A Hyaluronan Hydrogel Scaffold for Culture of Human Oral Mucosal Epithelial Cells in Limbal Stem-Cell Therapy
Abstract
1. Introduction
2. Materials and Methods
2.1. Chemicals
2.2. Ethical Considerations
2.3. Cell Isolation and Culture Conditions
2.4. Preparation of HA Hydrogels
2.5. Experimental Design
2.6. Cell Morphology
2.7. Cell Metaboilc Activity
2.8. RNA Extraction, cDNA Synthesis, Real-Time Quantitative Polymerase Chain Reaction (RT-qPCR) and Data Analysis
2.9. Statistical Analysis
3. Results
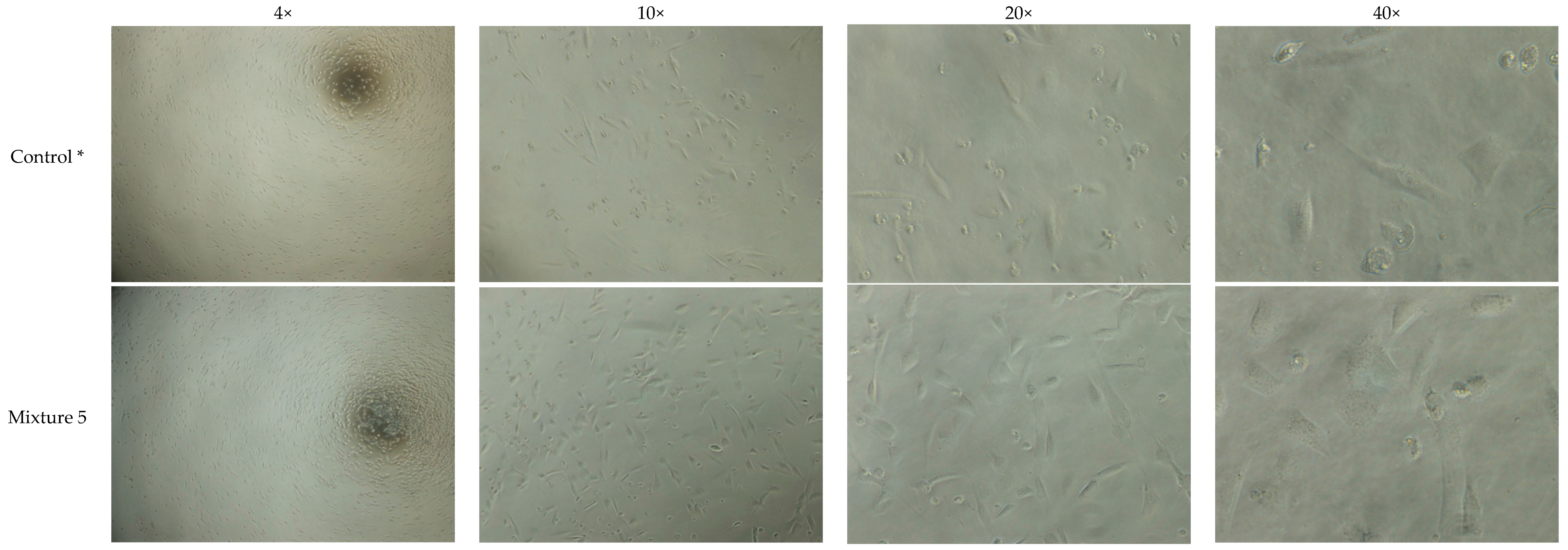
3.1. Observation of Cells on Initial HA Hydrogel Mixtures Indicate Cytotoxic Effect of Crosslinker
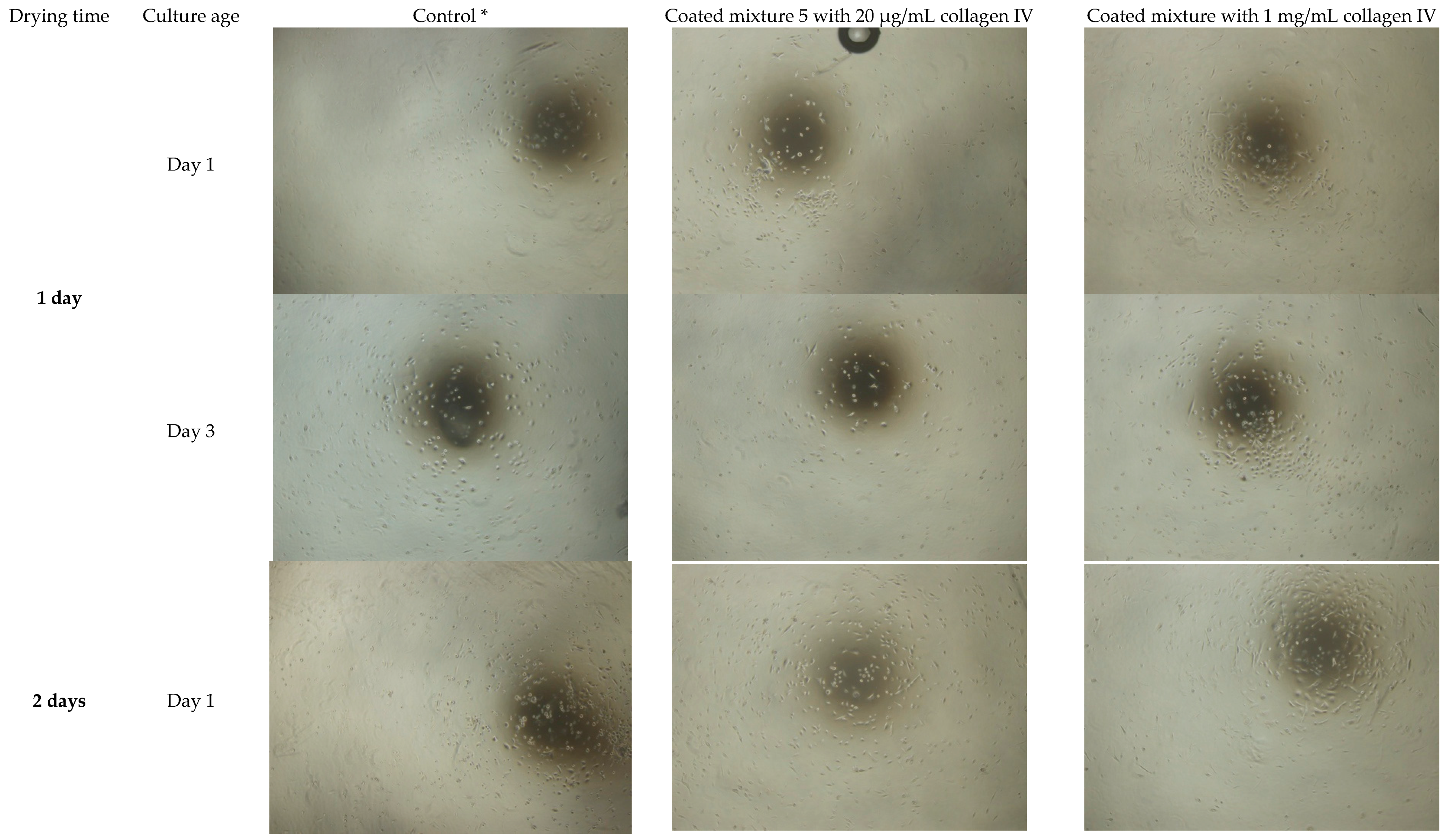
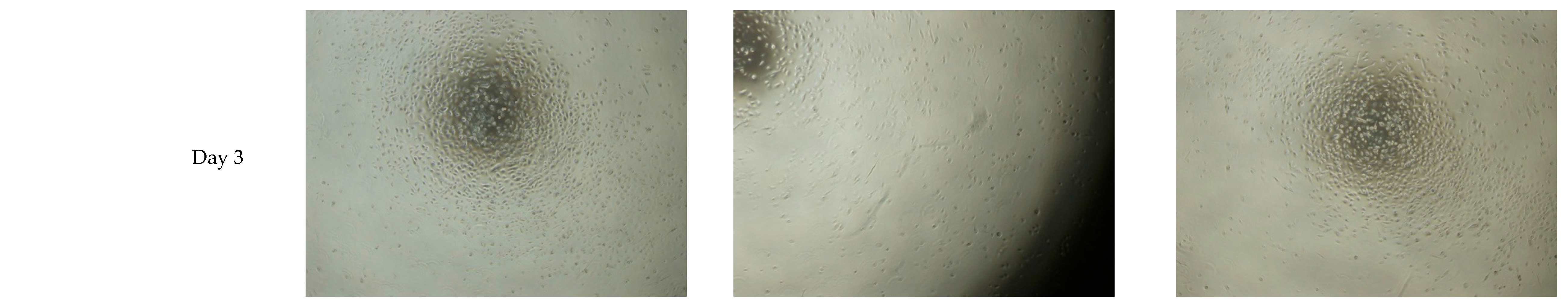
3.2. Extended Drying Time Improves the Integrity of HA Hydrogel Mixture 5
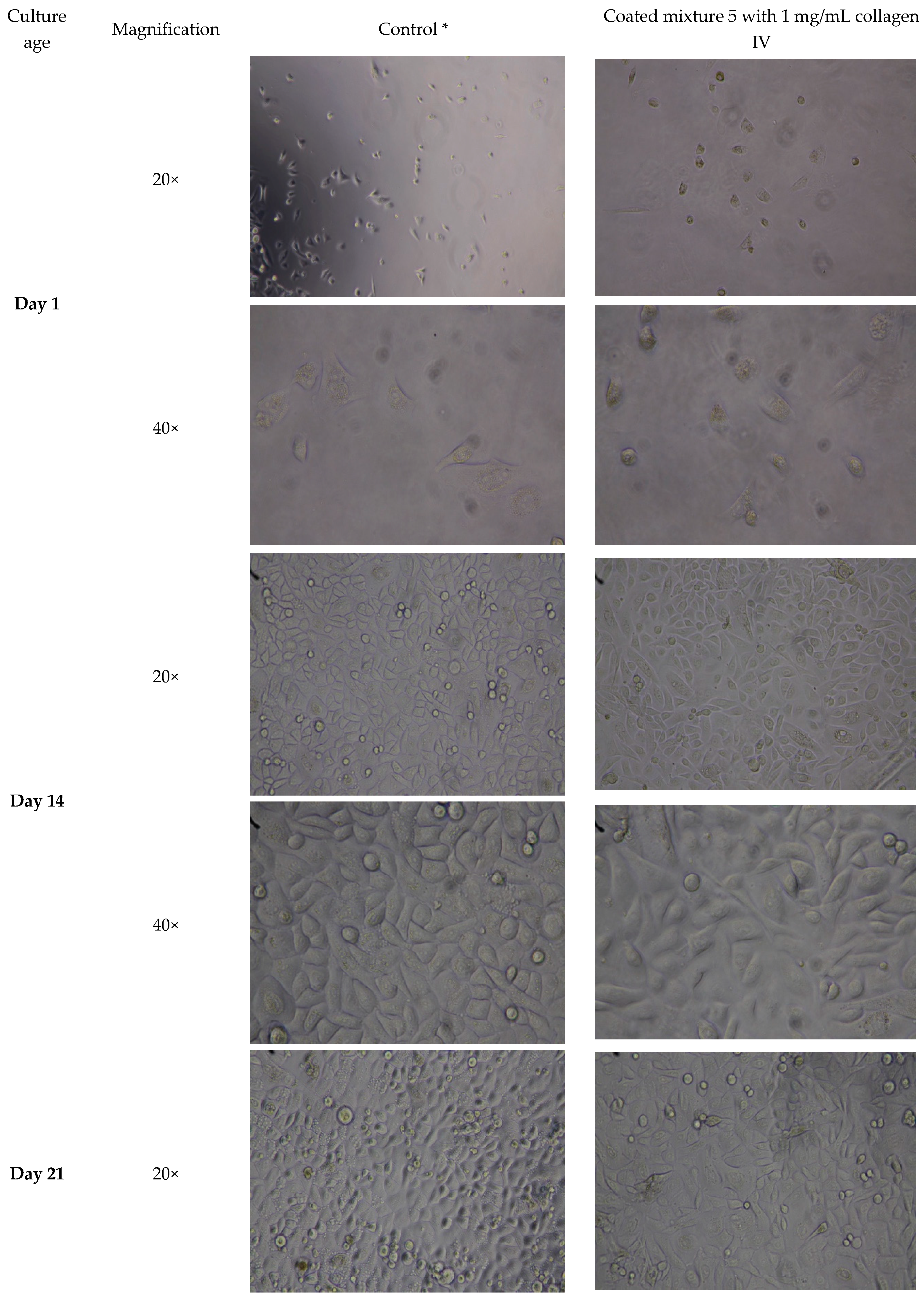
3.3. HA Hydrogel Supports Cell Adhesion of Three-Week Culture of Oral Mucosal Cells
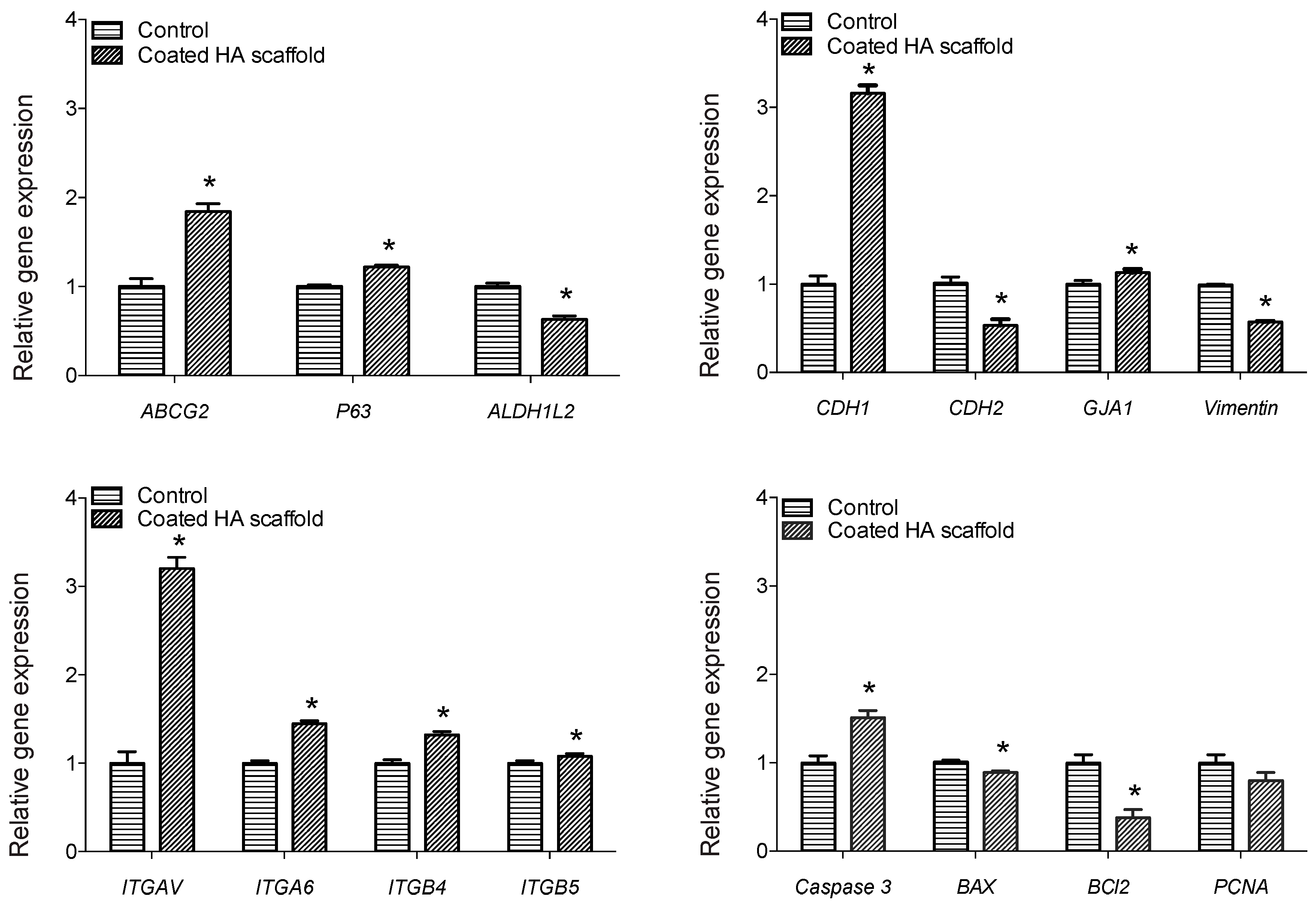
3.4. HA Hydrogel Promoted Increased Expression of Stem Cell Markers and Cell Adhesion Genes in Three-Week Cultures of Oral Mucosal Cells
4. Discussion
Supplementary Materials
Author Contributions
Funding
Conflicts of Interest
References
- Sejpal, K.; Bakhtiari, P.; Deng, S.X. Presentation, diagnosis and management of limbal stem cell deficiency. Middle East Afr. J. Ophthalmol. 2013, 20, 5. [Google Scholar] [PubMed]
- Ahmad, S. Concise review: Limbal stem cell deficiency, dysfunction, and distress. Stem Cell Transl. Med. 2012, 1, 110–115. [Google Scholar] [CrossRef] [PubMed]
- Pellegrini, G.; Traverso, C.E.; Franzi, A.T.; Zingirian, M.; Cancedda, R.; De Luca, M. Long-term restoration of damaged corneal surfaces with autologous cultivated corneal epithelium. Lancet 1997, 349, 990–993. [Google Scholar] [CrossRef]
- Nakamura, T.; Endo, K.-I.; Cooper, L.J.; Fullwood, N.J.; Tanifuji, N.; Tsuzuki, M.; Koizumi, N.; Inatomi, T.; Sano, Y.; Kinoshita, S. The successful culture and autologous transplantation of rabbit oral mucosal epithelial cells on amniotic membrane. Investig. Ophthalmol. Vis. Sci. 2003, 44, 106–116. [Google Scholar] [CrossRef] [PubMed]
- Nishida, K.; Yamato, M.; Hayashida, Y.; Watanabe, K.; Yamamoto, K.; Adachi, E.; Nagai, S.; Kikuchi, A.; Maeda, N.; Watanabe, H. Corneal reconstruction with tissue-engineered cell sheets composed of autologous oral mucosal epithelium. N. Engl. J. Med. 2004, 351, 1187–1196. [Google Scholar] [CrossRef]
- Homma, R.; Yoshikawa, H.; Takeno, M.; Kurokawa, M.S.; Masuda, C.; Takada, E.; Tsubota, K.; Ueno, S.; Suzuki, N. Induction of epithelial progenitors in vitro from mouse embryonic stem cells and application for reconstruction of damaged cornea in mice. Investig. Ophthalmol. Vis. Sci. 2004, 45, 4320–4326. [Google Scholar] [CrossRef]
- Tanioka, H.; Kawasaki, S.; Yamasaki, K.; Ang, L.P.; Koizumi, N.; Nakamura, T.; Yokoi, N.; Komuro, A.; Inatomi, T.; Kinoshita, S. Establishment of a cultivated human conjunctival epithelium as an alternative tissue source for autologous corneal epithelial transplantation. Investig. Ophthalmol. Vis. Sci. 2006, 47, 3820–3827. [Google Scholar] [CrossRef]
- Ma, Y.; Xu, Y.; Xiao, Z.; Yang, W.; Zhang, C.; Song, E.; Du, Y.; Li, L. Reconstruction of chemically burned rat corneal surface by bone marrow–derived human mesenchymal stem cells. Stem Cells 2006, 24, 315–321. [Google Scholar] [CrossRef]
- Yang, X.; Qu, L.; Wang, X.; Zhao, M.; Li, W.; Hua, J.; Shi, M.; Moldovan, N.; Wang, H.; Dou, Z. Plasticity of epidermal adult stem cells derived from adult goat ear skin. Mol. Reprod. Dev. Inc. Gamete Res. 2007, 74, 386–396. [Google Scholar] [CrossRef]
- Monteiro, B.; Serafim, R.; Melo, G.; Silva, M.; Lizier, N.; Maranduba, C.; Smith, R.; Kerkis, A.; Cerruti, H.; Gomes, J. Human immature dental pulp stem cells share key characteristic features with limbal stem cells. Cell Prolif. 2009, 42, 587–594. [Google Scholar] [CrossRef]
- Meyer-Blazejewska, E.A.; Call, M.K.; Yamanaka, O.; Liu, H.; Schlötzer-Schrehardt, U.; Kruse, F.E.; Kao, W.W. From hair to cornea: Toward the therapeutic use of hair follicle-derived stem cells in the treatment of limbal stem cell deficiency. Stem Cells 2011, 29, 57–66. [Google Scholar] [CrossRef] [PubMed]
- Reza, H.M.; Ng, B.-Y.; Gimeno, F.L.; Phan, T.T.; Ang, L.P.-K. Umbilical cord lining stem cells as a novel and promising source for ocular surface regeneration. Stem Cell Rev. Rep. 2011, 7, 935–947. [Google Scholar] [CrossRef] [PubMed]
- He, H.; Yiu, S.C. Stem cell-based therapy for treating limbal stem cells deficiency: A review of different strategies. Saudi J. Ophthalmol. 2014, 28, 188–194. [Google Scholar] [CrossRef]
- Rama, P.; Matuska, S.; Paganoni, G.; Spinelli, A.; De Luca, M.; Pellegrini, G. Limbal stem-cell therapy and long-term corneal regeneration. N. Engl. J. Med. 2010, 363, 147–155. [Google Scholar] [CrossRef] [PubMed]
- Nguyen, K.N.; Bobba, S.; Richardson, A.; Park, M.; Watson, S.L.; Wakefield, D.; Di Girolamo, N. Native and synthetic scaffolds for limbal epithelial stem cell transplantation. Acta Biomater. 2017. [Google Scholar] [CrossRef] [PubMed]
- Fiorica, C.; Senior, R.A.; Pitarresi, G.; Palumbo, F.S.; Giammona, G.; Deshpande, P.; MacNeil, S. Biocompatible hydrogels based on hyaluronic acid cross-linked with a polyaspartamide derivative as delivery systems for epithelial limbal cells. Int. J. Pharm. 2011, 414, 104–111. [Google Scholar] [CrossRef] [PubMed]
- Kiiskinen, J. Co-Culture of Corneal Epithelial Cells and Adipose Stem Cells-towards the Use of Hydrogels in Ocular Surface Reconstruction. Master Thesis, University of Tampere, Tampere, Finland, 2016. [Google Scholar]
- Chen, D.; Qu, Y.; Hua, X.; Zhang, L.; Liu, Z.; Pflugfelder, S.; Li, D. A hyaluronan hydrogel scaffold-based xeno-free culture system for ex vivo expansion of human corneal epithelial stem cells. Eye 2017, 31, 962. [Google Scholar] [CrossRef]
- Mori, M.; Yamaguchi, M.; Sumitomo, S.; Takai, Y. Hyaluronan-based biomaterials in tissue engineering. Acta Histochem. Cytochem. 2004, 37, 1–5. [Google Scholar] [CrossRef]
- Necas, J.; Bartosikova, L.; Brauner, P.; Kolar, J. Hyaluronic acid (hyaluronan): A review. Vet. Med. 2008, 53, 397–411. [Google Scholar] [CrossRef]
- Day, A.J.; Prestwich, G.D. Hyaluronan-binding proteins: Tying up the giant. J. Biol. Chem. 2002, 277, 4585–4588. [Google Scholar] [CrossRef]
- Agarwal, A.; McAnulty, J.F.; Schurr, M.J.; Murphy, C.J.; Abbott, N.L. 8-Polymeric materials for chronic wound and burn dressings A2-Farrar, David. In Advanced Wound Repair Therapies; Woodhead Publishing: Sawston, UK, 2011; pp. 186–208. [Google Scholar] [CrossRef]
- Burdick, J.A.; Prestwich, G.D. Hyaluronic acid hydrogels for biomedical applications. Adv. Mater. 2011, 23, H41–H56. [Google Scholar] [CrossRef]
- Yazdani, M.; Shahdadfar, A.; Jackson, C.J.; Utheim, T.P. Hyaluronan-Based Hydrogel Scaffolds for Limbal Stem Cell Transplantation: A Review. Cells 2019, 8, 245. [Google Scholar] [CrossRef] [PubMed]
- Roberts, J.J.; Elder, R.M.; Neumann, A.J.; Jayaraman, A.; Bryant, S.J. Interaction of hyaluronan binding peptides with glycosaminoglycans in poly (ethylene glycol) hydrogels. Biomacromolecules 2014, 15, 1132–1141. [Google Scholar] [CrossRef]
- Segura, T.; Anderson, B.C.; Chung, P.H.; Webber, R.E.; Shull, K.R.; Shea, L.D. Crosslinked hyaluronic acid hydrogels: A strategy to functionalize and pattern. Biomaterials 2005, 26, 359–371. [Google Scholar] [CrossRef] [PubMed]
- Khunmanee, S.; Jeong, Y.; Park, H. Crosslinking method of hyaluronic-based hydrogel for biomedical applications. J. Tssue Eng. 2017, 8, 2041731417726464. [Google Scholar] [CrossRef] [PubMed]
- Collins, M.N.; Birkinshaw, C. Hyaluronic acid based scaffolds for tissue engineering: A review. Carbohydr. Polym. 2013, 92, 1262–1279. [Google Scholar] [CrossRef] [PubMed]
- Rouillard, A.D.; Berglund, C.M.; Lee, J.Y.; Polacheck, W.J.; Tsui, Y.; Bonassar, L.J.; Kirby, B.J. Methods for photocrosslinking alginate hydrogel scaffolds with high cell viability. Tissue Eng. Part C Methods 2010, 17, 173–179. [Google Scholar] [CrossRef] [PubMed]
- Borzacchiello, A.; Russo, L.; Malle, B.M.; Schwach-Abdellaoui, K.; Ambrosio, L. Hyaluronic acid based hydrogels for regenerative medicine applications. BioMed Res. Int. 2015, 2015. [Google Scholar] [CrossRef]
- Lai, J.-Y.; Tu, I.-H. Adhesion, phenotypic expression, and biosynthetic capacity of corneal keratocytes on surfaces coated with hyaluronic acid of different molecular weights. Acta Biomater. 2012, 8, 1068–1079. [Google Scholar] [CrossRef]
- Liu, W.; Saint, D.A. A new quantitative method of real time reverse transcription polymerase chain reaction assay based on simulation of polymerase chain reaction kinetics. Anal Biochem. 2002, 302, 52–59. [Google Scholar] [CrossRef]
- Pfaffl, M.W.; Horgan, G.W.; Dempfle, L. Relative expression software tool (REST©) for group-wise comparison and statistical analysis of relative expression results in real-time PCR. Nucleic Acids Res. 2002, 30, 1–10. [Google Scholar] [CrossRef] [PubMed]
- Zar, J.H. Biostatistical Analysis, 15th ed.; Prentice-Hall/Pearson: Upper Saddle River, NJ, USA, 2010. [Google Scholar]
- Sokal, R.R.; Rohlf, F.J. Biometry; the Principles and Practice of Statistics in Biological Research; W. H. Freeman: New York, NY, USA, 1969. [Google Scholar]
- Lai, J.-Y. Hyaluronic acid concentration-mediated changes in structure and function of porous carriers for corneal endothelial cell sheet delivery. Mater. Sci. Eng. C 2016, 59, 411–419. [Google Scholar] [CrossRef] [PubMed]
- Gomes, J.; Amankwah, R.; Powell-Richards, A.; Dua, H. Sodium hyaluronate (hyaluronic acid) promotes migration of human corneal epithelial cells in vitro. Br. J. Ophthalmol. 2004, 88, 821–825. [Google Scholar] [CrossRef] [PubMed]
- Rozario, T.; DeSimone, D.W. The extracellular matrix in development and morphogenesis: A dynamic view. Dev. Biol. 2010, 341, 126–140. [Google Scholar] [CrossRef] [PubMed]
- Bhuvanesh, T.; Machatschek, R.; Lysyakova, L.; Kratz, K.; Schulz, B.; Ma, N.; Lendlein, A. Collagen type-IV Langmuir and Langmuir-Schäfer layers as model biointerfaces to direct stem cell adhesion. Biomed. Mater. 2018. [Google Scholar] [CrossRef]
- Cheng, W.; Yan-hua, R.; Fang-gang, N.; Guo-an, Z. The content and ratio of type I and III collagen in skin differ with age and injury. Afr. J. Biotechnol. 2011, 10, 2524–2529. [Google Scholar]
- Popova, S.; Lundgren-Akerlund, E.; Wiig, H.; Gullberg, D. Physiology and pathology of collagen receptors. Acta Physiol. (Oxf. Engl.) 2007, 190, 179–187. [Google Scholar] [CrossRef]
- Khoshnoodi, J.; Pedchenko, V.; Hudson, B.G. Mammalian collagen IV. Microsc. Res. Tech. 2008, 71, 357–370. [Google Scholar] [CrossRef]
- Stein, E.; Blaimauer, K.; Bauer, S.; Erovic, B.M.; Turhani, D.; Thurnher, D. High expression of integrin β1 correlates with high proliferation capacity in oral keratinocytes. Wien. Klin. Wochenschr. 2007, 119, 318–322. [Google Scholar] [CrossRef]
- Igarashi, T.; Shimmura, S.; Yoshida, S.; Tonogi, M.; Shinozaki, N.; Yamane, G.Y. Isolation of oral epithelial progenitors using collagen IV. Oral Dis. 2008, 14, 413–418. [Google Scholar] [CrossRef]
- Jones, P.H.; Watt, F.M. Separation of human epidermal stem cells from transit amplifying cells on the basis of differences in integrin function and expression. Cell 1993, 73, 713–724. [Google Scholar] [CrossRef]
- Jones, P.H.; Harper, S.; Watt, F.M. Stem cell patterning and fate in human epidermis. Cell 1995, 80, 83–93. [Google Scholar] [CrossRef]
- Watt, F.M. Role of integrins in regulating epidermal adhesion, growth and differentiation. EMBO J. 2002, 21, 3919–3926. [Google Scholar] [CrossRef] [PubMed]
- Coelho, N.; Llopis-Hernández, V.; Salmerón-Sánchez, M.; Altankov, G. Dynamic Reorganization and Enzymatic Remodeling of Type IV Collagen at Cell–Biomaterial Interface. In Advances in Protein Chemistry and Structural Biology; Elsevier: Amsterdam, The Netherlands, 2016; Volume 105, pp. 81–104. [Google Scholar]
- Coelho, N.M.; Salmerón-Sánchez, M.; Altankov, G. Fibroblasts remodeling of type IV collagen at a biomaterials interface. Biomater. Sci. 2013, 1, 494–502. [Google Scholar] [CrossRef]
- Berx, G.; Staes, K.; van Hengel, J.; Molemans, F.; Bussemakers, M.J.; van Bokhoven, A.; Van Roy, F. Cloning and characterization of the human invasion suppressor gene E-cadherin (CDH1). Genomics 1995, 26, 281–289. [Google Scholar] [CrossRef]
- Madhira, S.L.; Vemuganti, G.; Bhaduri, A.; Gaddipati, S.; Sangwan, V.S.; Ghanekar, Y. Culture and characterization of oral mucosal epithelial cells on human amniotic membrane for ocular surface reconstruction. Mol. Vis. 2008, 14, 189–196. [Google Scholar] [PubMed]
- Dhamodaran, K.; Subramani, M.; Jeyabalan, N.; Ponnalagu, M.; Chevour, P.; Shetty, R.; Matalia, H.; Shetty, R.; Prince, S.E.; Das, D. Characterization of ex vivo cultured limbal, conjunctival, and oral mucosal cells: A comparative study with implications in transplantation medicine. Mol. Vis. 2015, 21, 828. [Google Scholar]
- Vassalli, G. Aldehyde Dehydrogenases: Not Just Markers, but Functional Regulators of Stem Cells. Stem Cells Int. 2019. [Google Scholar] [CrossRef]
- Li, L.; Bennett, S.A.; Wang, L. Role of E-cadherin and other cell adhesion molecules in survival and differentiation of human pluripotent stem cells. Cell Adhes. Migr. 2012, 6, 59–73. [Google Scholar] [CrossRef]
- Bardag-Gorce, F.; Hoft, R.H.; Wood, A.; Oliva, J.; Niihara, H.; Makalinao, A.; Thropay, J.; Pan, D.; Meepe, I.; Tiger, K. The Role of E-Cadherin in maintaining the barrier function of corneal epithelium after treatment with cultured autologous oral mucosa epithelial cell sheet grafts for limbal stem deficiency. J. Ophthalmol. 2016, 2016. [Google Scholar] [CrossRef]
- Eslami, A.; Gallant-Behm, C.L.; Hart, D.A.; Wiebe, C.; Honardoust, D.; Gardner, H.; Hakkinen, L.; Larjava, H.S. Expression of integrin alphavbeta6 and TGF-beta in scarless vs scar-forming wound healing. J. Histochem. Cytochem. 2009, 57, 543–557. [Google Scholar] [CrossRef] [PubMed]

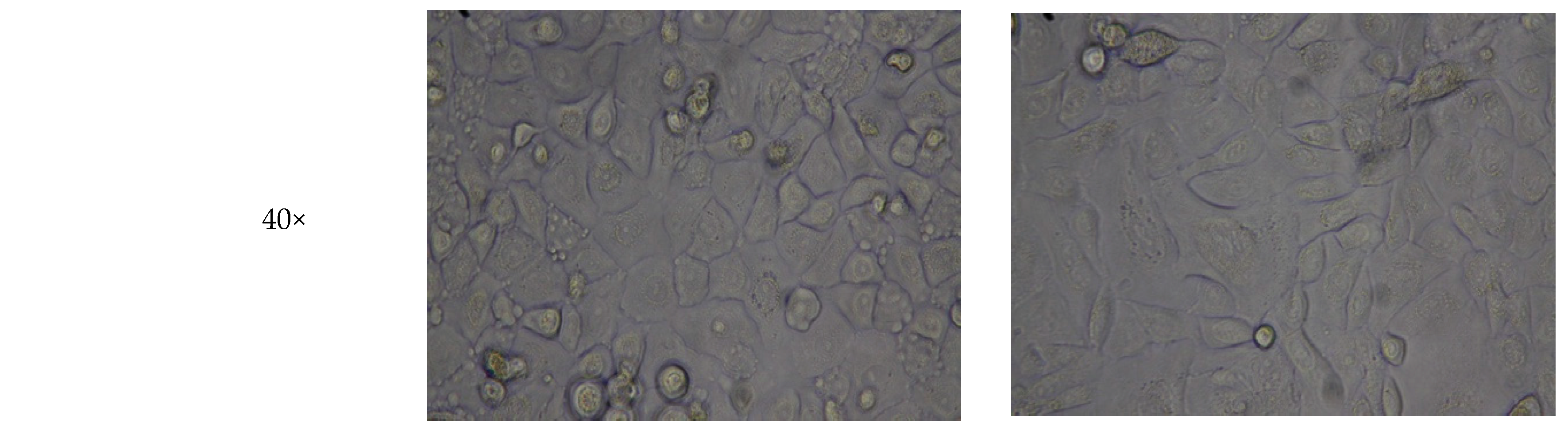

| Mixture 1 | Mixture 2 | Mixture 3 | Mixture 4 | Mixture 5 | |
|---|---|---|---|---|---|
| Glycosil | 80% | 60% | 40% | 20% | 100% |
| Extralink | 20% | 40% | 60% | 80% | 0 |
| Gelation time * | 30 min | 30 min | 30 min | ~6h | ~24h |
| Appearance | Firm | Firm | Firm | Little floppy | Floppy |
| Study No. | Objective | Endpoint | Examination/Assay | Hyaluronan (HA) Scaffold | Culture Age | ||
|---|---|---|---|---|---|---|---|
| Formula * | Drying Time | Collagen Coating | |||||
| 1) | To study toxic effects of crosslinker and compare collagen IV coatings | Cell attachment and morphology | Light microscope | Mixtures 1–5 | 30 min to ~1 day | Uncoated and coated (1 mg/mL and 20 µg/mL) | 1 day |
| 2) | To study effects of 1- and 2-day old collagen coated HA scaffold on oral mucosal epithelial cells (OMECs) culture within 3 day | Cell attachment and morphology | Light microscope | Mixture 5 | 1 and 2 days | Coated (1 mg/mL and 20 µg/mL) | 1 and 3 days |
| 3) | To study effects of 3- to 14-day-old collagen coated HA scaffold on OMECs cultured for 2 weeks | Cell attachment and morphology | Light microscope | Mixture 5 | 3, 7, 10 and 14 days | Coated (1 mg/mL) | 1, 3, 7, 10 and 14 days |
| 4) | To study effects of 3-day old collagen-coated HA scaffold on OMECs cultured for 3 weeks | Morphology, metabolic activity and gene expression | Light microscope, ATPlite luminescence and real-time quantitative polymerase chain reaction (RT-qPCR) ≠ | Mixture 5 | 3 days | Coated (1 mg/mL) | 1, 3, 7, 10, 14 and 21 days ≠ |
| Gene Symbol | Gene Name | Alias | Taqman Assay ID | E-value |
|---|---|---|---|---|
| GAPDH | Glyceraldehyde-3-phosphate dehydrogenase | G3PD, HEL-S-162eP | Hs99999905_m1 | 2.00 |
| ABCG2 | ATP-binding cassette sub-family G member 2 | ABC15, ABCP | Hs01053790_m1 | 2.07 |
| ΔNp63α | Tumor protein p63 | TP63, TP53L | Hs00978343_m1 | 1.91 |
| ALDH1L2 | Aldehyde dehydrogenase 1 family member L2 | mtFDH | Hs00402876_m1 | 2.00 |
| CDH1 | Cadherin 1, type 1, E-cadherin | CD324, CDH1 | Hs01023894_m1 | 2.02 |
| CDH2 | Cadherin 2, type 1, N-cadherin | CD325, NCAD | Hs00983056_m1 | 2.00 |
| GJA1 (Connexin 43) | Gap junction protein, alpha 1, 43kDa | CX43, GJAL | Hs00748445_m1 | 2.07 |
| Vimentin | Vimentin | Vim, FLJ36605 | Hs00185584_m1 | 1.97 |
| ITGAV | Integrin alpha V | CD51, MSK8 | Hs00233808_m1 | 2.00 |
| ITGA6 | Integrin alpha 6 | CD49f, VLA-6 | Hs01041011_m1 | 1.89 |
| ITGB4 | Integrin beta 4 | CD104 | Hs00236216_m1 | 1.84 |
| ITGB5 | Integrin beta 5 | - | Hs00174435_m1 | 2.00 |
| CASP3 | Caspase 3, apoptosis-related cysteine peptidase | CPP32, CPP32B | Hs00234387_m1 | 2.04 |
| BAX2 | BCL2-associated X protein | BCL2L4 | Hs00180269_m1 | 2.00 |
| BCL2 | BCL2, apoptosis regulator | Bcl-2, BCL2 | Hs99999018_m1 | 2.05 |
| PCNA | Proliferating cell nuclear antigen | MGC8367 | Hs00696862_m1 | 1.95 |
| Gene Symbol | Regulation | Fold Change |
|---|---|---|
| ABCG2 | UP | 1.84 |
| P63 | Up | 1.22 |
| ALDH1L2 | Down | 1.59 |
| CDH1 | Up | 3.16 |
| CDH2 | Down | 1.90 |
| GJA1 | Up | 1.13 |
| Vimentin | Down | 1.74 |
| ITGAV | Up | 3.20 |
| ITGA6 | Up | 1.45 |
| ITGB4 | Up | 1.32 |
| ITGB5 | Up | 1.08 |
| CASP3 | Up | 1.51 |
| BAX2 | Down | 1.13 |
| BCL2 | Down | 2.63 |
© 2019 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (http://creativecommons.org/licenses/by/4.0/).
Share and Cite
Yazdani, M.; Shahdadfar, A.; Jackson, C.J.; Utheim, T.P. A Hyaluronan Hydrogel Scaffold for Culture of Human Oral Mucosal Epithelial Cells in Limbal Stem-Cell Therapy. Bioengineering 2019, 6, 97. https://doi.org/10.3390/bioengineering6040097
Yazdani M, Shahdadfar A, Jackson CJ, Utheim TP. A Hyaluronan Hydrogel Scaffold for Culture of Human Oral Mucosal Epithelial Cells in Limbal Stem-Cell Therapy. Bioengineering. 2019; 6(4):97. https://doi.org/10.3390/bioengineering6040097
Chicago/Turabian StyleYazdani, Mazyar, Aboulghassem Shahdadfar, Catherine Joan Jackson, and Tor Paaske Utheim. 2019. "A Hyaluronan Hydrogel Scaffold for Culture of Human Oral Mucosal Epithelial Cells in Limbal Stem-Cell Therapy" Bioengineering 6, no. 4: 97. https://doi.org/10.3390/bioengineering6040097
APA StyleYazdani, M., Shahdadfar, A., Jackson, C. J., & Utheim, T. P. (2019). A Hyaluronan Hydrogel Scaffold for Culture of Human Oral Mucosal Epithelial Cells in Limbal Stem-Cell Therapy. Bioengineering, 6(4), 97. https://doi.org/10.3390/bioengineering6040097






