Current Practice in Preoperative Virtual and Physical Simulation in Neurosurgery
Abstract
1. Introduction
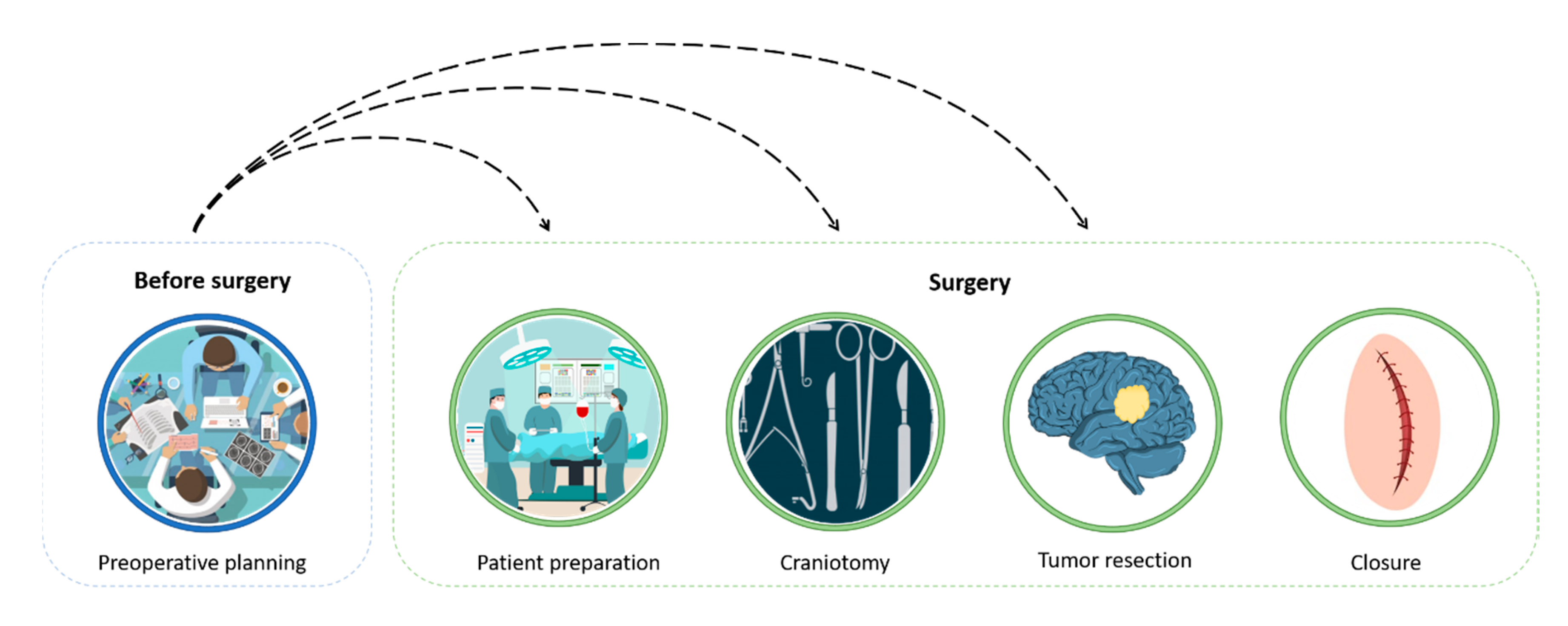
2. The Surgical Procedure and Planning
2.1. CT/MRI-Based Presurgical Planning
2.2. Virtual and Physical 3D Presurgical Planning
- (1)
- 3D reconstruction of the patient’s anatomy;
- (2)
- Surgery virtual planning;
- (3)
- Fabrication of the bio-model;
- (4)
- Surgery simulation (the surgeon uses a hands-on bio-model to simulate the surgery).
3. Simulation Process
3.1. 3D Reconstruction
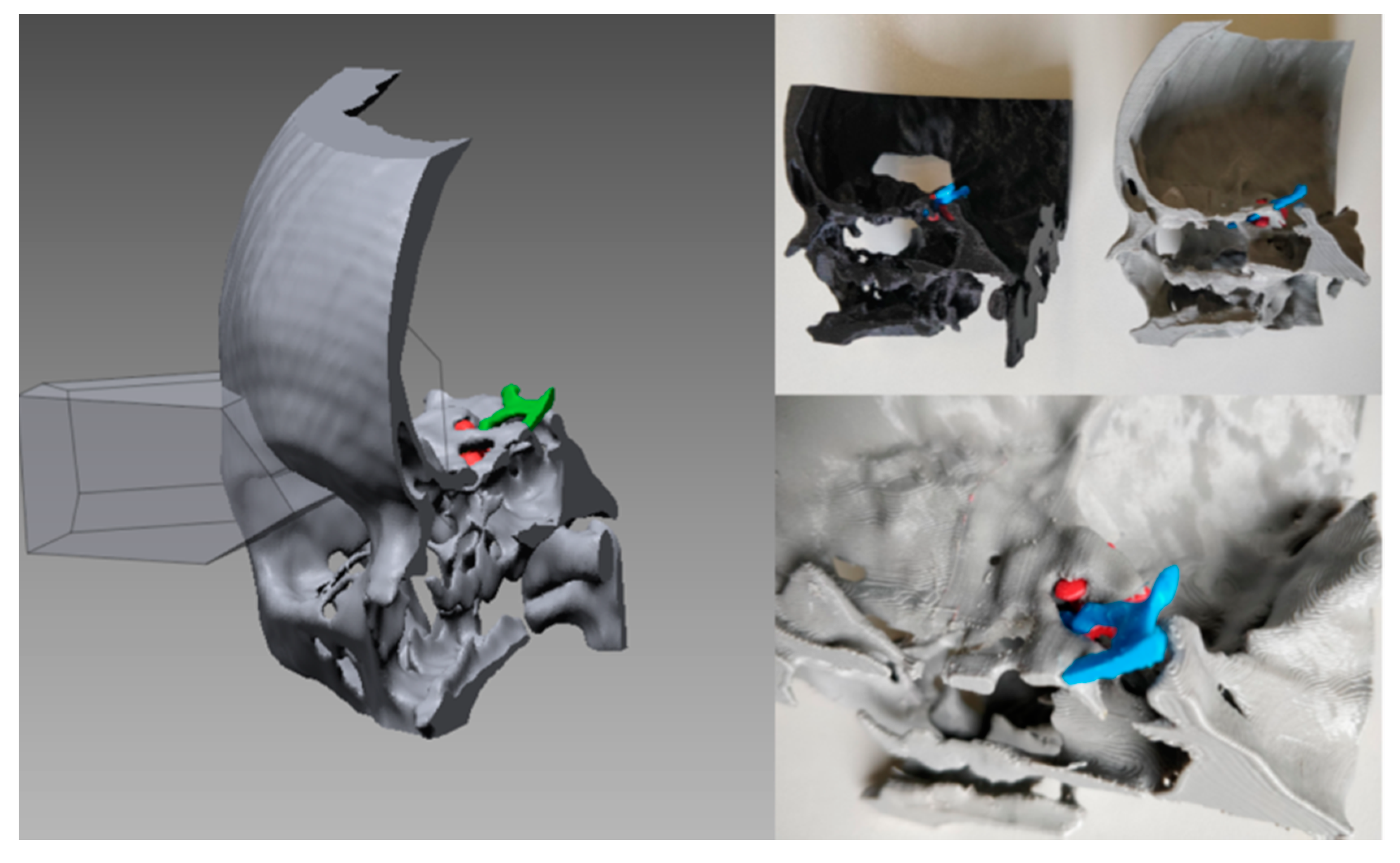
3.2. Virtual Planning
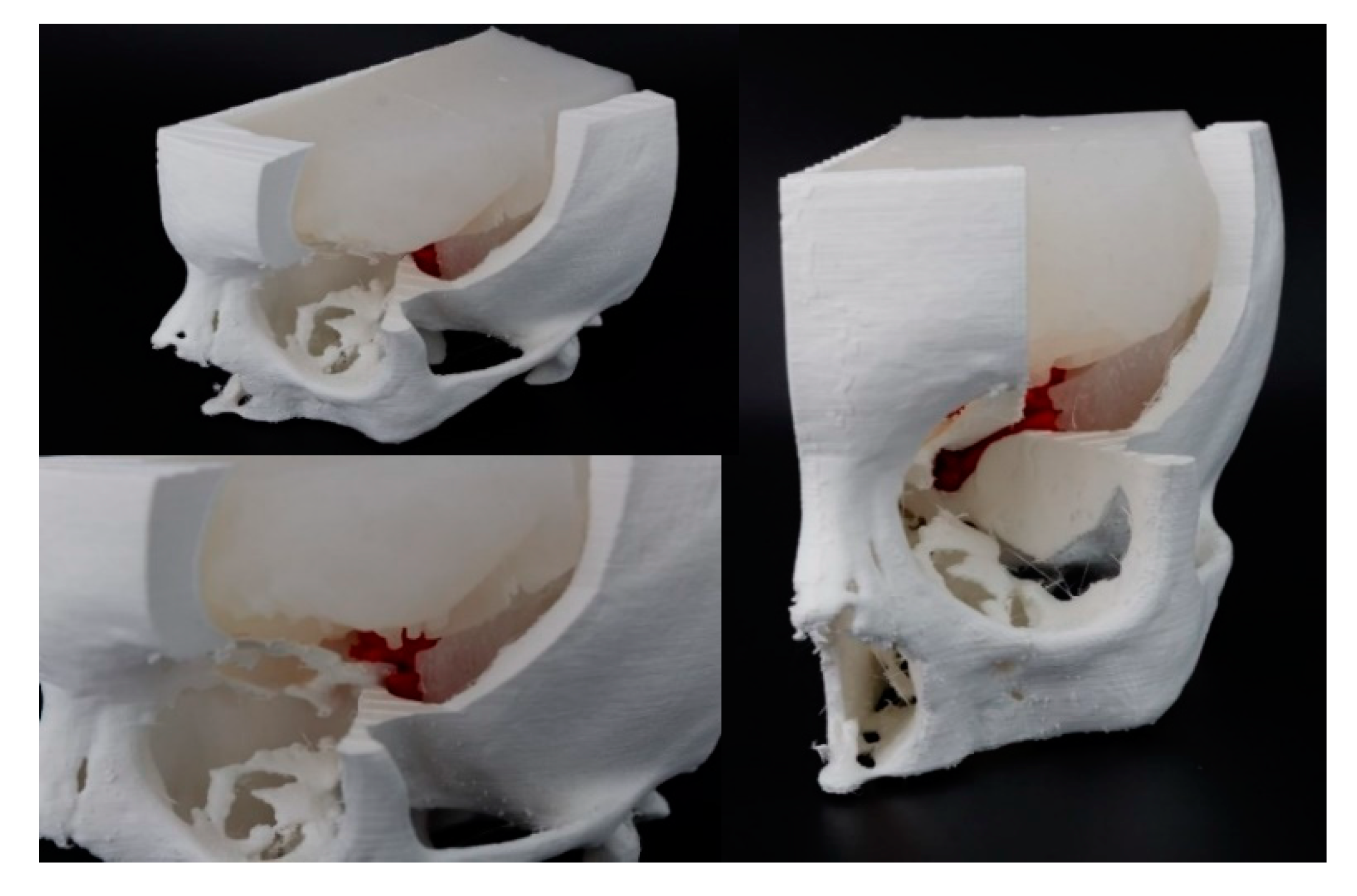
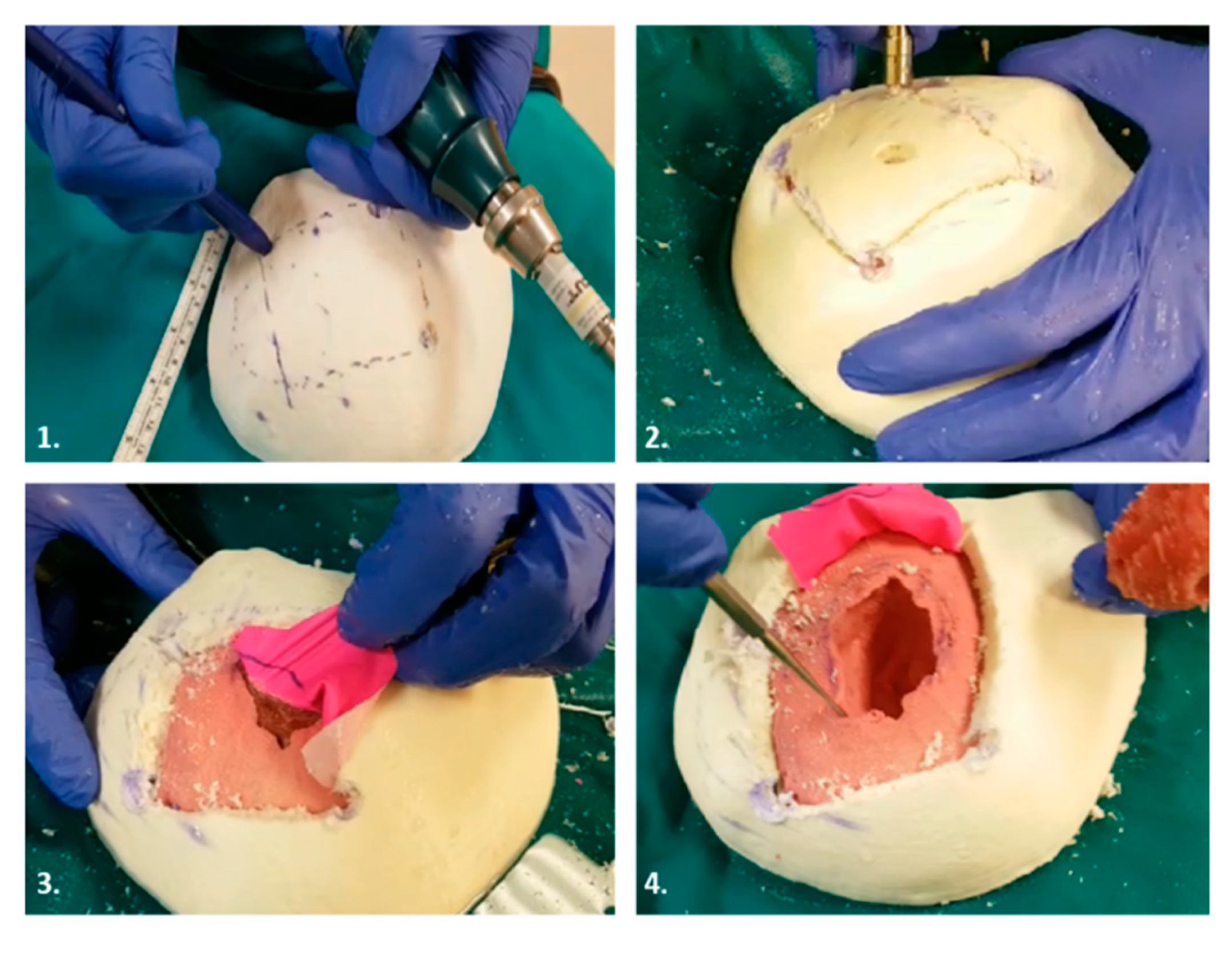
3.3. Fabrication
3.3.1. Rigid Parts Fabrication
3.3.2. Soft Tissues Fabrication
4. Case Studies
4.1. Case 1
4.2. Case 2
4.3. Case 3
4.4. Case 4
5. Costs and Timings Analysis
6. Conclusions
Author Contributions
Funding
Conflicts of Interest
References
- Rorke, L.B.; Gilles, F.H.; Davis, R.L.; Becker, L.E. Revision of the world health organization classification of brain tumors for childhood brain tumors. Cancer 1985, 56, 1869–1886. [Google Scholar] [CrossRef]
- Guo, N.; Leu, M.C. Additive manufacturing: Technology, applications and research needs. Front. Mech. Eng. 2013, 8, 215–243. [Google Scholar] [CrossRef]
- Ngo, T.D.; Kashani, A.; Imbalzano, G.; Nguyen, K.T.Q.; Hui, D. Additive manufacturing (3D printing): A review of materials, methods, applications and challenges. Compos. Part B Eng. 2018, 143, 172–196. [Google Scholar] [CrossRef]
- Tai, B.L.; Rooney, D.; Stephenson, F.; Liao, P.S.; Sagher, O.; Shih, A.J.; Savastano, L.E. Development of a 3D-printed external ventricular drain placement simulator: Technical note. J. Neurosurg. 2015, 123, 1070–1076. [Google Scholar] [CrossRef] [PubMed]
- Oishi, M.; Fukuda, M.; Yajima, N.; Yoshida, K.; Takahashi, M.; Hiraishi, T.; Takao, T.; Saito, A.; Fujii, Y. Interactive presurgical simulation applying advanced 3D imaging and modeling techniques for skull base and deep tumors: Clinical article. J. Neurosurg. 2013, 119, 94–105. [Google Scholar] [CrossRef]
- Waran, V.; Narayanan, V.; Karuppiah, R.; Owen, S.L.F.; Aziz, T. Utility of multimaterial 3D printers in creating models with pathological entities to enhance the training experience of neurosurgeons: Technical note. J. Neurosurg. 2014, 120, 489–492. [Google Scholar] [CrossRef]
- Tejo-Otero, A.; Buj-Corral, I.; Fenollosa-Artés, F. 3D Printing in Medicine for Preoperative Surgical Planning: A Review. Ann. Biomed. Eng. 2019, 1–20. [Google Scholar] [CrossRef]
- Mukherjee, S.; Pringle, C.; Crocker, M. A nine-year review of medicolegal claims in neurosurgery. Ann. R. Coll. Surg. Engl. 2014, 96, 266–270. [Google Scholar] [CrossRef]
- Nocerino, E.; Remondino, F.; Uccheddu, F.; Gallo, M.; Gerosa, G. 3D Modelling and rapid prototyping for cardiovascular surgical planning-Two case studies. In International Archives of the Photogrammetry, Remote Sensing and Spatial Information Sciences-ISPRS Archives; International Society for Photogrammetry and Remote Sensing: Christian Heipke, Germany, 2016; Volume 41, pp. 887–893. [Google Scholar]
- Ploch, C.C.; Mansi, C.S.S.A.; Jayamohan, J.; Kuhl, E. Using 3D printing to create personalized Brain models for neurosurgical training and preoperative planning. World Neurosurg. 2016, 90, 668–674. [Google Scholar] [CrossRef]
- Ottlakan, A.; Borda, B.; Morvay, Z.; Maraz, A.; Furak, J. The effect of diagnostic imaging on surgical treatment planning in diseases of the thymus. Contrast Media Mol. Imaging 2017, 2017, 9307292. [Google Scholar] [CrossRef]
- Grau, S.; Kellermann, S.; Faust, M.; Perrech, M.; Beutner, D.; Drzezga, A.; Zöller, J. Repair of cerebrospinal fluid leakage using a transfrontal, radial adipofascial flap: An individual approach supported by Three-dimensional printing for surgical planning. World Neurosurg. 2018, 110, 315–318. [Google Scholar] [CrossRef] [PubMed]
- Hoang, D.; Perrault, D.; Stevanovic, M.; Ghiassi, A. Today surgical applications of three-dimensional printing: A review of the current literature & how to get started. Ann. Transl. Med. 2016, 4, 456. [Google Scholar] [PubMed]
- Ferroli, P.; Tringali, G.; Acerbi, F.; Schiariti, M.; Broggi, M.; Aquino, D.; Broggi, G. Advanced 3-dimensional planning in neurosurgery. Neurosurgery 2013, 72, A54–A62. [Google Scholar] [CrossRef] [PubMed]
- Javia, L.; Sardesai, M.G. Physical Models and Virtual Reality Simulators in Otolaryngology. Otolaryngol. Clin. N. Am. 2017, 50, 875–891. [Google Scholar] [CrossRef]
- Mamone, V.; Viglialoro, R.M.; Cutolo, F.; Cavallo, F.; Guadagni, S.; Ferrari, V. Robust laparoscopic instruments tracking using colored strips. In Lecture Notes in Computer Science (Including Subseries Lecture Notes in Artificial Intelligence and Lecture Notes in Bioinformatics); Springer: Berlin/Heidelberg, Germany, 2017; Volume 10325, pp. 129–143. [Google Scholar]
- Pelagotti, A.; Del Mastio, A.; Uccheddu, F.; Remondino, F. Automated multispectral texture mapping of 3D models. In Proceedings of the 17th European Signal Processing Conference, Glasgow, UK, 24–28 August 2009; pp. 1215–1219. [Google Scholar]
- Viergever, M.A.; Maintz, J.B.A.; Klein, S.; Murphy, K.; Staring, M.; Pluim, J.P.W. A survey of medical image registration-under review. Med. Image Anal. 2016, 33, 140–144. [Google Scholar] [CrossRef]
- Miao, S.; Wang, Z.J.; Liao, R. A CNN Regression Approach for Real-Time 2D/3D Registration. IEEE Trans. Med. Imaging 2016, 35, 1352–1363. [Google Scholar] [CrossRef]
- Hajnal, J.V.; Hill, D.L.G.; Hawkes, D.J. Medical Image Registration; CRC Press: Boca Raton, FL, USA, 2001; ISBN 9781420042474. [Google Scholar]
- Besl, P.J.; McKay, N.D. A method for registration of 3-D shapes. IEEE Trans. Pattern Anal. Mach. Intell. 1992, 14, 239–256. [Google Scholar] [CrossRef]
- Zhou, Q.Y.; Park, J.; Koltun, V. Fast global registration. In Lecture Notes in Computer Science (Including Subseries Lecture Notes in Artificial Intelligence and Lecture Notes in Bioinformatics); Springer: Berlin/Heidelberg, Germany, 2016; Volume 9906 LNCS, pp. 766–782. [Google Scholar]
- Chen, D.-Y.; Ouhyoung, M. A 3D Model Alignment and Retrieval System. In Proceedings of the International Computer Symposium, Workshop on Multimedia Technologies, Hualien, Taiwan, 30 September 2002. [Google Scholar]
- Bücking, T.M.; Hill, E.R.; Robertson, J.L.; Maneas, E.; Plumb, A.A.; Nikitichev, D.I. From medical imaging data to 3D printed anatomical models. PLoS ONE 2017, 12. [Google Scholar] [CrossRef]
- Balafar, M.A.; Ramli, A.R.; Saripan, M.I.; Mashohor, S. Review of brain MRI image segmentation methods. Artif. Intell. Rev. 2010, 33, 261–274. [Google Scholar] [CrossRef]
- Buonamici, F.; Guariento, L.; Volpe, Y. 3D Digital Surgical Planning: An Investigation of Low-Cost Software Tools for Concurrent Design. In International Conference on Design, Simulation, Manufacturing: The Innovation Exchange; Springer: Berlin/Heidelberg, Germany, 2019; pp. 765–775. [Google Scholar]
- Ebel, E.; RTejournal, T.S. Fabrication of FDM 3D objects with ABS and PLA and determination of their mechanical properties. Rapid Technol. 2014, 2014, 4–22. [Google Scholar]
- Buonamici, F.; Carfagni, M.; Furferi, R.; Governi, L.; Saccardi, M.; Volpe, Y. Optimizing fabrication outcome in low-cost FDM machines. Part 1-Metrics. Manuf. Technol. 2018, 18, 372–378. [Google Scholar] [CrossRef]
- Witowski, J.S.; Pędziwiatr, M.; Major, P.; Budzyński, A. Cost-effective, personalized, 3D-printed liver model for preoperative planning before laparoscopic liver hemihepatectomy for colorectal cancer metastases. Int. J. Comput. Assist. Radiol. Surg. 2017, 12, 2047–2054. [Google Scholar] [CrossRef] [PubMed]
- Mussi, E.; Furferi, R.; Volpe, Y.; Facchini, F.; McGreevy, K.S.; Uccheddu, F. Ear Reconstruction Simulation: From Handcrafting to 3D Printing. Bioengineering 2019, 6, 14. [Google Scholar] [CrossRef] [PubMed]
- Bootsma, K.; Dimbath, E.; Berberich, J.; Sparks, J.L. Materials Used as Tissue Phantoms in Medical Simulation. In Studies in Mechanobiology Tissue Engineering and Biomaterials; Springer: Berlin/Heidelberg, Germany, 2017; pp. 1–48. [Google Scholar]
- Kuo, C.-C.; Lai, M.-Y. Development of an automatic vacuum degassing system and parameters optimization for degassing process. Indian J. Eng. Mater. Sci. 2011, 18, 405–410. [Google Scholar]
- Jiménez, M.; Romero, L.; Domínguez, I.A.; Espinosa, M.D.M.; Domínguez, M. Additive Manufacturing Technologies: An Overview about 3D Printing Methods and Future Prospects. Complexity 2019, 2019, 9656938. [Google Scholar] [CrossRef]
- Volpe, Y.; Furferi, R.; Governi, L.; Uccheddu, F.; Carfagni, M.; Mussa, F.; Scagnet, M.; Genitori, L. Surgery of complex craniofacial defects: A single-step AM-based methodology. Comput. Methods Programs Biomed. 2018, 165, 225–233. [Google Scholar] [CrossRef]


| Phase | Day | Working Hours | Professional Figures |
|---|---|---|---|
| 3D reconstruction | 1 | 4–8 | Radiologist, Engineer |
| Virtual planning | 2 | 1–5 | Engineer, Surgeon |
| Fabrication | 2–3 | 2–16 | Technician |
| Simulation | 4 | 1–3 | Surgeon |
| Surgery | 5 | 4–8 | Surgeon |
| Costs Analysis for the Prototyping of Items in A 3D Printer | |
|---|---|
| Machine Depreciation Data | |
| Price of Machine (€) | 2500 |
| Yearly maintenance cost (€) | 250 |
| Years of depreciation | 4 |
| Cost of Material Data | |
| Cost of material: ABS filament (€/kg) | ~20 |
| Cost of material: PLA filament (€/kg) | ~22 |
| Cost of material: wood-loaded PLA filament (€/kg) | ~30 |
| Cost of Technical Analysis Data | |
| Cost of technical model analysis (€/h) | 20 |
| Material | Cost |
|---|---|
| Silicone Rubbers | ~35 €/kg |
| Release Agents | ~12 € one-time fee |
| Colorants | ~15 € one-time fee |
| Silicone additives | ~ 40 €/kg |
| 3D printing coating | ~25 € one-time fee |
© 2020 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (http://creativecommons.org/licenses/by/4.0/).
Share and Cite
Mussi, E.; Mussa, F.; Santarelli, C.; Scagnet, M.; Uccheddu, F.; Furferi, R.; Volpe, Y.; Genitori, L. Current Practice in Preoperative Virtual and Physical Simulation in Neurosurgery. Bioengineering 2020, 7, 7. https://doi.org/10.3390/bioengineering7010007
Mussi E, Mussa F, Santarelli C, Scagnet M, Uccheddu F, Furferi R, Volpe Y, Genitori L. Current Practice in Preoperative Virtual and Physical Simulation in Neurosurgery. Bioengineering. 2020; 7(1):7. https://doi.org/10.3390/bioengineering7010007
Chicago/Turabian StyleMussi, Elisa, Federico Mussa, Chiara Santarelli, Mirko Scagnet, Francesca Uccheddu, Rocco Furferi, Yary Volpe, and Lorenzo Genitori. 2020. "Current Practice in Preoperative Virtual and Physical Simulation in Neurosurgery" Bioengineering 7, no. 1: 7. https://doi.org/10.3390/bioengineering7010007
APA StyleMussi, E., Mussa, F., Santarelli, C., Scagnet, M., Uccheddu, F., Furferi, R., Volpe, Y., & Genitori, L. (2020). Current Practice in Preoperative Virtual and Physical Simulation in Neurosurgery. Bioengineering, 7(1), 7. https://doi.org/10.3390/bioengineering7010007







