Strontium- and Zinc-Containing Bioactive Glass and Alginates Scaffolds
Abstract
1. Introduction
2. Materials and Methods
2.1. Glass Synthesis and Characterization
2.1.1. Bone Scaffold Synthesis and Characterization
2.1.2. Statistical Analysis
3. Results and Discussion
3.1. Characterization of Bioactive Glass
3.1.1. Particle Size Analysis
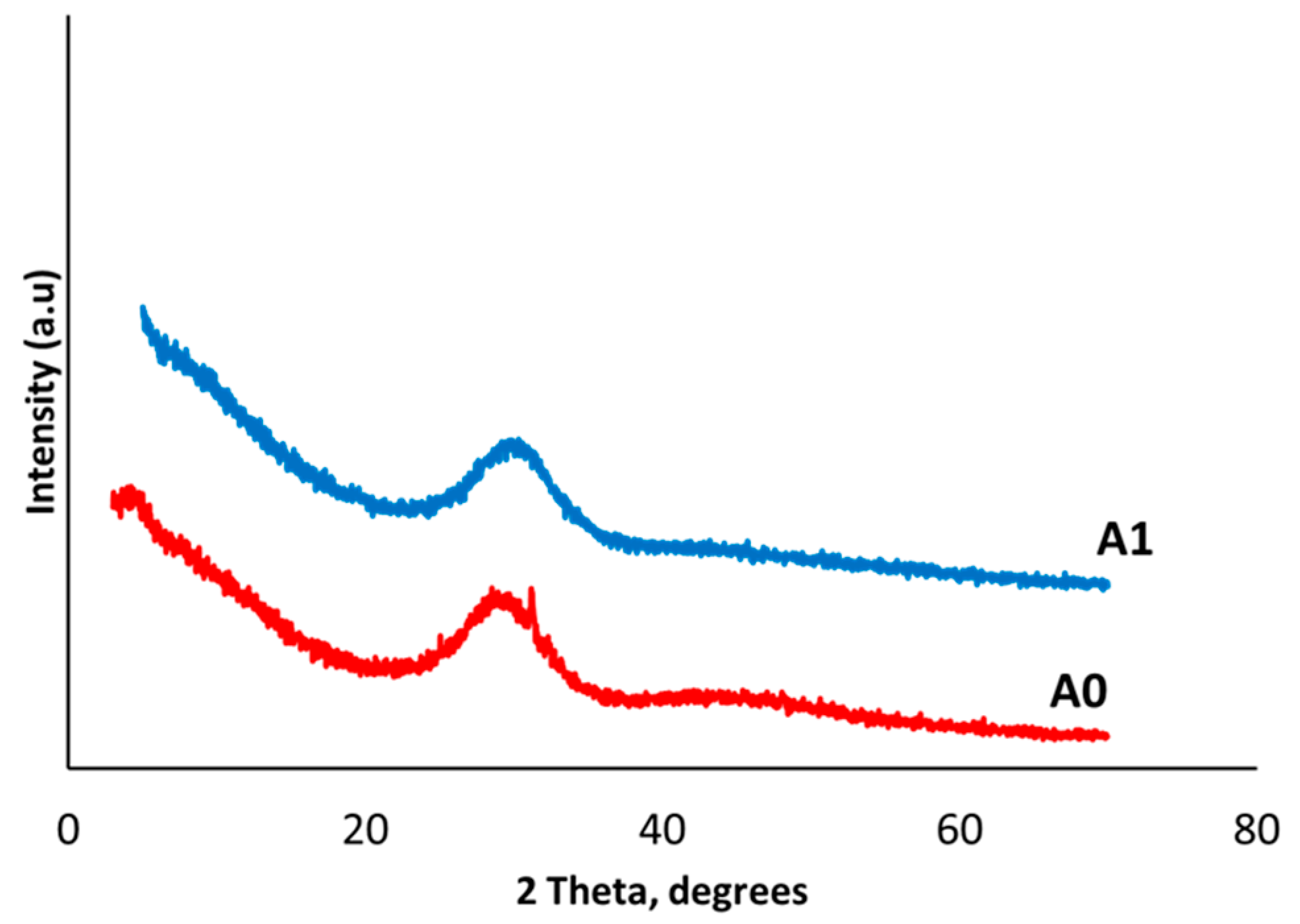
3.1.2. X-Ray Diffraction Analysis (XRD)
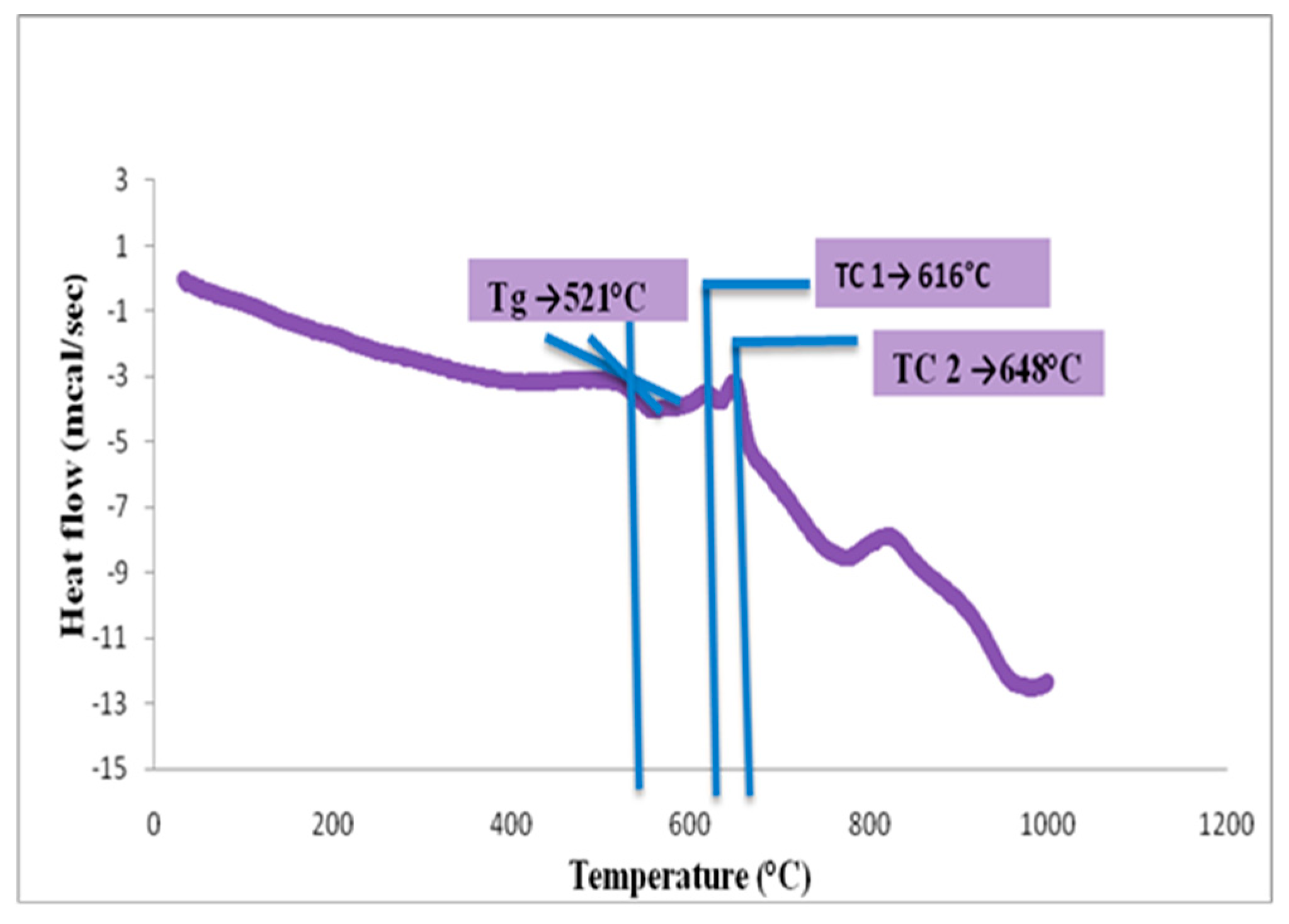
3.1.3. DSC Analysis of Glass Powder
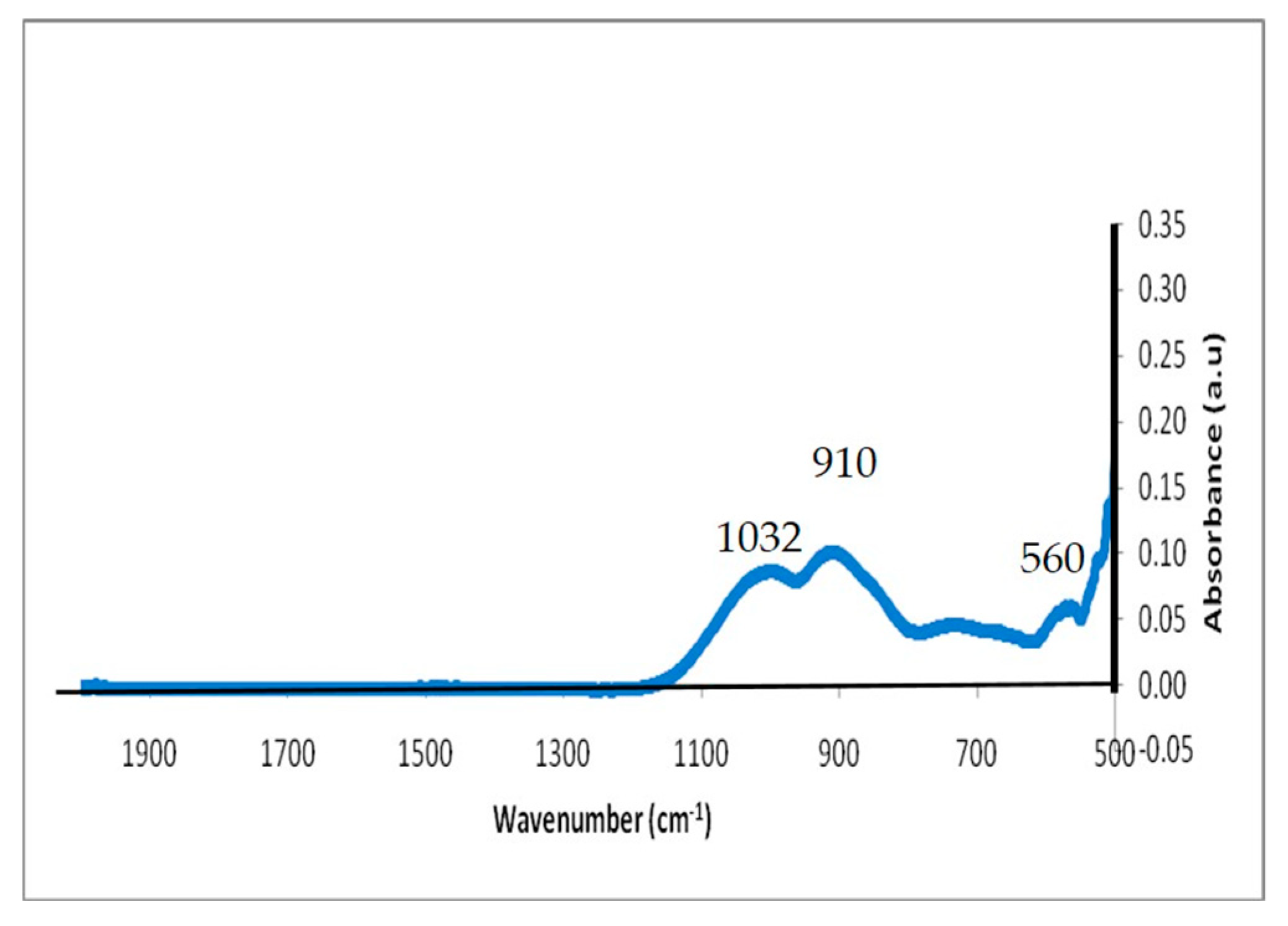
3.1.4. Fourier Transform Infrared (FTIR) Spectroscopy Analysis
3.2. Characterization of Scaffolds
3.2.1. FTIR Study of the Bone Scaffolds
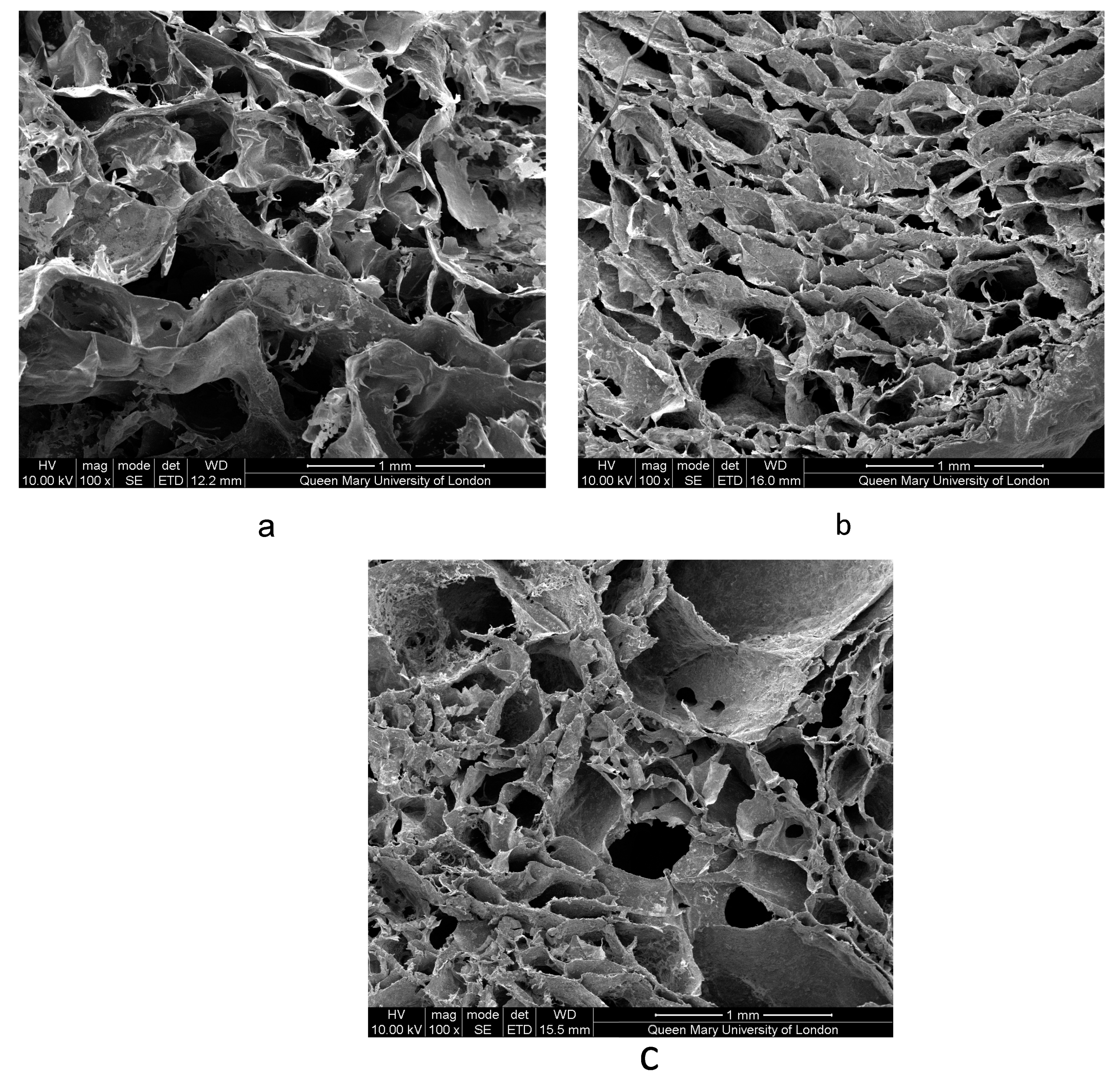
3.2.2. Scanning Electron Microscopy Imaging of Scaffolds
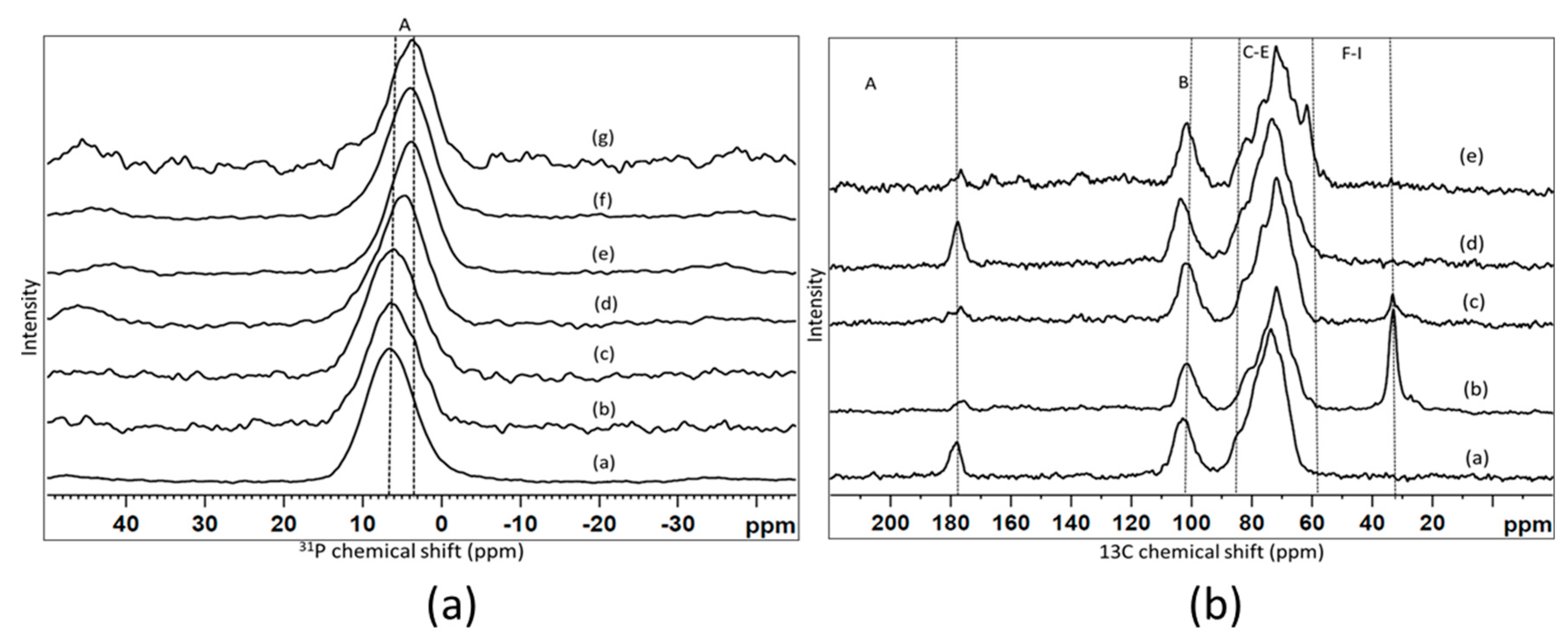
3.2.3. Nuclear Magnetic Resonance (NMR)
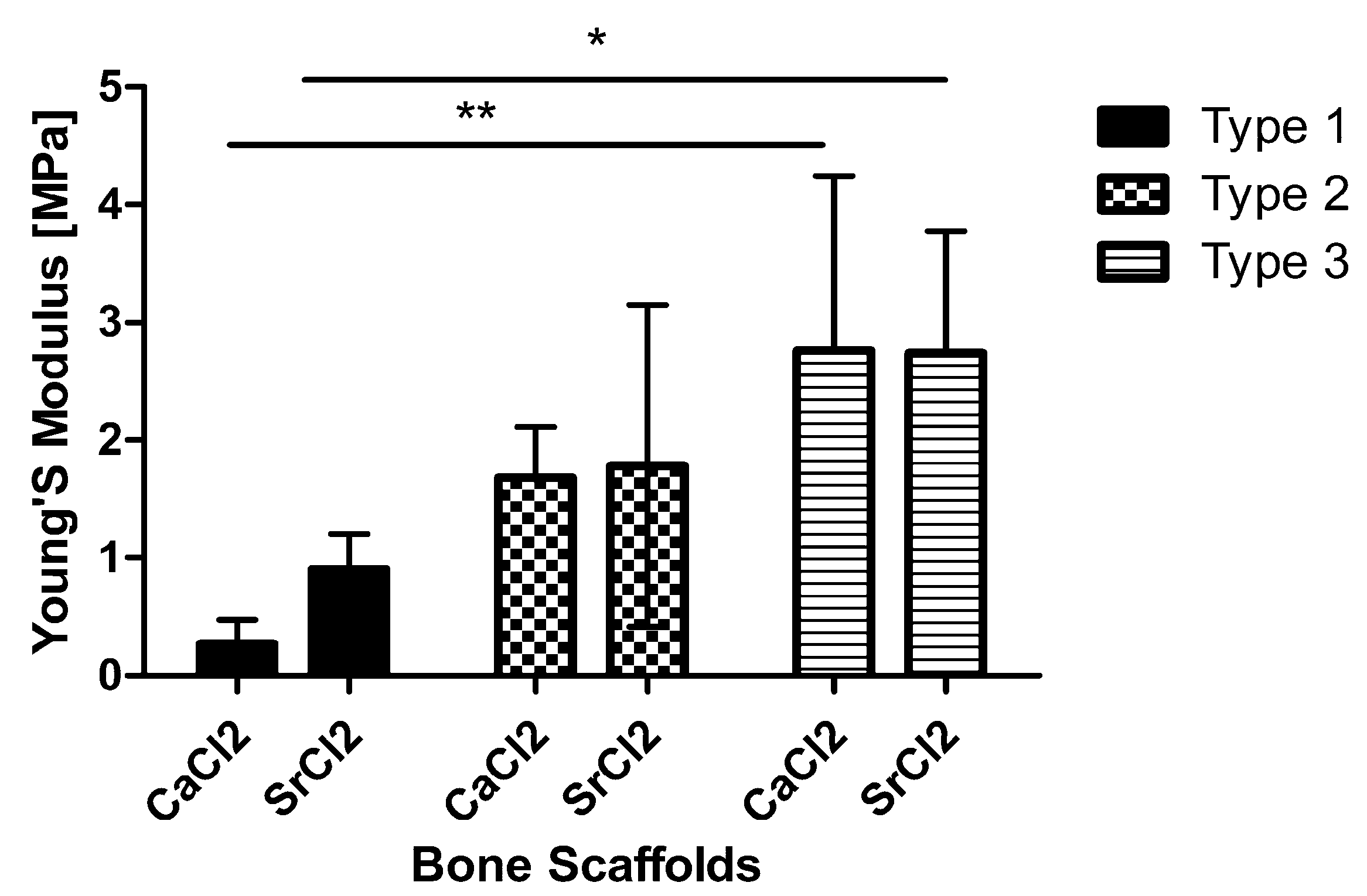
3.2.4. Compression Testing of Scaffolds
3.3. Tris-Buffer Study
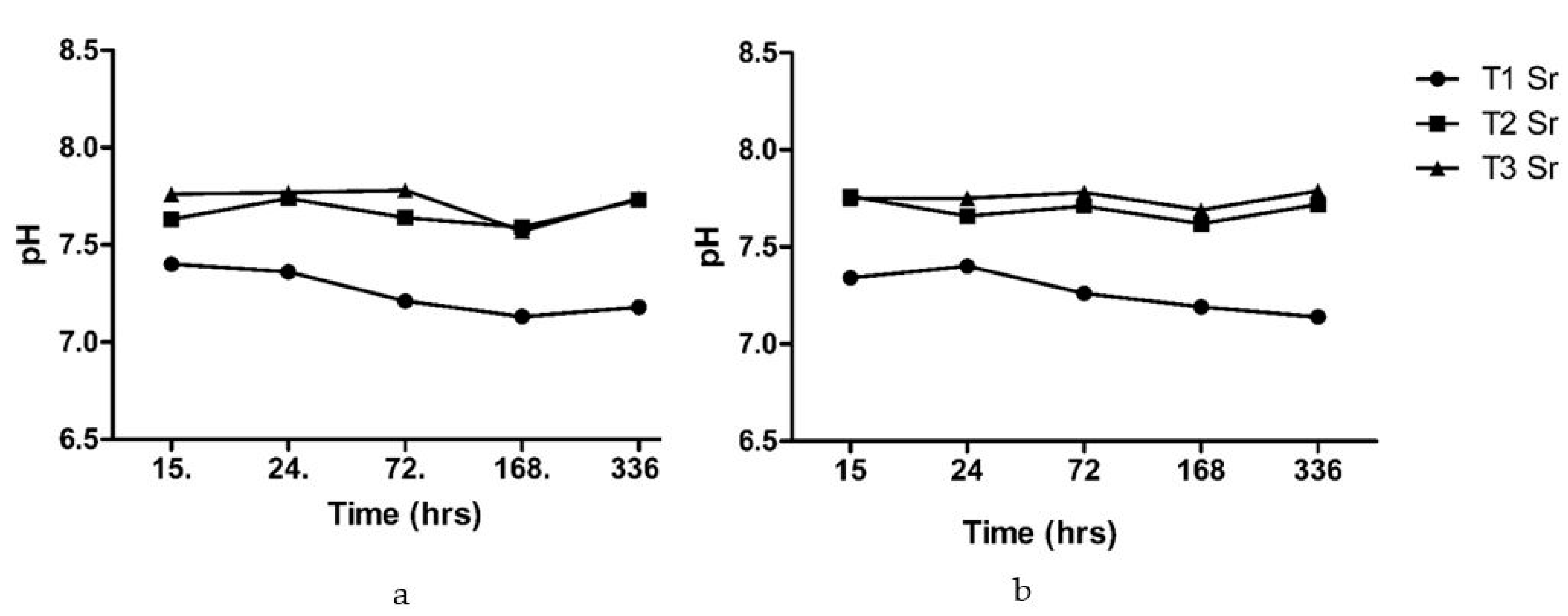
3.3.1. pH Measurements
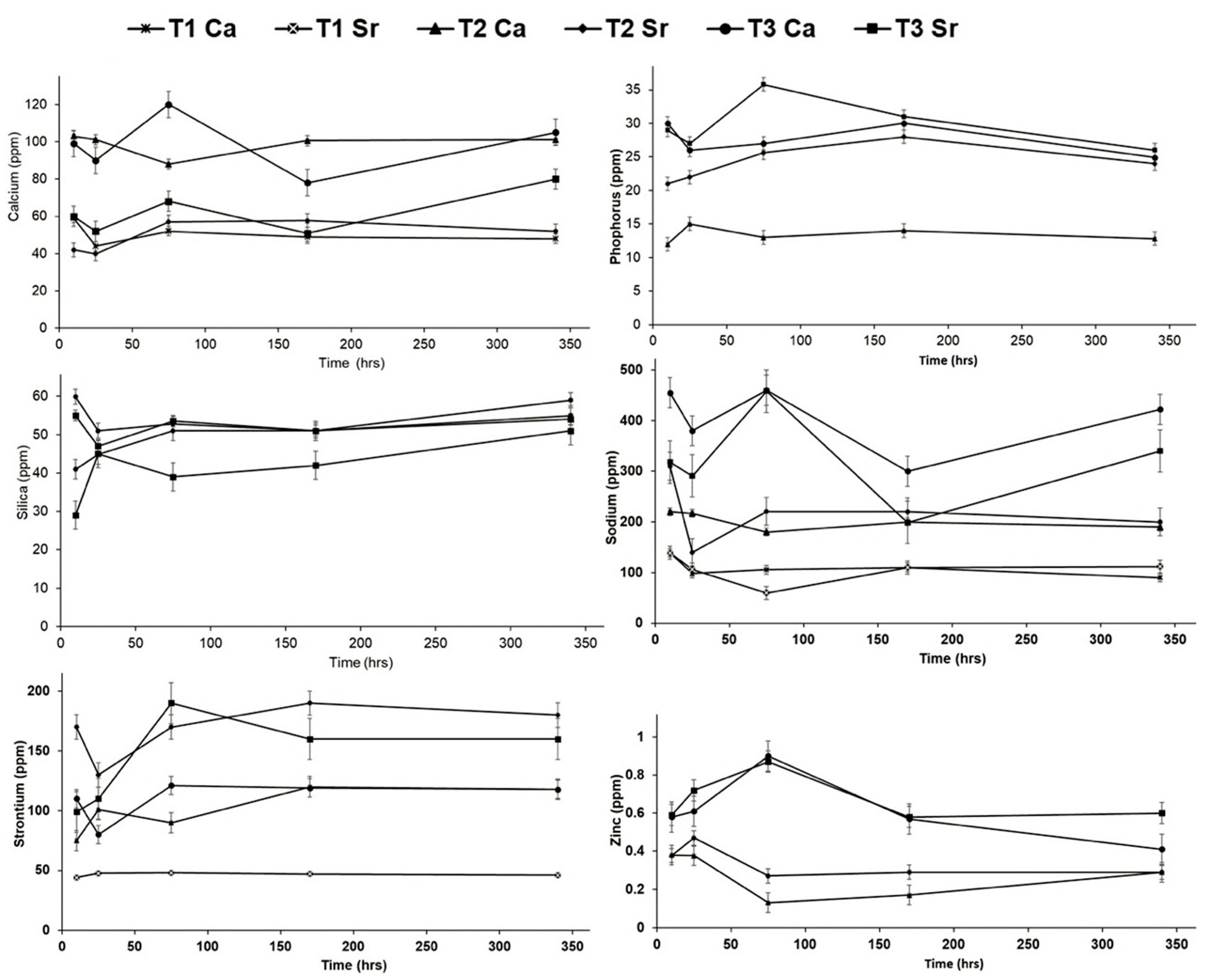
3.3.2. Degradation Study Using Inductive Coupled Plasma-Optical Emission Spectrometry (ICP-OES)
3.4. Characterization of Scaffolds after Immersion in Tris-Buffer
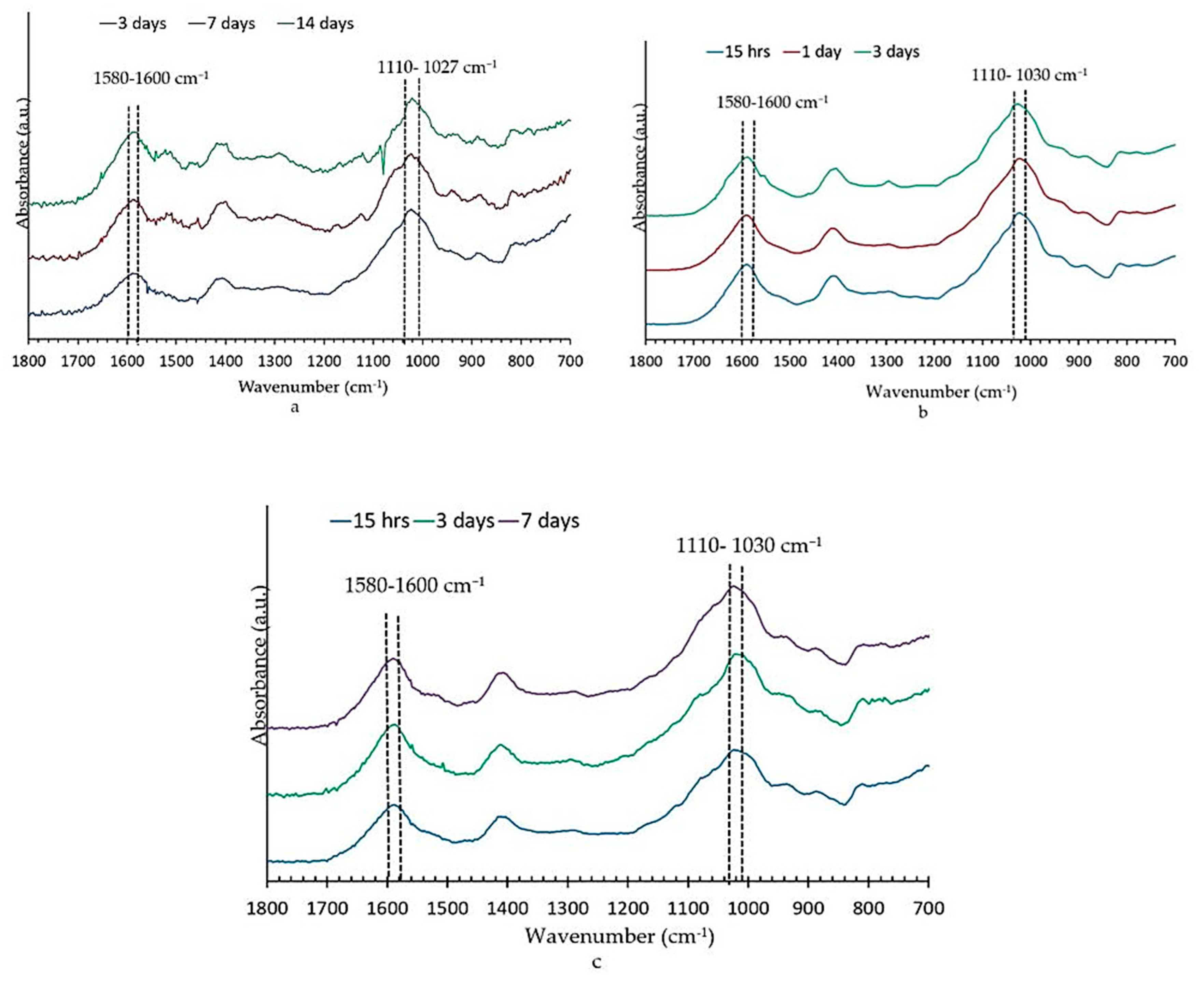
3.4.1. Fourier Transform Infrared Spectroscopy Analysis
3.4.2. Scanning Electron Microscopy
3.4.3. Nuclear Magnetic Resonance (NMR) of Scaffolds after Immersion in Tris-Buffer
4. Conclusions
Author Contributions
Funding
Acknowledgments
Conflicts of Interest
References
- Giannoudis, P.V.; Dinopoulos, H.; Tsiridis, E. Bone substitutes: An update. Injury 2005, 36, 20–27. [Google Scholar] [CrossRef]
- Bhatt, R.A.; Rozental, T.D. Bone graft substitutes. Hand Clin. 2012, 28, 457–468. [Google Scholar] [CrossRef]
- Royal College of Physicians. National Hip Fracture Database Annual Report 2017; RCP: London, UK, 2017; Available online: https://www.nhfd.co.uk/files/2017ReportFiles/NHFD-AnnualReport2017.pdf (accessed on 1 January 2020).
- Hutmacher, D.W. Scaffolds in tissue engineering bone and cartilage. Biomaterials 2000, 21, 2529–2543. [Google Scholar] [CrossRef]
- Polo-Corrales, L.; Latorre-Esteves, M.; Ramirez-Vick, J.E. Scaffold design for bone regeneration. J. Nanosci. Nanotechnol. 2014, 14, 15–56. [Google Scholar] [CrossRef] [PubMed]
- Ghassemi, T.; Shahroodi, A.; Ebrahimzadeh, M.H.; Mousavian, A.; Movaffagh, J.; Moradi, A. Current concepts in scaffolding for bone tissue engineering. Arch. Bone Joint Surg. 2018, 6, 90–99. [Google Scholar] [PubMed]
- Turco, G.; Marsich, E.; Bellomo, F.; Semeraro, S.; Donati, I.; Brun, F.; Grandolfo, M.; Accardo, A.; Paoletti, S. Alginate/hydroxyapatite biocomposite for bone ingrowth: A trabecular structure with high and isotropic connectivity. Biomacromolecules 2009, 10, 1575–1583. [Google Scholar] [CrossRef] [PubMed]
- Saurez-Gonzales, D.; Barnhart, K.; Saito, E.; Vanderby, R., Jr.; Hollister, S.J.; Murphy, W.L. Controlled nucleation of hydroxyapatite on alginate scaffolds for stem cell-based bone tissue engineering. J. Biomed. Mater. Res. A. 2010, 95, 222–234. [Google Scholar] [CrossRef]
- Hench, L.L. The story of Bioglass. J. Mater. Sci. Mater. Med. 2006, 17, 967–978. [Google Scholar] [CrossRef]
- Hench, L.L.; Splinter, R.J.; Allen, W.C.; Greenlee, T.K. Bonding mechanisms at the interface of ceramic prosthetic materials. J. Biomed. Mater. Res. 1971, 5, 117–141. [Google Scholar] [CrossRef]
- Mouriño, V.; Newby, P.; Boccaccini, A.R. Preparation and characterization of gallium releasing 3-D alginate coated 45S5 Bioglass® based scaffolds for bone tissue engineering. Adv. Eng. Mater. 2010, 12, B283–B291. [Google Scholar] [CrossRef]
- Hatton, J.; Davis, G.R.; Mourad, A.-H.; Cherupurakal, N.; Hill, R.G.; Mohsin, S. Fabrication of porous bone scaffolds using alginate and bioactive glass. J. Funct. Biomater 2019, 10, 15. [Google Scholar] [CrossRef] [PubMed]
- Jell, G.; Notingher, I.; Tsigkou, O.; Notingher, P.; Polak, J.M.; Hench, L.L.; Stevens, M.M. Bioactive glass-induced osteoblast differentiation: A noninvasive spectroscopic study. J. Biomed. Mater. Res. A 2008, 86, 31–40. [Google Scholar] [CrossRef] [PubMed]
- Hench, L.L. Genetic design of bioactive glass. J. Eur. Ceram. Soc. 2009, 29, 1257–1265. [Google Scholar] [CrossRef]
- Xynos, I.D.; Edgar, A.J.; Buttery, L.D.; Hench, L.L.; Polak, J.M. Gene-expression profiling of human osteoblasts following treatment with the ionic products of Bioglass 45S5 dissolution. J. Biomed. Mater. Res. 2001, 55, 151–157. [Google Scholar] [CrossRef]
- Bonnelye, E.; Chabadel, A.; Saltel, F.; Jurdic, P. Dual effect of strontium ranelate: Stimulation of osteoblast differentiation and inhibition of osteoclast formation and resorption in vitro. Bone 2008, 42, 129–138. [Google Scholar] [CrossRef] [PubMed]
- Yamaguchi, M. Role of nutritional zinc in the prevention of osteoporosis. Mol. Cell Biochem. 2010, 338, 241–254. [Google Scholar] [CrossRef]
- Reginster, J.Y.; Badurski, J.; Bellamy, N.; Bensen, W.; Chapurlat, R.; Chevalier, X.; Christiansen, C.; Genant, H.; Navarro, F.; Nasonov, E.; et al. Efficacy and safety of strontium ranelate in the treatment of knee osteoarthritis: Results of a double-blind, randomised placebo-controlled trial. Ann. Rheum. Dis. 2013, 72, 179–186. [Google Scholar] [CrossRef]
- Fredholm, Y.C.; Karpukhina, N.; Brauer, D.S.; Jones, J.R.; Law, R.V.; Hill, R.G. Influence of strontium for calcium substitution in bioactive glasses on degradation, ion release and apatite formation. J. R. Soc. Interface 2012, 9, 880–889. [Google Scholar] [CrossRef]
- Reginster, J.Y. Cardiac concerns associated with strontium ranelate. Expert Opin. Drug Saf. 2014, 13, 1209–1213. [Google Scholar] [CrossRef]
- Vestergaard, P.; Jorgensen, N.R.; Schwarz, P.; Mosekilde, L. Effects of treatment with fluoride on bone mineral density and fracture risk—a meta-analysis. Osteoporos. Int. 2008, 19, 257. [Google Scholar] [CrossRef]
- Elgayar, I.; Aliev, A.E.; Boccaccini, A.R.; Hill, R.G. Structural analysis of bioactive glasses. J. Non-Cryst. Solids 2005, 351, 173–183. [Google Scholar] [CrossRef]
- Mohan, N.; Nair, P.D. Novel porous, polysaccharide scaffolds for tissue engineering applications. Trends Biomater. Artif. Organs. 2005, 18, 219–224. [Google Scholar]
- Al-Khafaji, T.J.; Wong, F.; Fleming, P.S.; Karpukhina, N.; Hill, R. Novel fluoride and strontium-containing bioactive glasses for dental varnishes-design and bioactivity in Tris buffer solution. J. Non-Cryst. Solids 2019, 503, 120–130. [Google Scholar] [CrossRef]
- Al-Eesa, N.A.; Karpukhina, N.; Hill, R.G.; Johal, A.; Wong, F.S.L. Bioactive glass composite for orthodontic adhesives - Formation and characterisation of apatites using MAS-NMR and SEM. Dent. Mater. 2019, 35, 597–605. [Google Scholar] [CrossRef] [PubMed]
- Wheeler, D.L.; Stokes, K.E.; Hoellrich, R.G.; Chamberland, D.L.; McLoughlin, S.W. Effect of bioactive glass particle size on osseous regeneration of cancellous defects. J. Biomed. Mater. Res. 1998, 41, 527–533. [Google Scholar] [CrossRef]
- Massera, J.; Mayran, M.; Rocherulle, J. Crystallization behaviour of phosphate glasses and its impact on the glasses’ bioactivity. J. Mater. Sci. 2015, 50, 3091–3102. [Google Scholar] [CrossRef]
- Huang, S.; Ingber, D.E. The structural and mechanical complexity of cell-growth control. Nat. Cell Biol. 1999, 1, E131–E138. [Google Scholar] [CrossRef]
- Serra, J.; González, P.; Liste, S.; Serra, C.; Chiussi, S.; León, B.; Pérez-Amor, M.; Ylänen, H.O.; Hupa, M. FTIR and XPS studies of bioactive silica based glasses. J. Non-Cryst. Solids 2003, 332, 20–27. [Google Scholar] [CrossRef]
- Vallet-Regi, M.; Romero, A.M.; Ragel, C.V.; LeGeros, R.Z. XRD, SEM-EDS, and FTIR studies of in vitro growth of an apatite-like layer on sol-gel glasses. J. Biomed. Mater. Res. 1999, 44, 416–421. [Google Scholar] [CrossRef]
- Filgueiras, M.R.; La Torre, G.; Hench, L.L. Solution effects on the surface reactions of three bioactive glass compositions. J. Biomed. Mater. Res. 1993, 27, 1485–1493. [Google Scholar] [CrossRef]
- Peitl, O.; Zanotto, E.D.; Hench, L.L. Highly bioactive P2O5-Na2O-CaO-SiO2 glass-ceramics. J. Non-Cryst. Solids 2001, 292, 115–126. [Google Scholar] [CrossRef]
- Peitl, O.; LaTorre, G.P.; Hench, L.L. Effect of crystallization on apatite-layer formation of bioactive glass 45S5. J. Biomed. Mater. Res. 1996, 30, 509–514. [Google Scholar]
- Saarai, A.; Kasparkova, V.; Sedlacek, T.; Saha, P. On the development and characterisation of crosslinked sodium alginate/gelatine hydrogels. J. Mech. Behav. Biomed. Mater. 2013, 18, 152–166. [Google Scholar] [CrossRef] [PubMed]
- Jay, S.M.; Saltzman, W.M. Controlled delivery of VEGF via modulation of alginate microparticle ionic crosslinking. J. Control. Release 2009, 134, 26–34. [Google Scholar] [CrossRef]
- Draget, K.I.; Skjåk-Bræk, G.; Stokke, B.T. Similarities and differences between alginic acid gels and ionically crosslinked alginate gels. Food Hydrocoll. 2006, 20, 170–175. [Google Scholar] [CrossRef]
- Loh, L.Q.; Choong, C. Three-dimensional scaffolds for tissue engineering applications: Role of porosity and pore size. Tissue Eng. Part. B Rev. 2013, 19, 485–502. [Google Scholar] [CrossRef]
- Ceccaldi, C.; Fullana, S.G.; Alfarano, C.; Lairez, O.; Calise, D.; Cussac, D.; Parini, A.; Sallerin, B. Alginate scaffolds for mesenchymal stem cell cardiac therapy: Influence of alginate composition. Cell Transplant. 2012, 21, 1969–1984. [Google Scholar] [CrossRef]
- Zamani, D.; Moztarzadeh, F.; Bizari, D. Alginate-bioactive glass containing Zn and Mg composite scaffolds for bone tissue engineering. Int. J. Biol. Macromol. 2019, 137, 1256–1267. [Google Scholar] [CrossRef]
- Mneimne, M.; Hill, R.G.; Bushby, A.J.; Brauer, D.S. High phosphate content significantly increases apatite formation of fluoride-containing bioactive glasses. Acta Biomater. 2011, 7, 1827–1834. [Google Scholar] [CrossRef]
- Brauer, D.S.; Karpukhina, N.; O’Donnell, M.D.; Law, R.V.; Hill, R.G. Fluoride-containing bioactive glasses: Effect of glass design and structure on degradation, pH and apatite formation in simulated body fluid. Acta Biomater. 2010, 6, 3275–3282. [Google Scholar] [CrossRef]
- Mollica, G.; Ziarelli, F.; Lack, S.; Brunel, F.; Viel, S. Characterization of insoluble calcium alginates by solid-state NMR. Carbohydr. Polym. 2012, 87, 383–391. [Google Scholar] [CrossRef]
- Shapiro, L.; Cohen, S. Novel alginate sponges for cell culture and transplantation. Biomaterials 1997, 18, 583–590. [Google Scholar] [CrossRef]
- Espinosa, E.; Filgueira, D.; Rodríguez, A.; Chinga-Carrasco, G. Nanocellulose-Based inks—Effect of alginate content on the water absorption of 3d printed constructs. Bioengineering 2019, 6, 65. [Google Scholar] [CrossRef] [PubMed]
- Chakkalakal, D.A.; Mashoof, A.A.; Novak, J.; Strates, B.S.; McGuire, M.H. Mineralization and pH relationships in healing skeletal defects grafted with demineralized bone matrix. J. Biomed. Mater. Res. 1994, 28, 1439–1443. [Google Scholar] [CrossRef]
- Dashnyam, K.; El-Fiqi, A.; Buitrago, J.O.; Perez, R.A.; Knowles, J.C.; Kim, H.W. A mini review focused on the proangiogenic role of silicate ions released from silicon-containing biomaterials. J. Tissue Eng. 2017, 8, 2041731417707339. [Google Scholar] [CrossRef]
- Lafon, J.P.; Champion, E.; Bernache-Assollant, D. Processing of AB-Type carbonated hydroxyapatite Ca10−x(PO4)6−x(CO3)x(OH)2−x−2y(CO3)y ceramics with controlled composition. J. Eur. Ceram. Soc. 2008, 28, 139–147. [Google Scholar] [CrossRef]
- Misra, S.K.; Mohn, D.; Brunner, T.J.; Stark, W.J.; Philip, S.E.; Roy, I.; Salih, V.; Knowles, J.C.; Boccaccini, A.R. Comparison of nanoscale and microscale bioactive glass on the properties of P(3HB)/bioglass composites. Biomaterials 2008, 29, 1750–1761. [Google Scholar] [CrossRef]
- Rohanová, D.; Boccaccini, A.; Yunos, D.; Horkavcová, D.; Březovská, I.; Helebrant, A. Tris buffer in simulated body fluid distorts the assessment of glass-ceramic scaffold bioactivity. Acta Biomater. 2011, 7, 2623–2630. [Google Scholar] [CrossRef]
- Lin, K.S.; Tseng, Y.H.; Mou, Y.; Hsu, Y.C.; Yang, C.M.; Chan, J.C.C. Mechanistic study of apatite formation on bioactive glass surface using 31p solid-state NMR spectroscopy. Chem. Mater. 2005, 17, 4493–4501. [Google Scholar] [CrossRef]
- Sriranganathan, D.; Kanwal, N.; Hing, K.A.; Hill, R.G. Strontium substituted bioactive glasses for tissue-engineered scaffolds: The importance of octacalcium phosphate. J. Mater. Sci. Mater. Med. 2016, 27, 39. [Google Scholar] [CrossRef]
- Brauer, D.S.; Mneimne, M.; Hill, R.G. Fluoride-containing bioactive glasses: Fluoride loss during melting and ion release in tris buffer solution. J. Non-Cryst. Solids 2011, 357, 3328–3333. [Google Scholar] [CrossRef]
- Brown, W.E.; Eidelman, N.; Tomazic, B. Octacalcium phosphate as a precursor in biomineral formation. Adv. Dent. Res. 1987, 1, 306–313. [Google Scholar] [CrossRef] [PubMed]


| SiO2 | P2O5 | CaO | CaF2 | SrO | SrF2 | Na2O | K2O | ZnO | Melting Temperature (°C) | |
|---|---|---|---|---|---|---|---|---|---|---|
| A0 | 36.4 | 6.0 | 26.5 | 2.2 | 26.5 | 2.2 | 0 | 0 | 0 | 1500 |
| A1 | 44.0 | 5.0 | 15.0 | 0 | 15.0 | 0 | 10.0 | 10.0 | 1.0 | 1420 |
| Bone Scaffold | Alginate: BG | Mass of Alginate (g) | Mass of BG (g) |
|---|---|---|---|
| Type 1 (T1) | 1:0 | 3 | 0 |
| Type 2 (T2) | 1:1 | 3 | 3 |
| Type 3 (T3) | 2:1 | 6 | 3 |
| % of Particles | Particle Size <38 (µm) | Particle Size >38 (µm) |
|---|---|---|
| D(v,0.9) (90%) | 13.74 | 65.75 |
| D(v,0.1) (10%) | 1.21 | 1.51 |
| D(v0.5) (50%) | 4.20 | 22.06 |
| Sample Type | Pore Size (µm) |
|---|---|
| (T1) Scaffold (only alginate) | 133 ± 10.5 |
| (T2) Scaffold (alginate and bioactive glass 1:1) | 100 ± 8.5 |
| (T3) Scaffold (alginate and bioactive glass 2:1) | 107 ± 8.1 |
| Sample Name | 31P Chemical Shift at A Region (ppm) | |
|---|---|---|
| (a) | Bio glass | 6.6 |
| (b) | Type 2 before cross linking | 6.5 |
| (c) | Type 2 after cross linking with SrCl2 | 6.0 |
| (d) | Glass after 15 hrs in Tris/HCl | 4.7 |
| (e) | Type 2 Srcl2 after 15 hrs in Tris/HCl | 3.8 |
| (f) | Type 2 CaCl2 after 15 hrs in Tris/HCl | 3.9 |
| (g) | Type 2 CaCl2 after 14 days in Tris/HCl | 3.8 |
| Sample Name | 13C Chemical Shift at Region (ppm) | |||||||||
|---|---|---|---|---|---|---|---|---|---|---|
| A | B | C | D | E | F | G | H | I | ||
| (a) | Sodium alginate | 178 | 102.98 | 84 | 78 | 71 | ||||
| (b) | Type 1after cross linking with SrCl2 | 175.8 | 101.3 | 81 | 76 | 71.8 | 33 | |||
| (c) | Type 3 after cross linking with Cacl2 | 176.7 | 102 | 83 | 76.3 | 71.8 | 33.2 | |||
| (d) | Type 3 cross-linked with SrCl2 after 15 hrs in Tris | 177.7 | 103.7 | 82 | 77 | 73.4 | ||||
| (e) | Type 3 cross-linked with SrCl2 after 14 days in Tris | 176.8 | 101. 8 | 81.9 | 76.3 | 72 | 68.6 | 66.2 | 61.7 | |
© 2020 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (http://creativecommons.org/licenses/by/4.0/).
Share and Cite
Haider, A.; Waseem, A.; Karpukhina, N.; Mohsin, S. Strontium- and Zinc-Containing Bioactive Glass and Alginates Scaffolds. Bioengineering 2020, 7, 10. https://doi.org/10.3390/bioengineering7010010
Haider A, Waseem A, Karpukhina N, Mohsin S. Strontium- and Zinc-Containing Bioactive Glass and Alginates Scaffolds. Bioengineering. 2020; 7(1):10. https://doi.org/10.3390/bioengineering7010010
Chicago/Turabian StyleHaider, Asfia, Ahmad Waseem, Natalia Karpukhina, and Sahar Mohsin. 2020. "Strontium- and Zinc-Containing Bioactive Glass and Alginates Scaffolds" Bioengineering 7, no. 1: 10. https://doi.org/10.3390/bioengineering7010010
APA StyleHaider, A., Waseem, A., Karpukhina, N., & Mohsin, S. (2020). Strontium- and Zinc-Containing Bioactive Glass and Alginates Scaffolds. Bioengineering, 7(1), 10. https://doi.org/10.3390/bioengineering7010010






