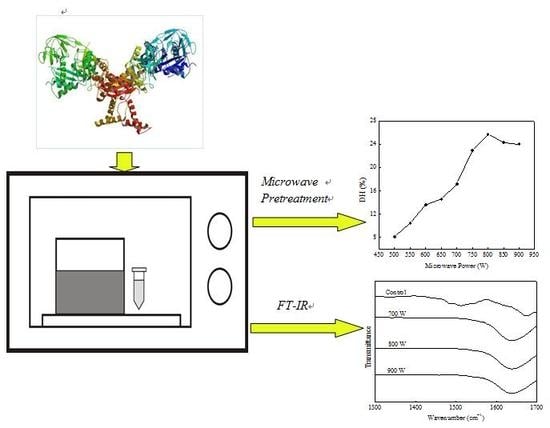
Microwave Pretreatment and Enzymolysis Optimization of the Lotus Seed Protein
Abstract
:1. Introduction
2. Experimental
2.1. Materials
2.2. Microwave Pretreatments
2.3. Enzymolysis of the Pretreated Protein
2.4. ATR-FTIR Spectroscopy
2.5. Statistical Analysis
3. Results and Discussion
3.1. Microwave Pretreatment
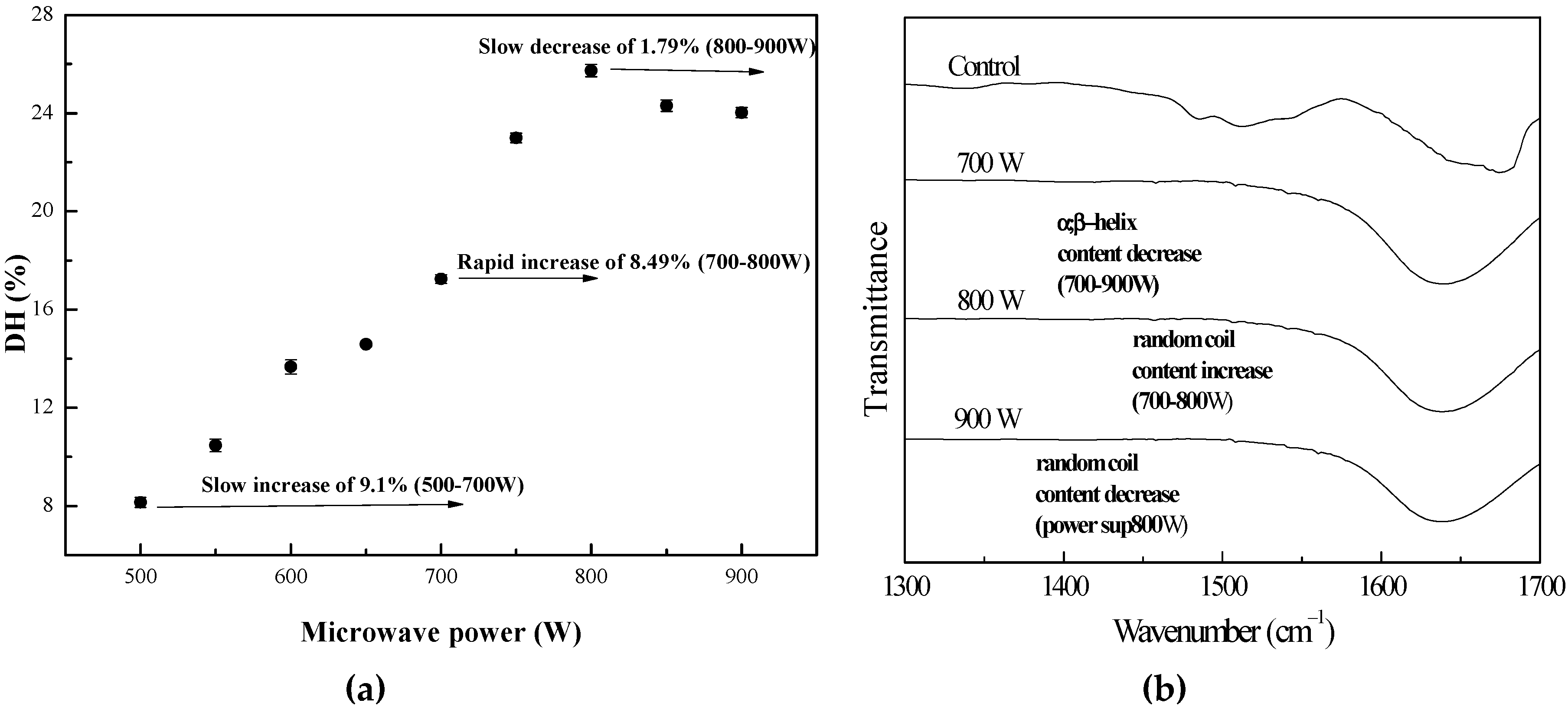
3.1.1. Influence of Microwave Power
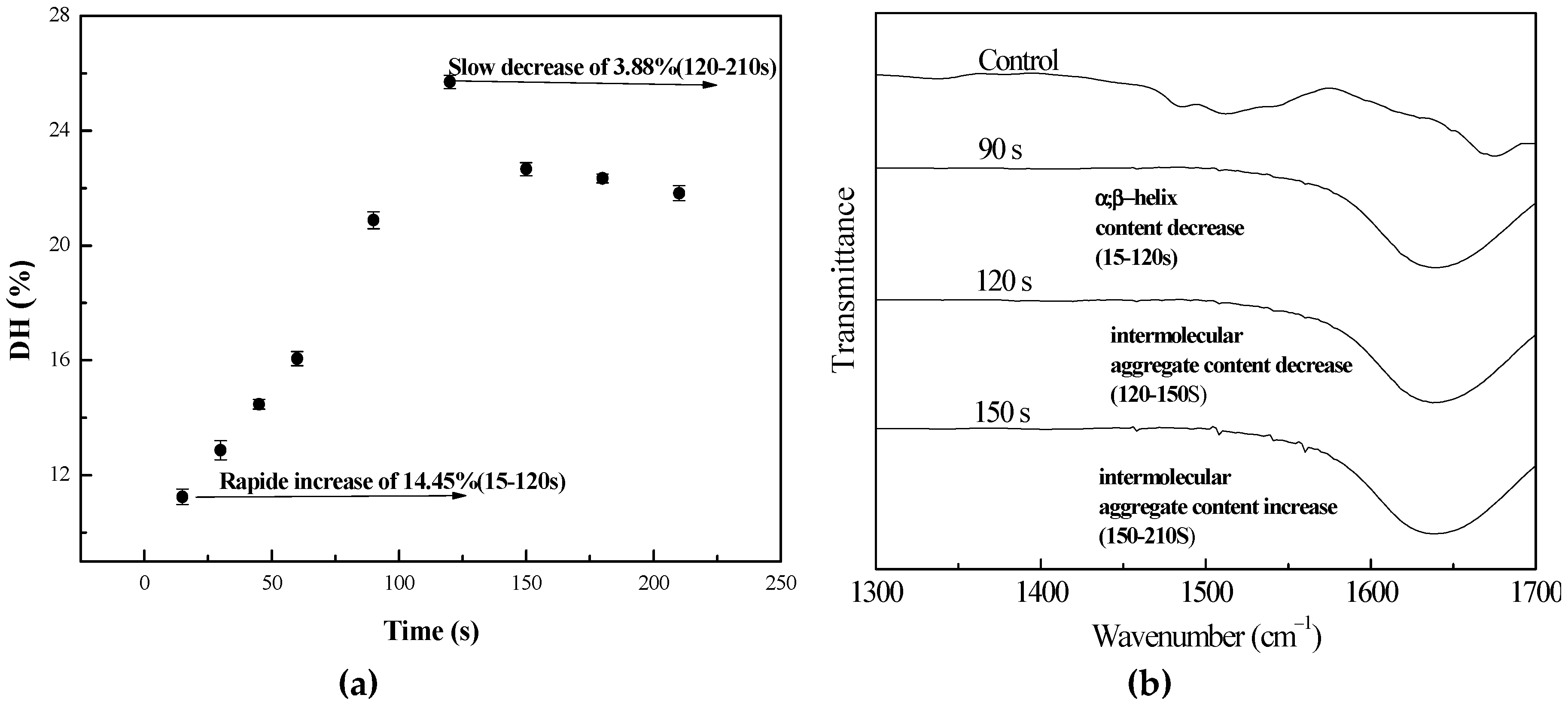
3.1.2. Influence of Microwave Time
3.2. Enzymolysis
3.2.1. Central composite design (CCD) analysis
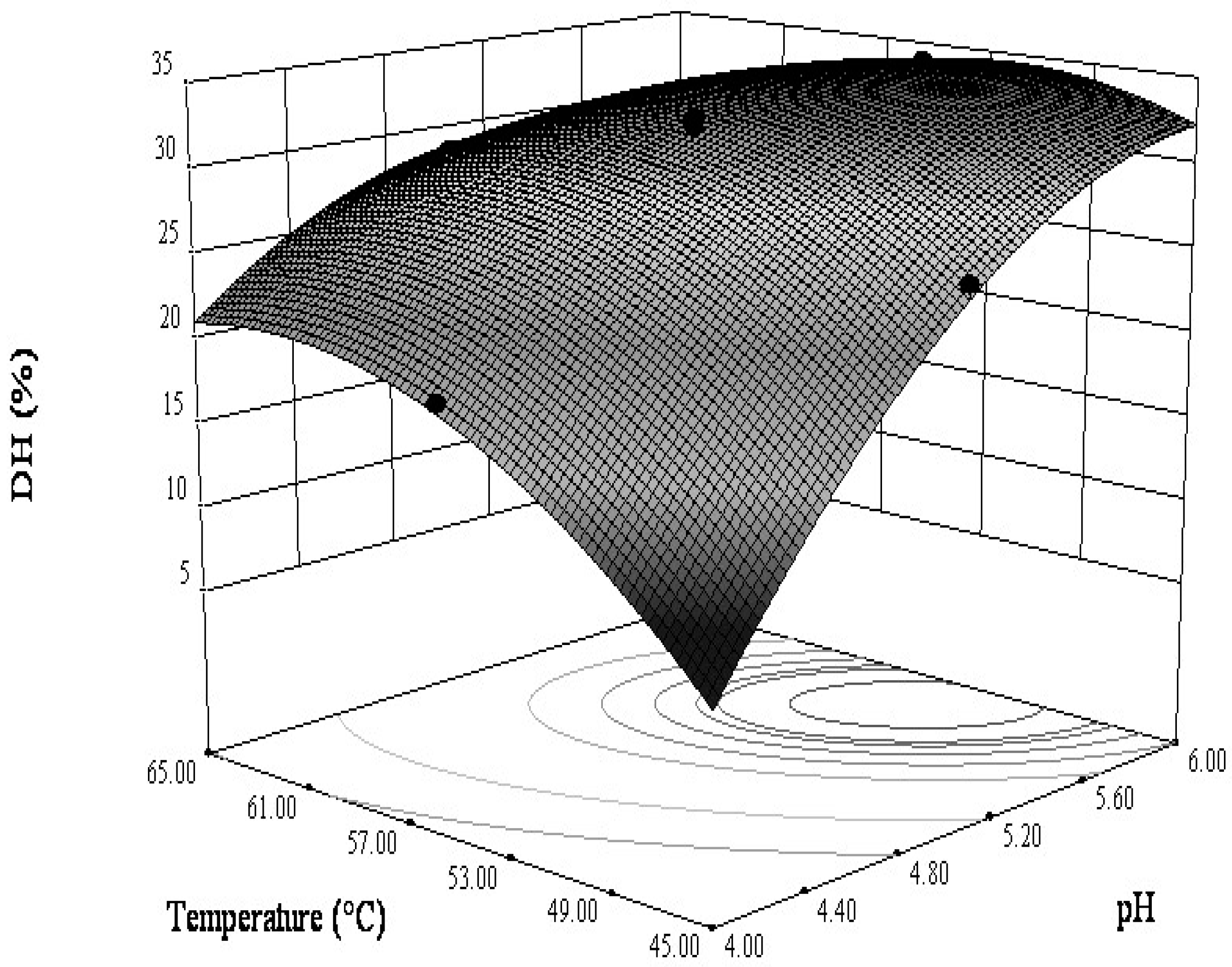
3.2.2. Interactions between the Variables
3.2.3. Optimization Analysis
4. Conclusions
Author Contributions
Funding
Conflicts of Interest
References
- Liu, X.R.; Xie, R.P.; Fan, Y.W.; Hu, J.N.; Luo, T.; Li, H.Y.; Deng, Z.Y. Polymerization of proanthocyanidins catalyzed by polyphenol oxidase from lotus seedpod. Eur. Food Res. Technol. 2014, 238, 727–739. [Google Scholar] [CrossRef]
- Qi, S.; Zhou, D. Lotus seed epicarp extract as potential antioxidant and anti-obesity additive in Chinese Cantonese Sausage. Meat Sci. 2013, 93, 257–262. [Google Scholar] [CrossRef]
- Liu, Y.; Ma, S.S.; Ibrahim, S.A.; Li, E.H.; Yang, H.; Huang, W. Identification and antioxidant properties of polyphenols in lotus seed epicarp at different ripening stages. Food Chem. 2015, 185, 159–164. [Google Scholar] [CrossRef]
- Mani, S.; Subramanian, I.; Pillai, S.; Muthusamy, K. Evaluation of hypoglycemic activity of inorganic constituents in Nelumbo nucifera seeds on streptozotocin-induced diabetes in rats. Biol. Trace Elem. Res. 2010, 138, 226–237. [Google Scholar] [CrossRef]
- Guo, J.B.; Zhang, W.J.; Wu, H.; Du, L.M. Microwave-assisted decomposition coupled with acidic food condiment as an efficient technology for ginger (Zingiber officinale Roscoe) Processing. Sep. Purif. Technology 2015, 146, 219–226. [Google Scholar] [CrossRef]
- Li, X.; Long, J.; Hua, Y.; Chen, Y.; Kong, X.; Zhang, C. Protein recovery and anti-nutritional factor removal from soybean wastewater by complexing with a high concentration of polysaccharides in a novel quick-shearing system. J. Food Eng. 2019, 241, 1–9. [Google Scholar] [CrossRef]
- Barratt, M.E.J.; Strachan, J.P.; Porte, P. Immunologically mediated nutritional disturbances associated with soya protein antigens. Proc. Nutr. Soc. 1979, 38, 143. [Google Scholar] [CrossRef]
- Lakemond, C.M.M.; Vereijken, J.M. Soybean proteins: Structure and function. In Industrial Proteins in Perspective. Progress in Biotechnology; Aalbersberg, W.Y., Hamer, R.J., Jasperse, P., de Jongh, H.H.J., de Kruif, C.G., Walstra, P., de Wolf, F.A., Eds.; Elsevier Science: Amsterdam, The Netherlands, 2003; pp. 55–62. [Google Scholar]
- Yu, Z.; Liu, L.; Xu, Y.; Wang, L.; Teng, X.; Li, X.; Dai, J. Characterization and biological activities of a novel polysaccharide isolated from raspberry (Rubus idaeus L.) fruits. Carbohyd. Polym. 2015, 132, 180–186. [Google Scholar] [CrossRef]
- Nimalaratne, C.; Bandara, N.; Wu, J. Purification and characterization of antioxidant peptides from enzymatically hydrolyzed chicken egg white. Food Chem. 2015, 188, 467–472. [Google Scholar] [CrossRef]
- Pan, A.D.; Zeng, H.Y.; Alain, G.B.F.C.; Feng, B. Heat-pretreatment and enzymolysis behavior of the lotus seed protein. Food Chem. 2016, 201, 230–236. [Google Scholar] [CrossRef]
- Barbosa, J.T.P.; Santos, C.M.M.; Peralva, V.N.; Flores, E.M.M.; Korn, M.; Nóbrega, J.A.; Korn, M.G.A. Microwave-assisted diluted acid digestion for trace elements analysis of edible soybean products. Food Chem. 2015, 175, 212–217. [Google Scholar] [CrossRef]
- Chen, Z.; Li, Y.; Lin, S.; Wei, M.; Du, F.; Ruan, G. Development of continuous microwave-assisted protein digestion with immobilized enzyme. Biochem. Biophys. Res. Commun. 2014, 445, 491–496. [Google Scholar] [CrossRef]
- Saha, M.; Eskicioglu, C.; Marin, J. Microwave, ultrasonic and chemo-mechanical pretreatments for enhancing methane potential of pulp mill wastewater treatment sludge. Bioresour. Technol. 2011, 102, 7815–7826. [Google Scholar] [CrossRef]
- Farzaneh, V.; Carvalho, S.I. Modelling of Microwave Assisted Extraction (MAE) of Anthocyanins (TMA). Journal of Applied Research on Medicinal and Aromatic Plants. J. Appl. Res. Med. Aromat. Plants 2017, 6, 92–100. [Google Scholar]
- Uluko, H.; Zhang, S.; Liu, L.; Chen, J.; Sun, Y.; Su, Y.; Li, H.; Cui, W.; Lv, J. Effects of microwave and ultrasound pretreatments on enzymolysis of milk protein concentrate with different enzymes. Int. J. Food Sci. Technol. 2013, 48, 2250–2257. [Google Scholar] [CrossRef]
- Wang, Z.; Zhou, J.; Wang, X.X.; Zhang, N.; Sun, X.X.; Ma, Z.S. The Effects of ultrasonic/microwave assisted treatment on the properties of soy protein isolate/microcrystalline wheat-bran cellulose film. J. Food Eng. 2013, 114, 183–191. [Google Scholar] [CrossRef]
- Sun, B.; Duan, L.; Peng, G.; Li, X.; Xu, A. Efficient production of glucose by microwave-assisted acid hydrolysis of cellulose hydrogel. Bioresour. Technol. 2015, 192, 253–256. [Google Scholar] [CrossRef]
- Da Silva, S.V.; Picoloto, R.S.; Flores, E.M.M.; Wagner, R.; Richards, N.S.D.; Barin, J.S. Evaluation of bromine and iodine content of milk whey proteins combining digestion by microwave-induced combustion and ICP-MS determination. Food Chem. 2016, 190, 364–367. [Google Scholar] [CrossRef]
- Zhai, Y.; Sun, S.; Wang, Z.; Cheng, J.; Sun, Y.; Wang, L. Microwave extraction of essential oils from dried fruits of Illicium verum Hook. f. and Cuminum cyminum L. using ionic liquid as the microwave absorption medium. J. Sep. Sci. 2009, 32, 3544–3549. [Google Scholar] [CrossRef]
- Buldini, P.L.; Ricci, L.; Sharma, J.L. Recent applications of sample preparation techniques in food analysis. J. Chromatogr. A 2002, 975, 47–70. [Google Scholar] [CrossRef]
- Moghimi, M.; Farzaneh, V.; Bakhshabadi, H. The effect of ultrasound pretreatment on some selected physicochemical properties of black cumin (Nigella Sativa). Nutrire 2018, 43, 18. [Google Scholar] [CrossRef]
- Zeng, H.Y.; Cai, L.H.; Cai, X.L.; Wang, Y.J.; Li, Y.Q. Amino acid profiles and quality from lotus seed proteins. J. Sci. Food Agric. 2013, 93, 1070–1075. [Google Scholar] [CrossRef]
- Kim, S.Y.; Park, P.S.; Rhee, K.C. Functional properties of proteolytic enzyme modified soy protein isolate. J. Agric. Food Chem. 1990, 38, 651–656. [Google Scholar] [CrossRef]
- Dolatabadi, Z.; Rad, A.H.E.; Farzaneh, V.; Feizabad, S.H.A.; Estiri, S.H.; Bakhshabadi, H. Modeling of the lycopene extraction from tomato pulps. Food Chem. 2016, 190, 968–973. [Google Scholar] [CrossRef]
- Horikoshi, S.; Nakamura, T.; Kawaguchi, M.; Serpone, N. Enzymatic proteolysis of peptide bonds by a metallo-endoproteinase under precise temperature control with 5.8-GHz microwave radiation. J. Mol. Catal. B Enzym. 2015, 116, 52–59. [Google Scholar] [CrossRef]
- Bakhshabadi, H.; Mirzaei, H.; Ghodsvali, A.; Jafari, S.M.; Ziaiifar, A.M.; Farzaneh, V. The effect of microwave pretreatment on some physico-chemical properties and 2 bioactivity of Black cumin seeds’ oil. Ind. Crops. Prod. 2017, 97, 1–9. [Google Scholar] [CrossRef]
- Franco, A.P.; Yamamoto, L.Y.; Tadini, C.C.; Gut, J.A.W. Dielectric properties of green coconut water relevant to microwave processing: Effect of temperature and field frequency. J. Food Eng. 2015, 155, 69–78. [Google Scholar] [CrossRef]
- Ni, J.; Teng, N.; Chen, H.; Wang, J.; Zhu, J.; Na, H. Hydrolysis behavior of regenerated celluloses with different degree of polymerization under microwave radiation. Bioresour. Technol. 2015, 191, 229–233. [Google Scholar] [CrossRef]
- Kong, J.; Yu, S. Fourier transform infrared spectroscopic analysis of protein secondary structures. Acta Biochim. Biophys. Sin. 2007, 39, 549–559. [Google Scholar] [CrossRef]
- Zhao, X.; Chen, F.; Xue, W.; Lee, L. FTIR spectra studies on the secondary structures of 7S and 11S globulins from soybean proteins using AOT reverse micellar extraction. Food Hydrocoll. 2008, 22, 568–575. [Google Scholar] [CrossRef]
- Carbonaro, M.; Maselli, P.; Dore, P.; Nucara, A. Application of Fourier transform infrared spectroscopy to legume seed flour analysis. Food Chem. 2008, 108, 361–368. [Google Scholar] [CrossRef]
- Llerena-Suster, C.R.; José, C.; Collins, S.E.; Briand, L.E.; Morcelle, S.R. Investigation of the structure and proteolytic activity of papain in aqueous miscible organic media. Process Biochem. 2012, 47, 47–56. [Google Scholar] [CrossRef]
- Fan, J.; De bruyn, M.; Budarin, V.L.; Gronnow, M.J.; Shuttleworth, P.S.; Breeden, S.; Macquarrie, D.J.; Clark, J.H. Direct microwave-assisted hydrothermal depolymerization of cellulose. J. Am. Chem. Soc. 2013, 135, 11728–11731. [Google Scholar] [CrossRef]
- Banik, S.; Bandyopadhyay, S.; Ganguly, S. Bioeffects of microwave-a brief review. Bioresour. Technol. 2003, 87, 155–159. [Google Scholar] [CrossRef]
- Guo, Z.; Zeng, S.; Lu, X.; Zhou, M.; Zheng, M.; Zheng, B. Structural and physicochemical properties of lotus seed starch treated with ultra-high pressure. Food Chem. 2015, 186, 223–230. [Google Scholar] [CrossRef]
- Jalili, F.; Jafari, S.M.; Emam-Djomeh, Z.; Malekjani, N.; Farzaneh, V. Optimization of ultrasound assisted extraction of oil from Canola seeds with the use of response surface methodology. Food Anal. Methods 2018, 11, 598–612. [Google Scholar] [CrossRef]
- Rostami, M.; Farzaneh, V.; Boujmehrani, A.; Mohammadi, M.; Bakhshabadi, H. Optimizing the extraction process of sesame seed’s oil using response surface method on the industrial scale. Ind. Crops. Prod. 2014, 58, 160–165. [Google Scholar] [CrossRef]
- Ganje, M.; Jafari, S.M.; Farzaneh, V.; Malekjani, N. Kinetics modelling of color deterioration during thermal processing of tomato paste with the use of response surface methodology. Heat Mass Transf. 2018, 54, 3663–3671. [Google Scholar] [CrossRef]
- Farzaneh, V.; Ghodsvali, A.; Bakhshabadi, H.; Dolatabadi, Z.; Carvalho, I.S. Screening of the alterations in qualitative characteristics of grape under the impacts of storage and harvest times using Artificial Neural Network. Evol. Syst. 2018, 9, 81–89. [Google Scholar] [CrossRef]
- Yuan, X.; Liu, J.; Zeng, G.; Shi, J.; Tong, J.; Huang, G. Optimization of conversion of waste rapeseed oil with high FFA to biodiesel using response surface methodology. Renew. Energy 2008, 33, 1678–1684. [Google Scholar] [CrossRef]

| Independent Variables | Symbols | Units | Code Levels | ||||
|---|---|---|---|---|---|---|---|
| −2 | −1 | 0 | +1 | +2 | |||
| Substrate concentration | S | g/L | 7.0 | 13.0 | 20.0 | 27.0 | 34.0 |
| pH | 4.0 | 4.5 | 5.0 | 5.5 | 6.0 | ||
| Hydrolysis temperature | T | °C | 45 | 50 | 55 | 60 | 65 |
| Papain amount | M | g/L | 0.3 | 0.4 | 0.5 | 0.6 | 0.7 |
| Assignment | Peak Centers (cm−1) | Control | Time (s) | Power (W) | ||||
|---|---|---|---|---|---|---|---|---|
| 90 | 120 | 150 | 700 | 800 | 900 | |||
| Intermolecular aggregate | 1615 | 12.97 | 10.71 | 6.00 | 9.24 | 13.89 | 5.23 | 11.88 |
| β-Sheet | 1625 | 13.00 | 24.25 | 26.21 | 34.45 | 29.57 | 24.51 | 26.43 |
| 1640 | ||||||||
| α-Helix | 1655 | 27.2 | 22.51 | 13.70 | 11.05 | 19.97 | 18.02 | 17.12 |
| Random | 1645 | 14.63 | 19.02 | 29.17 | 17.95 | 15.87 | 28.57 | 15.23 |
| β-Turn | 1668 | 32.19 | 23.49 | 24.93 | 27.31 | 20.68 | 23.66 | 29.32 |
| 1688 | ||||||||
| Run | Experimental Variables | Response Y (%) | ||||
|---|---|---|---|---|---|---|
| S (g/L) | P | T (°C) | E (g/L) | Expt. | Predicted | |
| 1 | 13 | 4.5 | 50 | 0.4 | 22.25 | 23.13 |
| 2 | 27 | 4.5 | 50 | 0.4 | 17.74 | 17.75 |
| 3 | 13 | 5.5 | 50 | 0.4 | 31.36 | 32.21 |
| 4 | 27 | 5.5 | 50 | 0.4 | 29.52 | 28.77 |
| 5 | 13 | 4.5 | 60 | 0.4 | 29.43 | 29.15 |
| 6 | 27 | 4.5 | 60 | 0.4 | 16.30 | 17.65 |
| 7 | 13 | 5.5 | 60 | 0.4 | 33.72 | 32.99 |
| 8 | 27 | 5.5 | 60 | 0.4 | 23.95 | 23.43 |
| 9 | 13 | 4.5 | 50 | 0.6 | 22.57 | 22.30 |
| 10 | 27 | 4.5 | 50 | 0.6 | 14.38 | 15.13 |
| 11 | 13 | 5.5 | 50 | 0.6 | 31.47 | 31.38 |
| 12 | 27 | 5.5 | 50 | 0.6 | 25.41 | 26.15 |
| 13 | 13 | 4.5 | 60 | 0.6 | 30.56 | 31.33 |
| 14 | 27 | 4.5 | 60 | 0.6 | 19.68 | 18.04 |
| 15 | 13 | 5.5 | 60 | 0.6 | 34.72 | 35.17 |
| 16 | 27 | 5.5 | 60 | 0.6 | 23.43 | 23.81 |
| 17 | 6 | 5.0 | 55 | 0.5 | 28.55 | 27.99 |
| 18 | 34 | 5.0 | 55 | 0.5 | 11.19 | 11.27 |
| 19 | 20 | 4.0 | 55 | 0.5 | 19.62 | 19.07 |
| 20 | 20 | 6.0 | 55 | 0.5 | 33.85 | 33.93 |
| 21 | 20 | 5.0 | 45 | 0.5 | 26.02 | 25.2 |
| 22 | 20 | 5.0 | 65 | 0.5 | 28.54 | 28.89 |
| 23 | 20 | 5.0 | 55 | 0.3 | 29.31 | 29.14 |
| 24 | 20 | 5.0 | 55 | 0.7 | 29.01 | 28.70 |
| 25 | 20 | 5.0 | 55 | 0.5 | 32.51 | 32.12 |
| 26 | 20 | 5.0 | 55 | 0.5 | 32.71 | 32.12 |
| 27 | 20 | 5.0 | 55 | 0.5 | 32.64 | 32.12 |
| 28 | 20 | 5.0 | 55 | 0.5 | 32.28 | 32.12 |
| 29 | 20 | 5.0 | 55 | 0.5 | 31.69 | 32.12 |
| 30 | 20 | 5.0 | 55 | 0.5 | 30.87 | 32.12 |
| Source | Sum of Squares | DF | Mean Square | F | p-Value |
|---|---|---|---|---|---|
| Model | 1160.19 | 13 | 89.25 | 104.43 | <0.0001 |
| S | 419.92 | 1 | 419.92 | 491.39 | <0.0001 |
| P | 331.01 | 1 | 331.01 | 387.34 | <0.0001 |
| T | 20.41 | 1 | 20.41 | 23.88 | 0.0002 |
| E | 0.29 | 1 | 0.29 | 0.34 | 0.5666 |
| SP | 3.75 | 1 | 3.75 | 4.39 | 0.0523 |
| ST | 37.42 | 1 | 37.42 | 43.79 | <0.0001 |
| SE | 3.21 | 1 | 3.21 | 3.76 | 0.0703 |
| PT | 27.48 | 1 | 27.48 | 32.16 | <0.0001 |
| TE | 9.05 | 1 | 9.05 | 10.58 | 0.0050 |
| S2 | 267.16 | 1 | 267.16 | 312.63 | <0.0001 |
| P2 | 54.12 | 1 | 54.12 | 63.33 | <0.0001 |
| T2 | 44.13 | 1 | 44.13 | 51.64 | <0.0001 |
| E2 | 17.49 | 1 | 17.49 | 20.46 | 0.0003 |
| Residual | 13.67 | 16 | 0.85 | - | - |
| Lack of Fit | 11.13 | 11 | 1.01 | 1.99 | 0.2315 |
| Pure Error | 2.54 | 5 | 0.51 | - | - |
| Cor Total | 1173.86 | 29 | - | - | - |
| R2 | 0.9884 | ||||
| Adjusted R2 | 0.9789 | ||||
| Predicted R2 | 0.9537 | ||||
| Adeq precision | 37.846 | ||||
| CV | 3.44% | ||||
© 2019 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (http://creativecommons.org/licenses/by/4.0/).
Share and Cite
Gohi, B.F.C.A.; Du, J.; Zeng, H.-Y.; Cao, X.-j.; Zou, K.m. Microwave Pretreatment and Enzymolysis Optimization of the Lotus Seed Protein. Bioengineering 2019, 6, 28. https://doi.org/10.3390/bioengineering6020028
Gohi BFCA, Du J, Zeng H-Y, Cao X-j, Zou Km. Microwave Pretreatment and Enzymolysis Optimization of the Lotus Seed Protein. Bioengineering. 2019; 6(2):28. https://doi.org/10.3390/bioengineering6020028
Chicago/Turabian StyleGohi, Bi Foua Claude Alain, Jinze Du, Hong-Yan Zeng, Xiao-ju Cao, and Kai min Zou. 2019. "Microwave Pretreatment and Enzymolysis Optimization of the Lotus Seed Protein" Bioengineering 6, no. 2: 28. https://doi.org/10.3390/bioengineering6020028
APA StyleGohi, B. F. C. A., Du, J., Zeng, H.-Y., Cao, X.-j., & Zou, K. m. (2019). Microwave Pretreatment and Enzymolysis Optimization of the Lotus Seed Protein. Bioengineering, 6(2), 28. https://doi.org/10.3390/bioengineering6020028