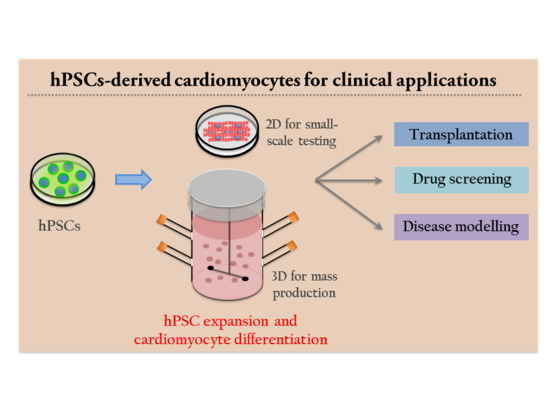
Expansion Culture of Human Pluripotent Stem Cells and Production of Cardiomyocytes
Abstract
1. Introduction
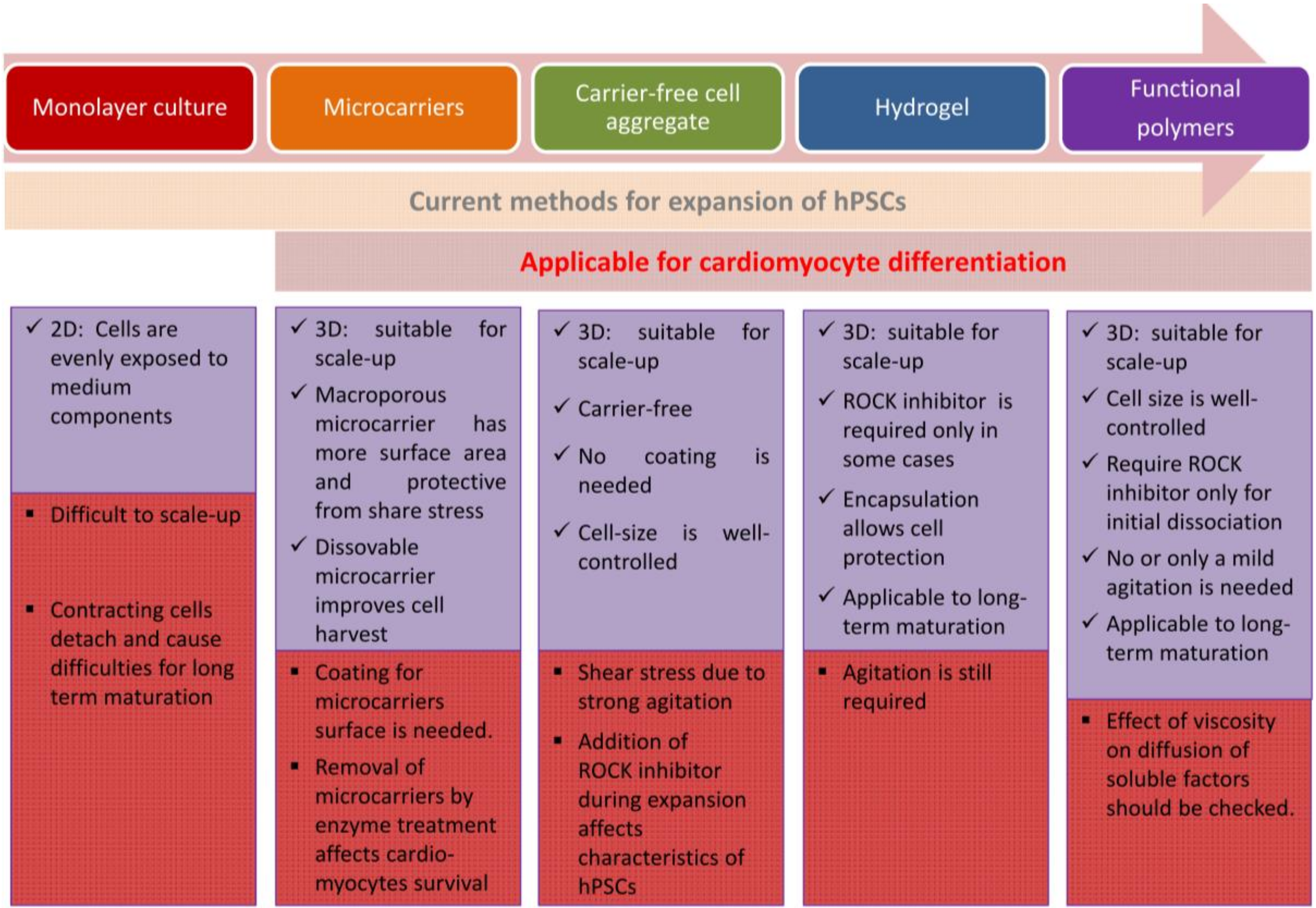
2. Large Scale Expansion Culture of Human Pluripotent Stem Cells
2.1. Adherent Culture of Human Pluripotent Stem Cells
2.2. Suspension Culture of Human Pluripotent Stem Cells
2.2.1. Application of Microcarriers
2.2.2. Formation of Carrier-Free 3D Aggregates
2.2.3. Hydrogels
2.2.4. Functional Polymers for Suspension Culture of hiPSCs without Agitation
3. Application of Human Pluripotent Stem Cells for Cardiomyocyte Differentiation
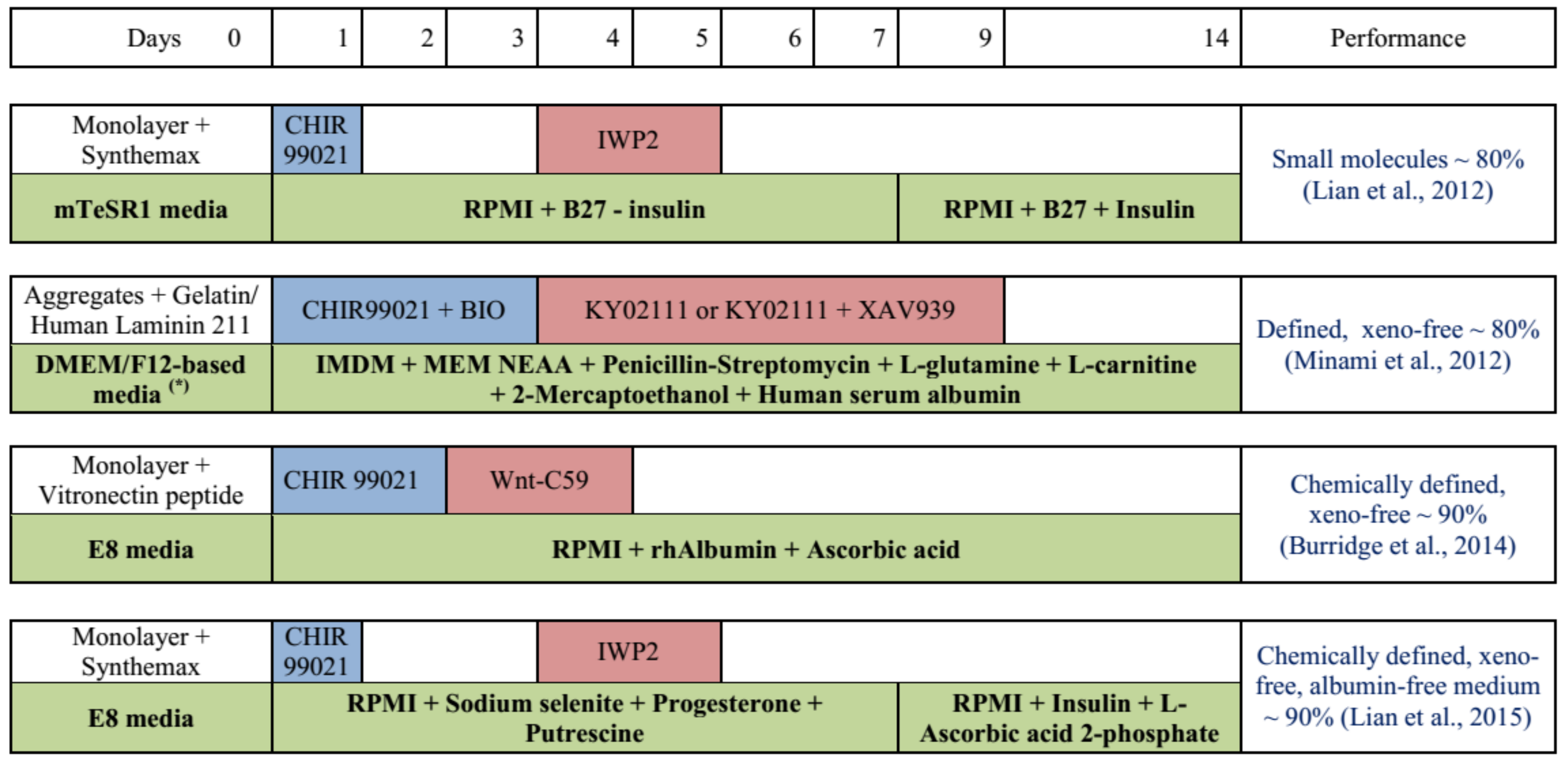
3.1. Cardiomyocyte Differentiation as Monolayer Culture
3.2. Cardiomyocyte Differentiation by Suspension Culture Using Microcarriers
3.3. Cardiomyocyte Differentiation by Applying Carrier-Free Cell Aggregates
3.4. Cardiomyocyte Differentiation by Applying Hydrogels
4. Strategies for Cardiomyocyte Maturation
5. Conclusions and Future Perspective
Author Contributions
Funding
Conflicts of Interest
References
- Wang, H. Pluripotent Stem Cells and Repair of Myocardial Infarction. Trop. Med. Surg. 2015. [Google Scholar] [CrossRef]
- Chow, A.; Stuckey, D.J.; Kidher, E.; Rocco, M.; Jabbour, R.J.; Mansfield, C.A.; Darzi, A.; Harding, S.E.; Stevens, M.M.; Athanasiou, T. Human Induced Pluripotent Stem Cell-Derived Cardiomyocyte Encapsulating Bioactive Hydrogels Improve Rat Heart Function Post Myocardial Infarction. Stem Cell Rep. 2017, 9, 1415–1422. [Google Scholar] [CrossRef] [PubMed]
- Thomson, J.A.; Itskovitz-Eldor, J.; Shapiro, S.S.; Waknitz, M.A.; Swiergiel, J.J.; Marshall, V.S.; Jones, J.M. Embryonic stem cell lines derived from human blastocysts. Science 1998, 282, 1145–1147. [Google Scholar] [CrossRef] [PubMed]
- Takahashi, K.; Tanabe, K.; Ohnuki, M.; Narita, M.; Ichisaka, T.; Tomoda, K.; Yamanaka, S. Induction of pluripotent stem cells from adult human fibroblasts by defined factors. Cell 2007, 131, 861–872. [Google Scholar] [CrossRef] [PubMed]
- Nelson, T.J.; Martinez-Fernandez, A.; Yamada, S.; Perez-Terzic, C.; Ikeda, Y.; Terzic, A. Repair of acute myocardial infarction by human stemness factors induced pluripotent stem cells. Circulation 2009, 120, 408–416. [Google Scholar] [CrossRef]
- Singla, D.K.; Long, X.; Glass, C.; Singla, R.D.; Yan, B. Induced pluripotent stem (iPS) cells repair and regenerate infarcted myocardium. Mol. Pharm. 2011, 8, 1573–1581. [Google Scholar] [CrossRef]
- Ahmed, R.P.; Ashraf, M.; Buccini, S.; Shujia, J.; Haider, H. Cardiac tumorigenic potential of induced pluripotent stem cells in an immunocompetent host with myocardial infarction. Regen. Med. 2011, 6, 171–178. [Google Scholar] [CrossRef] [PubMed]
- Lalit, P.A.; Hei, D.J.; Raval, A.N.; Kamp, T.J. Induced pluripotent stem cells for post-myocardial infarction repair: Remarkable opportunities and challenges. Circ. Res. 2014, 114, 1328–1345. [Google Scholar] [CrossRef] [PubMed]
- Liu, Y.W.; Chen, B.; Yang, X.; Fugate, J.A.; Kalucki, F.A.; Futakuchi-Tsuchida, A.; Couture, L.; Vogel, K.W.; Astley, C.A.; Baldessari, A.; et al. Human embryonic stem cell-derived cardiomyocytes restore function in infarcted hearts of non-human primates. Nat. Biotechnol. 2018, 36, 597–605. [Google Scholar] [CrossRef] [PubMed]
- Osaka University to Begin World’s First iPS Cell-Based Therapy for the Heart. Available online: http://www.med.osaka-u.ac.jp/eng/archives/3739 (accessed on 20 April 2019).
- Halbach, M.; Krausgrill, B.; Hannes, T.; Wiedey, M.; Peinkofer, G.; Baumgartner, S.; AliSahito, R.G.; Pfannkuche, K.; Pillekamp, F.; Reppel, M.; et al. Time-course of the electrophysiological maturation and integration of transplanted cardiomyocytes. J. Mol. Cell. Cardiol. 2012, 53, 401–408. [Google Scholar] [CrossRef] [PubMed]
- Yang, X.; Pabon, L.; Murry, C.E. Engineering adolescence: Maturation of human pluripotent stem cell-derived cardiomyocytes. Circ. Res. 2014, 114, 511–523. [Google Scholar] [CrossRef] [PubMed]
- Li, T.S.; Cheng, K.; Malliaras, K.; Smith, R.R.; Zhang, Y.; Sun, B.; Matsushita, N.; Blusztajn, A.; Terrovitis, J.; Kusuoka, H.; et al. Direct comparison of different stem cell types and subpopulations reveals superior paracrine potency and myocardial repair efficacy with cardiosphere-derived cells. J. Am. Coll. Cardiol. 2012, 59, 942–953. [Google Scholar] [CrossRef] [PubMed]
- Miyagawa, S.; Domae, K.; Yoshikawa, Y.; Fukushima, S.; Nakamura, T.; Saito, A.; Sakata, Y.; Hamada, S.; Toda, K.; Pak, K.; et al. Phase I Clinical Trial of Autologous Stem Cell-Sheet Transplantation Therapy for Treating Cardiomyopathy. J. Am. Heart Assoc. 2017, 6. [Google Scholar] [CrossRef]
- Chen, K.G.; Mallon, B.S.; McKay, R.D.; Robey, P.G. Human pluripotent stem cell culture: Considerations for maintenance, expansion, and therapeutics. Cell Stem Cell 2014, 14, 13–26. [Google Scholar] [CrossRef] [PubMed]
- Dakhore, S.; Nayer, B.; Hasegawa, K. Human Pluripotent Stem Cell Culture: Current Status, Challenges, and Advancement. Stem Cells Int. 2018, 2018, 7396905. [Google Scholar] [CrossRef] [PubMed]
- Hartman, M.E.; Dai, D.F.; Laflamme, M.A. Human pluripotent stem cells: Prospects and challenges as a source of cardiomyocytes for in vitro modeling and cell-based cardiac repair. Adv. Drug Deliv. Rev. 2016, 96, 3–17. [Google Scholar] [CrossRef]
- Denning, C.; Borgdorff, V.; Crutchley, J.; Firth, K.S.; George, V.; Kalra, S.; Kondrashov, A.; Hoang, M.D.; Mosqueira, D.; Patel, A.; et al. Cardiomyocytes from human pluripotent stem cells: From laboratory curiosity to industrial biomedical platform. Biochim. Biophys. Acta 2016, 1863, 1728–1748. [Google Scholar] [CrossRef]
- Aigha, I.; Raynaud, C. Maturation of pluripotent stem cell derived cardiomyocytes: The new challenge. Glob. Cardiol. Sci. Pract. 2016, 2016, e201606. [Google Scholar] [CrossRef] [PubMed]
- Scuderi, G.J.; Butcher, J. Naturally Engineered Maturation of Cardiomyocytes. Front. Cell Dev. Biol. 2017, 5, 50. [Google Scholar] [CrossRef] [PubMed]
- Kempf, H.; Andree, B.; Zweigerdt, R. Large-scale production of human pluripotent stem cell derived cardiomyocytes. Adv. Drug Deliv. Rev. 2016, 96, 18–30. [Google Scholar] [CrossRef]
- Tsutsui, H.; Valamehr, B.; Hindoyan, A.; Qiao, R.; Ding, X.; Guo, S.; Witte, O.N.; Liu, X.; Ho, C.M.; Wu, H. An optimized small molecule inhibitor cocktail supports long-term maintenance of human embryonic stem cells. Nat. Commun. 2011, 2, 167. [Google Scholar] [CrossRef]
- Chen, G.; Gulbranson, D.R.; Hou, Z.; Bolin, J.M.; Ruotti, V.; Probasco, M.D.; Smuga-Otto, K.; Howden, S.E.; Diol, N.R.; Propson, N.E.; et al. Chemically defined conditions for human iPSC derivation and culture. Nat. Methods 2011, 8, 424–429. [Google Scholar] [CrossRef]
- Miyazaki, T.; Isobe, T.; Nakatsuji, N.; Suemori, H. Efficient Adhesion Culture of Human Pluripotent Stem Cells Using Laminin Fragments in an Uncoated Manner. Sci. Rep. 2017, 7, 41165. [Google Scholar] [CrossRef]
- Melkoumian, Z.; Weber, J.L.; Weber, D.M.; Fadeev, A.G.; Zhou, Y.; Dolley-Sonneville, P.; Yang, J.; Qiu, L.; Priest, C.A.; Shogbon, C.; et al. Synthetic peptide-acrylate surfaces for long-term self-renewal and cardiomyocyte differentiation of human embryonic stem cells. Nat. Biotechnol. 2010, 28, 606–610. [Google Scholar] [CrossRef] [PubMed]
- Jin, S.; Yao, H.; Weber, J.L.; Melkoumian, Z.K.; Ye, K. A synthetic, xeno-free peptide surface for expansion and directed differentiation of human induced pluripotent stem cells. PLoS ONE 2012, 7, e50880. [Google Scholar] [CrossRef] [PubMed]
- Brafman, D.A.; Chang, C.W.; Fernandez, A.; Willert, K.; Varghese, S.; Chien, S. Long-term human pluripotent stem cell self-renewal on synthetic polymer surfaces. Biomaterials 2010, 31, 9135–9144. [Google Scholar] [CrossRef] [PubMed]
- Mei, Y.; Krishanu, S.; Bogatyrev, S.R.; Yang, J.; Hook, A.L.; Kalcioglu, Z.I.; Cho, S.W.; Mitalipova, M.; Pyzocha, N.; Roja, F.; et al. Combinatorial development of biomaterials for clonal growth of human pluripotent stem cells. Nat. Mater. 2010, 9, 768–778. [Google Scholar] [CrossRef]
- Villa-Diaz, L.G.; Nandivada, H.; Ding, J.; Nogueira-de-Souza, N.C.; Krebsbach, P.H.; O’Shea, K.S.; Lahann, J.; Smith, G.D. Synthetic polymer coatings for long-term growth of human embryonic stem cells. Nat. Biotechnol. 2010, 28, 581–583. [Google Scholar] [CrossRef]
- Irwin, E.F.; Gupta, R.; Dashti, D.C.; Healy, K.E. Engineered polymer-media interfaces for the long-term self-renewal of human embryonic stem cells. Biomaterials 2011, 32, 6912–6919. [Google Scholar] [CrossRef]
- Ross, A.M.; Nandivada, H.; Ryan, A.L.; Lahann, J. Synthetic substrates for long-term stem cell culture. Polymer 2012, 53, 2533–2539. [Google Scholar] [CrossRef]
- Celiz, A.D.; Smith, J.G.; Patel, A.K.; Hook, A.L.; Rajamohan, D.; George, V.T.; Flatt, L.; Patel, M.J.; Epa, V.C.; Singh, T.; et al. Discovery of a Novel Polymer for Human Pluripotent Stem Cell Expansion and Multilineage Differentiation. Adv. Mater. 2015, 27, 4006–4012. [Google Scholar] [CrossRef]
- Yasuda, S.-y.; Ikeda, T.; Shahsavarani, H.; Yoshida, N.; Nayer, B.; Hino, M.; Vartak-Sharma, N.; Suemori, H.; Hasegawa, K. Chemically defined and growth-factor-free culture system for the expansion and derivation of human pluripotent stem cells. Nat. Biomed. Eng. 2018, 2, 173–182. [Google Scholar] [CrossRef]
- Microcarrier Cell Culture—Principles and Methods. Available online: http://www.gelifesciences.co.kr/wp-content/uploads/2016/07/023.8_Microcarrier-Cell-Culture.pdf (accessed on 20 April 2019).
- Chen, A.K.; Chen, X.; Choo, A.B.; Reuveny, S.; Oh, S.K. Critical microcarrier properties affecting the expansion of undifferentiated human embryonic stem cells. Stem Cell Res. 2011, 7, 97–111. [Google Scholar] [CrossRef] [PubMed]
- Silva, M.M.; Rodrigues, A.F.; Correia, C.; Sousa, M.F.; Brito, C.; Coroadinha, A.S.; Serra, M.; Alves, P.M. Robust Expansion of Human Pluripotent Stem Cells: Integration of Bioprocess Design with Transcriptomic and Metabolomic Characterization. Stem Cells Transl. Med. 2015, 4, 731–742. [Google Scholar] [CrossRef] [PubMed]
- Rodrigues, C.A.V.; Silva, T.P.; Nogueira, D.E.S.; Fernandes, T.G.; Hashimura, Y.; Wesselschmidt, R.; Diogo, M.M.; Lee, B.; Cabral, J.M.S. Scalable culture of human induced pluripotent cells on microcarriers under xeno-free conditions using single-use vertical-wheel™ bioreactors. J. Chem. Technol. Biotechnol. 2018, 93, 3597–3606. [Google Scholar] [CrossRef]
- Lecina, M.; Ting, S.; Choo, A.; Reuveny, S.; Oh, S. Scalable platform for human embryonic stem cell differentiation to cardiomyocytes in suspended microcarrier cultures. Tissue Eng. Part C Methods 2010, 16, 1609–1619. [Google Scholar] [CrossRef]
- Merten, O.W. Advances in cell culture: Anchorage dependence. Philos. Trans. R. Soc. London. Ser. Bbiol. Sci. 2015, 370, 20140040. [Google Scholar] [CrossRef]
- Farrell, C.J.; Cicalese, S.M.; Davis, H.B.; Dogdas, B.; Shah, T.; Culp, T.; Hoang, V.M. Cell confluency analysis on microcarriers by micro-flow imaging. Cytotechnology 2016, 68, 2469–2478. [Google Scholar] [CrossRef]
- Phillips, B.W.; Horne, R.; Lay, T.S.; Rust, W.L.; Teck, T.T.; Crook, J.M. Attachment and growth of human embryonic stem cells on microcarriers. J. Biotechnol. 2008, 138, 24–32. [Google Scholar] [CrossRef] [PubMed]
- Oh, S.K.; Chen, A.K.; Mok, Y.; Chen, X.; Lim, U.M.; Chin, A.; Choo, A.B.; Reuveny, S. Long-term microcarrier suspension cultures of human embryonic stem cells. Stem Cell Res. 2009, 2, 219–230. [Google Scholar] [CrossRef] [PubMed]
- Badenes, S.M.; Fernandes, T.G.; Cordeiro, C.S.; Boucher, S.; Kuninger, D.; Vemuri, M.C.; Diogo, M.M.; Cabral, J.M. Defined Essential 8 Medium and Vitronectin Efficiently Support Scalable Xeno-Free Expansion of Human Induced Pluripotent Stem Cells in Stirred Microcarrier Culture Systems. PLoS ONE 2016, 11, e0151264. [Google Scholar] [CrossRef]
- Stathopoulos, N.A.; Hellums, J.D. Shear stress effects on human embryonic kidney cells in Vitro. Biotechnol. Bioeng. 1985, 27, 1021–1026. [Google Scholar] [CrossRef]
- Cormier, J.T.; Nieden, N.I.Z.; Rancourt, D.E.; Kallos, M.S. Expansion of Undifferentiated Murine Embryonic Stem Cells as Aggregates in Suspension Culture Bioreactors. Tissue Eng. 2006, 12, 3233–3245. [Google Scholar] [CrossRef] [PubMed]
- Badenes, S.M.; Fernandes, T.G.; Miranda, C.C.; Pusch-Klein, A.; Haupt, S.; Rodrigues, C.A.V.; Diogo, M.M.; Brüstle, O.; Cabral, J.M.S. Long-term expansion of human induced pluripotent stem cells in a microcarrier-based dynamic system. J. Chem. Technol. Biotechnol. 2017, 92, 492–503. [Google Scholar] [CrossRef]
- Shekaran, A.; Lam, A.; Sim, E.; Jialing, L.; Jian, L.; Wen, J.T.P.; Chan, J.K.Y.; Choolani, M.; Reuveny, S.; Birch, W.; et al. Biodegradable ECM-coated PCL microcarriers support scalable human early MSC expansion and in vivo bone formation. Cytotherapy 2016, 18, 1332–1344. [Google Scholar] [CrossRef]
- Wang, Y.; Yuan, X.; Yu, K.; Meng, H.; Zheng, Y.; Peng, J.; Lu, S.; Liu, X.; Xie, Y.; Qiao, K. Fabrication of nanofibrous microcarriers mimicking extracellular matrix for functional microtissue formation and cartilage regeneration. Biomaterials 2018, 183, 171–172. [Google Scholar] [CrossRef]
- Rodrigues, A.L.; Rodrigues, C.A.V.; Gomes, A.R.; Vieira, S.F.; Badenes, S.M.; Diogo, M.M.; Cabral, J.M.S. Dissolvable Microcarriers Allow Scalable Expansion And Harvesting Of Human Induced Pluripotent Stem Cells Under Xeno-Free Conditions. Biotechnol. J. 2019, 14, e1800461. [Google Scholar] [CrossRef]
- Amit, M.; Chebath, J.; Margulets, V.; Laevsky, I.; Miropolsky, Y.; Shariki, K.; Peri, M.; Blais, I.; Slutsky, G.; Revel, M.; et al. Suspension Culture of Undifferentiated Human Embryonic and Induced Pluripotent Stem Cells. Stem Cell Rev. Rep. 2010, 6, 248–259. [Google Scholar] [CrossRef]
- Olmer, R.; Haase, A.; Merkert, S.; Cui, W.; Palecek, J.; Ran, C.; Kirschning, A.; Scheper, T.; Glage, S.; Miller, K.; et al. Long term expansion of undifferentiated human iPS and ES cells in suspension culture using a defined medium. Stem Cell Res. 2010, 5, 51–64. [Google Scholar] [CrossRef] [PubMed]
- Abbasalizadeh, S.; Larijani, M.R.; Samadian, A.; Baharvand, H. Bioprocess Development for Mass Production of Size-Controlled Human Pluripotent Stem Cell Aggregates in Stirred Suspension Bioreactor. Tissue Eng. Part C 2012, 18. [Google Scholar] [CrossRef]
- Chen, V.C.; Couture, S.M.; Ye, J.; Lin, Z.; Hua, G.; Huang, H.I.; Wu, J.; Hsu, D.; Carpenter, M.K.; Couture, L.A. Scalable GMP compliant suspension culture system for human ES cells. Stem Cell Res. 2012, 8, 388–402. [Google Scholar] [CrossRef]
- Hookway, T.A.; Butts, J.C.; Lee, E.; Tang, H.; McDevitt, T.C. Aggregate formation and suspension culture of human pluripotent stem cells and differentiated progeny. Methods 2016, 101, 11–20. [Google Scholar] [CrossRef]
- Bauwens, C.L.; Toms, D.; Ungrin, M. Aggregate Size Optimization in Microwells for Suspension-based Cardiac Differentiation of Human Pluripotent Stem Cells. J. Vis. Exp. JOVE 2016. [Google Scholar] [CrossRef] [PubMed]
- Amit, M.; Laevsky, I.; Miropolsky, Y.; Shariki, K.; Peri, M.; Itskovitz-Eldor, J. Dynamic suspension culture for scalable expansion of undifferentiated human pluripotent stem cells. Nat. Protoc. 2011, 6, 572–579. [Google Scholar] [CrossRef]
- Kwok, C.K.; Ueda, Y.; Kadari, A.; Gunther, K.; Ergun, S.; Heron, A.; Schnitzler, A.C.; Rook, M.; Edenhofer, F. Scalable stirred suspension culture for the generation of billions of human induced pluripotent stem cells using single-use bioreactors. J. Tissue Eng. Regen. Med. 2017, 12, e1076–e1087. [Google Scholar] [CrossRef]
- Wang, Y.; Chou, B.K.; Dowey, S.; He, C.; Gerecht, S.; Cheng, L. Scalable expansion of human induced pluripotent stem cells in the defined xeno-free E8 medium under adherent and suspension culture conditions. Stem Cell Res. 2013, 11, 1103–1116. [Google Scholar] [CrossRef]
- Kim, M.H.; Takeuchi, K.; Kino-Oka, M. Role of cell-secreted extracellular matrix formation in aggregate formation and stability of human induced pluripotent stem cells in suspension culture. J. Biosci. Bioeng. 2018, 127, 372–380. [Google Scholar] [CrossRef]
- Vernardis, S.; Terzoudis, K.; Panoskaltsis, N.; Mantalaris, A. Human embryonic and induced pluripotent stem cells maintain phenotype but alter their metabolism after exposure to ROCK inhibitor. Sci. Rep. 2017, 7, 42138. [Google Scholar] [CrossRef]
- Bai, Q.; Ramirez, J.M.; Becker, F.; Pantesco, V.; Lavabre-Bertrand, T.; Hovatta, O.; Lemaitre, J.M.; Pellestor, F.; De Vos, J. Temporal analysis of genome alterations induced by single-cell passaging in human embryonic stem cells. Stem Cells Dev. 2015, 24, 653–662. [Google Scholar] [CrossRef]
- Garitaonandia, I.; Amir, H.; Boscolo, F.S.; Wambua, G.K.; Schultheisz, H.L.; Sabatini, K.; Morey, R.; Waltz, S.; Wang, Y.C.; Tran, H.; et al. Increased risk of genetic and epigenetic instability in human embryonic stem cells associated with specific culture conditions. PLoS ONE 2015, 10, e0118307. [Google Scholar] [CrossRef]
- Maldonado, M.; Luu, R.J.; Ramos, M.E.P.; Nam, J. ROCK inhibitor primes human induced pluripotent stemcells to selectively differentiate towardsmesendodermal lineage via epithelial-mesenchymal transition-likemodulation. Stem Cell Res. 2016, 17, 222–227. [Google Scholar] [CrossRef] [PubMed]
- Caliari, S.R.; Burdick, J.A. A practical guide to hydrogels for cell culture. Nat. Methods 2016, 13, 405–414. [Google Scholar] [CrossRef]
- Kopeček, J. Hydrogel Biomaterials: A Smart Future? Biomaterials 2007, 28, 5185–5192. [Google Scholar] [CrossRef]
- Ahmed, E.M. Hydrogel: Preparation, characterization, and applications: A review. J. Adv. Res. 2015, 6, 105–121. [Google Scholar] [CrossRef]
- Tsou, Y.H.; Khoneisser, J.; Huang, P.C.; Xu, X. Hydrogel as a bioactive material to regulate stem cell fate. Bioact. Mater. 2016, 1, 39–55. [Google Scholar] [CrossRef]
- Siti-Ismail, N.; Bishop, A.E.; Polak, J.M.; Mantalaris, A. The benefit of human embryonic stem cell encapsulation for prolonged feeder-free maintenance. Biomaterials 2008, 29, 3946–3952. [Google Scholar] [CrossRef] [PubMed]
- Lou, Y.R.; Kanninen, L.; Kuisma, T.; Niklander, J.; Noon, L.A.; Burks, D.; Urtti, A.; Yliperttula, M. The use of nanofibrillar cellulose hydrogel as a flexible three-dimensional model to culture human pluripotent stem cells. Stem Cells Dev. 2014, 23, 380–392. [Google Scholar] [CrossRef]
- Lei, Y.; Schaffer, D.V. A fully defined and scalable 3D culture system for human pluripotent stem cell expansion and differentiation. Proc. Natl. Acad. Sci. USA 2013, 110, E5039–E5048. [Google Scholar] [CrossRef]
- Otsuji, T.G.; Bin, J.; Yoshimura, A.; Tomura, M.; Tateyama, D.; Minami, I.; Yoshikawa, Y.; Aiba, K.; Heuser, J.E.; Nishino, T.; et al. A 3D Sphere Culture System Containing Functional Polymers for Large-Scale Human Pluripotent Stem Cell Production. Stem Cell Rep. 2014, 2, 734–745. [Google Scholar] [CrossRef]
- Zhu, Z.W.; Chen, L.; Liu, J.X.; Huang, J.W.; Wu, G.; Zheng, Y.F.; Yao, K.T. A novel three-dimensional tumorsphere culture system for the efficient and low-cost enrichment of cancer stem cells with natural polymers. Exp. Ther. Med. 2018, 15, 85–92. [Google Scholar] [CrossRef]
- Miyazaki, T.; Futaki, S.; Hasegawa, K.; Kawasaki, M.; Sanzen, N.; Hayashi, M.; Kawase, E.; Sekiguchi, K.; Nakatsuji, N.; Suemori, H. Recombinant human laminin isoforms can support the undifferentiated growth of human embryonic stem cells. Biochem. Biophys. Res. Commun. 2008, 375, 27–32. [Google Scholar] [CrossRef]
- Lian, X.; Hsiao, C.; Wilson, G.; Zhu, K.; Hazeltine, L.B.; Azarin, S.M.; Raval, K.K.; Zhang, J.; Kamp, T.J.; Palecek, S.P. Robust cardiomyocyte differentiation from human pluripotent stem cells via temporal modulation of canonical Wnt signaling. Proc. Natl. Acad. Sci. USA 2012, 109, E1848–E1857. [Google Scholar] [CrossRef]
- Minami, I.; Yamada, K.; Otsuji, T.G.; Yamamoto, T.; Shen, Y.; Otsuka, S.; Kadota, S.; Morone, N.; Barve, M.; Asai, Y.; et al. A small molecule that promotes cardiac differentiation of human pluripotent stem cells under defined, cytokine- and xeno-free conditions. Cell Rep. 2012, 2, 1448–1460. [Google Scholar] [CrossRef] [PubMed]
- Burridge, P.W.; Matsa, E.; Shukla, P.; Lin, Z.C.; Churko, J.M.; Ebert, A.D.; Lan, F.; Diecke, S.; Huber, B.; Mordwinkin, N.M.; et al. Chemically defined generation of human cardiomyocytes. Nat. Methods 2014, 11, 855–860. [Google Scholar] [CrossRef] [PubMed]
- Lian, X.; Bao, X.; Zilberter, M.; Westman, M.; Fisahn, A.; Hsiao, C.; Hazeltine, L.B.; Dunn, K.K.; Kamp, T.J.; Palecek., S.P. Chemically defined, albumin-free human cardiomyocyte generation. Nat. Methods 2015, 12, 595–596. [Google Scholar] [CrossRef] [PubMed]
- Tohyama, S.; Fujita, J.; Fujita, C.; Yamaguchi, M.; Kanaami, S.; Ohno, R.; Sakamoto, K.; Kodama, M.; Kurokawa, J.; Kanazawa, H.; et al. Efficient Large-Scale 2D Culture System for Human Induced Pluripotent Stem Cells and Differentiated Cardiomyocytes. Stem Cell Rep. 2017, 9, 1406–1414. [Google Scholar] [CrossRef] [PubMed]
- Vohwinkel, C.U.; Lecuona, E.; Sun, H.; Sommer, N.; Vadasz, I.; Chandel, N.S.; Sznajder, J.I. Elevated CO2 levels cause mitochondrial dysfunction and impair cell proliferation. J. Biol. Chem. 2011, 286, 37067–37076. [Google Scholar] [CrossRef] [PubMed]
- Pasquet, S.; Sovalat, H.; Henon, P.; Bischoff, N.; Arkam, Y.; Ojeda-Uribe, M.; Bouar, R.; Rimelen, V.; Brink, I.; Dallemand, R.; et al. Long-term benefit of intracardiac delivery of autologous granulocyte-colony-stimulating factor-mobilized blood CD34+ cells containing cardiac progenitors on regional heart structure and function after myocardial infarct. Cytotherapy 2009, 11, 1002–1015. [Google Scholar] [CrossRef]
- Ogasawara, T.; Okano, S.; Ichimura, H.; Kadota, S.; Tanaka, Y.; Minami, I.; Uesugi, M.; Wada, Y.; Saito, N.; Okada, K.; et al. Impact of extracellular matrix on engraftment and maturation of pluripotent stem cell-derived cardiomyocytes in a rat myocardial infarct model. Sci. Rep. 2017, 7, 8630. [Google Scholar] [CrossRef] [PubMed]
- Hsieh, P.C.; Davis, M.E.; Lisowski, L.K.; Lee, R.T. Endothelial-cardiomyocyte interactions in cardiac development and repair. Annu. Rev. Physiol. 2006, 68, 51–66. [Google Scholar] [CrossRef]
- Chen, K.; Bai, H.; Arzigian, M.; Gao, Y.X.; Bao, J.; Wu, W.S.; Shen, W.F.; Wu, L.; Wang, Z.Z. Endothelial cells regulate cardiomyocyte development from embryonic stem cells. J. Cell. Biochem. 2010, 111, 29–39. [Google Scholar] [CrossRef] [PubMed]
- Giacomelli, E.; Bellin, M.; Sala, L.; van Meer, B.J.; Tertoolen, L.G.; Orlova, V.V.; Mummery, C.L. Three-dimensional cardiac microtissues composed of cardiomyocytes and endothelial cells co-differentiated from human pluripotent stem cells. Development 2017, 144, 1008–1017. [Google Scholar] [CrossRef] [PubMed]
- Paige, S.L.; Osugi, T.; Afanasiev, O.K.; Pabon, L.; Reinecke, H.; Murry, C.E. Endogenous Wnt/beta-catenin signaling is required for cardiac differentiation in human embryonic stem cells. PLoS ONE 2010, 5, e11134. [Google Scholar] [CrossRef]
- Lian, X.; Zhang, J.; Azarin, S.M.; Zhu, K.; Hazeltine, L.B.; Bao, X.; Hsiao, C.; Kamp, T.J.; Palecek, S.P. Directed cardiomyocyte differentiation from human pluripotent stem cells by modulating Wnt/beta-catenin signaling under fully defined conditions. Nat. Protoc. 2013, 8, 162–175. [Google Scholar] [CrossRef] [PubMed]
- Estarás, C.; Hsu, H.; Huang, L.; Jones, K. YAP repression of the WNT3 gene controls hESC differentiation along the cardiac mesoderm lineage. Genes Dev. 2017, 31, 2250–2263. [Google Scholar] [CrossRef]
- Le, M.N.T.; Takahi, M.; Maruyama, K.; Kurisaki, A.; Ohnuma, K. Cardiac differentiation at an initial low density of human-induced pluripotent stem cells. Vitr. Cell. Dev. Biol. Anim. 2018, 54, 513–522. [Google Scholar] [CrossRef]
- Lundy, S.D.; Zhu, W.Z.; Regnier, M.; Laflamme, M.A. Structural and functional maturation of cardiomyocytes derived from human pluripotent stem cells. Stem Cells Dev. 2013, 22, 1991–2002. [Google Scholar] [CrossRef]
- Kamakura, T.; Makiyama, T.; Sasaki, K.; Yoshida, Y.; Wuriyanghai, Y.; Chen, J.; Hattori, T.; Ohno, S.; Kita, T.; Horie, M.; et al. Ultrastructural Maturation of Human-Induced Pluripotent Stem Cell-Derived Cardiomyocytes in a Long-Term Culture. Circ. J. 2013, 77, 1307–1314. [Google Scholar] [CrossRef]
- Oh, S.; Chen, A.; Ting, S.; Wei, H.; Reuveny, S. Unified microcarrier process for human pluripotent stem cell expansion, and cardiomyocyte differentiation and purification in a 500 mL bioreactor. Cytotherapy 2017, 19, e22. [Google Scholar] [CrossRef]
- Braam, S.R.; Nauw, R.; Oostwaard, D.W.; Mummery, C.; Passier, R. Inhibition of ROCK improves survival of human embryonic stem cell–derived cardiomyocytes after dissociation. Ann. N. Y. Acad. Sci. 2010, 1188, 52–57. [Google Scholar] [CrossRef]
- Rao, J.; Pfeiffer, M.J.; Frank, S.; Adachi, K.; Piccini, I.; Quaranta, R.; Arauzo-Bravo, M.; Schwarz, J.; Schade, D.; Leidel, S.; et al. Stepwise Clearance of Repressive Roadblocks Drives Cardiac Induction in Human ESCs. Cell Stem Cell 2016, 18, 341–353. [Google Scholar] [CrossRef] [PubMed]
- Kempf, H.; Olmer, R.; Kropp, C.; Ruckert, M.; Jara-Avaca, M.; Robles-Diaz, D.; Franke, A.; Elliott, D.A.; Wojciechowski, D.; Fischer, M.; et al. Controlling expansion and cardiomyogenic differentiation of human pluripotent stem cells in scalable suspension culture. Stem Cell Rep. 2014, 3, 1132–1146. [Google Scholar] [CrossRef]
- Chen, V.C.; Ye, J.; Shukla, P.; Hua, G.; Chen, D.; Lin, Z.; Liu, J.C.; Chai, J.; Gold, J.; Wu, J.; et al. Development of a scalable suspension culture for cardiac differentiation from human pluripotent stem cells. Stem Cell Res. 2015, 15, 365–375. [Google Scholar] [CrossRef]
- Hartung, S.; Schwanke, K.; Haase, A.; David, R.; Franz, W.M.; Martin, U.; Zweigerdt, R. Directing cardiomyogenic differentiation of human pluripotent stem cells by plasmid-based transient overexpression of cardiac transcription factors. Stem Cells Dev. 2013, 22, 1112–1125. [Google Scholar] [CrossRef]
- Kempf, H.; Olmer, R.; Haase, A.; Franke, A.; Bolesani, E.; Schwanke, K.; Robles-Diaz, D.; Coffee, M.; Gohring, G.; Drager, G.; et al. Bulk cell density and Wnt/TGFbeta signalling regulate mesendodermal patterning of human pluripotent stem cells. Nat. Commun. 2016, 7, 13602. [Google Scholar] [CrossRef] [PubMed]
- Laco, F.; Woo, T.L.; Zhong, Q.; Szmyd, R.; Ting, S.; Khan, F.J.; Chai, C.L.L.; Reuveny, S.; Chen, A.; Oh, S. Unraveling the Inconsistencies of Cardiac Differentiation Efficiency Induced by the GSK3beta Inhibitor CHIR99021 in Human Pluripotent Stem Cells. Stem Cell Rep. 2018, 10, 1851–1866. [Google Scholar] [CrossRef]
- Jacobs, K.; Zambelli, F.; Mertzanidou, A.; Smolders, I.; Geens, M.; Nguyen, H.T.; Barbe, L.; Sermon, K.; Spits, C. Higher-Density Culture in Human Embryonic Stem Cells Results in DNA Damage and Genome Instability. Stem Cell Rep. 2016, 6, 330–341. [Google Scholar] [CrossRef]
- Nguyen, D.C.; Hookway, T.A.; Wu, Q.; Jha, R.; Preininger, M.K.; Chen, X.; Easley, C.A.; Spearman, P.; Deshpande, S.R.; Maher, K.; et al. Microscale generation of cardiospheres promotes robust enrichment of cardiomyocytes derived from human pluripotent stem cells. Stem Cell Rep. 2014, 3, 260–268. [Google Scholar] [CrossRef]
- Correia, C.; Koshkin, A.; Duarte, P.; Hu, D.; Carido, M.; Sebastião, M.J.; Gomes-Alves, P.; Elliott, D.A.; Domian, I.J.; Teixeira, A.P.; et al. 3D aggregate culture improves metabolic maturation of human pluripotent stem cell derived cardiomyocytes. Biotechnol. Bioeng. 2018, 115, 630–644. [Google Scholar] [CrossRef]
- Kerscher, P.; Turnbull, I.C.; Hodge, A.J.; Kim, J.; Seliktar, D.; Easley, C.J.; Costa, K.D.; Lipke, E.A. Direct hydrogel encapsulation of pluripotent stem cells enables ontomimetic differentiation and growth of engineered human heart tissues. Biomaterials 2016, 83, 383–395. [Google Scholar] [CrossRef]
- Ting, S.; Chen, A.; Reuveny, S.; Oh, S. An intermittent rocking platform for integrated expansion and differentiation of human pluripotent stem cells to cardiomyocytes in suspended microcarrier cultures. Stem Cell Res. 2014, 13, 202–213. [Google Scholar] [CrossRef]
- Horning, M.; Kidoaki, S.; Kawano, T.; Yoshikawa, K. Rigidity matching between cells and the extracellular matrix leads to the stabilization of cardiac conduction. Biophys. J. 2012, 102, 379–387. [Google Scholar] [CrossRef] [PubMed]
- Mitsuhi Hirata, T.Y. Effect of stem cell niche elasticity/ECM protein on the self-beating cardiomyocyte differentiation of induced pluripotent stem (iPS) cells at different stages. Acta Biomater. 2018, 65, 44–52. [Google Scholar] [CrossRef]
- Nunes, S.S.; Miklas, J.W.; Liu, J.; Aschar-Sobbi, R.; Xiao, Y.; Zhang, B.; Jiang, J.; Masse, S.; Gagliardi, M.; Hsieh, A.; et al. Biowire: A platform for maturation of human pluripotent stem cell-derived cardiomyocytes. Nat. Methods 2013, 10, 781–787. [Google Scholar] [CrossRef]
- Mihic, A.; Li, J.; Miyagi, Y.; Gagliardi, M.; Li, S.H.; Zu, J.; Weisel, R.D.; Keller, G.; Li, R.K. The effect of cyclic stretch on maturation and 3D tissue formation of human embryonic stem cell-derived cardiomyocytes. Biomaterials 2014, 35, 2798–2808. [Google Scholar] [CrossRef]
- Ruan, J.L.; Tulloch, N.L.; Razumova, M.V.; Saiget, M.; Muskheli, V.; Pabon, L.; Reinecke, H.; Regnier, M.; Murry, C.E. Mechanical Stress Conditioning and Electrical Stimulation Promote Contractility and Force Maturation of Induced Pluripotent Stem Cell-Derived Human Cardiac Tissue. Circulation 2016, 134, 1557–1567. [Google Scholar] [CrossRef]
- Stout, D.A.; Yoo, J.; Santiago-Miranda, A.N.; Webster, T.J. Mechanisms of greater cardiomyocyte functions on conductive nanoengineered composites for cardiovascular application. Int. J. Nanomed. 2012, 7, 5653–5669. [Google Scholar] [CrossRef]
- Martins, A.M.; Eng, G.; Caridade, S.G.; Mano, J.F.; Reis, R.L.; Vunjak-Novakovic, G. Electrically Conductive Chitosan/Carbon Scaffolds for Cardiac Tissue Engineering. Biomacromolecules 2014, 15, 635–643. [Google Scholar] [CrossRef] [PubMed]
- Shen, N.; Knopf, A.; Westendorf, C.; Kraushaar, U.; Riedl, J.; Bauer, H.; Poschel, S.; Layland, S.L.; Holeiter, M.; Knolle, S.; et al. Steps toward Maturation of Embryonic Stem Cell-Derived Cardiomyocytes by Defined Physical Signals. Stem Cell Rep. 2017, 9, 122–135. [Google Scholar] [CrossRef]
- Olmer, R.; Lange, A.; Selzer, S.; Kasper, C.; Haverich, A.; Martin, U.; Zweigerdt, R. Suspension culture of human pluripotent stem cells in controlled, stirred bioreactors. Tissue Eng. Part C Methods 2012, 18, 772–784. [Google Scholar] [CrossRef]
- Kempf, H.; Kropp, C.; Olmer, R.; Martin, U.; Zweigerdt, R. Cardiac differentiation of human pluripotent stem cells in scalable suspension culture. Nat. Protoc. 2015, 10, 1345–1361. [Google Scholar] [CrossRef]
- Dvir, T.; Levy, O.; Shachar, M.; Granot, Y.; Cohen, S. Activation of the ERK1/2 cascade via pulsatile interstitial fluid flow promotes cardiac tissue assembly. Tissue Eng. 2007, 13, 2185–2193. [Google Scholar] [CrossRef]
- Cheng, M.; Moretti, M.; Engelmayr, G.C.; Freed, L.E. Insulin-like growth factor-I and slow, bi-directional perfusion enhance the formation of tissue-engineered cardiac grafts. Tissue Eng. Part A 2009, 15, 645–653. [Google Scholar] [CrossRef] [PubMed]
- Jackman, C.P.; Carlson, A.L.; Bursac, N. Dynamic culture yields engineered myocardium with near-adult functional output. Biomaterials 2016, 111, 66–79. [Google Scholar] [CrossRef] [PubMed]
- Shafa, M.; Krawetz, R.; Zhang, Y.; Rattner, J.B.; Godollei1, A.; Duff, H.J.; Rancourt, D.E. Impact of stirred suspension bioreactor culture on the differentiation of murine embryonic stem cells into cardiomyocytes. BMC Cell Biol. 2011, 12, 53. [Google Scholar] [CrossRef] [PubMed]
- Mogi, A.; Takei, S.; Shimizu, H.; Miura, H.; Tomotsune, D.; Sasaki, K. Effects of Fluid Dynamic Forces Created by Rotary Orbital Suspension Culture on Cardiomyogenic Differentiation of Human Embryonic Stem Cells. J. Med. Biol. Eng. 2014, 34, 101. [Google Scholar] [CrossRef]
- Takahashi, K.; Kakimoto, Y.; Toda, K.; Naruse, K. Mechanobiology in cardiac physiology and diseases. J. Cell. Mol. Med. 2013, 17, 225–232. [Google Scholar] [CrossRef]
- Tallawi, M.; Rai, R.; Boccaccini, A.R.; Aifantis, K.E. Effect of substrate mechanics on cardiomyocyte maturation and growth. Tissue Eng. Part B Rev. 2015, 21, 157–165. [Google Scholar] [CrossRef]
- Noor, N.; Shapira, A.; Edri, R.; Gal, I.; Wertheim, L.; Dvir, T. 3D Printing of Personalized Thick and Perfusable Cardiac Patches and Hearts. Adv. Sci. 2019. [Google Scholar] [CrossRef]
- Jing, D.; Parikh, A.; Tzanakakis, E.S. Cardiac cell generation from encapsulated embryonic stem cells in static and scalable culture systems. Cell Transplant. 2010, 19, 1397–1412. [Google Scholar] [CrossRef] [PubMed]
- Cheng, Y.T.; Yeih, D.F.; Liang, S.M.; Chien, C.Y.; Yu, Y.L.; Ko, B.S.; Jan, Y.J.; Kuo, C.C.; Sung, L.Y.; Shyue, S.K.; et al. Rho-associated kinase inhibitors promote the cardiac differentiation of embryonic and induced pluripotent stem cells. Int. J. Cardiol. 2015, 201, 441–448. [Google Scholar] [CrossRef] [PubMed]
- Yoshida, S.; Miyagawa, S.; Fukushima, S.; Kawamura, F.; Kashiyama, N.; Ohashi, F.; Toyofuku, T.; Toda, K.; Sawa, Y. Maturation of Human Induced Pluripotent Stem Cell-Derived Cardiomyocytes by Soluble Factors from Human Mesenchymal Stem Cells. Mol. Ther. 2018, 26, 2681–2695. [Google Scholar] [CrossRef]
- Ribeiro, A.J.; Ang, Y.S.; Fu, J.D.; Rivas, R.N.; Mohamed, T.M.; Higgs, G.C.; Srivastava, D.; Pruitt, B.L. Contractility of single cardiomyocytes differentiated from pluripotent stem cells depends on physiological shape and substrate stiffness. Proc. Natl. Acad. Sci. USA 2015, 112, 12705–12710. [Google Scholar] [CrossRef] [PubMed]
- Horning, M.; Nakahata, M.; Linke, P.; Yamamoto, A.; Veschgini, M.; Kaufmann, S.; Takashima, Y.; Harada, A.; Tanaka, M. Dynamic Mechano-Regulation of Myoblast Cells on Supramolecular Hydrogels Cross-Linked by Reversible Host-Guest Interactions. Sci. Rep. 2017, 7, 7660. [Google Scholar] [CrossRef] [PubMed]
- Higuchi, A.; Kao, S.H.; Ling, Q.D.; Chen, Y.M.; Li, H.F.; Alarfaj, A.A.; Munusamy, M.A.; Murugan, K.; Chang, S.C.; Lee, H.C.; et al. Long-term xeno-free culture of human pluripotent stem cells on hydrogels with optimal elasticity. Sci. Rep. 2015, 5, 18136. [Google Scholar] [CrossRef]
| hPSCs Culture Format | Characteristics | Method | Advantage | Disadvantage | Efficiency | References |
|---|---|---|---|---|---|---|
| Microcarrier | Matrigel-coated cellulose microcarriers for human embryonic stem cells (hESCs) culture |
|
|
| Achieved 3.5 × 106 cells/mL in 50-mL spinner flask compared to 1.5 × 106 cells/mL in static microcarrier culture and 0.8 × 106 cells/mL in 2D colony cultures | Oh et al., 2009 [42] |
| Microcarrier | A xeno-free subtract (human vitronectin) and chemically defined medium (Essential 8) culture system for human induced pluripotent stem cells (hiPSCs) |
|
|
| Achieved 1.4 × 106 cells/mL after 10 days | Badenes et al., 2016 [43] |
| Microcarrier | Dissolvable microcarriers for scalable expansion of hiPSCs under xeno-free conditions |
|
|
| Achieved maximum 8.81 × 105 cells/mL using dissolvable Synthemax coated microcarriers at high recovery rate of 92% | Rodrigues et al., 2019 [49] |
| Carrier-free cell aggregates (3D sphere) | Suspension culture of hPSCs in static and dynamic culture, using interleukin and bFGF with serum-free media |
|
|
| 25-fold in 10 days | Amit et al., 2010 and 2011 [50,56] |
| Carrier-free cell aggregates (3D sphere) | Sphere culture of hPSCs with single cell dissociation |
|
|
| Cell numbers increase by six-fold within 4 days | Olmer et al., 2010 [51] |
| Carrier-free cell aggregates (3D sphere) | A scalable GMP compliant suspension culture system for hESCs |
|
|
| An average expansion rate of 4-fold can be obtained per passage of 3–4 days | Chen et al., 2012 [53] |
| Hydrogel | Use a thermo-responsive hydrogel to culture hPSCs |
|
|
| In single cell passage, cell fold increase is 10 after 4 days | Lei et al., 2013 [70] |
| Hydrogel | Apply plant-derived nanofibrillar cellulose (NFC) hydrogel to culture hPSCs |
|
|
| N/A | Lou et al., 2014 [69] |
| Functional polymers | Apply two functional polymers to change the viscosity of medium in the culture hPSCs |
|
| Seeding at 13.2 × 106 cells/bag, yielded 1.4 × 108 cells/bag which corresponds to a 12.5-fold | Otsuji et al., 2014 [71] |
| hPSCs Culture Format | Characteristics | Culture Vessel | Medium for hPSCs Expansion | Yield | Culture Medium and Format for CMs Differentiation | Efficiency of CMs Differentiation | Advantage | Disadvantage | References |
|---|---|---|---|---|---|---|---|---|---|
| Monolayer culture |
| 4-layer or 10-layer of 632 cm2 culture plates |
| Seeding of 1 × 106 hiPSCs per layer yielded 7.2 × 108 hiPSCs in 4-layer and 1.7 × 109 hiPSCs in 10-layer culture plates |
|
| High efficiency in generation of pure hiPSCs-CMs since all cells are evenly exposed to purification medium. |
| Tohyama et al., 2017 [78] |
| Monolayer culture |
| N/A |
| N/A |
|
| Approach by co-differentiation to get cardiac identity-endothelial cells prior to microtissue formation helps promote CMs maturation |
| Giacomelli et al., 2017 [84] |
| Microcarriers | Expansion of hESC followed by CMs differentiation in a homogenous process | Ultra low attachment T-25 flask with rocker culture |
| Seeding of 2 × 105 cells/mL yielded 3.74 × 106 cells/mL after 7 days |
| Yield 2.45 × 106 CM/mL with 65.73% expression of cTnT after 12 days differentiation | Integrate hPSCs expansion and CMs differentiation in a continuous process | CMs were separated from microcarrier by enzymatic dissociation and filter through 40 µm cell strainer | Ting et al., 2014 [103] |
| Microcarriers | hPSC expansion, differentiation, and purification using microcarriers | 500 mL controlled bioreactor |
| 3.66 × 106 cells/mL after 7 days culture (18-fold increase) |
| 1.33 × 106 CMs/mL with 83.1% expression of cTnT after 23 days culture and purification | A high yield of CMs can be obtained using a large volume bioreactor | Removal of microcarriers before further applying CMs for transplantation, drug screening and disease modeling | Steve Oh et al., 2017 [91] |
| Carrier-free cell aggregates (3D sphere) | Two methods to form aggregates
|
|
| 60 × 106 cells in 100 mL bioreactor |
| In bioreactor, efficiencies are 85%, 54%, 68% (n = 3) after 10 days of differentiation Erlenmeyer ~ 60.4% | Applying carrier-free cell aggregates facilitates cell harvesting compared to microcarriers | Maintenance of cell aggregates required ROCK inhibitor during culture process may change hPSCs metabolome profile. | Kempf et al., 2014 [94] |
| Carrier-free cell aggregates (3D sphere) | The culture process is defined and standardized in compliance with GMP regulations. | 6-well plate; 125, 500, and 1000 mL spinner flasks |
| Seeding at 2.5 × 105 cells/mL yielded 1 × 106 cells/mL after 3 days |
| >90% CM purity after 25 days of differentiation Yield 1.5 to 2 × 109 CM/L |
| Maintenance of cell aggregates required ROCK inhibitor during culture process may change hPSCs metabolome profile. | Chen et al., 2012;Chen et al., 2015 [95] |
| Hydrogel | hiPSCs were encapsulated in PEG-fibrinogen hydrogels and differentiated into CMs continuously | Prepare PDMS mold on acrylated glass, put in 6-well plate | mTeSR1 with ROCK inhibitor supplemented for the first 24 h | Cells were seeded at 5.5 × 105 hiPSCs per tissue |
|
|
| Kerscher et al., 2016 [102] |
© 2019 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (http://creativecommons.org/licenses/by/4.0/).
Share and Cite
Le, M.N.T.; Hasegawa, K. Expansion Culture of Human Pluripotent Stem Cells and Production of Cardiomyocytes. Bioengineering 2019, 6, 48. https://doi.org/10.3390/bioengineering6020048
Le MNT, Hasegawa K. Expansion Culture of Human Pluripotent Stem Cells and Production of Cardiomyocytes. Bioengineering. 2019; 6(2):48. https://doi.org/10.3390/bioengineering6020048
Chicago/Turabian StyleLe, Minh Nguyen Tuyet, and Kouichi Hasegawa. 2019. "Expansion Culture of Human Pluripotent Stem Cells and Production of Cardiomyocytes" Bioengineering 6, no. 2: 48. https://doi.org/10.3390/bioengineering6020048
APA StyleLe, M. N. T., & Hasegawa, K. (2019). Expansion Culture of Human Pluripotent Stem Cells and Production of Cardiomyocytes. Bioengineering, 6(2), 48. https://doi.org/10.3390/bioengineering6020048