Low Temperature Dissolution of Yeast Chitin-Glucan Complex and Characterization of the Regenerated Polymer
Abstract
:1. Introduction
2. Materials and Methods
2.1. Materials
2.2. CGC Dissolution in NaOH/Urea Solvent Systems
2.3. Polymer Regeneration
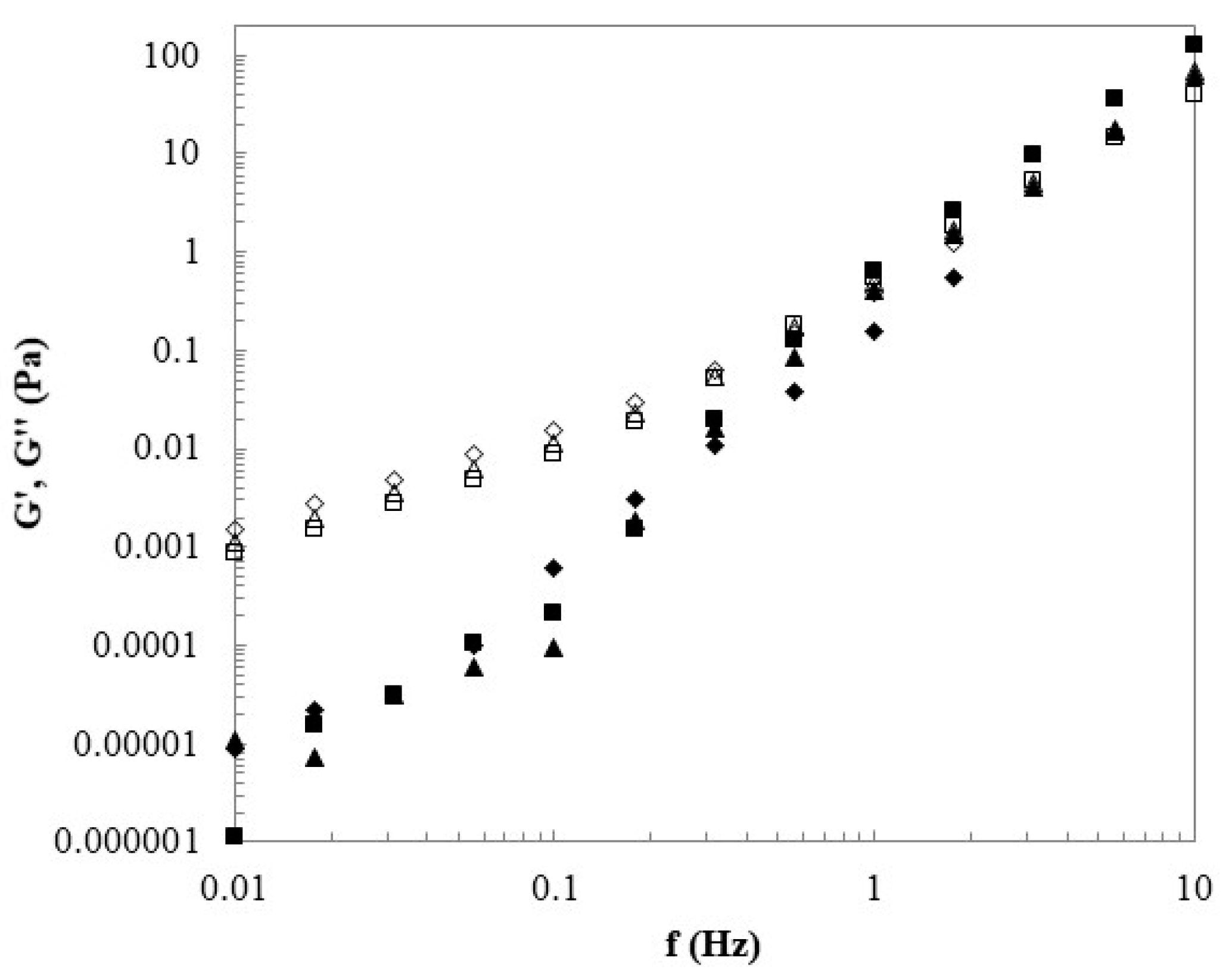
2.4. Rheological Properties of the Polymer Solutions
2.5. Characterization of the Regenerated Polymer
2.5.1. Elemental Analysis and Degree of Acetylation
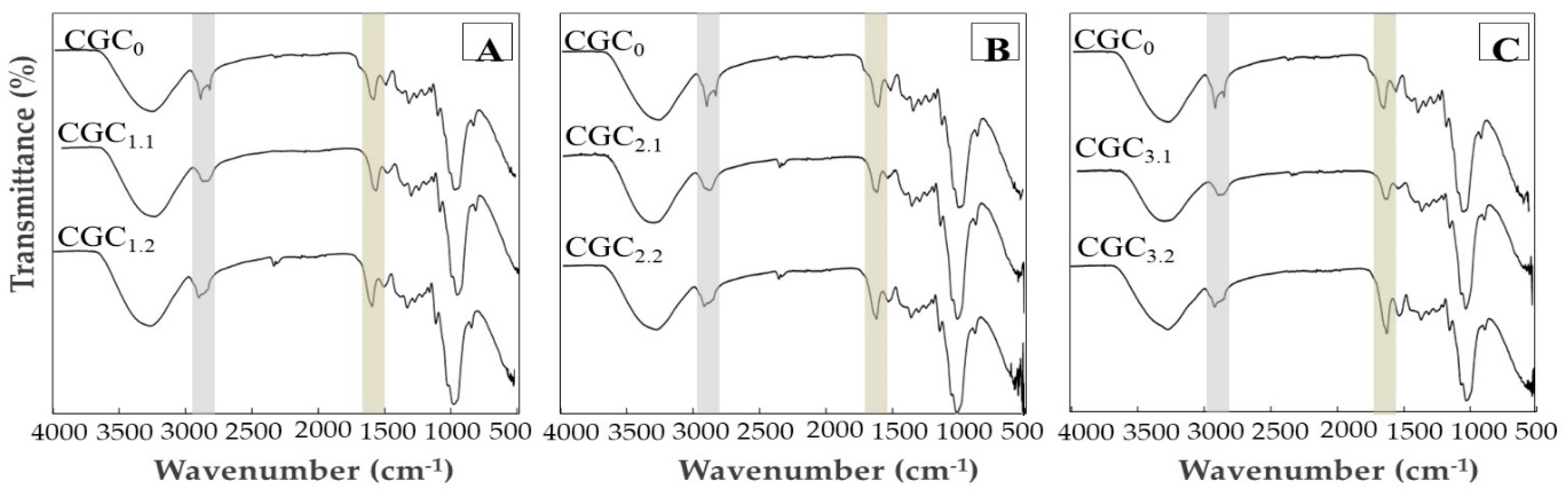
2.5.2. Fourier Transform Infrared Spectroscopy
2.5.3. X-ray Diffraction Profiles
2.5.4. Thermal Properties
3. Results and Discussion
3.1. Preparation of CGC Solutions in NaOH/urea Solvent Systems
3.2. Rheological Behavior of the Solutions
3.3. Characterization of the Regenerated Polymer
3.3.1. Elemental Analysis and Degree of Acetylation
3.3.2. Fourier Transform Infrared Spectroscopy
3.3.3. XRD Analysis
3.3.4. Thermal Properties
4. Conclusions
Author Contributions
Funding
Conflicts of Interest
References
- Meichik, N.R.; Vorob’ev, D.V. Chitin-glucan complex in cell walls of Peltigera aphthosa lichen. Appl. Biochem. Microbiol. 2012, 48, 307–311. [Google Scholar] [CrossRef]
- Gautier, S.; Xhauflaire-Uhoda, E.; Gonry, P.; Pierard, G.E. Chitin-glucan, a natural cell scaffold for skin moisturization and rejuvenation. Int. J. Cosmet. Sci. 2008, 30, 459–469. [Google Scholar] [CrossRef]
- Roca, C.; Chagas, B.; Farinha, I.; Freitas, F.; Mafra, L.; Aguiar, F.; Oliveira, R.; Reis, M.A.M. Production of yeast chitin-glucan complex from biodiesel industry byproduct. Proc. Biochem. 2012, 47, 1670–1675. [Google Scholar] [CrossRef]
- Wu, T.; Zivanovic, S.; Draughon, F.A.; Conway, W.S.; Sams, C.E. Physicochemical properties and bioactivity of fungal chitin and chitosan. J. Agric. Food Chem. 2005, 53, 3888–3894. [Google Scholar] [CrossRef] [PubMed]
- Berecochea-Lopez, A.; Decordé, K.; Ventura, E.; Godard, M.; Bornet, A.; Teissèdre, P.L.; Cristol, J.P.; Rouanet, J.M. Fungal chitin-glucan from Aspergillus niger efficiently reduces aortic fatty streak accumulation in the high-fat fed hamster, an animal model of nutritionally induced atherosclerosis. J. Agric. Food Chem. 2009, 57, 1093–1098. [Google Scholar] [CrossRef]
- Feofilova, E.P. The fungal cell wall: Modern concepts of its composition and biological function. Microbiology 2010, 79, 711–720. [Google Scholar] [CrossRef]
- Kulev, D.; Negrutsa, I. Chitin-glucan complex-food additive with sorbent properties. J. Hyg. Eng. Des. 2015, 11, 53–56. [Google Scholar]
- Mogosanu, G.D.; Grumezescu, A.M. Natural and synthetic polymers for wounds and burns dressing. Int. J. Pharm. 2014, 463, 127–136. [Google Scholar] [CrossRef]
- Neyrinck, A.M.; Possemiers, S.; Verstraete, W.; Backer, F.D.; Cani, P.D.; Delzenne, N.M. Dietary modulation of clostridial cluster XIVa gut bacteria (Roseburia spp.) by chitin–glucan fiber improves host metabolic alterations induced by high-fat diet in mice. J. Nutr. Biochem. 2012, 23, 51–59. [Google Scholar] [CrossRef]
- Mislovicová, D.; Masárová, J.; Bendzálová, K.; Soltés, L.; Machová, E. Sonication of chitin-glucan, preparation of water-soluble fractions and characterization by HPLC. Ultrason. Sonochem. 2000, 7, 63–68. [Google Scholar] [CrossRef]
- Cabib, E.; Blanco, N.; Arroyo, J. Presence of a large β(1–3)glucan linked to chitin at the Saccharomyces cerevisiae mother-bud neck suggests involvement in localized growth control. Eukaryot. Cell 2012, 11, 388–400. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Reis, M.A.; Oliveira, R.M.; Freitas, M.F.; Chagas, B.F.; Cruz, A.L.; Cunha, A.E.; Clemente, J.J. Process for the Co-Production of Chitin, Its Derivates and Polymers Containing Glucose, Mannose and/or Galactose by the Fermentation of the Yeast Pichia pastoris. International Patent WO2010/013174, 4 February 2010. [Google Scholar]
- Tarabukina, E.B.; Kalinina, N.A.; Adamov, A.V.; Petrova, V.A.; Nud’ga, L.A.; Klenin, S.I. Molecular characteristics and supramolecular organization of chitin–glucan complexes in solutions. Polym. Sci. Ser. A 2005, 47, 462–468. [Google Scholar]
- Siestma, J.H.; Wessels, J.G.H. Solubility of (1-3)-β-D/(1-6)-β-D-Glucan in fungal walls: Importance of presumed linkage between glucan and chitin. J. Gen. Microbiol. 1981, 125, 209–212. [Google Scholar]
- Gamayurova, V.S.; Kotlyar, M.N.; Shabrukova, N.V.; Khalitov, F.G. Synthesis of soluble derivatives of chitin-glucan complex. Thematic course: Natural Polysaccharides. Part I Chem. Comput. Simul. Commun. 1998, 1, 73–76. [Google Scholar]
- Skorik, Y.A.; Pestov, A.V.; Yatluk, Y.G. Evaluation of various chitin-glucan derivates from Aspergillus niger as transition metal adsorbents. Bioresour. Technol. 2010, 101, 1769–1775. [Google Scholar] [CrossRef]
- Oliveira, A.F.R. Development of Chitin-Glucan Polymeric Structures Using Biocompatible Ionic Liquids. Master’s Thesis, Chemical and Biochemical Engineering, NOVA School of Science and Technology, Caparica, Portugal, 2016. [Google Scholar]
- Hu, X.; Du, Y.; Tang, Y.; Wang, Q.; Feng, T.; Yang, J.; Kennedy, J.F. Solubility and property of chitin in NaOH/urea aqueous solution. Carbohydr. Polym. 2007, 70, 451–458. [Google Scholar] [CrossRef]
- Gong, P.; Wang, J.; Liu, B.; Ru, G.; Feng, J. Dissolution of chitin in aqueous KOH. Cellulose 2016, 23, 1705–1711. [Google Scholar] [CrossRef]
- Chen, X.; Xu, X.; Zhang, L.; Kennedy, J.F. Flexible chain conformation of (1–3)-β-d-glucan from Poriacocos sclerotium in NaOH/urea aqueous solution. Carbohydr. Polym. 2009, 75, 586–591. [Google Scholar] [CrossRef]
- Yoshida, T.; Honda, Y.; Tujimoto, T.; Uyama, H.; Azuma, J. Freeze-thaw treatment in 2% w/w NaOH-6M urea enhanced extraction of β-(1,3;1,4)-glucan from corn pericarp. Macromol. Symp. 2015, 353, 205–211. [Google Scholar] [CrossRef]
- Chen, X.; Chen, J.; You, T.; Wang, K.; Xu, F. Effects of polymorphs on dissolution of cellulose in NaOH/urea aqueous solution. Carbohydr. Polym. 2015, 125, 85–91. [Google Scholar] [CrossRef]
- Mense, A.; Shi, Y.C. Dissolution of wheat bran by NaOH/urea solutions and structure of soluble materials. ACS Sustain. Chem Eng 2018, 6, 4264–4271. [Google Scholar] [CrossRef]
- Araújo, D.; Alves, V.D.; Lima, S.A.C.; Reis, S.; Freitas, F.; Reis, M.A.M. Novel hydrogels based on yeast chitin-glucan complex: Characterization and safety assessment. Int. J. Biol. Macromol. 2020, in press. [Google Scholar] [CrossRef]
- Roy, J.C.; Salaun, F.; Giraud, S.; Ferri, A.; Chen, G.; Guan, J. Solubility of chitin: Solvents, solution behaviors and their related mechanisms. In Solubility of Polysaccharides; Xu, Z., Ed.; IntechOpen: London, UK, 2017; pp. 100–127. [Google Scholar]
- Zhou, J.; Zhang, L. Solubility of cellulose in NaOH/Urea aqueous solution. Polym. J. 2000, 32, 866–870. [Google Scholar] [CrossRef] [Green Version]
- Cai, J.; Zhang, L.; Chang, C.; Cheng, G.; Chen, X.; Chu, B. Hydrogen-bond-induced inclusion complex in aqueous cellulose/LiOH/urea solution at low temperature. ChemPhysChem 2007, 8, 1572–1579. [Google Scholar] [CrossRef]
- Farinha, I.; Duarte, P.; Pimentel, A.; Plotnikova, E.; Chagas, B.; Mafra, L.; Grandfils, C.; Freitas, F.; Fortunato, E.; Reis, M.A.M. Chitin-glucan complex production by Komagataella pastoris: Downstream optimization and product characterization. Carbohydr. Polym. 2015, 130, 455–464. [Google Scholar] [CrossRef]
- Araújo, D.; Freitas, F.; Sevrin, C.; Grandfils, C.; Reis, M.A.M. Co-production of chitin-glucan complex and xylitol by Komagatella pastoris using glucose and xylose mixtures as carbon source. Carbohydr. Polym. 2017, 166, 24–30. [Google Scholar] [CrossRef]
- Wang, Y.; Li, Y.; Liu, S.; Li, B. Fabrication of chitin microspheres and their multipurpose application as catalyst support and adsorbent. Carbohydr. Polym. 2015, 120, 53–59. [Google Scholar] [CrossRef]
- Shang, Y.; Ding, F.; Xiao, L.; Deng, H.; Du, Y.; Shi, X. Chitin-based fast responsive pH sensitive microspheres for controlled drug release. Carbohydr. Polym. 2014, 102, 413–418. [Google Scholar] [CrossRef]
- Alves, L.; Medronho, B.; Filipe, A.; Antunes, F.E.; Lindman, B.; Topgaard, D.; Davidovich, I.; Talmon, Y. New insights on the role of urea on the dissolution and thermally-induced gelation of cellulose in aqueous alkali. Gels 2018, 4, 87. [Google Scholar] [CrossRef] [Green Version]
- Barrera, G.E.; Beltran, L.M.; Diosa, J.A. Chitin-Glucan Complexes and Method for the Preparation Thereof from Chitin-Rich Biomaterials. U.S. Patent US2016/0122444, 5 May 2016. [Google Scholar]
- Xiong, B.; Zhao, P.; Hu, K.; Zhang, L.; Cheng, G. Dissolution of cellulose in aqueous NaOH/urea solution: Role of urea. Cellulose 2014, 21, 1183–1192. [Google Scholar] [CrossRef]
- Terbojevich, S.; Carraro, C.; Cosani, A. Solution studies of the chitin-lithium chloride-N,N-Dimethylacetamide system. Carbohydr. Res. 1988, 180, 73–86. [Google Scholar] [CrossRef]
- Sharma, M.; Mukesh, C.; Mondal, D.; Prasad, K. Dissolution of α-chitin in deep eutectic solvents. RSC Adv. 2013, 3, 18149–18155. [Google Scholar] [CrossRef]
- Jaworska, M.M.; Górak, A.; Zdunek, J. Modification of chitin particles with ionic liquids containing ethyl substituent in a cation. Adv. Mater. Sci. Eng. 2017, 2017, 1–9. [Google Scholar] [CrossRef] [Green Version]
- Huber, T.; Starling, K.; Cen, W.S.; Fee, C.; Dimartino, S. Effect of urea concentration on the viscosity and thermal stability of aqueous NaOH/urea cellulose solutions. J. Polym. 2016, 2016, 9. [Google Scholar] [CrossRef] [Green Version]
- Hu, X.; Tang, Y.; Wang, Q.; Li, Y.; Yang, J.; Du, Y.; Kennedy, J.F. Rheological behaviour of chitin in NaOH/urea aqueous solution. Carbohydr. Polym. 2011, 83, 1128–1133. [Google Scholar] [CrossRef]
- Pillai, C.K.S.; Paul, W.; Sharma, C.P. Chitin and chitosan polymers: Chemistry, solubility and fiber formation. Prog. Polym. Sci. 2009, 34, 641–678. [Google Scholar] [CrossRef]
- Zargar, V.; Asghari, M.; Dashti, A. A review on chitin and chitosan polymers: Structure, chemistry, solubility, derivatives and applications. ChemBioEng Rev. 2015, 2, 204–226. [Google Scholar] [CrossRef]
- Cárdenas, G.; Cabrera, G.; Taboada, E.; Miranda, P. Chitin characterization by SEM, FTIR, XRD and 13C cross polarization/mass angle spinning NMR. J. Appl. Polym. Sci. 2004, 93, 1876–1885. [Google Scholar] [CrossRef]
- Synytsya, A.; Novak, M. Structural analysis of glucans. Ann. Transl. Med. 2014, 2, 17. [Google Scholar]
- Kumari, S.; Rath, P.; Kumar, A.S.H.; Tiwari, T.N. Extraction and characterization of chitin and chitosan from fishery waste by chemical method. Environ. Technol. Innov. 2015, 3, 77–85. [Google Scholar] [CrossRef]
- Jalal, A.F.; Risheed, C.M.; Ibrahim, B.M. Optimization of chitin extraction from chicken feet. J. Anal. Bioanal. Tech. 2012, 3, 145–150. [Google Scholar] [CrossRef]
- Young, A.L. Powder X-ray diffraction and its applications to biotherapeutic formulation development. Am. Pharm. Rev. 2012, 15, 74. [Google Scholar]
- Kumirska, J.; Czerwicka, M.; Kaczyński, Z.; Bychowska, A.; Brzozowski, K.; Thöming, J.; Stepnowski, P. Application of spectroscopic methods for structural analysis of chitin and chitosan. Mar. Drugs 2010, 8, 1567. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Seoudi, R.; Nada, A.M.A. Molecular structure and dielectric properties studies of chitin treated by acid, base and hypochlorite. Carbohydr. Polym. 2007, 68, 728–733. [Google Scholar] [CrossRef]
- Tang, Z.; Li, W.; Lin, X.; Xiao, H.; Miao, Q.; Huang, L.; Chen, L.; Wu, H. TEMPO-Oxidized cellulose with high degree of oxidation. Polymers 2017, 9, 421. [Google Scholar] [CrossRef] [Green Version]
- Wanjun, T.; Cunxin, W.; Donghua, C. Kinetic studies on the pyrolysis of chitin and chitosan. Polym. Degrad. Stab. 2005, 87, 389–394. [Google Scholar] [CrossRef]
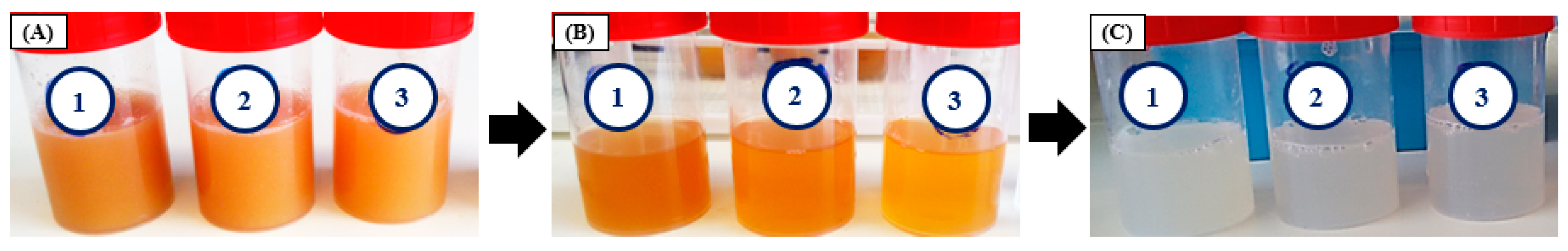



| Solvent System | NaOH:Urea (wt%:wt%) | Starting Polymer Mass (mg) | Fraction Solution | Solubilized Polymer Mass (mg) | Polymer Concentration (wt%) | Solubility (%) | Overall Solubility (%) |
|---|---|---|---|---|---|---|---|
| 1 | 6:8 | 504.4 ± 5.1 | CGC1.1 CGC1.2 | 275.8 ± 4.1 67.2 ± 0.9 | 13.8 ± 0.2 3.4 ± 0.1 | 54.7 ± 1.4 13.3 ± 0.3 | 68.0 ± 1.7 |
| 2 | 8:4 | 506.0 ± 4.7 | CGC2.1 CGC2.2 | 275.4 ± 6.0 54.4 ± 10.8 | 13.8 ± 0.3 2.7 ± 0.5 | 54.4 ± 1.7 10.7 ± 2.0 | 65.2 ± 0.3 |
| 3 | 11:4 | 502.7 ± 1.2 | CGC3.1 CGC3.2 | 258.3 ± 22.3 57.8 ± 7.5 | 12.9 ± 1.1 2.9 ± 0.4 | 51.4 ± 4.6 11.5 ± 1.5 | 62.9 ± 3.1 |
| Sample | Elemental Analysis (%) | Chitin Content (%) | DA (%) | ||
|---|---|---|---|---|---|
| C | H | N | |||
| CGC0 | 43.6 ± 0.05 | 7.2 ± 0.05 | 1.7 ± 0.01 | 23.8 ± 0.10 | 61.3 ± 0.41 |
| CGC1.1 | 40.4 ± 0.17 | 7.2 ± 0.05 | 1.3 ± 0.15 | 18.8 ± 2.11 | 34.5 ± 1.40 |
| CGC1.2 | 42.0 ± 0.09 | 7.2 ± 0.10 | 1.5 ± 0.03 | 21.7 ± 0.40 | 47.6 ± 0.76 |
| CGC2.1 | 40.3 ± 0.12 | 7.2 ± 0.09 | 1.5 ± 0.03 | 20.7 ± 0.40 | 33.9 ± 0.99 |
| CGC2.2 | 41.6 ± 0.35 | 7.0 ± 0.18 | 1.9 ± 0.16 | 26.5 ± 2.31 | 44.8 ± 2.86 |
| CGC3.1 | 41.2 ± 0.22 | 7.1 ± 0.06 | 1.7 ± 0.18 | 23.4 ± 2.61 | 41.6 ± 1.81 |
| CGC3.2 | 42.3 ± 0.12 | 6.9 ± 0.06 | 2.3 ± 0.11 | 32.8 ± 1.61 | 50.6 ± 0.99 |
| Sample | CGC0 | CGC1.1 | CGC1.2 | CGC2.1 | CGC2.2 | CGC3.1 | CGC3.2 |
|---|---|---|---|---|---|---|---|
| Tdeg (°C) | 302 | 250 | 293 | 256 | 300 | 267 | 302 |
| CI (%) | 35 | 28 | 30 | 32 | 32 | 23 | 25 |
© 2020 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (http://creativecommons.org/licenses/by/4.0/).
Share and Cite
Araújo, D.; Alves, V.D.; Marques, A.C.; Fortunato, E.; Reis, M.A.M.; Freitas, F. Low Temperature Dissolution of Yeast Chitin-Glucan Complex and Characterization of the Regenerated Polymer. Bioengineering 2020, 7, 28. https://doi.org/10.3390/bioengineering7010028
Araújo D, Alves VD, Marques AC, Fortunato E, Reis MAM, Freitas F. Low Temperature Dissolution of Yeast Chitin-Glucan Complex and Characterization of the Regenerated Polymer. Bioengineering. 2020; 7(1):28. https://doi.org/10.3390/bioengineering7010028
Chicago/Turabian StyleAraújo, Diana, Vítor D. Alves, Ana C. Marques, Elvira Fortunato, Maria A. M. Reis, and Filomena Freitas. 2020. "Low Temperature Dissolution of Yeast Chitin-Glucan Complex and Characterization of the Regenerated Polymer" Bioengineering 7, no. 1: 28. https://doi.org/10.3390/bioengineering7010028
APA StyleAraújo, D., Alves, V. D., Marques, A. C., Fortunato, E., Reis, M. A. M., & Freitas, F. (2020). Low Temperature Dissolution of Yeast Chitin-Glucan Complex and Characterization of the Regenerated Polymer. Bioengineering, 7(1), 28. https://doi.org/10.3390/bioengineering7010028