Extracellular Vesicles in the Oviduct: Progress, Challenges and Implications for the Reproductive Success
Abstract
1. Introduction
2. Studies on Oviductal EVs
2.1. Analysis of oEVs Molecular Content
2.2. Oviductal EVs and Their Functional Effect on Gametes
2.3. Oviductal EVs and Their Functional Effect on Embryo(s)
3. Oviductal EVs: Current Knowledge about Their Molecular Content
3.1. Oviductal EVs and Proteins
3.2. Oviductal EVs and mRNA
3.3. Oviductal EVs and miRNA
3.4. Oviductal EVs and other Molecular Cargo
3.5. Regulation of the Dynamic and Complex Oviductal EVs Molecular Cargo
4. Oviductal EVs and Their Potential as Therapeutic Vectors
5. Challenges and Future Directions for oEVs Research
6. Concluding Remarks
Author Contributions
Funding
Conflicts of Interest
References
- Wolf, E.; Arnold, G.J.; Bauersachs, S.; Beier, H.M.; Blum, H.; Einspanier, R.; Frohlich, T.; Herrler, A.; Hiendleder, S.; Kolle, S.; et al. Embryo-maternal communication in bovine—Strategies for deciphering a complex cross-talk. Reprod. Domest. Anim. 2003, 38, 276–289. [Google Scholar] [CrossRef]
- Fazeli, A. Maternal communication with gametes and embryos. Theriogenology 2008, 70, 1182–1187. [Google Scholar] [CrossRef]
- Fazeli, A.; Affara, N.A.; Hubank, M.; Holt, W.V. Sperm-induced modification of the oviductal gene expression profile after natural insemination in mice. Biol. Reprod. 2004, 71, 60–65. [Google Scholar] [CrossRef] [PubMed]
- Georgiou, A.S.; Sostaric, E.; Wong, C.H.; Snijders, A.P.; Wright, P.C.; Moore, H.D.; Fazeli, A. Gametes alter the oviductal secretory proteome. Mol. Cell. Proteom. 2005, 4, 1785–1796. [Google Scholar] [CrossRef] [PubMed]
- Lee, K.F.; Yao, Y.Q.; Kwok, K.L.; Xu, J.S.; Yeung, W.S. Early developing embryos affect the gene expression patterns in the mouse oviduct. Biochem. Biophys. Res. Commun. 2002, 292, 564–570. [Google Scholar] [CrossRef] [PubMed]
- Alminana, C.; Heath, P.R.; Wilkinson, S.; Sanchez-Osorio, J.; Cuello, C.; Parrilla, I.; Gil, M.A.; Vazquez, J.L.; Vazquez, J.M.; Roca, J.; et al. Early developing pig embryos mediate their own environment in the maternal tract. PLoS ONE 2012, 7, e33625. [Google Scholar] [CrossRef] [PubMed]
- Maillo, V.; Gaora, P.O.; Forde, N.; Besenfelder, U.; Havlicek, V.; Burns, G.W.; Spencer, T.E.; Gutierrez-Adan, A.; Lonergan, P.; Rizos, D. Oviduct-Embryo Interactions in Cattle: Two-Way Traffic or a One-Way Street? Biol. Reprod. 2015, 92, 144. [Google Scholar] [CrossRef]
- Hunter, R.H. Fallopian tube physiology: Preliminaries to monospermic fertilization and cellular events post-fertilization. In Proceedings of the Ernst Schering Research Foundation Workshop, Kobe, Japan, 1–3 September 2005; pp. 245–261. [Google Scholar]
- Holt, W.V.; Fazeli, A. The oviduct as a complex mediator of mammalian sperm function and selection. Mol. Reprod. Dev. 2010, 77, 934–943. [Google Scholar] [CrossRef]
- Perez-Cerezales, S.; Ramos-Ibeas, P.; Acuna, O.S.; Aviles, M.; Coy, P.; Rizos, D.; Gutierrez-Adan, A. The oviduct: From sperm selection to the epigenetic landscape of the embryo. Biol. Reprod. 2018, 98, 262–276. [Google Scholar] [CrossRef]
- Lazaraviciute, G.; Kauser, M.; Bhattacharya, S.; Haggarty, P.; Bhattacharya, S. A systematic review and meta-analysis of DNA methylation levels and imprinting disorders in children conceived by IVF/ICSI compared with children conceived spontaneously. Hum. Reprod. Update 2014, 20, 840–852. [Google Scholar] [CrossRef]
- Duranthon, V.; Chavatte-Palmer, P. Long term effects of ART: What do animals tell us? Mol. Reprod. Dev. 2018, 85, 348–368. [Google Scholar] [CrossRef] [PubMed]
- Georgiou, A.S.; Snijders, A.P.; Sostaric, E.; Aflatoonian, R.; Vazquez, J.L.; Vazquez, J.M.; Roca, J.; Martinez, E.A.; Wright, P.C.; Fazeli, A. Modulation of the oviductal environment by gametes. J. Proteome Res. 2007, 6, 4656–4666. [Google Scholar] [CrossRef]
- Schmaltz-Panneau, B.; Cordova, A.; Dhorne-Pollet, S.; Hennequet-Antier, C.; Uzbekova, S.; Martinot, E.; Doret, S.; Martin, P.; Mermillod, P.; Locatelli, Y. Early bovine embryos regulate oviduct epithelial cell gene expression during in vitro co-culture. Anim. Reprod. Sci. 2014, 149, 103–116. [Google Scholar] [CrossRef]
- Bauersachs, S.; Blum, H.; Mallok, S.; Wenigerkind, H.; Rief, S.; Prelle, K.; Wolf, E. Regulation of ipsilateral and contralateral bovine oviduct epithelial cell function in the postovulation period: A transcriptomics approach. Boil. Reprod. 2003, 68, 1170–1177. [Google Scholar] [CrossRef] [PubMed]
- Bauersachs, S.; Rehfeld, S.; Ulbrich, S.E.; Mallok, S.; Prelle, K.; Wenigerkind, H.; Einspanier, R.; Blum, H.; Wolf, E. Monitoring gene expression changes in bovine oviduct epithelial cells during the oestrous cycle. J. Mol. Endocrinol. 2004, 32, 449–466. [Google Scholar] [CrossRef] [PubMed]
- Alminana, C.; Caballero, I.; Heath, P.R.; Maleki-Dizaji, S.; Parrilla, I.; Cuello, C.; Gil, M.A.; Vazquez, J.L.; Vazquez, J.M.; Roca, J.; et al. The battle of the sexes starts in the oviduct: Modulation of oviductal transcriptome by X and Y-bearing spermatozoa. BMC Genom. 2014, 15, 293. [Google Scholar] [CrossRef]
- Buhi, W.C.; Alvarez, I.M.; Kouba, A.J. Secreted proteins of the oviduct. Cells Tissues Organs 2000, 166, 165–179. [Google Scholar] [CrossRef]
- Killian, G.J. Evidence for the role of oviduct secretions in sperm function, fertilization and embryo development. Anim. Reprod. Sci. 2004, 82–83, 141–153. [Google Scholar] [CrossRef]
- Lamy, J.; Labas, V.; Harichaux, G.; Tsikis, G.; Mermillod, P.; Saint-Dizier, M. Regulation of the bovine oviductal fluid proteome. Reproduction 2016, 152, 629–644. [Google Scholar] [CrossRef]
- Pillai, V.V.; Weber, D.M.; Phinney, B.S.; Selvaraj, V. Profiling of proteins secreted in the bovine oviduct reveals diverse functions of this luminal microenvironment. PLoS ONE 2017, 12, e0188105. [Google Scholar] [CrossRef]
- Al-Dossary, A.A.; Strehler, E.E.; Martin-Deleon, P.A. Expression and secretion of plasma membrane Ca2+-ATPase 4a (PMCA4a) during murine estrus: Association with oviductal exosomes and uptake in sperm. PLoS ONE 2013, 8, e80181. [Google Scholar] [CrossRef]
- Lopera-Vasquez, R.; Hamdi, M.; Fernandez-Fuertes, B.; Maillo, V.; Beltran-Brena, P.; Calle, A.; Redruello, A.; Lopez-Martin, S.; Gutierrez-Adan, A.; Yanez-Mo, M.; et al. Extracellular Vesicles from BOEC in In Vitro Embryo Development and Quality. PLoS ONE 2016, 11, e0148083. [Google Scholar] [CrossRef] [PubMed]
- Alminana, C.; Corbin, E.; Tsikis, G.; Alcantara-Neto, A.S.; Labas, V.; Reynaud, K.; Galio, L.; Uzbekov, R.; Garanina, A.S.; Druart, X.; et al. Oviduct extracellular vesicles protein content and their role during oviduct-embryo cross-talk. Reproduction 2017, 154, 153–168. [Google Scholar] [CrossRef] [PubMed]
- Colombo, M.; Raposo, G.; Thery, C. Biogenesis, secretion, and intercellular interactions of exosomes and other extracellular vesicles. Annu. Rev. Cell Dev. Biol. 2014, 30, 255–289. [Google Scholar] [CrossRef] [PubMed]
- Simon, C.; Greening, D.W.; Bolumar, D.; Balaguer, N.; Salamonsen, L.A.; Vilella, F. Extracellular Vesicles in Human Reproduction in Health and Disease. Endocr. Rev. 2018, 39, 292–332. [Google Scholar] [CrossRef] [PubMed]
- Van Niel, G.; D’Angelo, G.; Raposo, G. Shedding light on the cell biology of extracellular vesicles. Nat. Rev. Mol. Cell Biol. 2018, 19, 213–228. [Google Scholar] [CrossRef] [PubMed]
- Tannetta, D.; Dragovic, R.; Alyahyaei, Z.; Southcombe, J. Extracellular vesicles and reproduction-promotion of successful pregnancy. Cell. Mol. Immunol. 2014, 11, 548–563. [Google Scholar] [CrossRef]
- Barkalina, N.; Jones, C.; Wood, M.J.; Coward, K. Extracellular vesicle-mediated delivery of molecular compounds into gametes and embryos: Learning from nature. Hum. Reprod. Update 2015, 21, 627–639. [Google Scholar] [CrossRef]
- Nguyen, H.P.; Simpson, R.J.; Salamonsen, L.A.; Greening, D.W. Extracellular Vesicles in the Intrauterine Environment: Challenges and Potential Functions. Biol. Reprod. 2016, 95, 109. [Google Scholar] [CrossRef] [PubMed]
- Fereshteh, Z.; Schmidt, S.A.; Al-Dossary, A.A.; Accerbi, M.; Arighi, C.; Cowart, J.; Song, J.L.; Green, P.J.; Choi, K.; Yoo, S.; et al. Murine Oviductosomes (OVS) microRNA profiling during the estrous cycle: Delivery of OVS-borne microRNAs to sperm where miR-34c-5p localizes at the centrosome. Sci. Rep. 2018, 8, 16094. [Google Scholar] [CrossRef]
- Bathala, P.; Fereshteh, Z.; Li, K.; Al-Dossary, A.A.; Galileo, D.S.; Martin-DeLeon, P.A. Oviductal extracellular vesicles (oviductosomes, OVS) are conserved in humans: Murine OVS play a pivotal role in sperm capacitation and fertility. Mol. Hum. Reprod. 2018, 24, 143–157. [Google Scholar] [CrossRef]
- Huang, A.; Isobe, N.; Yoshimura, Y. Changes in localization and density of CD63-positive exosome-like substances in the hen oviduct with artificial insemination and their effect on sperm viability. Theriogenology 2017, 101, 135–143. [Google Scholar] [CrossRef] [PubMed]
- Lange-Consiglio, A.; Perrini, C.; Albini, G.; Modina, S.; Lodde, V.; Orsini, E.; Esposti, P.; Cremonesi, F. Oviductal microvesicles and their effect on in vitro maturation of canine oocytes. Reproduction 2017, 154, 167–180. [Google Scholar] [CrossRef] [PubMed]
- Waqas, M.Y.; Zhang, Q.; Ahmed, N.; Yang, P.; Xing, G.; Akhtar, M.; Basit, A.; Liu, T.; Hong, C.; Arshad, M.; et al. Cellular Evidence of Exosomes in the Reproductive Tract of Chinese Soft-Shelled Turtle Pelodiscus sinensis. J. Exp. Zool. A 2017, 327, 18–27. [Google Scholar] [CrossRef] [PubMed]
- Qu, P.; Zhao, Y.; Wang, R.; Zhang, Y.; Li, L.; Fan, J.; Liu, E. Extracellular vesicles derived from donor oviduct fluid improved birth rates after embryo transfer in mice. Reprod. Fertil. Dev. 2018, 31, 324–332. [Google Scholar] [CrossRef]
- Alminana, C.; Tsikis, G.; Labas, V.; Uzbekov, R.; da Silveira, J.C.; Bauersachs, S.; Mermillod, P. Deciphering the oviductal extracellular vesicles content across the estrous cycle: Implications for the gametes-oviduct interactions and the environment of the potential embryo. BMC Genom. 2018, 19, 622. [Google Scholar] [CrossRef]
- Harding, C.; Heuser, J.; Stahl, P. Receptor-Mediated Endocytosis of Transferrin and Recycling of the Transferrin Receptor in Rat Reticulocytes. J. Cell Boil. 1983, 97, 329–339. [Google Scholar] [CrossRef]
- Fabiani, R.; Johansson, L.; Lundkvist, O.; Ulmsten, U.; Ronquist, G. Promotive effect by prostasomes on normal human spermatozoa exhibiting no forward motility due to buffer washings. Eur. J. Obstet. Gynecol. Reprod. Biol. 1994, 57, 181–188. [Google Scholar] [CrossRef]
- Sullivan, R.; Saez, F.; Girouard, J.; Frenette, G. Role of exosomes in sperm maturation during the transit along the male reproductive tract. Blood Cells Mol. Dis. 2005, 35, 1–10. [Google Scholar] [CrossRef]
- Griffiths, G.S.; Galileo, D.S.; Reese, K.; Martin-Deleon, P.A. Investigating the role of murine epididymosomes and uterosomes in GPI-linked protein transfer to sperm using SPAM1 as a model. Mol. Reprod. Dev. 2008, 75, 1627–1636. [Google Scholar] [CrossRef]
- Ezzati, M.; Djahanbakhch, O.; Arian, S.; Carr, B.R. Tubal transport of gametes and embryos: A review of physiology and pathophysiology. J. Assist. Reprod. Genet. 2014, 31, 1337–1347. [Google Scholar] [CrossRef] [PubMed]
- Maillo, V.; Lopera-Vasquez, R.; Hamdi, M.; Gutierrez-Adan, A.; Lonergan, P.; Rizos, D. Maternal-embryo interaction in the bovine oviduct: Evidence from in vivo and in vitro studies. Theriogenology 2016, 86, 443–450. [Google Scholar] [CrossRef] [PubMed]
- Rizos, D.; Ramirez, M.A.; Pintado, B.; Lonergan, P.; Gutierrez-Adan, A. Culture of bovine embryos in intermediate host oviducts with emphasis on the isolated mouse oviduct. Theriogenology 2010, 73, 777–785. [Google Scholar] [CrossRef] [PubMed]
- Ferreira, A.D.F.; Gomes, D.A. Stem Cell Extracellular Vesicles in Skin Repair. Bioengineering 2018, 6, 4. [Google Scholar] [CrossRef]
- Van Deun, J.; Mestdagh, P.; Sormunen, R.; Cocquyt, V.; Vermaelen, K.; Vandesompele, J.; Bracke, M.; De Wever, O.; Hendrix, A. The impact of disparate isolation methods for extracellular vesicles on downstream RNA profiling. J. Extracell. Vesicles 2014, 3, 24858. [Google Scholar] [CrossRef]
- Taylor, D.D.; Shah, S. Methods of isolating extracellular vesicles impact down-stream analyses of their cargoes. Methods 2015, 87, 3–10. [Google Scholar] [CrossRef]
- Lobb, R.J.; Becker, M.; Wen, S.W.; Wong, C.S.; Wiegmans, A.P.; Leimgruber, A.; Moller, A. Optimized exosome isolation protocol for cell culture supernatant and human plasma. J. Extracell. Vesicles 2015, 4, 27031. [Google Scholar] [CrossRef]
- Vergauwen, G.; Dhondt, B.; Van Deun, J.; De Smedt, E.; Berx, G.; Timmerman, E.; Gevaert, K.; Miinalainen, I.; Cocquyt, V.; Braems, G.; et al. Confounding factors of ultrafiltration and protein analysis in extracellular vesicle research. Sci. Rep. 2017, 7, 2704. [Google Scholar] [CrossRef]
- Kowal, J.; Arras, G.; Colombo, M.; Jouve, M.; Morath, J.P.; Primdal-Bengtson, B.; Dingli, F.; Loew, D.; Tkach, M.; Thery, C. Proteomic comparison defines novel markers to characterize heterogeneous populations of extracellular vesicle subtypes. Proc. Natl. Acad. Sci. USA 2016, 113, E968–E977. [Google Scholar] [CrossRef]
- Campoy, I.; Lanau, L.; Altadill, T.; Sequeiros, T.; Cabrera, S.; Cubo-Abert, M.; Perez-Benavente, A.; Garcia, A.; Borros, S.; Santamaria, A.; et al. Exosome-like vesicles in uterine aspirates: A comparison of ultracentrifugation-based isolation protocols. J. Transl. Med. 2016, 14, 180. [Google Scholar] [CrossRef]
- Bosch, S.; de Beaurepaire, L.; Allard, M.; Mosser, M.; Heichette, C.; Chretien, D.; Jegou, D.; Bach, J.M. Trehalose prevents aggregation of exosomes and cryodamage. Sci. Rep. 2016, 6, 36162. [Google Scholar] [CrossRef] [PubMed]
- Mateescu, B.; Kowal, E.J.; van Balkom, B.W.; Bartel, S.; Bhattacharyya, S.N.; Buzas, E.I.; Buck, A.H.; de Candia, P.; Chow, F.W.; Das, S.; et al. Obstacles and opportunities in the functional analysis of extracellular vesicle RNA—An ISEV position paper. J. Extracell. Vesicles 2017, 6, 1286095. [Google Scholar] [CrossRef] [PubMed]
- Antimisiaris, S.G.; Mourtas, S.; Marazioti, A. Exosomes and Exosome-Inspired Vesicles for Targeted Drug Delivery. Pharmaceutics 2018, 10, 218. [Google Scholar] [CrossRef] [PubMed]
- Thery, C.; Witwer, K.W.; Aikawa, E.; Alcaraz, M.J.; Anderson, J.D.; Andriantsitohaina, R.; Antoniou, A.; Arab, T.; Archer, F.; Atkin-Smith, G.K.; et al. Minimal information for studies of extracellular vesicles 2018 (MISEV2018): A position statement of the International Society for Extracellular Vesicles and update of the MISEV2014 guidelines. J. Extracell. Vesicles 2018, 7, 1535750. [Google Scholar] [CrossRef]
- Jeppesen, D.K.; Hvam, M.L.; Primdahl-Bengtson, B.; Boysen, A.T.; Whitehead, B.; Dyrskjot, L.; Orntoft, T.F.; Howard, K.A.; Ostenfeld, M.S. Comparative analysis of discrete exosome fractions obtained by differential centrifugation. J. Extracell. Vesicles 2014, 3, 25011. [Google Scholar] [CrossRef]
- Lotvall, J.; Hill, A.F.; Hochberg, F.; Buzas, E.I.; Di Vizio, D.; Gardiner, C.; Gho, Y.S.; Kurochkin, I.V.; Mathivanan, S.; Quesenberry, P.; et al. Minimal experimental requirements for definition of extracellular vesicles and their functions: A position statement from the International Society for Extracellular Vesicles. J. Extracell. Vesicles 2014, 3, 26913. [Google Scholar] [CrossRef] [PubMed]
- Al-Dossary, A.A.; Bathala, P.; Caplan, J.L.; Martin-DeLeon, P.A. Oviductosome-Sperm Membrane Interaction in Cargo Delivery: Detection of fusion and underlying molecular players using three-dimensional super-resolution structured illumination microscopy (SR-SIM). J. Biol. Chem. 2015, 290, 17710–17723. [Google Scholar] [CrossRef]
- Lopera-Vasquez, R.; Hamdi, M.; Maillo, V.; Gutierrez-Adan, A.; Bermejo-Alvarez, P.; Ramirez, M.A.; Yanez-Mo, M.; Rizos, D. Effect of bovine oviductal extracellular vesicles on embryo development and quality in vitro. Reproduction 2017, 153, 461–470. [Google Scholar] [CrossRef]
- Nakano, S.; Yamamoto, S.; Okada, A.; Nakajima, T.; Sato, M.; Takagi, T.; Tomooka, Y. Role of extracellular vesicles in the interaction between epithelial and mesenchymal cells during oviductal ciliogenesis. Biochem. Biophys. Res. Commun. 2017, 483, 245–251. [Google Scholar] [CrossRef]
- Wennemuth, G.; Blocher, S.; Schill, W.B.; Aumuller, G.; Monsees, T.K. Bradykinin increases intracellular calcium levels in rat testis peritubular cells via the B2 receptor subtype. Br. J. Pharmacol. 2003, 138, 351–358. [Google Scholar] [CrossRef][Green Version]
- Schuh, K.; Cartwright, E.J.; Jankevics, E.; Bundschu, K.; Liebermann, J.; Williams, J.C.; Armesilla, A.L.; Emerson, M.; Oceandy, D.; Knobeloch, K.P.; et al. Plasma membrane Ca2+ ATPase 4 is required for sperm motility and male fertility. J. Biol. Chem. 2004, 279, 28220–28226. [Google Scholar] [CrossRef]
- Okunade, G.W.; Miller, M.L.; Pyne, G.J.; Sutliff, R.L.; O’Connor, K.T.; Neumann, J.C.; Andringa, A.; Miller, D.A.; Prasad, V.; Doetschman, T.; et al. Targeted ablation of plasma membrane Ca2+-ATPase (PMCA) 1 and 4 indicates a major housekeeping function for PMCA1 and a critical role in hyperactivated sperm motility and male fertility for PMCA4. J. Biol. Chem. 2004, 279, 33742–33750. [Google Scholar] [CrossRef] [PubMed]
- Da Silveira, J.C.; Andrade, G.M.; Del Collado, M.; Sampaio, R.V.; Sangalli, J.R.; Silva, L.A.; Pinaffi, F.V.L.; Jardim, I.B.; Cesar, M.C.; Nogueira, M.F.G.; et al. Supplementation with small-extracellular vesicles from ovarian follicular fluid during in vitro production modulates bovine embryo development. PLoS ONE 2017, 12, e0179451. [Google Scholar] [CrossRef]
- Wrenzycki, C.; Herrmann, D.; Keskintepe, L.; Martins, A., Jr.; Sirisathien, S.; Brackett, B.; Niemann, H. Effects of culture system and protein supplementation on mRNA expression in pre-implantation bovine embryos. Hum. Reprod. 2001, 16, 893–901. [Google Scholar] [CrossRef] [PubMed]
- Machado, G.M.; Ferreira, A.R.; Pivato, I.; Fidelis, A.; Spricigo, J.F.; Paulini, F.; Lucci, C.M.; Franco, M.M.; Dode, M.A. Post-hatching development of in vitro bovine embryos from day 7 to 14 in vivo versus in vitro. Mol. Reprod. Dev. 2013, 80, 936–947. [Google Scholar] [CrossRef] [PubMed]
- Neuer, A.; Spandorfer, S.D.; Giraldo, P.; Dieterle, S.; Rosenwaks, Z.; Witkin, S.S. The role of heat shock proteins in reproduction. Hum. Reprod. Update 2000, 6, 149–159. [Google Scholar] [CrossRef] [PubMed]
- Huang, L.; Min, J.N.; Masters, S.; Mivechi, N.F.; Moskophidis, D. Insights into function and regulation of small heat shock protein 25 (HSPB1) in a mouse model with targeted gene disruption. Genesis 2007, 45, 487–501. [Google Scholar] [CrossRef]
- Ignotz, G.G.; Cho, M.Y.; Suarez, S.S. Annexins are candidate oviductal receptors for bovine sperm surface proteins and thus may serve to hold bovine sperm in the oviductal reservoir. Biol. Reprod. 2007, 77, 906–913. [Google Scholar] [CrossRef] [PubMed]
- Hunter, R.H.; Rodriguez-Martinez, H. Capacitation of mammalian spermatozoa in vivo, with a specific focus on events in the Fallopian tubes. Mol. Reprod. Dev. 2004, 67, 243–250. [Google Scholar] [CrossRef]
- Hunter, R.H. Components of oviduct physiology in eutherian mammals. Biol. Rev. 2012, 87, 244–255. [Google Scholar] [CrossRef]
- Griffiths, K.L.; O’Neill, H.C. Dendritic cells as immune regulators: The mouse model. J. Cell. Mol. Med. 2008, 12, 1909–1914. [Google Scholar] [CrossRef]
- Elliott, R.M.; Lloyd, R.E.; Fazeli, A.; Sostaric, E.; Georgiou, A.S.; Satake, N.; Watson, P.F.; Holt, W.V. Effects of HSPA8, an evolutionarily conserved oviductal protein, on boar and bull spermatozoa. Reproduction 2009, 137, 191–203. [Google Scholar] [CrossRef]
- Moein-Vaziri, N.; Phillips, I.; Smith, S.; Alminana, C.; Maside, C.; Gil, M.A.; Roca, J.; Martinez, E.A.; Holt, W.V.; Pockley, A.G.; et al. Heat-shock protein A8 restores sperm membrane integrity by increasing plasma membrane fluidity. Reproduction 2014, 147, 719–732. [Google Scholar] [CrossRef] [PubMed]
- Zhou, X.; Liao, W.J.; Liao, J.M.; Liao, P.; Lu, H. Ribosomal proteins: Functions beyond the ribosome. J. Mol. Cell Biol. 2015, 7, 92–104. [Google Scholar] [CrossRef] [PubMed]
- Salilew-Wondim, D.; Saeed-Zidane, M.; Hoelker, M.; Gebremedhn, S.; Poirier, M.; Pandey, H.O.; Tholen, E.; Neuhoff, C.; Held, E.; Besenfelder, U.; et al. Genome-wide DNA methylation patterns of bovine blastocysts derived from in vivo embryos subjected to in vitro culture before, during or after embryonic genome activation. BMC Genom. 2018, 19, 424. [Google Scholar] [CrossRef]
- Koziol, M.J.; Garrett, N.; Gurdon, J.B. Tpt1 activates transcription of oct4 and nanog in transplanted somatic nuclei. Curr. Biol. 2007, 17, 801–807. [Google Scholar] [CrossRef]
- Demant, M.; Deutsch, D.R.; Frohlich, T.; Wolf, E.; Arnold, G.J. Proteome analysis of early lineage specification in bovine embryos. Proteomics 2015, 15, 688–701. [Google Scholar] [CrossRef]
- Corcoran, D.; Fair, T.; Park, S.; Rizos, D.; Patel, O.V.; Smith, G.W.; Coussens, P.M.; Ireland, J.J.; Boland, M.P.; Evans, A.C.; et al. Suppressed expression of genes involved in transcription and translation in in vitro compared with in vivo cultured bovine embryos. Reproduction 2006, 131, 651–660. [Google Scholar] [CrossRef] [PubMed]
- Gutierrez-Adan, A.; Rizos, D.; Fair, T.; Moreira, P.N.; Pintado, B.; de la Fuente, J.; Boland, M.P.; Lonergan, P. Effect of speed of development on mRNA expression pattern in early bovine embryos cultured in vivo or in vitro. Mol. Reprod. Dev. 2004, 68, 441–448. [Google Scholar] [CrossRef]
- Bauersachs, S.; Wolf, E. Uterine Responses to the Preattachment Embryo in Domestic Ungulates: Recognition of Pregnancy and Preparation for Implantation. Annu. Rev. Anim. Biosci. 2015, 3, 489–511. [Google Scholar] [CrossRef] [PubMed]
- Hafner, M.; Renwick, N.; Brown, M.; Mihailovic, A.; Holoch, D.; Lin, C.; Pena, J.T.; Nusbaum, J.D.; Morozov, P.; Ludwig, J.; et al. RNA-ligase-dependent biases in miRNA representation in deep-sequenced small RNA cDNA libraries. RNA 2011, 17, 1697–1712. [Google Scholar] [CrossRef] [PubMed]
- Hinton, A.; Afrikanova, I.; Wilson, M.; King, C.C.; Maurer, B.; Yeo, G.W.; Hayek, A.; Pasquinelli, A.E. A distinct microRNA signature for definitive endoderm derived from human embryonic stem cells. Stem Cells Dev. 2010, 19, 797–807. [Google Scholar] [CrossRef] [PubMed]
- Fan, Y.; Habib, M.; Xia, J. Xeno-miRNet: A comprehensive database and analytics platform to explore xeno-miRNAs and their potential targets. PeerJ 2018, 6, e5650. [Google Scholar] [CrossRef] [PubMed]
- Liu, W.M.; Pang, R.T.; Chiu, P.C.; Wong, B.P.; Lao, K.; Lee, K.F.; Yeung, W.S. Sperm-borne microRNA-34c is required for the first cleavage division in mouse. Proc. Natl. Acad. Sci. USA 2012, 109, 490–494. [Google Scholar] [CrossRef]
- Wang, M.; Gao, Y.; Qu, P.; Qing, S.; Qiao, F.; Zhang, Y.; Mager, J.; Wang, Y. Sperm-borne miR-449b influences cleavage, epigenetic reprogramming and apoptosis of SCNT embryos in bovine. Sci. Rep. 2017, 7, 13403. [Google Scholar] [CrossRef] [PubMed]
- Hu, S.J.; Ren, G.; Liu, J.L.; Zhao, Z.A.; Yu, Y.S.; Su, R.W.; Ma, X.H.; Ni, H.; Lei, W.; Yang, Z.M. MicroRNA expression and regulation in mouse uterus during embryo implantation. J. Biol. Chem. 2008, 283, 23473–23484. [Google Scholar] [CrossRef]
- Vilella, F.; Moreno-Moya, J.M.; Balaguer, N.; Grasso, A.; Herrero, M.; Martinez, S.; Marcilla, A.; Simon, C. Hsa-miR-30d, secreted by the human endometrium, is taken up by the pre-implantation embryo and might modify its transcriptome. Development 2015, 142, 3210–3221. [Google Scholar] [CrossRef] [PubMed]
- Kim, D.K.; Lee, J.; Simpson, R.J.; Lotvall, J.; Gho, Y.S. EVpedia: A community web resource for prokaryotic and eukaryotic extracellular vesicles research. Semin. Cell Dev. Biol. 2015, 40, 4–7. [Google Scholar] [CrossRef] [PubMed]
- Palomo, L.; Casal, E.; Royo, F.; Cabrera, D.; van-Liempd, S.; Falcon-Perez, J.M. Considerations for applying metabolomics to the analysis of extracellular vesicles. Front. Immunol. 2014, 5, 651. [Google Scholar] [CrossRef]
- Lamy, J.; Gatien, J.; Dubuisson, F.; Nadal-Desbarats, L.; Salvetti, P.; Mermillod, P.; Saint-Dizier, M. Metabolomic profiling of bovine oviductal fluid across the oestrous cycle using proton nuclear magnetic resonance spectroscopy. Reprod. Fertil. Dev. 2018, 30, 1021–1028. [Google Scholar] [CrossRef]
- Belaz, K.R.; Tata, A.; Franca, M.R.; Santos da Silva, M.I.; Vendramini, P.H.; Fernandes, A.M.; D’Alexandri, F.L.; Eberlin, M.N.; Binelli, M. Phospholipid Profile and Distribution in the Receptive Oviduct and Uterus During Early Diestrus in Cattle. Biol. Reprod. 2016, 95, 127. [Google Scholar] [CrossRef] [PubMed]
- McRae, C.; Sharma, V.; Fisher, J. Metabolite Profiling in the Pursuit of Biomarkers for IVF Outcome: The Case for Metabolomics Studies. Int. J. Reprod. Med. 2013, 2013, 603167. [Google Scholar] [CrossRef]
- Bracewell-Milnes, T.; Saso, S.; Abdalla, H.; Nikolau, D.; Norman-Taylor, J.; Johnson, M.; Holmes, E.; Thum, M.Y. Metabolomics as a tool to identify biomarkers to predict and improve outcomes in reproductive medicine: A systematic review. Hum. Reprod. Update 2017, 23, 723–736. [Google Scholar] [CrossRef] [PubMed]
- Sagini, K.; Costanzi, E.; Emiliani, C.; Buratta, S.; Urbanelli, L. Extracellular Vesicles as Conveyors of Membrane-Derived Bioactive Lipids in Immune System. Int. J. Mol. Sci. 2018, 19, 1227. [Google Scholar] [CrossRef]
- Weems, C.W.; Weems, Y.S.; Randel, R.D. Prostaglandins and reproduction in female farm animals. Vet. J. 2006, 171, 206–228. [Google Scholar] [CrossRef] [PubMed]
- Skotland, T.; Ekroos, K.; Kauhanen, D.; Simolin, H.; Seierstad, T.; Berge, V.; Sandvig, K.; Llorente, A. Molecular lipid species in urinary exosomes as potential prostate cancer biomarkers. Eur. J. Cancer 2017, 70, 122–132. [Google Scholar] [CrossRef]
- Acuna, O.S.; Aviles, M.; Lopez-Ubeda, R.; Guillen-Martinez, A.; Soriano-Ubeda, C.; Torrecillas, A.; Coy, P.; Izquierdo-Rico, M.J. Differential gene expression in porcine oviduct during the oestrous cycle. Reprod. Fertil. Dev. 2017, 29, 2387–2399. [Google Scholar] [CrossRef]
- Seytanoglu, A.; Georgiou, A.S.; Sostaric, E.; Watson, P.F.; Holt, W.V.; Fazeli, A. Oviductal cell proteome alterations during the reproductive cycle in pigs. J. Proteome Res. 2008, 7, 2825–2833. [Google Scholar] [CrossRef]
- Cerny, K.L.; Garrett, E.; Walton, A.J.; Anderson, L.H.; Bridges, P.J. A transcriptomal analysis of bovine oviductal epithelial cells collected during the follicular phase versus the luteal phase of the estrous cycle. Reprod. Biol. Endocrinol. 2015, 13, 84. [Google Scholar] [CrossRef]
- Soleilhavoup, C.; Riou, C.; Tsikis, G.; Labas, V.; Harichaux, G.; Kohnke, P.; Reynaud, K.; de Graaf, S.P.; Gerard, N.; Druart, X. Proteomes of the Female Genital Tract During the Oestrous Cycle. Mol. Cell. Proteom. 2016, 15, 93–108. [Google Scholar] [CrossRef]
- Greening, D.W.; Nguyen, H.P.; Elgass, K.; Simpson, R.J.; Salamonsen, L.A. Human Endometrial Exosomes Contain Hormone-Specific Cargo Modulating Trophoblast Adhesive Capacity: Insights into Endometrial-Embryo Interactions. Biol. Reprod. 2016, 94, 38. [Google Scholar] [CrossRef]
- Burns, G.W.; Brooks, K.E.; O’Neil, E.V.; Hagen, D.E.; Behura, S.K.; Spencer, T.E. Progesterone Effects on Extracellular Vesicles in the Sheep Uterus. Biol. Reprod. 2018, 98, 612–622. [Google Scholar] [CrossRef]
- Burns, G.; Brooks, K.; Wildung, M.; Navakanitworakul, R.; Christenson, L.K.; Spencer, T.E. Extracellular vesicles in luminal fluid of the ovine uterus. PLoS ONE 2014, 9, e90913. [Google Scholar] [CrossRef]
- Saadeldin, I.M.; Oh, H.J.; Lee, B.C. Embryonic-maternal cross-talk via exosomes: Potential implications. Stem Cells Cloning 2015, 8, 103–107. [Google Scholar] [CrossRef]
- Machtinger, R.; Laurent, L.C.; Baccarelli, A.A. Extracellular vesicles: Roles in gamete maturation, fertilization and embryo implantation. Hum. Reprod. Update 2016, 22, 182–193. [Google Scholar] [CrossRef]
- Martin-DeLeon, P.A. Uterosomes: Exosomal cargo during the estrus cycle and interaction with sperm. Front. Biosci. 2016, 8, 115–122. [Google Scholar] [CrossRef][Green Version]
- Burnett, L.A.; Nowak, R.A. Exosomes mediate embryo and maternal interactions at implantation and during pregnancy. Front. Biosci. 2016, 8, 79–96. [Google Scholar]
- Homer, H.; Rice, G.E.; Salomon, C. Review: Embryo- and endometrium-derived exosomes and their potential role in assisted reproductive treatments-liquid biopsies for endometrial receptivity. Placenta 2017, 54, 89–94. [Google Scholar] [CrossRef]
- Ren, X.; Chen, X.; Wang, Z.; Wang, D. Is transcription in sperm stationary or dynamic? J. Reprod. Dev. 2017, 63, 439–443. [Google Scholar] [CrossRef]
- Malama, E.B.S.; Bick, J.; Janett, F.; Bollwein, H. The population of small RNAs in cryopreserved semen of fertile and subfertile bulls. Reprod. Domest. Anim. 2017, 52, 77. [Google Scholar]
- Capra, E.; Turri, F.; Lazzari, B.; Cremonesi, P.; Gliozzi, T.M.; Fojadelli, I.; Stella, A.; Pizzi, F. Small RNA sequencing of cryopreserved semen from single bull revealed altered miRNAs and piRNAs expression between High- and Low-motile sperm populations. BMC Genom. 2017, 18, 14. [Google Scholar] [CrossRef]
- Talbot, N.C.; Powell, A.M.; Caperna, T.J.; Garrett, W.M. Proteomic analysis of the major cellular proteins of bovine trophectoderm cell lines derived from IVP, parthenogenetic and nuclear transfer embryos: Reduced expression of annexins I and II in nuclear transfer-derived cell lines. Anim. Reprod. Sci. 2010, 120, 187–202. [Google Scholar] [CrossRef]
- Leahy, T.; Gadella, B.M. Sperm surface changes and physiological consequences induced by sperm handling and storage. Reproduction 2011, 142, 759–778. [Google Scholar] [CrossRef] [PubMed]
- Rizos, D.; Gutierrez-Adan, A.; Perez-Garnelo, S.; De La Fuente, J.; Boland, M.P.; Lonergan, P. Bovine embryo culture in the presence or absence of serum: Implications for blastocyst development, cryotolerance, and messenger RNA expression. Biol. Reprod. 2003, 68, 236–243. [Google Scholar] [CrossRef] [PubMed]
- Lonergan, P.; Rizos, D.; Gutierrez-Adan, A.; Fair, T.; Boland, M.P. Oocyte and embryo quality: Effect of origin, culture conditions and gene expression patterns. Reprod. Domest. Anim. 2003, 38, 259–267. [Google Scholar] [CrossRef]
- Lener, T.; Gimona, M.; Aigner, L.; Borger, V.; Buzas, E.; Camussi, G.; Chaput, N.; Chatterjee, D.; Court, F.A.; del Portillo, H.A.; et al. Applying extracellular vesicles based therapeutics in clinical trial—An ISEV position paper. J. Extracell. Vesicles 2015, 4, 30087. [Google Scholar] [CrossRef]
- Reiner, A.T.; Witwer, K.W.; van Balkom, B.W.M.; de Beer, J.; Brodie, C.; Corteling, R.L.; Gabrielsson, S.; Gimona, M.; Ibrahim, A.G.; de Kleijn, D.; et al. Concise Review: Developing Best-Practice Models for the Therapeutic Use of Extracellular Vesicles. Stem Cells Transl. Med. 2017, 6, 1730–1739. [Google Scholar] [CrossRef] [PubMed]
- Fuster-Matanzo, A.; Gessler, F.; Leonardi, T.; Iraci, N.; Pluchino, S. Acellular approaches for regenerative medicine: On the verge of clinical trials with extracellular membrane vesicles? Stem Cell Res. Ther. 2015, 6, 227. [Google Scholar] [CrossRef] [PubMed]
- Kao, C.Y.; Papoutsakis, E.T. Extracellular vesicles: Exosomes, microparticles, their parts, and their targets to enable their biomanufacturing and clinical applications. Curr. Opin. Biotechnol. 2019, 60, 89–98. [Google Scholar] [CrossRef] [PubMed]
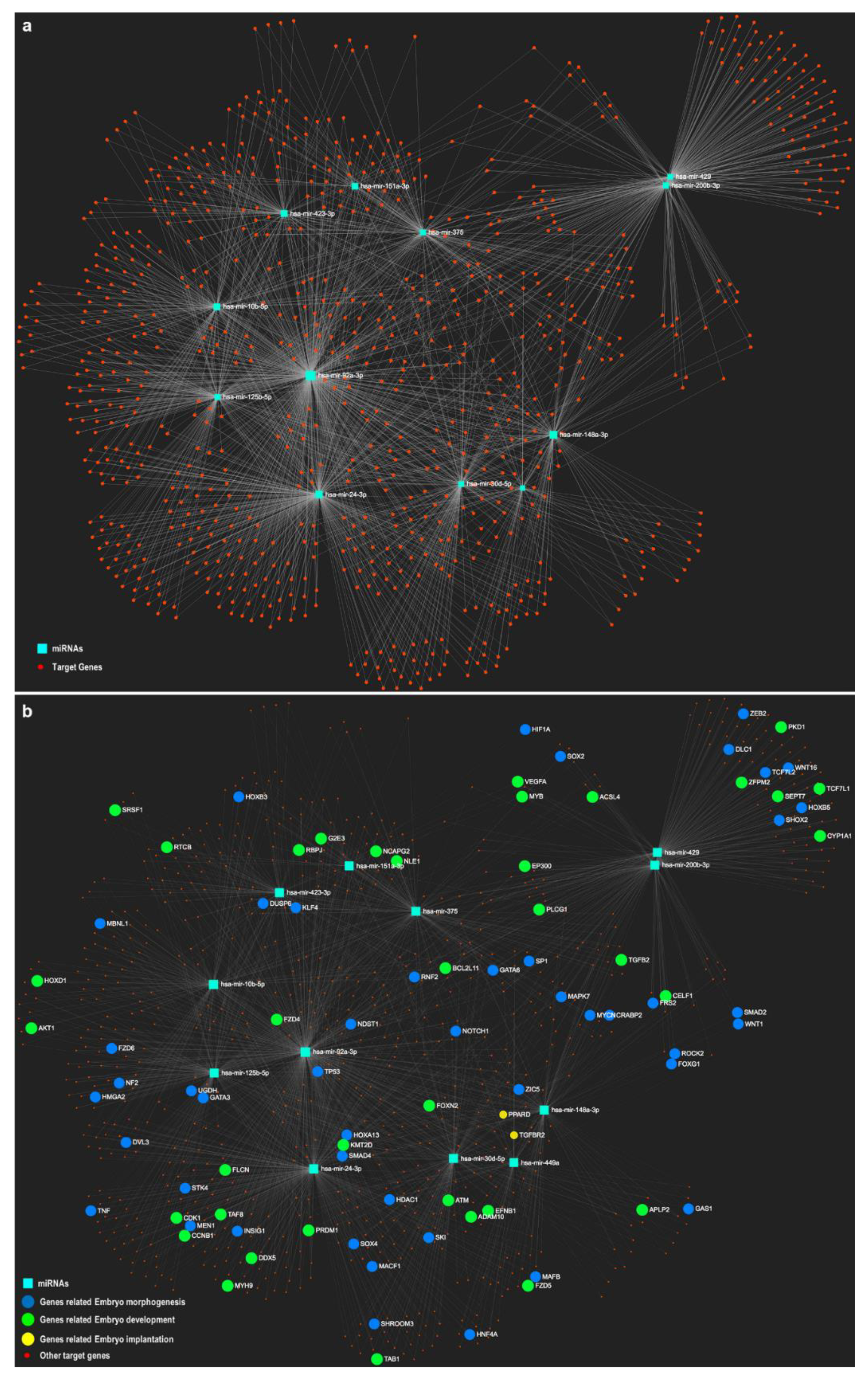
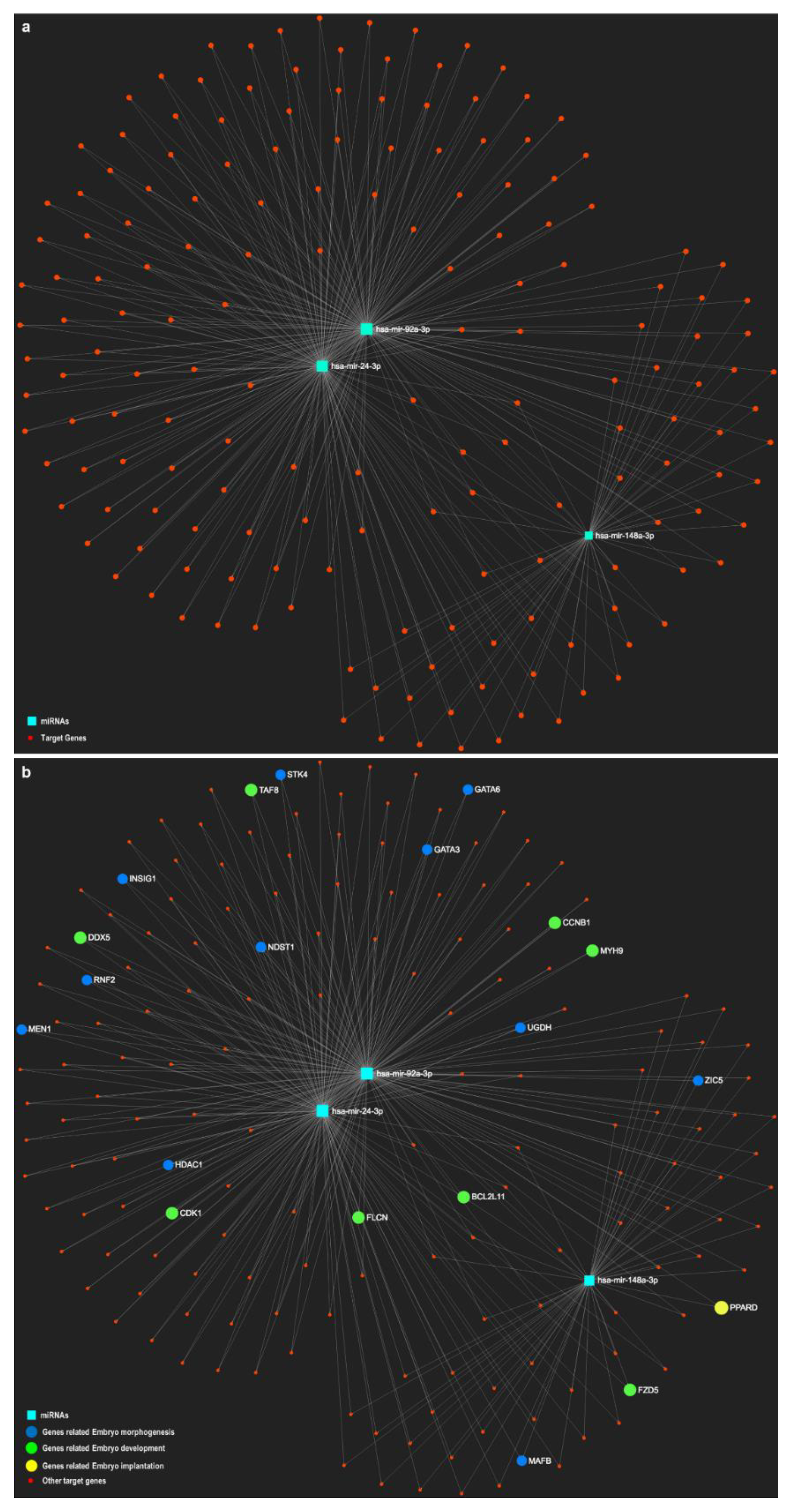
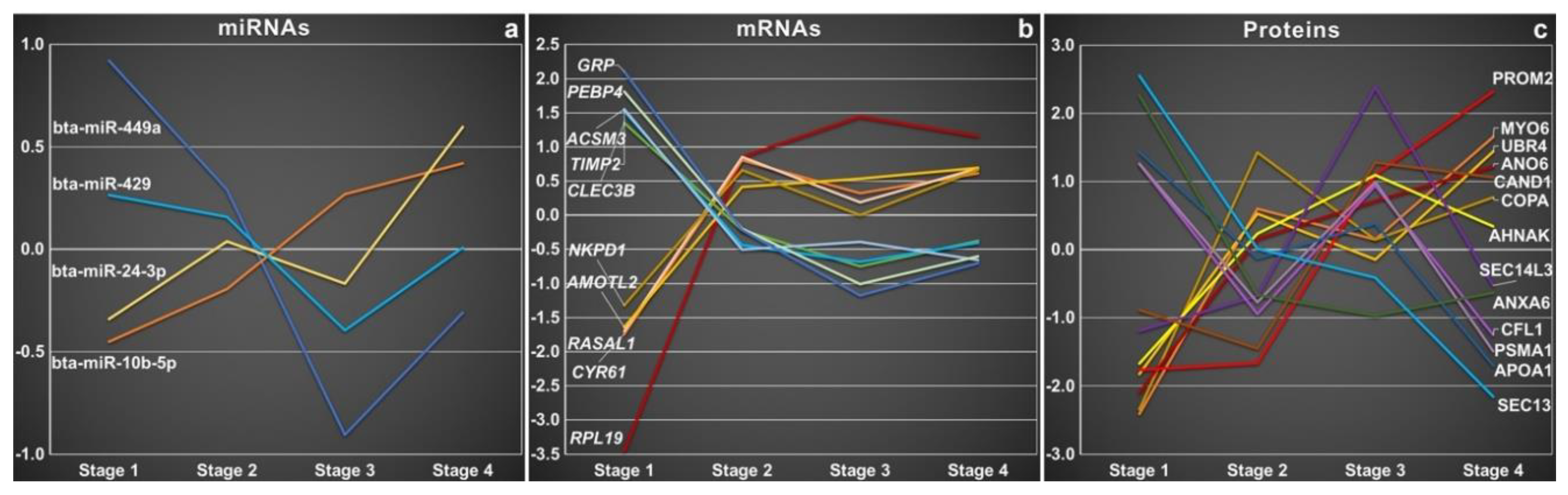
| Topic | Species | Year | Findings | Analyzed oEVS Content | Characterization Method | Exosomal Molecular Markers | Citation |
|---|---|---|---|---|---|---|---|
| Analysis of oEVs molecular content | Bovine | 2017 |
| Proteins | TEM and WB | HSP70 (WB) | Almiñana et al., 2017 Reproduction [24] |
| Bovine | 2018 |
| proteins, mRNA and small ncRNA | TEM and WB | HSP70 and ANXA1 (WB) | Almiñana et al., 2018 BMC Genomics [37] | |
| Murine | 2018 |
| miRNAs | TEM and WB | CD9 (WB) | Fereshteh et al., 2018 Scientific Reports [31] | |
| oEVs and their functional effects on gametes (sperm and oocyte) | Murine | 2013 |
| protein PMCA4a | TEM and WB | HSC70 and CD9 (WB); CD9 (TEM) | Al-Dossary et al., 2013 PLOS One [22] |
| Murine | 2015 |
| No | TEM and WB | PMCA4 (TEM) CD9 (WB) | Al-Dossary et al., 2015 J Biol Chem [58] | |
| Avian | 2017 |
| No | WB | CD63 (WB) | Huang et al., 2017 Theriogenology [33] | |
| Canine | 2017 |
| hsa-miR-30b, has-miR-375, cfa-miR-503 | NTA | _ | Lange-Consiglio et al., 2017 Reproduction [34] | |
| Turtle | 2017 |
| No | TEM and immunostaining | CD63 (Immunostaining) | Waqas et al., 2017 J. Exp. Zool. [35] | |
| Murine | 2018 |
| miRNAs | TEM and WB | CD9 (TEM) CD9 (WB) | Fereshteh et al., 2018 Sci Rep [31] | |
| Human/Murine | 2018 |
| proteins (PMCA1-4) | TEM anand WB | PMCA4 (TEM) HSC70 (WB) | Bathala et al., 2018 Mol Hum Reprod [32] | |
| oEVs and their functional effects on the embryo(s) | Bovine | 2016 |
| No | TEM, NTA and FC, WB | CD9 and CD63 (FC) CD9, ERM and TSG101 (WB) | Lopera-Vásquez et al., 2016 PLOS One [23] |
| Bovine | 2017 |
| proteins | TEM and WB | HSP70 (WB) | Almiñana et al., 2017 Reproduction [24] | |
| Bovine | 2017 |
| No | TEM, NTA and WB | CD9, ERM and TSG101 (WB) | Lopera-Vásquez et al., 2017 Reproduction [59] | |
| Murine | 2019 |
| No | TEM; NTA; WB; BCA | CD9 and HSP70 (WB) | Qu et al., 2019 Reproduction, Fertility and Development [36] | |
| Other oEVs functions | Murine | 2017 |
| mRNA (beta-actin, GAPDH and Vimentin) | TEM and WB | CD9 and CD81(WB) C81 (TEM) | Nakano et al., 2017 Biochem & Biophys Res Com [60] |
| Bovine | Murine | Human | |||
|---|---|---|---|---|---|
| Protein Description | Symbol | Protein Description | Symbol | Protein Description | Symbol |
| oviductal glycoprotein 1 | OVGP1 | Plasma membrane calcium-transporting ATPase 4 | PMCA4 | Plasma membrane calcium-transporting ATPase 4 | PMCA4 |
| annexin A1 | ANXA1 | Plasma membrane calcium-transporting ATPase 1 | PMCA1 | Plasma membrane calcium-transporting ATPase 1 | PMCA1 |
| tubulin, beta 2B class IIb | TUBB2B | Endothelial nitric oxide synthase | eNOS (NOS3) | Endothelial nitric oxide synthase | eNOS (NOS3) |
| annexin A2 | ANXA2 | neuronal nitric oxide synthase | nNOS (NOS1) | neuronal nitric oxide synthase | nNOS (NOS1) |
| annexin A4 | ANXA4 | calcium/calmodulin-dependent serine kinase | CASK | calcium/calmodulin-dependent serine kinase | CASK |
| heat shock protein family A (Hsp70) member 8 | HSPA8 | heat shock protein family A (Hsp70) member 8 | HSPA8 (HSC70) | heat shock protein family A (Hsp70) member 8 | HSPA8 (HSC70) |
| actin beta | ACTB | actin beta | ACTB | actin beta | ACTB |
| CD109 molecule | CD109 | ||||
| tubulin, alpha 3e | TUBA3E | ||||
| annexin A5 | ANXA5 | ||||
| heat shock 70kDa protein 1A | HSPA1A | ||||
| heat shock protein 90 alpha family class A member 1 | HSP90AA1 | ||||
| 5’-nucleotidase ecto | NT5E | ||||
| annexin A8-like 1 | ANXA8L1 | ||||
| ezrin | EZR | ||||
| clathrin heavy chain | CLTC | ||||
| glyceraldehyde-3-phosphate dehydrogenase | GAPDH | ||||
| stomatin | STOM | ||||
| mesothelin | MSLN | ||||
| major vault protein | MVP | ||||
| annexin A11 | ANXA11 | ||||
| ectonucleotide pyrophosphatase/phosphodiesterase 3 | ENPP3 | ||||
| heat shock protein family B (small) member 1 | HSPB1 | ||||
| clusterin | CLU | ||||
| RAB5C, member RAS oncogene family | RAB5C | ||||
© 2019 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (http://creativecommons.org/licenses/by/4.0/).
Share and Cite
Almiñana, C.; Bauersachs, S. Extracellular Vesicles in the Oviduct: Progress, Challenges and Implications for the Reproductive Success. Bioengineering 2019, 6, 32. https://doi.org/10.3390/bioengineering6020032
Almiñana C, Bauersachs S. Extracellular Vesicles in the Oviduct: Progress, Challenges and Implications for the Reproductive Success. Bioengineering. 2019; 6(2):32. https://doi.org/10.3390/bioengineering6020032
Chicago/Turabian StyleAlmiñana, Carmen, and Stefan Bauersachs. 2019. "Extracellular Vesicles in the Oviduct: Progress, Challenges and Implications for the Reproductive Success" Bioengineering 6, no. 2: 32. https://doi.org/10.3390/bioengineering6020032
APA StyleAlmiñana, C., & Bauersachs, S. (2019). Extracellular Vesicles in the Oviduct: Progress, Challenges and Implications for the Reproductive Success. Bioengineering, 6(2), 32. https://doi.org/10.3390/bioengineering6020032





