Tequila Agave Bagasse Hydrolysate for the Production of Polyhydroxybutyrate by Burkholderia sacchari
Abstract
1. Introduction
2. Materials and Methods
2.1. Characterization TAB
2.2. Chemical Hydrolysis of TAB and Hydrolysate Characterization
2.3. Microorganism and Culture Media
2.4. B. sacchari Growth Inhibition by TABH
2.5. B. sacchari Growth Inhibition by Model Toxic Compounds
2.6. Detoxification of TABH by Different Methods
2.7. PHB Production from Detoxified TABH by B. sacchari
2.8. PHB Characterization
3. Results and Discussions
3.1. Characterization of TAB
3.2. Chemical Hydrolysis of TAB
3.3. B. sacchari Growth Inhibition by Toxic Compounds Present in the TABH
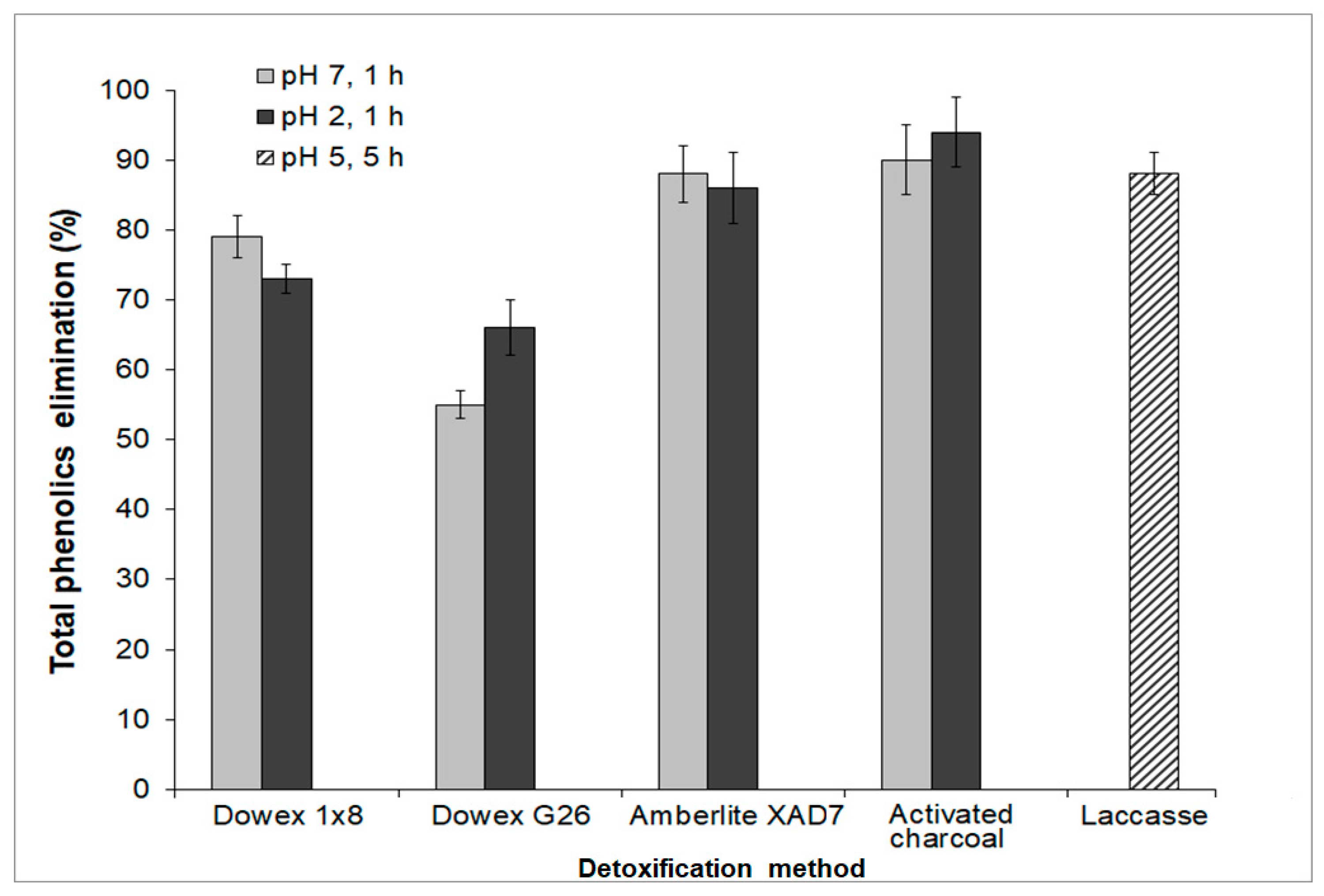
3.4. Elimination of Growth Inhibitory Compounds from TABH
3.5. B. sacchari Growth in TABH Detoxified by Different Methods
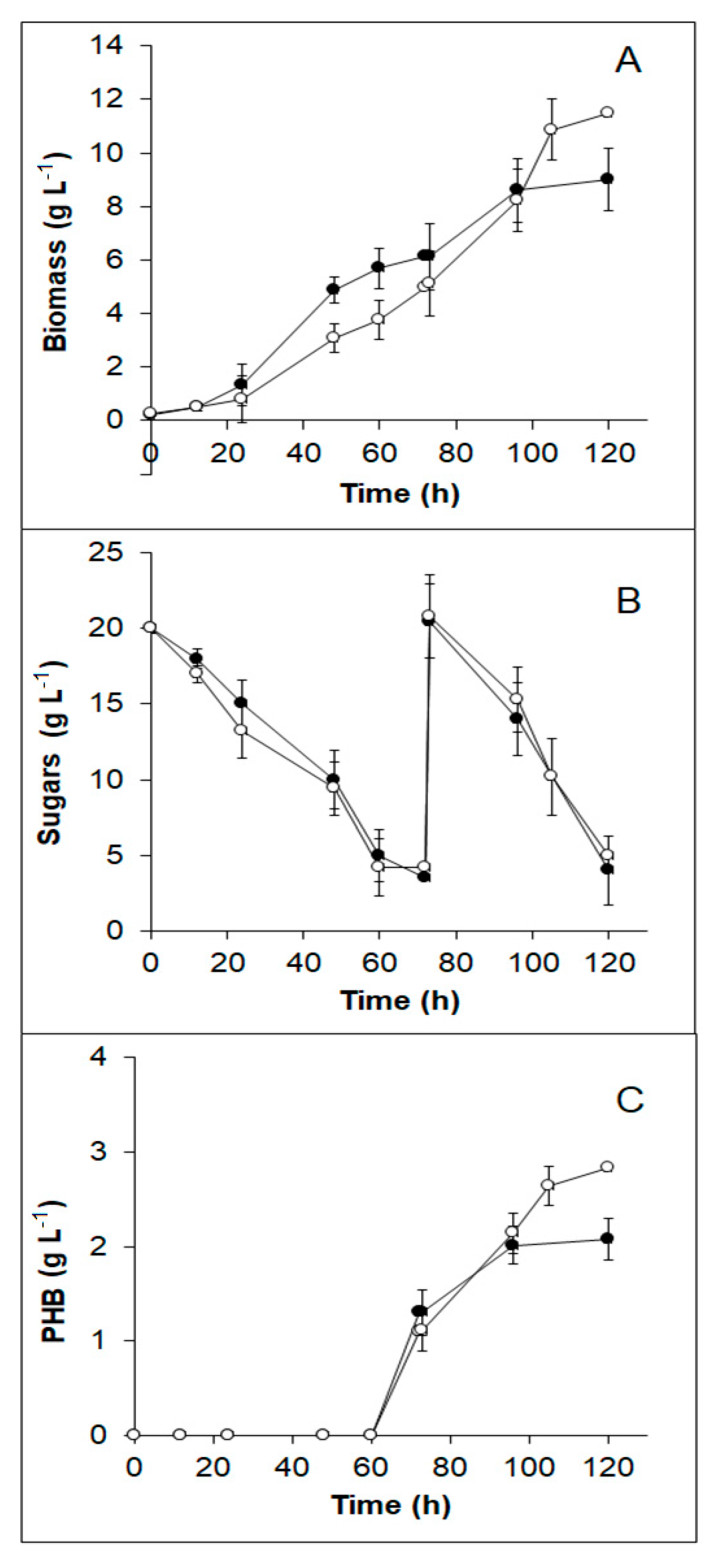
3.6. PHB Production from TABH Detoxified with Activated Charcoal
3.7. PHB Characterization
Author Contributions
Funding
Conflicts of Interest
References
- Tsang, Y.F.; Kumar, V.; Samadar, P.; Yang, Y.; Lee, J.; Ok, Y.S.; Song, H.; Kim, K.-H.; Kwon, E.E.; Jeon, Y.J. Production of bioplastic through food waste valorization. Environ. Int. 2019, 127, 625–644. [Google Scholar] [CrossRef]
- Salgaonkar, B.B.; Bragança, J.M. Utilization of Sugarcane Bagasse by Halogeometricum borinquense Strain E3 for Biosynthesis of Poly(3-hydroxybutyrate-co-3-hydroxyvalerate). Bioengineering 2017, 4, 50. [Google Scholar] [CrossRef]
- Sandhya, M.; Aravind, J.; Kanmani, P. Production of polyhydroxyalkanoates from Ralstonia eutropha using paddy straw as cheap substrate. Int. J. Environ. Sci. Technol. 2012, 10, 47–54. [Google Scholar] [CrossRef]
- Nielsen, C.; Rahman, A.; Rehman, A.U.; Walsh, M.K.; Miller, C.D. Food waste conversion to microbial polyhydroxyalkanoates. Microb. Biotechnol. 2017, 10, 1338–1352. [Google Scholar] [CrossRef] [PubMed]
- Saucedo-Luna, J.; Castro-Montoya, A.J.; Rico, J.L.; Campos-García, J. Optimization of acid hydrolysis of bagasse from Agave tequilana Weber. Rev. Mex. Ing. Quim. 2010, 9, 91–97. [Google Scholar]
- Aguilar, D.L.; Rodríguez-Jasso, R.M.; Zanuso, E.; de Rodríguez, D.J.; Amaya-Delgado, L.; Sanchez, A.; Ruiz, H.A. Scale-up and evaluation of hydrothermal pretreatment in isothermal and non-isothermal regimen for bioethanol production using agave bagasse. Bioresour. Technol. 2018, 263, 112–119. [Google Scholar] [CrossRef] [PubMed]
- Niehus, X.; Crutz-Le Coq, A.-M.; Sandoval, G.; Nicaud, J.-M.; Ledesma-Amaro, R. Engineering Yarrowia lipolytica to enhance lipid production from lignocellulosic materials. Biotechnol. Biofuels 2018, 11, 11. [Google Scholar] [CrossRef]
- Alva Munoz, L.E.; Riley, M.R. Utilization of cellulosic waste from tequila bagasse and production of polyhydroxyalkanoate (PHA) bioplastics by Saccharophagus degradans. Biotechnol. Bioeng. 2008, 100, 882–888. [Google Scholar] [CrossRef]
- Obruca, S.; Benesova, P.; Marsalek, L.; Marova, I. Use of lignocellulosic materials for PHA production. Chem. Biochem. Eng. Q. 2015, 29, 135–144. [Google Scholar] [CrossRef]
- Dubois, M.; Gilles, K.; Hamilton, J.; Rebers, P.; Smith, F. Colorimetric method based on phenol sulfuric acid. Anal. Chem. 1956, 28, 356. [Google Scholar]
- Miller, G.L. Use of dinitrosalicylic acid reagent for determination of reducing sugar. Anal. Chem. 1956, 31, 426–428. [Google Scholar] [CrossRef]
- Singleton, V.L.; Orthofer, R.; Lamuela-Raventos, R.M. Analysis of total phenols and other oxidation substrates and antioxidants by means of Folin–Ciocalteau reagent. Method. Enzymol. 1999, 299, 152–178. [Google Scholar]
- Chandel, A.K.; Kapoor, R.K.; Singh, A.; Kuhad, R.C. Detoxification of sugarcane bagasse hydrolysate improves ethanol production by Candida shehatae NCIM 3501. Bioresour. Technol. 2007, 98, 1947–1950. [Google Scholar] [CrossRef] [PubMed]
- Linton, E.; Rahman, A.; Viamajala, S.; Sims, R.C.; Miller, C.D. Polyhydroxyalkanoate quantification in organic wastes and pure cultures using a single-step extraction and 1H NMR analysis. Water Sci. Technol. 2012, 66, 1000–1006. [Google Scholar] [CrossRef] [PubMed]
- Núñez, H.M.; Rodríguez, L.F.; Khanna, M. Agave for tequila and biofuels: An economic assessment and potential opportunities. GCB Bioenergy 2011, 3, 43–57. [Google Scholar] [CrossRef]
- Mussatto, S.I.; Roberto, I.C. Alternatives for detoxification of diluted-acid lignocellulosic hydrolyzates for use in fermentative processes: A review. Bioresour. Technol. 2004, 93, 1–10. [Google Scholar] [CrossRef] [PubMed]
- Zamudio-Jaramillo, M.A.; Castro-Montoya, A.J.; Yescas, R.M.; Parga, M.D.C.C.; Hernández, J.C.G.; Luna, J.S. Optimization of particle size for hydrolysis of pine wood polysaccharides and its impact on milling energy. IJRER 2014, 4, 338–348. [Google Scholar]
- Laopaiboon, P.; Thani, A.; Leelavatcharamas, V.; Laopaiboon, L. Acid hydrolysis of sugarcane bagasse for lactic acid production. Bioresour. Technol. 2010, 101, 1036–1043. [Google Scholar] [CrossRef]
- Martinez, A.; Rodriguez, M.E.; Wells, M.L.; York, S.W.; Preston, J.F.; Ingram, L.O. Detoxification of dilute acid hydrolysates of lignocellulose with lime. Biotechnol. Prog. 2001, 17, 287–293. [Google Scholar] [CrossRef]
- Kamal, S.M.M.; Mohamad, N.L.; Abdullah, A.G.L.; Abdullah, N. Detoxification of sago trunk hydrolysate using activated charcoal for xylitol production. Procedia Food Sci. 2011, 1, 908–913. [Google Scholar] [CrossRef][Green Version]
- Chandel, A.K.; Singh, O.V.; Rao, L.V.; Chandrasekhar, G.; Narasu, M.L. Bioconversion of novel substrate Saccharum spontaneum, a weedy material, into ethanol by Pichia stipitis NCIM3498. Bioresour. Technol. 2011, 102, 1709–1714. [Google Scholar] [CrossRef] [PubMed]
- Palmqvist, E.; Hahn-Hägerdal, B. Fermentation of lignocellulosic hydrolysates. I: Inhibition and detoxification. Bioresour. Technol. 2000, 74, 17–24. [Google Scholar] [CrossRef]
- Silva, L.; Taciro, M.; Ramos, M.; Carter, J.; Pradella, J.; Gomez, G. Poly-3-hydroxybutyrate (P3HB) production by bacteria from xylose, glucose, and sugarcane bagasse hydrolysate. J. Ind. Microbiol. Biot. 2004, 31, 245–254. [Google Scholar] [CrossRef] [PubMed]
- Liu, R.; Liang, L.; Cao, W.; Mingke, W.; Chen, K.; Ma, J.; Jiang, M.; Wei, P.; Ouyang, P. Succinate production by metabolically engineered Escherichia coli using sugarcane bagasse hydrolysate as the carbon source. Bioresour. Technol. 2012, 135. [Google Scholar]
- Nigam, J.N. Ethanol production from wheat straw hemicellulose hydrolysate by Pichia stipitis. J. Biotechnol. 2001, 87, 17–27. [Google Scholar] [CrossRef]
- Palmqvist, E.; Hahn-Hägerdal, B. Fermentation of lignocellulosic hydrolysates. II: Inhibitors and mechanisms of inhibition. Bioresour. Technol. 2000, 74, 25–33. [Google Scholar] [CrossRef]
- Millati, R.; Niklasson, C.; Taherzadeh, M.J. Effect of pH, time and temperature of overliming on detoxification of dilute-acid hydrolyzates for fermentation by Saccharomyces cerevisiae. Proc. Biochem. 2002, 38, 515–522. [Google Scholar] [CrossRef]
- Monlau, F.; Sambusiti, C.; Barakat, A.; Quéméneur, M.; Trably, E.; Steyer, J.-P.; Carrere, H. Do furanic and phenolic compounds of lignocellulosic and algae biomass hydrolyzate inhibit anaerobic mixed cultures? A comprehensive review. Biotechnol. Adv. 2014, 32, 934–951. [Google Scholar] [CrossRef]
- Liu, X.; Fatehi, P.; Ni, Y. Removal of inhibitors from pre-hydrolysis liquor of kraft-based dissolving pulp production process using adsorption and flocculation processes. Bioresour. Technol. 2012, 116, 492–496. [Google Scholar] [CrossRef]
- Zhang, Y.; Xia, C.; Lu, M.; Tu, M. Effect of overliming and activated carbon detoxification on inhibitors removal and butanol fermentation of poplar prehydrolysates. Biotechnol. Biofuels. 2018, 11, 178. [Google Scholar] [CrossRef]
- Mateo, S.; Roberto, I.C.; Sánchez, S.; Moya, A.J. Detoxification of hemicellulosic hydrolyzate from olive tree pruning residue. Ind. Crop. Prod. 2013, 49, 196–203. [Google Scholar] [CrossRef]
- Sarawan, C.; Suinyuy, T.; Sewsynker-Sukai, Y.; Kana, E.B. Optimized activated charcoal detoxification of acid-pretreated lignocellulosic substrate and assessment for bioethanol production. Bioresour. Technol. 2019, 286, 121403. [Google Scholar] [CrossRef] [PubMed]
- Michailof, C.; Stavropoulos, G.G.; Panayiotou, C. Enhanced adsorption of phenolic compounds, commonly encountered in olive mill wastewaters, on olive husk derived activated carbons. Bioresour. Technol. 2008, 99, 6400–6408. [Google Scholar] [CrossRef] [PubMed]
- Costa, T.D.S.; Rogez, H.; Pena, R.D.S. Adsorption capacity of phenolic compounds onto cellulose and xylan. Food Sci. Technol. 2015, 35, 314–320. [Google Scholar] [CrossRef]
- Carvalheiro, F.; Duarte, L.C.; Lopes, S.; Parajó, J.C.; Pereira, H.; Gírio, F.M. Evaluation of the detoxification of brewery’s spent grain hydrolysate for xylitol production by Debaryomyces hansenii CCMI 941. Proc. Biochem. 2005, 40, 1215–1223. [Google Scholar] [CrossRef]
- Nilvebrant, N.-O.; Reimann, A.; Larsson, S.; Jönsson, L.J. Detoxification of lignocellulose hydrolysates with ion-exchange resins. Appl. Biochem. Biotechnol. 2001, 91, 35–49. [Google Scholar] [CrossRef]
- Mota, M.I.F.; Barbosa, S.; Pinto, P.C.R.; Ribeiro, A.M.; Ferreira, A.; Loureiro, J.M.; Rodrigues, A.E. Adsorption of vanillic and syringic acids onto a macroporous polymeric resin and recovery with ethanol:water (90:10 %V/V) solution. Sep. Purif. Technol. 2019, 217, 108–117. [Google Scholar] [CrossRef]
- Nitzsche, R.; Gröngröft, A.; Kraume, M. Separation of lignin from beech wood hydrolysate using polymeric resins and zeolites—Determination and application of adsorption isotherms. Sep. Purif. Technol. 2019, 209, 491–502. [Google Scholar] [CrossRef]
- Martos, N.; Sánchez, A.; Molina-Díaz, A. Comparative study of the retention of nine phenolic compounds on anionic exchanger resins. Chem. Pap. 2005, 59, 161. [Google Scholar]
- Bertin, L.; Ferri, F.; Scoma, A.; Marchetti, L.; Fava, F. Recovery of high added value natural polyphenols from actual olive mill wastewater through solid phase extraction. Chem. Eng. J. 2011, 171, 1287–1293. [Google Scholar] [CrossRef]
- Moreno, A.D.; Ibarra, D.; Fernández, J.L.; Ballesteros, M. Different laccase detoxification strategies for ethanol production from lignocellulosic biomass by the thermotolerant yeast Kluyveromyces marxianus CECT 10875. Bioresour. Technol. 2012, 106, 101–109. [Google Scholar] [CrossRef] [PubMed]
- Jurado, M.; Prieto, A.; Martínez-Alcalá, A.; Martínez, A.T.; Martínez, M.J. Laccase detoxification of steam-exploded wheat straw for second generation bioethanol. Bioresour. Technol. 2009, 100, 6378–6384. [Google Scholar] [CrossRef] [PubMed]
- Huang, C.; Wu, H.; Liu, Q.; Li, Y.; Zong, M. Effects of aldehydes on the growth and lipid accumulation of oleaginous yeast Trichosporon fermentans. J. Agric. Food Chem. 2011, 59, 4606–4613. [Google Scholar] [CrossRef] [PubMed]
- Guo, Z.; Olsson, L. Physiological response of Saccharomyces cerevisiae to weak acids present in lignocellulosic hydrolysate. FEMS Yeast Res. 2014, 14, 1234–1248. [Google Scholar] [CrossRef] [PubMed]
- Nikodinovic-Runic, J.; Guzik, M.; Kenny, S.T.; Babu, R.; Werker, A.; O Connor, K.E. Carbon-rich wastes as feedstocks for biodegradable polymer (Polyhydroxyalkanoate) production suing bacteria. In Advances in Applied Microbiology; Elsevier: Amsterdam, The Netherlands, 2013; Volume 84, pp. 139–200. ISBN 978-0-12-407673-0. [Google Scholar]
- Arcos-Hernandez, M.V.; Gurieff, N.; Pratt, S.; Magnusson, P.; Werker, A.; Vargas, A.; Lant, P. Rapid quantification of intracellular PHA using infrared spectroscopy: An application in mixed cultures. J. Biotechnol. 2010, 150, 372–379. [Google Scholar] [CrossRef]
- Lopes, M.S.G.; Gosset, G.; Rocha, R.C.S.; Gomez, J.G.C.; Ferreira da Silva, L. PHB biosynthesis in catabolite repression mutant of Burkholderia sacchari. Curr. Microbiol. 2011, 63, 319–326. [Google Scholar] [CrossRef] [PubMed]



| Component | Content (%) |
|---|---|
| Cellulose | 50.1 ± 2.1 |
| Hemicellulose | 21.1 ± 2.4 |
| Lignin | 13.1 ± 1.3 |
| Extractable | 8.0 ± 1.1 |
| Ashes | 0.8 ± 0.1 |
| Humidity | 7.0 ± 0.9 |
| Compound | Fiber Size | |
|---|---|---|
| Mixed (125–420 μm) | 60 mesh (250 μm) | |
| Total sugars (g·L−1) | 25.5 ± 1.5 | 23.9 ± 1.9 |
| Reducing sugars (g·L−1) | 20.61 ± 0.92 | 19.14 ± 1.03 |
| Xylose (%) 1 | 72 | 71 |
| Glucose (%) | 28 | 29 |
| Total phenolic compounds (g·L−1) | 1.7 ± 0.12 | 1.6 ± 0.13 |
| Lignocellulosic Material | Reducing Sugars (g·L−1) | Phenolic Compounds (g·L−1) | Reference |
|---|---|---|---|
| TAB | 24.9 | n.r. | [5] |
| Sugarcane bagasse | 25.38 | n.r. | [18] |
| Sugarcane bagasse | n.r. | 2.86 | [19] |
| Sago trunk cortex | 29.46 | 2.15 | [20] |
| Sugarcane bagasse | 30.29 | 2.75 | [13] |
| Saccharum spontaneum | 32.15 | 2.01 | [21] |
| Parameter | Control Medium (CM) | TABH (Detoxified) |
|---|---|---|
| Total biomass (g·L−1) | 8.78 ± 1.04 | 11.03 ± 1.14 |
| Residual biomass (g·L−1) a | 6.77 ± 1.09 | 8.36 ± 0.91 |
| PHB (g·L−1) | 2.01 ± 0.86 | 2.67 ± 0.96 |
| PHB (%) b | 22.91 ± 1.18 | 24.20 ± 1.26 |
| μmax (h−1) | 0.08 ± 0.01 | 0.11 ± 0.02 |
| YX/S (g·g−1) c | 0.23 ± 0.02 | 0.25 ± 0.02 |
| YP/S (g·g−1) d | 0.10 ± 0.01 | 0.10 ± 0.01 |
© 2019 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (http://creativecommons.org/licenses/by/4.0/).
Share and Cite
González-García, Y.; Grieve, J.; Meza-Contreras, J.C.; Clifton-García, B.; Silva-Guzman, J.A. Tequila Agave Bagasse Hydrolysate for the Production of Polyhydroxybutyrate by Burkholderia sacchari. Bioengineering 2019, 6, 115. https://doi.org/10.3390/bioengineering6040115
González-García Y, Grieve J, Meza-Contreras JC, Clifton-García B, Silva-Guzman JA. Tequila Agave Bagasse Hydrolysate for the Production of Polyhydroxybutyrate by Burkholderia sacchari. Bioengineering. 2019; 6(4):115. https://doi.org/10.3390/bioengineering6040115
Chicago/Turabian StyleGonzález-García, Yolanda, Janessa Grieve, Juan Carlos Meza-Contreras, Berenice Clifton-García, and José Antonio Silva-Guzman. 2019. "Tequila Agave Bagasse Hydrolysate for the Production of Polyhydroxybutyrate by Burkholderia sacchari" Bioengineering 6, no. 4: 115. https://doi.org/10.3390/bioengineering6040115
APA StyleGonzález-García, Y., Grieve, J., Meza-Contreras, J. C., Clifton-García, B., & Silva-Guzman, J. A. (2019). Tequila Agave Bagasse Hydrolysate for the Production of Polyhydroxybutyrate by Burkholderia sacchari. Bioengineering, 6(4), 115. https://doi.org/10.3390/bioengineering6040115





