Mass Spectrom. Rev. 2019. [Google Scholar] [CrossRef] [PubMed]
- O’Brien, F.J.; Harley, B.A.; Yannas, I.V.; Gibson, L. Influence of freezing rate on pore structure in freeze-dried collagen-GAG scaffolds. Biomaterials 2004, 25, 1077–1086. [Google Scholar] [CrossRef]
- Caliari, S.R.; Weisgerber, D.W.; Ramirez, M.A.; Kelkhoff, D.O.; Harley, B.A.C. The influence of collagen-glycosaminoglycan scaffold relative density and microstructural anisotropy on tenocyte bioactivity and transcriptomic stability. J. Mech. Behav. Biomed. Mater. 2012, 11, 27–40. [Google Scholar] [CrossRef] [PubMed]
- John, A.; Hong, L.; Ikada, Y.; Tabata, Y. A trial to prepare biodegradable collagen-hydroxyapatite composites for bone repair. J. Biomater. Sci. Polym. Ed. 2001, 12, 689–705. [Google Scholar] [CrossRef]
- Yannas, I.V.; Tzeranis, D.S.; So, P.T.C. Regeneration of injured skin and peripheral nerves requires control of wound contraction, not scar formation. Wound Repair Regen. 2017, 25, 177–191. [Google Scholar] [CrossRef]
- Yannas, I.V.; Tzeranis, D.S.; Harley, B.A.; So, P.T. Biologically active collagen-based scaffolds: Advances in processing and characterization. Philos. Trans. R. Soc. A Math. Phys. Eng. Sci. 2010, 368, 2123–2139. [Google Scholar] [CrossRef]
- Chamberlain, L.J.; Yannas, I.V.; Hsu, H.-P.; Strichartz, G.; Spector, M. Collagen-GAG substrate enhances the quality of nerve regeneration through collagen tubes up to level of autograft. Exp. Neurol. 1998, 154, 315–329. [Google Scholar] [CrossRef]
- Cascone, M.G.; Sim, B.; Sandra, D. Blends of synthetic and natural polymers as drug delivery systems for growth hormone. Biomaterials 1995, 16, 569–574. [Google Scholar] [CrossRef]
- Maeda, M.; Kadota, K.; Kajihara, M.; Sano, A.; Fujioka, K. Sustained release of human growth hormone (hGH) from collagen film and evaluation of effect on wound healing in db/db mice. J. Control. Release 2001, 77, 261–272. [Google Scholar] [CrossRef]
- Wang, Z.; Hu, S.; Wang, H. Scale-up preparation and characterization of collagen/sodium alginate blend films. J. Food Qual. 2017, 2017, 4954259. [Google Scholar] [CrossRef]
- Fuller, K.; Pandit, A.; Zeugolis, D.I. The multifaceted potential of electro-spinning in regenerative medicine. Pharm. Nanotechnol. 2014, 2, 23–34. [Google Scholar] [CrossRef]
- Zeugolis, D.I.; Khew, S.T.; Yew, E.S.Y.; Ekaputra, A.K.; Tong, Y.W.; Yung, L.-Y.L.; Hutmacher, D.W.; Sheppard, C.; Raghunath, M. Electro-spinning of pure collagen nano-fibres—Just an expensive way to make gelatin? Biomaterials 2008, 29, 2293–2305. [Google Scholar] [CrossRef]
- Ladd, M.R.; Lee, S.J.; Stitzel, J.D.; Atala, A.; Yoo, J.J. Co-electrospun dual scaffolding system with potential for muscle-tendon junction tissue engineering. Biomaterials 2011, 32, 1549–1559. [Google Scholar] [CrossRef] [PubMed]
- McClure, M.J.; Sell, S.A.; Simpson, D.G.; Walpoth, B.H.; Bowlin, G.L. A three-layered electrospun matrix to mimic native arterial architecture using polycaprolactone, elastin, and collagen: A preliminary study. Acta Biomater. 2010, 6, 2422–2433. [Google Scholar] [CrossRef] [PubMed]
- Ekaputra, A.K.; Prestwich, G.D.; Cool, S.M.; Hutmacher, D.W. Combining electrospun scaffolds with electrosprayed hydrogels leads to three-dimensional cellularization of hybrid constructs. Biomacromolecules 2008, 9, 2097–2103. [Google Scholar] [CrossRef]
- Dong, B.; Arnoult, O.; Smith, M.E.; Wnek, G.E. Electrospinning of collagen nanofiber scaffolds from benign solvents. Macromol. Rapid Commun. 2009, 30, 539–542. [Google Scholar] [CrossRef]
- Ozbolat, I.T.; Yu, Y. Bioprinting toward organ fabrication: Challenges and future trends. IEEE Trans. Biomed. Eng. 2013, 60, 691–699. [Google Scholar] [CrossRef]
- Kang, D.; Ahn, G.; Kim, D.; Kang, H.W.; Yun, S.; Yun, W.S.; Shim, J.H.; Jin, S. Pre-set extrusion bioprinting for multiscale heterogeneous tissue structure fabrication. Biofabrication 2018, 10, 35008. [Google Scholar] [CrossRef]
- Wu, Z.; Su, X.; Xu, Y.; Kong, B.; Sun, W.; Mi, S. Bioprinting three-dimensional cell-laden tissue constructs with controllable degradation. Sci. Rep. 2016, 6, 24474. [Google Scholar] [CrossRef]
- Zhu, K.; Chen, N.; Liu, X.; Mu, X.; Zhang, W.; Wang, C.; Zhang, Y.S. A General strategy for extrusion bioprinting of bio-macromolecular bioinks through alginate-templated dual-stage crosslinking. Macromol. Biosci. 2018, 18, e1800127. [Google Scholar] [CrossRef] [PubMed]
- Moncal, K.K.; Ozbolat, V.; Datta, P.; Heo, D.N.; Ozbolat, I.T. Thermally-controlled extrusion-based bioprinting of collagen. J. Mater. Sci. Mater. Med. 2019, 30, 55. [Google Scholar] [CrossRef]
- Isaacson, A.; Swioklo, S.; Connon, C.J. 3D bioprinting of a corneal stroma equivalent. Exp. Eye Res. 2018, 173, 188–193. [Google Scholar] [CrossRef] [PubMed]
- Diamantides, N.; Wang, L.; Pruiksma, T.; Siemiatkoski, J.; Dugopolski, C.; Shortkroff, S.; Kennedy, S.; Bonassar, L.J. Correlating rheological properties and printability of collagen bioinks: The effects of riboflavin photocrosslinking and pH. Biofabrication 2017, 9, 34102. [Google Scholar] [CrossRef]
- Kim, B.S.; Lee, J.S.; Gao, G.; Cho, D.W. Direct 3D cell-printing of human skin with functional transwell system. Biofabrication 2017, 9, 25034. [Google Scholar] [CrossRef] [PubMed]
- Kim, J.D.; Choi, J.S.; Kim, B.S.; Chan Choi, Y.; Cho, Y.W. Piezoelectric inkjet printing of polymers: Stem cell patterning on polymer substrates. Polymer 2010, 51, 2147–2154. [Google Scholar] [CrossRef]
- Duarte Campos, D.F.; Rohde, M.; Ross, M.; Anvari, P.; Blaeser, A.; Vogt, M.; Panfil, C.; Yam, G.H.; Mehta, J.S.; Fischer, H.; et al. Corneal bioprinting utilizing collagen-based bioinks and primary human keratocytes. J. Biomed. Mater. Res. Part A 2019, 107, 1945–1953. [Google Scholar] [CrossRef]
- Duarte Campos, D.F.; Blaeser, A.; Buellesbach, K.; Sen, K.S.; Xun, W.; Tillmann, W.; Fischer, H. Bioprinting organotypic hydrogels with improved mesenchymal stem cell remodeling and mineralization properties for bone tissue engineering. Adv. Healthc. Mater. 2016, 5, 1336–1345. [Google Scholar] [CrossRef]
- Roth, E.A.; Xu, T.; Das, M.; Gregory, C.; Hickman, J.J.; Boland, T. Inkjet printing for high-throughput cell patterning. Biomaterials 2004, 25, 3707–3715. [Google Scholar] [CrossRef]
- Sanjana, N.E.; Fuller, S.B. A fast flexible ink-jet printing method for patterning dissociated neurons in culture. J. Neurosci. Methods 2004, 136, 151–163. [Google Scholar] [CrossRef] [PubMed]
- Park, T.M.; Kang, D.; Jang, I.; Yun, W.S.; Shim, J.H.; Jeong, Y.H.; Kwak, J.Y.; Yoon, S.; Jin, S. Fabrication of in vitro cancer microtissue array on fibroblast-layered nanofibrous membrane by inkjet printing. Int. J. Mol. Sci. 2017, 18, 2348. [Google Scholar] [CrossRef]
- Boland, T.; Mironov, V.; Gutowska, A.; Roth, E.A.; Markwald, R.R. Cell and organ printing 2: Fusion of cell aggregates in three-dimensional gels. Anat. Rec. Part A Discov. Mol. Cell. Evol. Biol. 2003, 272A, 497–502. [Google Scholar] [CrossRef]
- Keriquel, V.; Oliveira, H.; Remy, M.; Ziane, S.; Delmond, S.; Rousseau, B.; Rey, S.; Catros, S.; Amedee, J.; Guillemot, F.; et al. In situ printing of mesenchymal stromal cells, by laser-assisted bioprinting, for in vivo bone regeneration applications. Sci. Rep. 2017, 7, 1778. [Google Scholar] [CrossRef]
- Guillotin, B.; Guillemot, F. Cell patterning technologies for organotypic tissue fabrication. Trends Biotechnol. 2011, 29, 183–190. [Google Scholar] [CrossRef] [PubMed]
- Guillotin, B.; Catros, S.; Guillemot, F. Laser assisted bio-printing (LAB) of Cells and Bio-materials Based on Laser Induced Forward Transfer (LIFT). In Laser Technology in Biomimetics; Biological and Medical Physics, Biomedical Engineering; Springer: Berlin/Heidelberg, Germany, 2013; pp. 193–209. ISBN1 978-3-642-41340-7. ISBN2 978-3-642-41341-4. [Google Scholar]
- Koch, L.; Deiwick, A.; Schlie, S.; Michael, S.; Gruene, M.; Coger, V.; Zychlinski, D.; Schambach, A.; Reimers, K.; Vogt, P.M.; et al. Skin tissue generation by laser cell printing. Biotechnol. Bioeng. 2012, 109, 1855–1863. [Google Scholar] [CrossRef] [PubMed]
- Michael, S.; Sorg, H.; Peck, C.T.; Koch, L.; Deiwick, A.; Chichkov, B.; Vogt, P.M.; Reimers, K. Tissue engineered skin substitutes created by laser-assisted bioprinting form skin-like structures in the dorsal skin fold chamber in mice. PLoS ONE 2013, 8, e57741. [Google Scholar] [CrossRef] [PubMed]
- Sorkio, A.; Koch, L.; Koivusalo, L.; Deiwick, A.; Miettinen, S.; Chichkov, B.; Skottman, H. Human stem cell based corneal tissue mimicking structures using laser-assisted 3D bioprinting and functional bioinks. Biomaterials 2018, 171, 57–71. [Google Scholar] [CrossRef]
- Knowlton, S.; Yenilmez, B.; Anand, S.; Tasoglu, S. Photocrosslinking-based bioprinting: Examining crosslinking schemes. Bioprinting 2017, 5, 10–18. [Google Scholar] [CrossRef]
- Bártolo, P.J. Stereolithography: Materials, Processes and Applications; Springer: New York, NY, USA, 2011; ISBN 978-0-387-92903-3. [Google Scholar]
- Drzewiecki, K.E.; Malavade, J.N.; Ahmed, I.; Lowe, C.J.; Shreiber, D.I. A thermoreversible, photocrosslinkable collagen bio-ink for free-form fabrication of scaffolds for regenerative medicine. Technology 2017, 5, 185–195. [Google Scholar] [CrossRef]
- Goldstein, T.A.; Epstein, C.J.; Schwartz, J.; Krush, A.; Lagalante, D.J.; Mercadante, K.P.; Zeltsman, D.; Smith, L.P.; Grande, D.A. Feasibility of bioprinting with a modified desktop 3D printer. Tissue Eng. Part C Methods 2016, 22, 1071–1076. [Google Scholar] [CrossRef]
- Murphy, S.V.; Skardal, A.; Atala, A. Evaluation of hydrogels for bio-printing applications. J. Biomed. Mater. Res. Part A 2013, 101A, 272–284. [Google Scholar] [CrossRef] [PubMed]
- Hollister, S.J.; Murphy, W.L. Scaffold translation: Barriers between concept and clinic. Tissue Eng. Part B. Rev. 2011, 17, 459–474. [Google Scholar] [CrossRef]
- Pashuck, E.T.; Stevens, M.M. Designing regenerative biomaterial therapies for the clinic. Sci. Transl. Med. 2012, 4, 160sr4. [Google Scholar] [CrossRef] [PubMed]
- Fernandez-Moure, J.S. Lost in Translation: The gap in scientific advancements and clinical application. Front. Bioeng. Biotechnol. 2016, 4, 43. [Google Scholar] [CrossRef] [PubMed]
- Ratcliffe, A. Difficulties in the translation of functionalized biomaterials into regenerative medicine clinical products. Biomaterials 2011, 32, 4215–4217. [Google Scholar] [CrossRef][Green Version]
- Ng, W.L.; Wang, S.; Yeong, W.Y.; Naing, M.W. Skin bioprinting: Impending reality or fantasy? Trends Biotechnol. 2016, 34, 689–699. [Google Scholar] [CrossRef] [PubMed]
- Hourd, P.; Medcalf, N.; Segal, J.; Williams, D.J. A 3D bioprinting exemplar of the consequences of the regulatory requirements on customized processes. Regen. Med. 2015, 10, 863–883. [Google Scholar] [CrossRef]
- Furth, M.E.; Atala, A.; van Dyke, M.E. Smart biomaterials design for tissue engineering and regenerative medicine. Biomaterials 2007, 28, 5068–5073. [Google Scholar] [CrossRef]
| Bioprinting Method | Collagen-Based Ink Formulation | Outcome | Ref. |
|---|---|---|---|
| Extrusion | Methacrylated type I collagen; Sodium alginate | Fabrication of structures that resembles native human corneal stroma with cell-laden bioink via extrusion bioprinting. | [116] |
| Extrusion | Collagen Type I; Alginic acid sodium salt from brown algae; CaCl2 solution | Core-sheath coaxial extrusion of alginate/collagen bioink with CaCl2 allows creation of scaffolds with low collagen centration despite its low viscosity. | [114] |
| Extrusion | Rat tail type I collagen; Gelatin (type A); Sodium alginate | Extrusion bioprinting of collagen scaffold via gelatin/alginate system with controllable degradation time based on amount of sodium citrate during incubation. | [113] |
| Extrusion | Type I collagen was extracted from tendons obtained from rat tails | Identified storage modulus as the best predictor of collagen bioink printability during deposition. | [117] |
| Extrusion | PureCol Purified Bovine Collagen Solution; Soldium alginate (low viscosity) | Fabrication of interwoven hard (PLLA) and soft (bioink) scaffolds which support cell attachment and proliferation using a modified desktop 3D printer. | [135] |
| Extrusion | Methacrylated COL I; Heprasil; Photoinitiator | Successful bioprinting of liver model. Printed primary hepatocytes retained function over 2 weeks exhibiting appropriate response to toxic drugs. | [41] |
| Extrusion | Lyophilized Atelo-collagen, Matrixen-PSP | Pre-set extrusion bioprinting technique is able to create heterogeneous, multicellular and multi-material structures which perform better than traditional bioprinting. | [112] |
| Extrusion | Collagen Type I extracted from rat tails; Pluronic® F127 | Fabrication of 3D constructs without chemical or photocrosslinking before and after printing via thermally-controlled extrusion. | [115] |
| Extrusion | Lyophilized sterile collagen, Viscoll | Formation of scaffolds which support spatial arrangement of tissue spheroids as well as support cell adhesion and proliferation. | [47] |
| Extrusion | Type-I collagen, Matrixen-PSP; Tannic acid | Fabrication of 3D porous structures which support cell migration and proliferation for long periods of culture. Determined optimal tannic acid crosslinking. | [67] |
| Extrusion | Collagen Type I; Sodium Alginate | Improved mechanical strength and bioactivity via the addition of collagen. Higher cartilage gene markers expressed, preservation of chondrocyte phenotype. | [42] |
| Extrusion | Type-1 collagen, Matrixen-PSP | Established a crosslinking process using tannic acid. High printed preosteoblast viability and well-defined pore size and strut dimensions for bone regeneration. | [68] |
| Extrusion | Type-I collagen, Matrixen-PSP; Decellularised extracellular matrix (dECM); Silk Fibroin(SF) | Hybrid collagen/dECM/SF scaffold with enhanced cellular activity and mechanical properties. Enhanced cell differentiation, mechanical properties, amenable for hard tissue regeneration. | [59] |
| Extrusion | Atelocollagen Type I powder | Novel self-assembly induced 3D printing to produce macro/nano porous collagen scaffolds with reasonable mechanical properties, excellent biocompatibility and mimicking native ECM. | [58] |
| Extrusion | Type-I collagen, Matrixen-PSP; Polycaprolactone (PCL); Hydroxyapatite (HA)/β-tricalcium-phosphate (TCP); Platelet-rich plasma(PRP) | Fabrication of collagen/PCL biocomposites loaded with bio-additives via 3D extrusion printing. Collagen/PCL biocomposites allow controlled release of HA/TCP bio-additives, which promote osteogenesis. PRP biocomposites demonstrate increased mineralisation. | [46] |
| Extrusion | Type-I collagen, Matrixen-PSP | Genipin crosslinking allowed fabrication of 3D cell-laden porous scaffold (Cellblock) with mechanical stability, pore size and osteogenic (bone tissue regeneration) potential. | [70] |
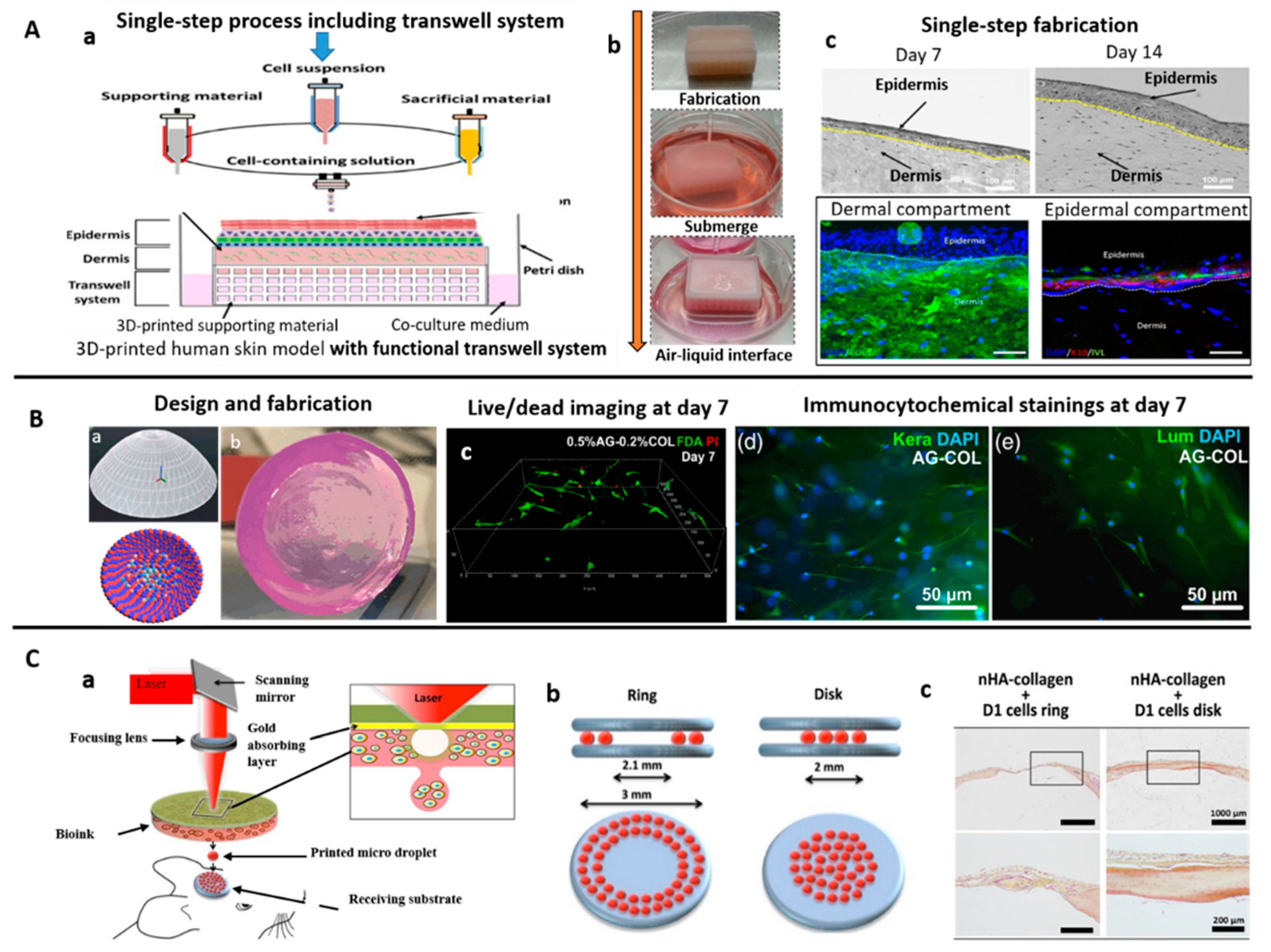
| Extrusion/Inkjet | Lyophilized collagen type 1 sponge derived from porcine skin | Development of a one-step process to produce a 3D human skin model with functional transwell system. Cost-effective compared to traditional transwell cultures. | [118] |
| Inkjet | Type I rat tail collagen; poly-d-lysine | Fabrication of neuron-adhesive patterns by printing cell-adhesive layers onto cell-repulsive substrates. | [123] |
| Inkjet | Collagen (Calf skin) | Cell aggregates printed between layers of collagen gels suitable for tissue engineering. | [125] |
| Inkjet | Collagen (rat-tail); collagen (calf skin) | Low-cost, high-throughput surface patterning with collagen and potentially, other proteins. | [122] |
| Inkjet | Collagen Type I | Fabrication of in vitro cancer microtissues via collagen inkjet printing. Four individual microtissues within one 96-well plate well, maintained for up to seven days. | [124] |
| Inkjet | Collagen: Type I rat tail collagen; Fibrinogen; Thrombin | Collagen bioinks and Fibrin/Collagen bioinks unsuitable for in situ inkjet bioprinting. | [136] |
| Inkjet | Type I acidic collagen; Agarose (low gelling temperature) | Fabrication of 3D corneal stromal structure with optically properties similar to native corneal stroma. Potential as a clinical or experimental model. | [120] |
| Inkjet | Acidic collagen solution; Agarose (low gelling temperature) | MSC branching, spreading and osteogenic differentiation controlled by collagen concentration; Osteogenic potential (bone tissue engineering). | [121] |
| Laser-assisted | Collagen Type I (Rat-tail) | Fabrication of cell-laden skin tissue using laser-assisted bioprinting, in vivo potential. Skin tissues consist of: a base matriderm layer, 20 layers of fibroblast and 20 layers of keratinocytes. | [130] |
| Laser-assisted | Collagen (Rat-tail) | Multicellular collagen skin tissue constructs printed using laser-assisted bioprinting. Keratinocyte and fibroblast layers did not intermix after 10 days. Mimics tissue-specific functions (e.g., gap-junction). | [129] |
| Laser-assisted | Type I collagen (rat) solution; Nano hydroxyapatite (nHA) | In situ printing of cell-laden collagen-based ink via laser assisted bioprinting allow bone regeneration (mouse calvaria defect model). Contact free printing method is sterile with clinical potential. | [126] |
| Laser-assisted | OptiCol™ human Col I; Ethylenediaminetetraacetic acid (EDTA) human female AB blood plasma; Thrombin from human plasma | Fabrication of 3D cornea tissue using novel human protein bioinks via laser assisted bioprinting. Novel bioink is biocompatible, without requiring additional crosslinking. First study to demonstrate laser-assisted bioprinting for corneal applications using human stem cells. | [131] |
| Stereolithography (SLA) | Collagen methacrylamide(CMA) synthesized using Type-I collagen; Irgacure (I2959) | Free-form photolithographic fabrication; photopatterned hydrogels retain structure after 24 h. CMA retains native collagen self-assembling properties; hydrogels biocompatible in vivo. | [134] |
© 2020 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (http://creativecommons.org/licenses/by/4.0/).
Share and Cite
Chan, W.W.; Yeo, D.C.L.; Tan, V.; Singh, S.; Choudhury, D.; Naing, M.W. Additive Biomanufacturing with Collagen Inks. Bioengineering 2020, 7, 66. https://doi.org/10.3390/bioengineering7030066
Chan WW, Yeo DCL, Tan V, Singh S, Choudhury D, Naing MW. Additive Biomanufacturing with Collagen Inks. Bioengineering. 2020; 7(3):66. https://doi.org/10.3390/bioengineering7030066
Chicago/Turabian StyleChan, Weng Wan, David Chen Loong Yeo, Vernice Tan, Satnam Singh, Deepak Choudhury, and May Win Naing. 2020. "Additive Biomanufacturing with Collagen Inks" Bioengineering 7, no. 3: 66. https://doi.org/10.3390/bioengineering7030066
APA StyleChan, W. W., Yeo, D. C. L., Tan, V., Singh, S., Choudhury, D., & Naing, M. W. (2020). Additive Biomanufacturing with Collagen Inks. Bioengineering, 7(3), 66. https://doi.org/10.3390/bioengineering7030066




