Optimization of Co-Culture Conditions for a Human Vascularized Adipose Tissue Model
Abstract
1. Introduction
2. Materials and Methods
2.1. Cell Culture and Differentiation
2.2. Cell Spheroid Formation and Encapsulation
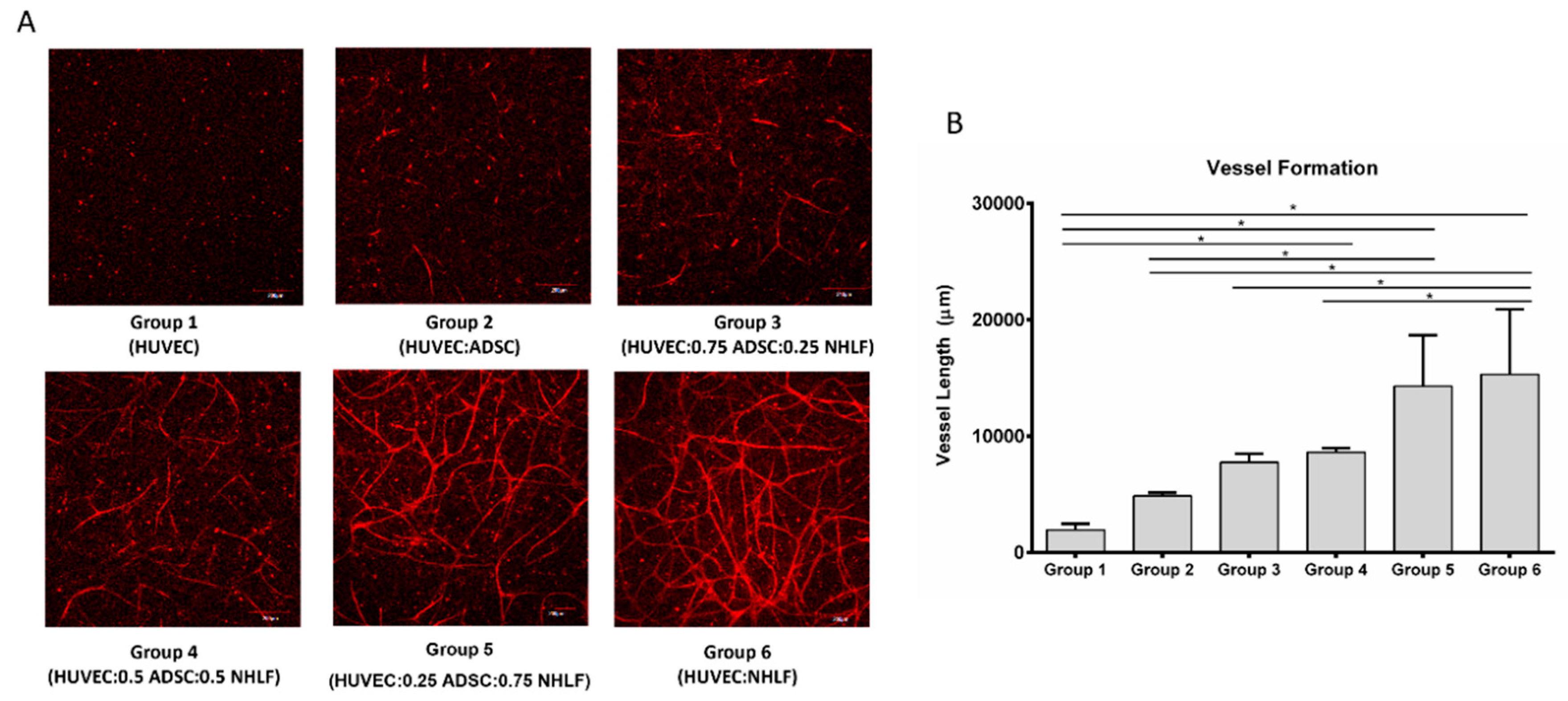
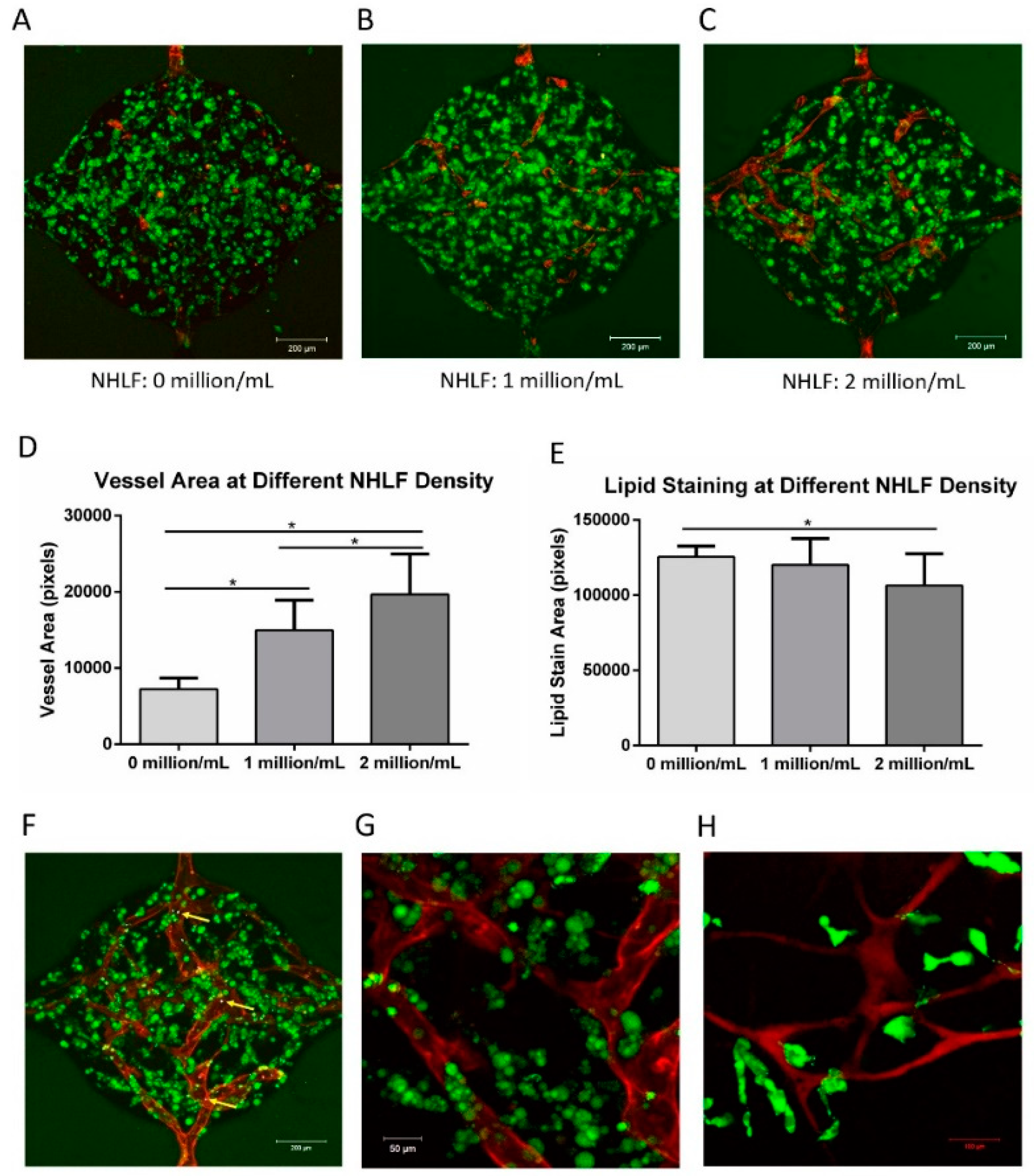
2.3. Identification of Supporting Cells for Vessel Formation
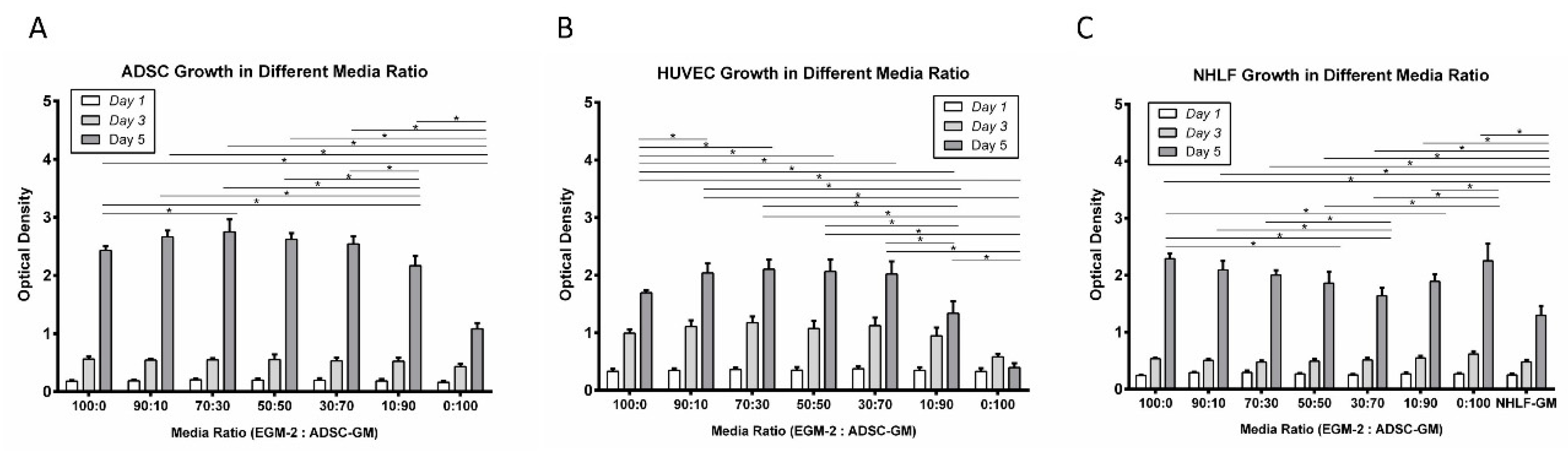
2.4. Cell Proliferation Assay
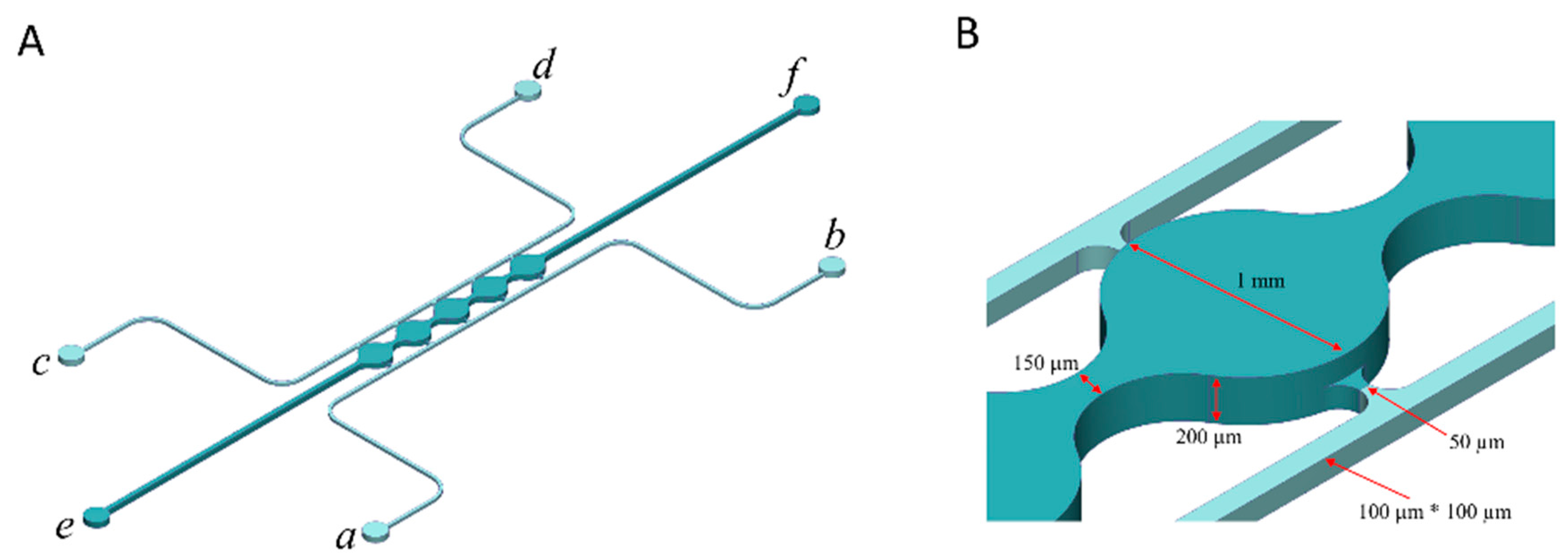
2.5. Fabrication of a Microfluidic Device
2.6. Cell Loading to the Microfluidic Device
2.7. Staining
2.8. Fatty Acid Uptake
2.9. Statistics
3. Results
3.1. Proliferation
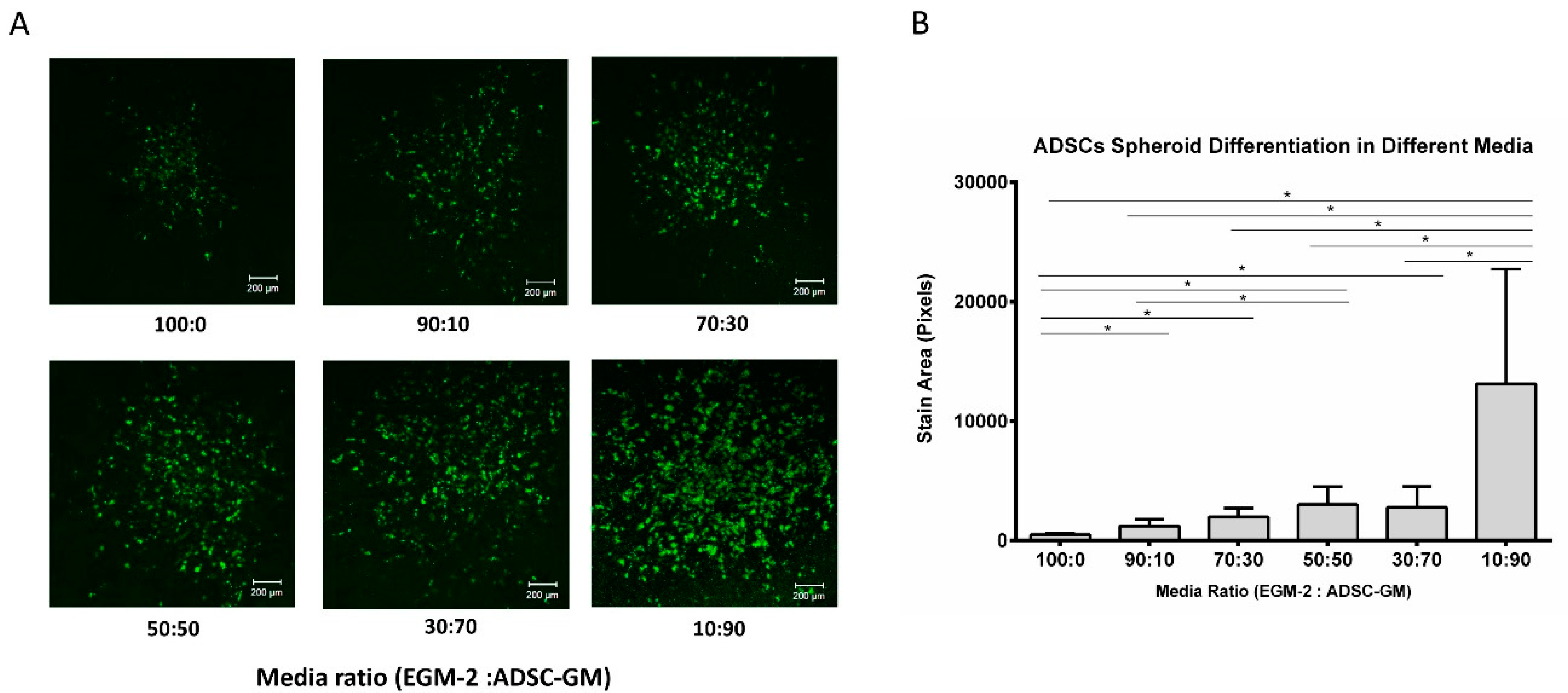
3.2. Adipogenic Differentiation
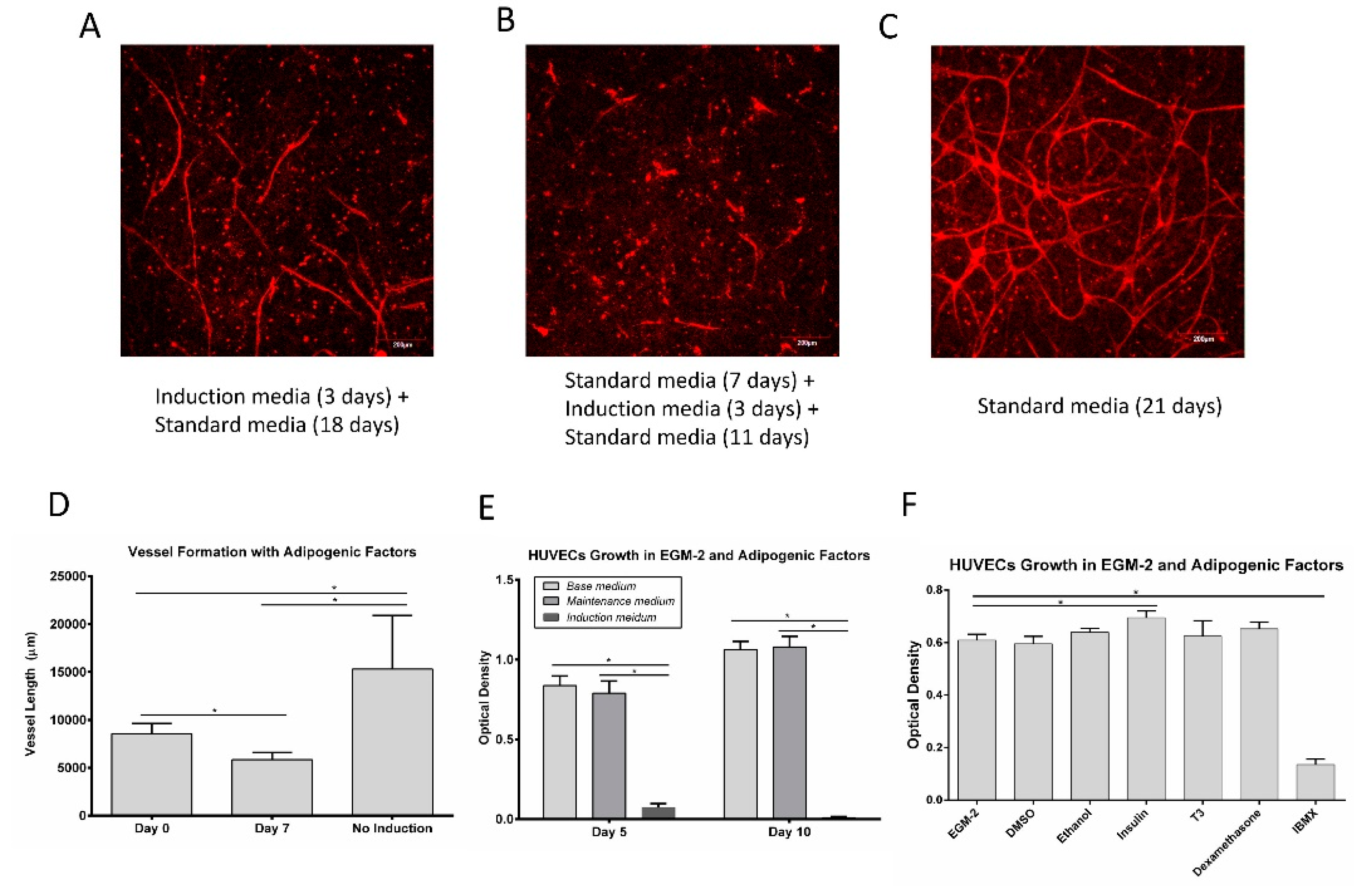
3.3. Vessel Formation
3.4. Vessel Assembly in the Presence of Adipogenic Factors
3.5. Adipogenic Induction of ADSC in a Microfluidic Device
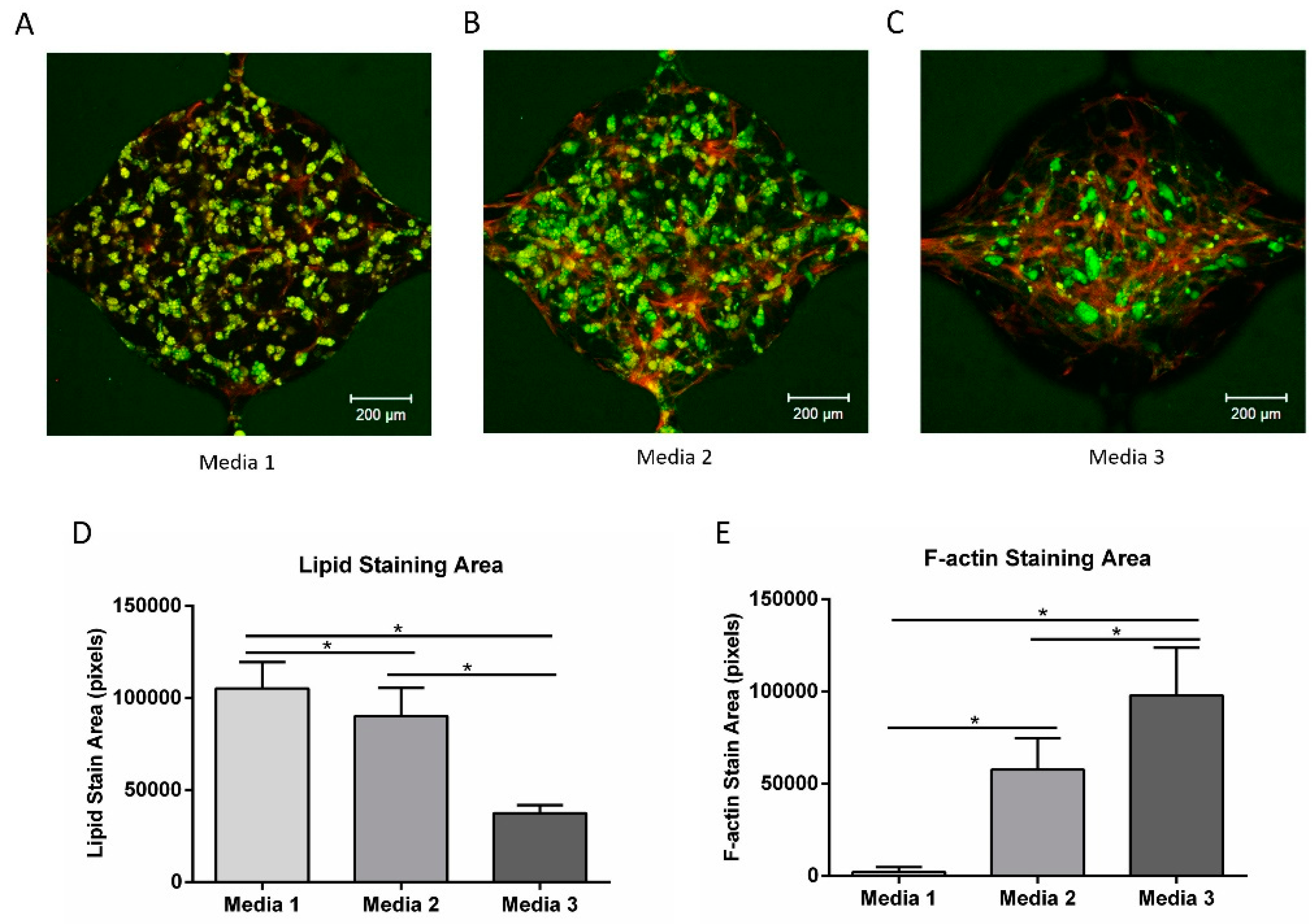
3.6. Pre-Induction of ADSC for Co-Culture
3.7. Vascular Network Formation Using Co-Culture Media
3.8. Vascularized Adipose Tissue in the Microfluidic Device
4. Discussion
5. Conclusions
Supplementary Materials
Author Contributions
Funding
Acknowledgments
Conflicts of Interest
References
- Luo, L.; Liu, M. Adipose tissue in control of metabolism. J. Endocrinol. 2016, 231, R77–R99. [Google Scholar] [CrossRef] [PubMed]
- Biener, A.; Cawley, J.; Meyerhoefer, C. The High and Rising Costs of Obesity to the US Health Care System. J. Gen. Intern. Med. 2017, 32, 6–8. [Google Scholar] [CrossRef] [PubMed]
- Dai, H.; Alsalhe, T.; Chalghaf, N.; Riccò, M.; Bragazzi, N.; Wu, J. chicaAC and territories, 1990–2017: An analysis of the Global Burden of Disease Study. PLoS Med. 2020, 17, e1003198. [Google Scholar] [CrossRef] [PubMed]
- Christiaens, V.; Lijnen, H.R. Angiogenesis and development of adipose tissue. Mol. Cell. Endocrinol. 2010, 318, 2–9. [Google Scholar] [CrossRef] [PubMed]
- Trayhurn, P. Hypoxia and adipose tissue function and dysfunction in obesity. Physiol. Rev. 2013, 93, 1–21. [Google Scholar] [CrossRef] [PubMed]
- Cao, Y. Adipose tissue angiogenesis as a therapeutic target for obesity and metabolic diseases. Nat. Rev. Drug Discov. 2010, 9, 107. [Google Scholar] [CrossRef]
- Goldberg, R.B. Cytokine and cytokine-like inflammation markers, endothelial dysfunction, and imbalanced coagulation in development of diabetes and its complications. J. Clin. Endocrinol. Metab. 2009, 94, 3171–3182. [Google Scholar] [CrossRef]
- Kayabolen, A.; Keskin, D.; Aykan, A.; Karslıoglu, Y.; Zor, F.; Tezcaner, A. Native extracellular matrix/fibroin hydrogels for adipose tissue engineering with enhanced vascularization. Biomed. Mater. 2017, 12, 35007. [Google Scholar] [CrossRef]
- Volz, A.; Hack, L.; Kluger, P.J. A cellulose-based material for vascularized adipose tissue engineering. J. Biomed. Mater. Res. Part B Appl. Biomater. 2019, 107, 1434–1439. [Google Scholar] [CrossRef]
- Yao, R.; Du, Y.; Zhang, R.; Lin, F.; Luan, J. A biomimetic physiological model for human adipose tissue by adipocytes and endothelial cell cocultures with spatially controlled distribution. Biomed. Mater. 2013, 8, 045005. [Google Scholar] [CrossRef]
- Wittmann, K.; Dietl, S.; Ludwig, N.; Berberich, O.; Hoefner, C.; Storck, K.; Blunk, T.; Bauer-Kreisel, P. Engineering Vascularized Adipose Tissue Using the Stromal-Vascular Fraction and Fibrin Hydrogels. Tissue Eng. Part A 2015, 21, 1343–1353. [Google Scholar] [CrossRef] [PubMed]
- Kang, J.H.; Gimble, J.M.; Kaplan, D.L. In Vitro 3D Model for Human Vascularized Adipose Tissue. Tissue Eng. Part A 2009, 15, 2227–2236. [Google Scholar] [CrossRef] [PubMed]
- Yao, R.; Zhang, R.; Lin, F.; Luan, J. Biomimetic injectable HUVEC-adipocytes/collagen/alginate microsphere co-cultures for adipose tissue engineering. Biotechnol. Bioeng. 2013, 110, 1430–1443. [Google Scholar] [CrossRef] [PubMed]
- Moreno-Navarrete, J.M.; Fernández-Real, J.M. Adipocyte differentiation. In Adipose Tissue Biology; Springer: Berlin/Heidelberg, Germany, 2017; pp. 69–90. [Google Scholar]
- Lee, M.J.; Wu, Y.Y.; Fried, S.K. A Modified Protocol to Maximize Differentiation of Human Preadipocytes and Improve Metabolic Phenotypes. Obesity 2012, 20, 2334–2340. [Google Scholar] [CrossRef] [PubMed]
- Danielsen, T.; Rofstad, E.K. VEGF, bFGF and EGF in the angiogenesis of human melanoma xenografts. Int. J. Cancer 1998, 76, 836–841. [Google Scholar] [CrossRef]
- Cross, M.J.; Claesson-Welsh, L. FGF and VEGF function in angiogenesis: Signalling pathways, biological responses and therapeutic inhibition. Trends Pharmacol. Sci. 2001, 22, 201–207. [Google Scholar] [CrossRef]
- Lequoy, P.; Murschel, F.; Liberelle, B.; Lerouge, S.; De Crescenzo, G. Controlled co-immobilization of EGF and VEGF to optimize vascular cell survival. Acta Biomater. 2016, 29, 239–247. [Google Scholar] [CrossRef]
- Volz, A.-C.; Huber, B.; Schwandt, A.M.; Kluger, P.J. EGF and hydrocortisone as critical factors for the co-culture of adipogenic differentiated ASCs and endothelial cells. Differentiation 2017, 95, 21–30. [Google Scholar] [CrossRef]
- Guilak, F.; Cohen, D.M.; Estes, B.T.; Gimble, J.M.; Liedtke, W.; Chen, C.S. Control of Stem Cell Fate by Physical Interactions with the Extracellular Matrix. Cell Stem Cell 2009, 5, 17–26. [Google Scholar] [CrossRef]
- Zhang, S.C.; Liu, P.; Chen, L.; Wang, Y.J.; Wang, Z.G.; Zhang, B. The effects of spheroid formation of adipose-derived stem cells in a microgravity bioreactor on sternness properties and therapeutic potential. Biomaterials 2015, 41, 15–25. [Google Scholar] [CrossRef]
- Cheng, N.-C.; Wang, S.; Young, T.-H. The influence of spheroid formation of human adipose-derived stem cells on chitosan films on stemness and differentiation capabilities. Biomaterials 2012, 33, 1748–1758. [Google Scholar] [CrossRef] [PubMed]
- Welti, J.; Loges, S.; Dimmeler, S.; Carmeliet, P. Recent molecular discoveries in angiogenesis and antiangiogenic therapies in cancer. J. Clin. Investig. 2013, 123, 3190–3200. [Google Scholar] [CrossRef] [PubMed]
- Kachgal, S.; Putnam, A.J. Mesenchymal stem cells from adipose and bone marrow promote angiogenesis via distinct cytokine and protease expression mechanisms. Angiogenesis 2011, 14, 47–59. [Google Scholar] [CrossRef] [PubMed]
- Freiman, A.; Shandalov, Y.; Rozenfeld, D.; Shor, E.; Segal, S.; Ben-David, D.; Meretzki, S.; Egozi, D.; Levenberg, S. Adipose-derived endothelial and mesenchymal stem cells enhance vascular network formation on three-dimensional constructs in vitro. Stem Cell Res. Ther. 2016, 7. [Google Scholar] [CrossRef] [PubMed]
- Moya, M.L.; Hsu, Y.H.; Lee, A.P.; Hughes, C.C.W.; George, S.C. In Vitro Perfused Human Capillary Networks. Tissue Eng. Part C-Methods 2013, 19, 730–737. [Google Scholar] [CrossRef]
- Wang, X.L.; Phan, D.T.T.; Sobrino, A.; George, S.C.; Hughes, C.C.W.; Lee, A.P. Engineering anastomosis between living capillary networks and endothelial cell-lined microfluidic channels. Lab Chip 2016, 16, 282–290. [Google Scholar] [CrossRef]
- Hsu, Y.-H.; Moya, M.L.; Abiri, P.; Hughes, C.C.W.; George, S.C.; Lee, A.P. Full range physiological mass transport control in 3D tissue cultures. Lab Chip 2013, 13, 81–89. [Google Scholar] [CrossRef]
- Kim, S.; Lee, H.; Chung, M.; Jeon, N.L. Engineering of functional, perfusable 3D microvascular networks on a chip. Lab Chip 2013, 13, 1489–1500. [Google Scholar] [CrossRef]
- Hsu, Y.-H.; Moya, M.L.; Hughes, C.C.W.; George, S.C.; Lee, A.P. A microfluidic platform for generating large-scale nearly identical human microphysiological vascularized tissue arrays. Lab Chip 2013, 13, 2990–2998. [Google Scholar] [CrossRef] [PubMed]
- Campisi, M.; Shin, Y.; Osaki, T.; Hajal, C.; Chiono, V.; Kamm, R.D. 3D self-organized microvascular model of the human blood-brain barrier with endothelial cells, pericytes and astrocytes. Biomaterials 2018, 180, 117–129. [Google Scholar] [CrossRef]
- Sobrino, A.; Phan, D.T.T.; Datta, R.; Wang, X.L.; Hachey, S.J.; Romero-Lopez, M.; Gratton, E.; Lee, A.P.; George, S.C.; Hughes, C.C.W. 3D microtumors in vitro supported by perfused vascular networks. Sci. Rep. 2016, 6, 31589. [Google Scholar] [CrossRef] [PubMed]
- Jeon, J.S.; Bersini, S.; Gilardi, M.; Dubini, G.; Charest, J.L.; Moretti, M.; Kamm, R.D. Human 3D vascularized organotypic microfluidic assays to study breast cancer cell extravasation. Proc. Natl. Acad. Sci. USA 2015, 112, 214–219. [Google Scholar] [CrossRef]
- Whisler, J.A.; Chen, M.B.; Kamm, R.D. Control of Perfusable Microvascular Network Morphology Using a Multiculture Microfluidic System. Tissue Eng. Part C-Methods 2014, 20, 543–552. [Google Scholar] [CrossRef]
- Namkoong, S.; Kim, C.K.; Cho, Y.L.; Kim, J.H.; Lee, H.; Ha, K.S.; Choe, J.; Kim, P.H.; Won, M.H.; Kwon, Y.G.; et al. Forskolin increases angiogenesis through the coordinated cross-talk of PKA-dependent VEGF expression and Epac-mediated PI3K/Akt/eNOS signaling. Cell. Signal. 2009, 21, 906–915. [Google Scholar] [CrossRef] [PubMed]
- Huber, B.; Kluger, P.J. Decelerating Mature Adipocyte Dedifferentiation by Media Composition. Tissue Eng. Part C Methods 2015, 21, 1237–1245. [Google Scholar] [CrossRef] [PubMed]
- Huber, B.; Maria Czaja, A.; Kluger, P.J.; Czaja, A.M.; Kluger, P.J. Influence of epidermal growth factor (EGF) and hydrocortisone on the co-culture of mature adipocytes and endothelial cells for vascularized adipose tissue engineering. Cell Biol. Int. 2016, 40, 569–578. [Google Scholar] [CrossRef]
- Hauner, H.; Rohrig, K.; Petruschke, T. Effects of epidermal growth factor (EGF), platelet-derived growth factor (PDGF) and fibroblast growth factor (FGF) on human adipocyte development and function. Eur. J. Clin. Investig. 1995, 25, 90–96. [Google Scholar] [CrossRef]
- Liao, Y.; Zeng, Z.; Lu, F.; Dong, Z.; Chang, Q.; Gao, J. In Vivo Dedifferentiation of Adult Adipose Cells. PLoS ONE 2015, 10, e0125254. [Google Scholar] [CrossRef]
- Le Blanc, S.; Simann, M.; Jakob, F.; Schütze, N.; Schilling, T. Fibroblast growth factors 1 and 2 inhibit adipogenesis of human bone marrow stromal cells in 3D collagen gels. Exp. Cell Res. 2015, 338, 136–148. [Google Scholar] [CrossRef]
- Liu, Y.; Berendsen, A.D.; Jia, S.; Lotinun, S.; Baron, R.; Ferrara, N.; Olsen, B.R. Intracellular VEGF regulates the balance between osteoblast and adipocyte differentiation. J. Clin. Investig. 2012, 122, 3101–3113. [Google Scholar] [CrossRef]
- Wilson, K.L.; Berk, J.M. The nuclear envelope at a glance. J. Cell Sci. 2010, 123, 1973–1978. [Google Scholar] [CrossRef] [PubMed]
- Obregon, M.-J. Thyroid hormone and adipocyte differentiation. Thyroid 2008, 18, 185–195. [Google Scholar] [CrossRef] [PubMed]
- Flores-Delgado, G.; Marsch-Moreno, M.; Kuri-Harcuch, W. Thyroid hormone stimulates adipocyte differentiation of 3T3 cells. Mol. Cell. Biochem. 1987, 76, 35–43. [Google Scholar] [CrossRef] [PubMed]
- Lee, M.J.; Pramyothin, P.; Karastergiou, K.; Fried, S.K. Deconstructing the roles of glucocorticoids in adipose tissue biology and the development of central obesity. Biochim. Biophys. Acta Mol. Basis Dis. 2014, 1842, 473–481. [Google Scholar] [CrossRef] [PubMed]
- Tomlinson, J.J.; Boudreau, A.; Wu, D.; Atlas, E.; Haché, R.J.G. Modulation of early human preadipocyte differentiation by glucocorticoids. Endocrinology 2006, 147, 5284–5293. [Google Scholar] [CrossRef]
- Wiper-Bergeron, N.; Salem, H.A.; Tomlinson, J.J.; Wu, D.; Hache, R.J.G. Glucocorticoid-stimulated preadipocyte differentiation is mediated through acetylation of C/EBPbeta by GCN5. Proc. Natl. Acad. Sci. USA 2007, 104, 2703–2708. [Google Scholar] [CrossRef]
- Zhang, Y.; Li, X.; Qian, S.; Guo, L.; Huang, H.; He, Q.; Liu, Y.; Ma, C.; Tang, Q.-Q. Down-Regulation of Type I Runx2 Mediated by Dexamethasone Is Required for 3T3-L1 Adipogenesis. Mol. Endocrinol. 2012, 26, 798–808. [Google Scholar] [CrossRef]
- Saltiel, A.R.; Olefsky, J.M. Thiazolidinediones in the treatment of insulin resistance and type II diabetes. Diabetes 1996, 45, 1661–1669. [Google Scholar] [CrossRef]
- Arnold, S.V.; Inzucchi, S.E.; Echouffo-Tcheugui, J.B.; Tang, F.; Lam, C.S.P.; Sperling, L.S.; Kosiborod, M. Understanding Contemporary Use of Thiazolidinediones: An Analysis From the Diabetes Collaborative Registry. Circ. Heart Fail. 2019, 12, e005855. [Google Scholar] [CrossRef]
- Benvenuti, S.; Cellai, I.; Luciani, P.; Deledda, C.; Baglioni, S.; Giuliani, C.; Saccardi, R.; Mazzanti, B.; Dal Pozzo, S.; Mannucci, E. Rosiglitazone stimulates adipogenesis and decreases osteoblastogenesis in human mesenchymal stem cells. J. Endocrinol. Investig. 2007, 30, RC26–RC30. [Google Scholar] [CrossRef] [PubMed]
- Kanne, H.; Burte, N.P.; Prasanna, V.; Gujjula, R. Extraction and elemental analysis of Coleus forskohlii extract. Pharmacogn. Res. 2015, 7, 237. [Google Scholar] [CrossRef]
- Sapio, L.; Gallo, M.; Illiano, M.; Chiosi, E.; Naviglio, D.; Spina, A.; Naviglio, S. The natural cAMP elevating compound forskolin in cancer therapy: Is it time? J. Cell. Physiol. 2017, 232, 922–927. [Google Scholar] [CrossRef] [PubMed]
- Petersen, R.K.; Madsen, L.; Pedersen, L.M.; Hallenborg, P.; Hagland, H.; Viste, K.; Doskeland, S.O.; Kristiansen, K. Cyclic AMP (cAMP)-mediated stimulation of adipocyte differentiation requires the synergistic action of Epac- and cAMP-dependent protein kinase-dependent processes. Mol. Cell. Biol. 2008, 28, 3804–3816. [Google Scholar] [CrossRef]
- Li, F.; Wang, D.; Zhou, Y.; Zhou, B.; Yang, Y.; Chen, H.; Song, J. Protein Kinase A suppresses the differentiation of 3T3-L1 preadipocytes. Cell Res. 2008, 18, 311–323. [Google Scholar] [CrossRef] [PubMed]
- Coppé, J.-P.; Kauser, K.; Campisi, J.; Beauséjour, C.M. Secretion of vascular endothelial growth factor by primary human fibroblasts at senescence. J. Biol. Chem. 2006, 281, 29568–29574. [Google Scholar] [CrossRef] [PubMed]
- Newman, A.C.; Nakatsu, M.N.; Chou, W.; Gershon, P.D.; Hughes, C.C.W. The requirement for fibroblasts in angiogenesis: Fibroblast-derived matrix proteins are essential for endothelial cell lumen formation. Mol. Biol. Cell 2011, 22, 3791–3800. [Google Scholar] [CrossRef]
- Balzan, S.; Del Carratore, R.; Nardulli, C.; Sabatino, L.; Lubrano, V.; Iervasi, G. The stimulative effect of T3 and T4 on human myocardial endothelial cell proliferation, migration, and angiogenesis. J. Clin. Exp. Cardiol. 2013, 4, 2. [Google Scholar]
- Jia, B.; Madsen, L.; Petersen, R.K.; Techer, N.; Kopperud, R.; Ma, T.; Døskeland, S.O.; Ailhaud, G.; Wang, J.; Amri, E.Z.; et al. Activation of protein kinase a and exchange protein directly activated by cAMP promotes adipocyte differentiation of human mesenchymal stem cells. PLoS ONE 2012, 7, e34114. [Google Scholar] [CrossRef]
- Perrot, C.Y.; Sawada, J.; Komatsu, M. Prolonged activation of cAMP signaling leads to endothelial barrier disruption via transcriptional repression of RRAS. FASEB J. 2018, 32, 5793–5812. [Google Scholar] [CrossRef] [PubMed]



| Groups | HUVEC | ADSC | NHLF |
|---|---|---|---|
| Group 1 (HUVEC) | 1 million/mL | 0 | 0 |
| Group 2 (HUVEC:ADSC) | 1 million/mL | 1 million/mL | 0 |
| Group 3 (HUVEC:0.75 ADSC:0.25 NHLF) | 1 million/mL | 0.75 million/mL | 0.25 million/mL |
| Group 4 (HUVEC:0.5 ADSC:0.5 NHLF) | 1 million/mL | 0.5 million/mL | 0.5 million/mL |
| Group 5 (HUVEC:0.25 ADSC:0.75 NHLF) | 1 million/mL | 0.25 million/mL | 0.75 million/mL |
| Group 6 (HUVEC:NHLF) | 1 million/mL | 0 | 1 million/mL |
| Groups | Media Composition |
|---|---|
| G01 | ADSC-GM |
| G02 | ADSC-GM + EBM-2 |
| G03 | ADSC-GM + EBM-2 + hEGF |
| G04 | ADSC-GM + EBM-2 + Hydrocortisone |
| G05 | ADSC-GM + EBM-2 + AA |
| G06 | ADSC-GM + EBM-2 + Heparin |
| G07 | ADSC-GM + EBM-2 + VEGF |
| G08 | ADSC-GM + EBM-2 + IGF |
| G09 | ADSC-GM + EBM-2 + hFGF-B |
| G10 | ADSC-GM + EBM-2 + VEGF + IGF + hFGF-B |
| G11 | ADSC-GM + EBM-2 + VEGF + IGF + hFGF-B + AA |
| G12 | ADSC-GM + EBM-2 + VEGF + IGF + hFGF-B + AA + Heparin |
| G13 | ADSC-GM + EBM-2 + VEGF + IGF + hFGF-B + AA + Heparin + hEGF |
| G14 | ADSC-GM + EBM-2 + VEGF + IGF + hFGF-B + AA + Heparin + Hydrocortisone |
| G15 | ADSC-GM + EBM-2 + VEGF + IGF + hFGF-B + AA + Heparin + Hegf + Hydrocortisone |
| Media | Media Composition | Adipogenic Factors |
|---|---|---|
| Media 1 | ADSC-GM + EBM-2+IGF + AA + Heparin | Insulin+T3+dexamethasone + rosiglitazone+forskolin |
| Media 2 | ADSC-GM + EBM-2 + IGF + AA + Heparin | Insulin+T3 + dexamethasone + rosiglitazone |
| Media 3 | ADSC-GM + EBM-2 + IGF + AA + Heparin | Insulin + T3 |
© 2020 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (http://creativecommons.org/licenses/by/4.0/).
Share and Cite
Yang, F.; Cohen, R.N.; Brey, E.M. Optimization of Co-Culture Conditions for a Human Vascularized Adipose Tissue Model. Bioengineering 2020, 7, 114. https://doi.org/10.3390/bioengineering7030114
Yang F, Cohen RN, Brey EM. Optimization of Co-Culture Conditions for a Human Vascularized Adipose Tissue Model. Bioengineering. 2020; 7(3):114. https://doi.org/10.3390/bioengineering7030114
Chicago/Turabian StyleYang, Feipeng, Ronald N. Cohen, and Eric M. Brey. 2020. "Optimization of Co-Culture Conditions for a Human Vascularized Adipose Tissue Model" Bioengineering 7, no. 3: 114. https://doi.org/10.3390/bioengineering7030114
APA StyleYang, F., Cohen, R. N., & Brey, E. M. (2020). Optimization of Co-Culture Conditions for a Human Vascularized Adipose Tissue Model. Bioengineering, 7(3), 114. https://doi.org/10.3390/bioengineering7030114





