Regenerative Medicine: A Review of the Evolution of Autologous Chondrocyte Implantation (ACI) Therapy
Abstract
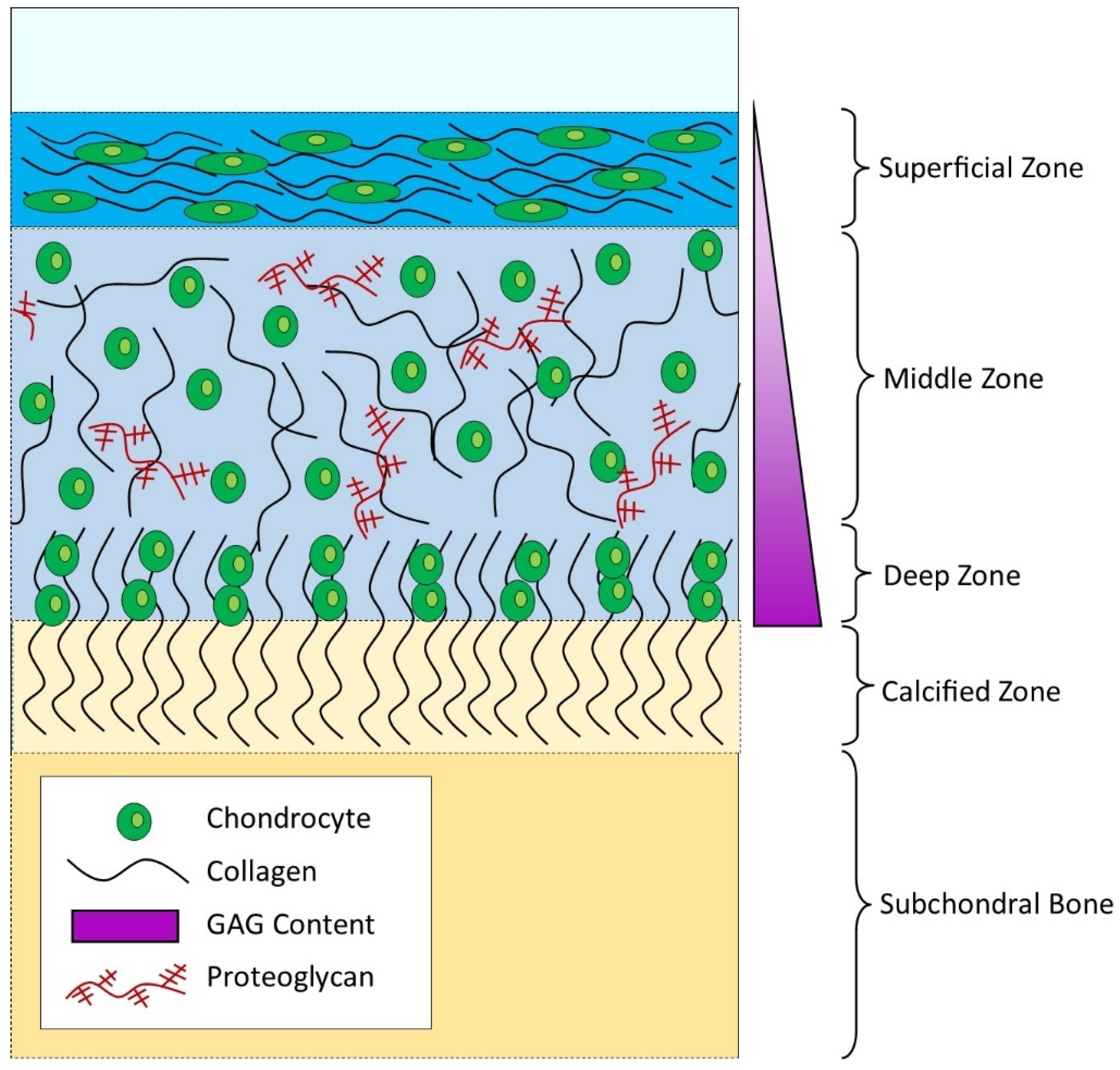
1. The Complexity of Adult Articular Cartilage
2. Articular Cartilage Injury as a Risk Factor for Osteoarthritis
3. The Surgical Strategies for Treating Cartilage Injury
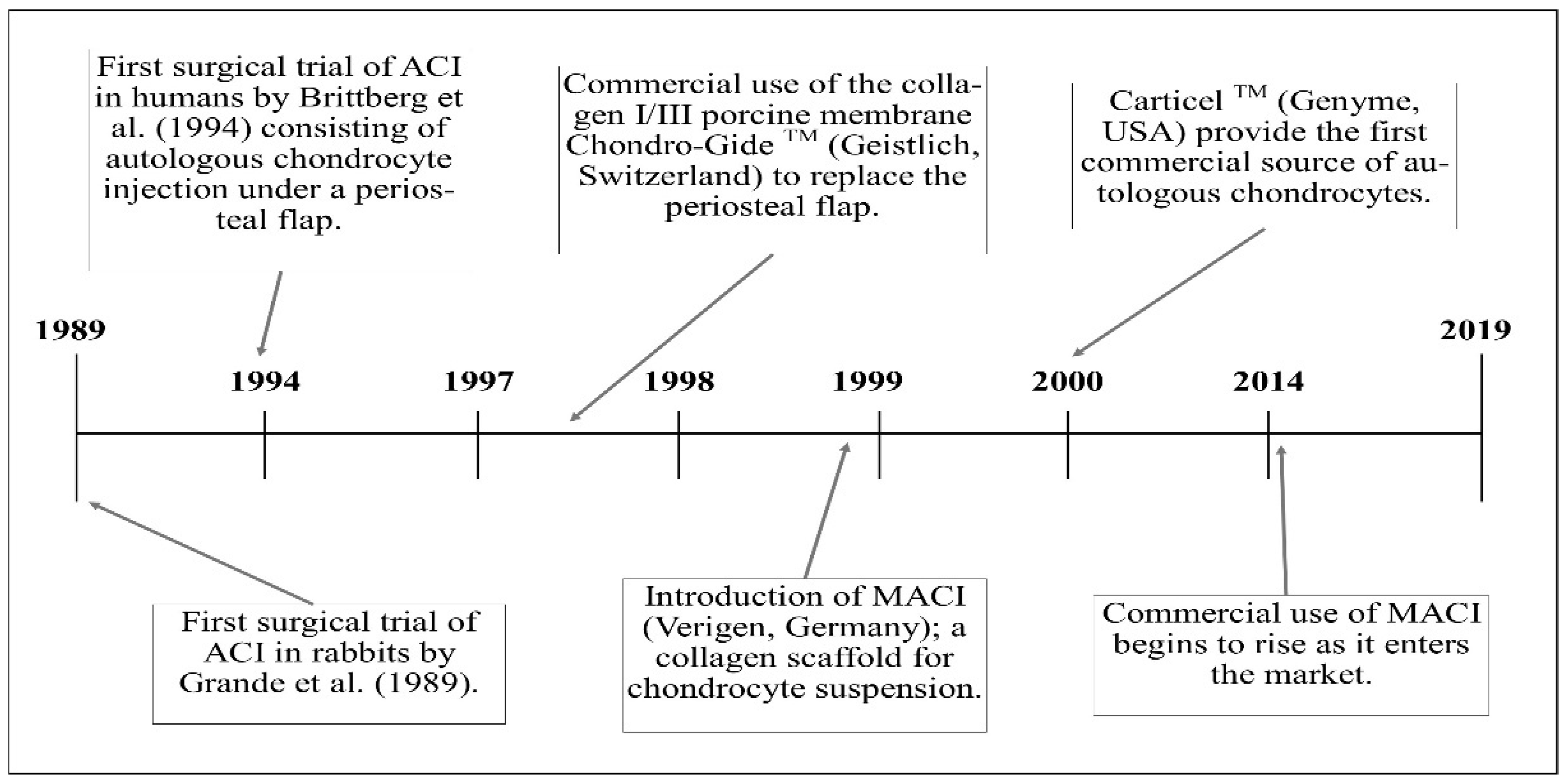
4. The 20-Year Evolution of ACI from Pilot Study to Long-Standing Surgical Technique
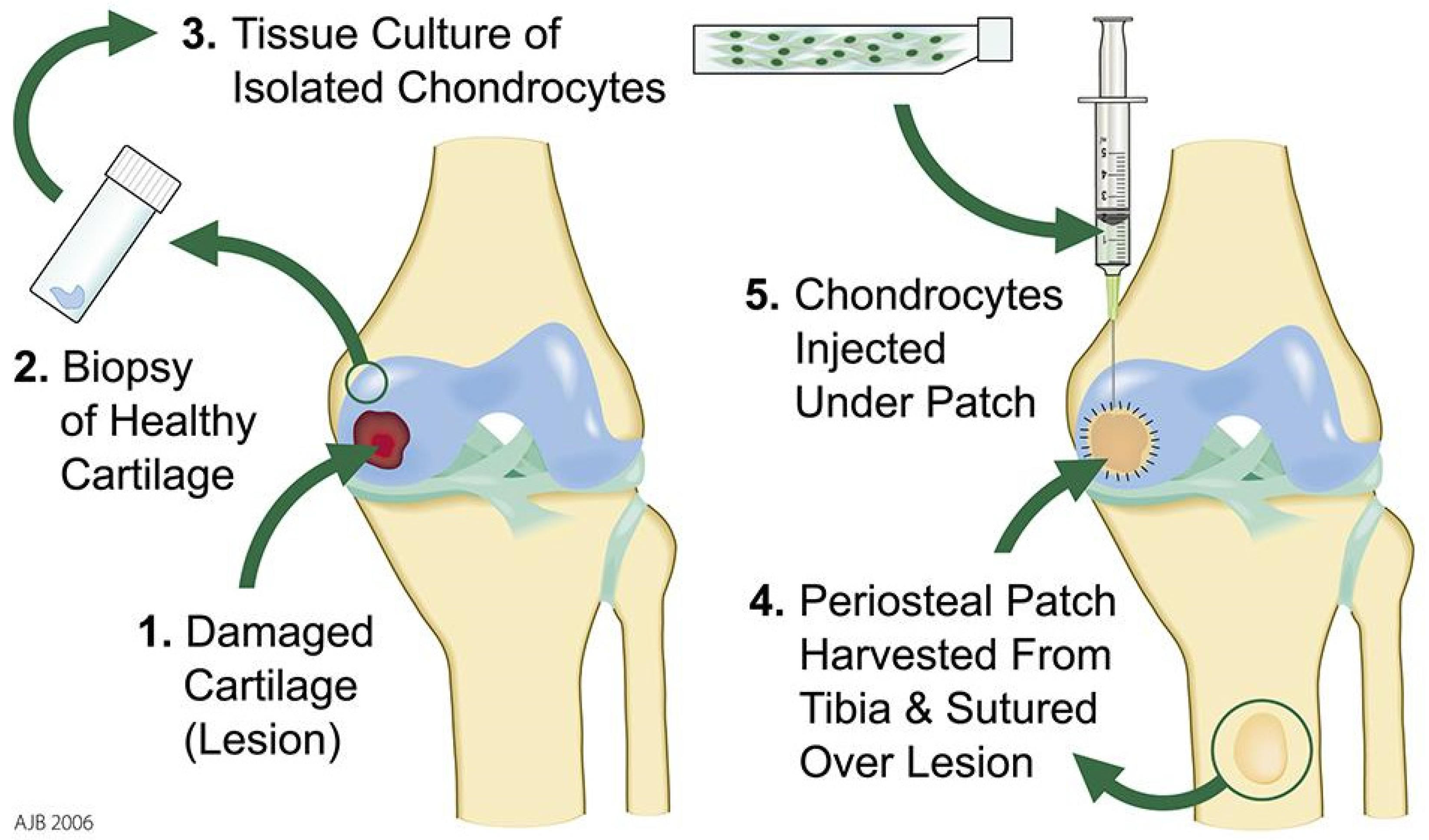
4.1. First Generation ACI
4.2. Second Generation ACI
4.3. Third Generation ACI
5. What Are the Key Limitations of ACI in Its Current Form?
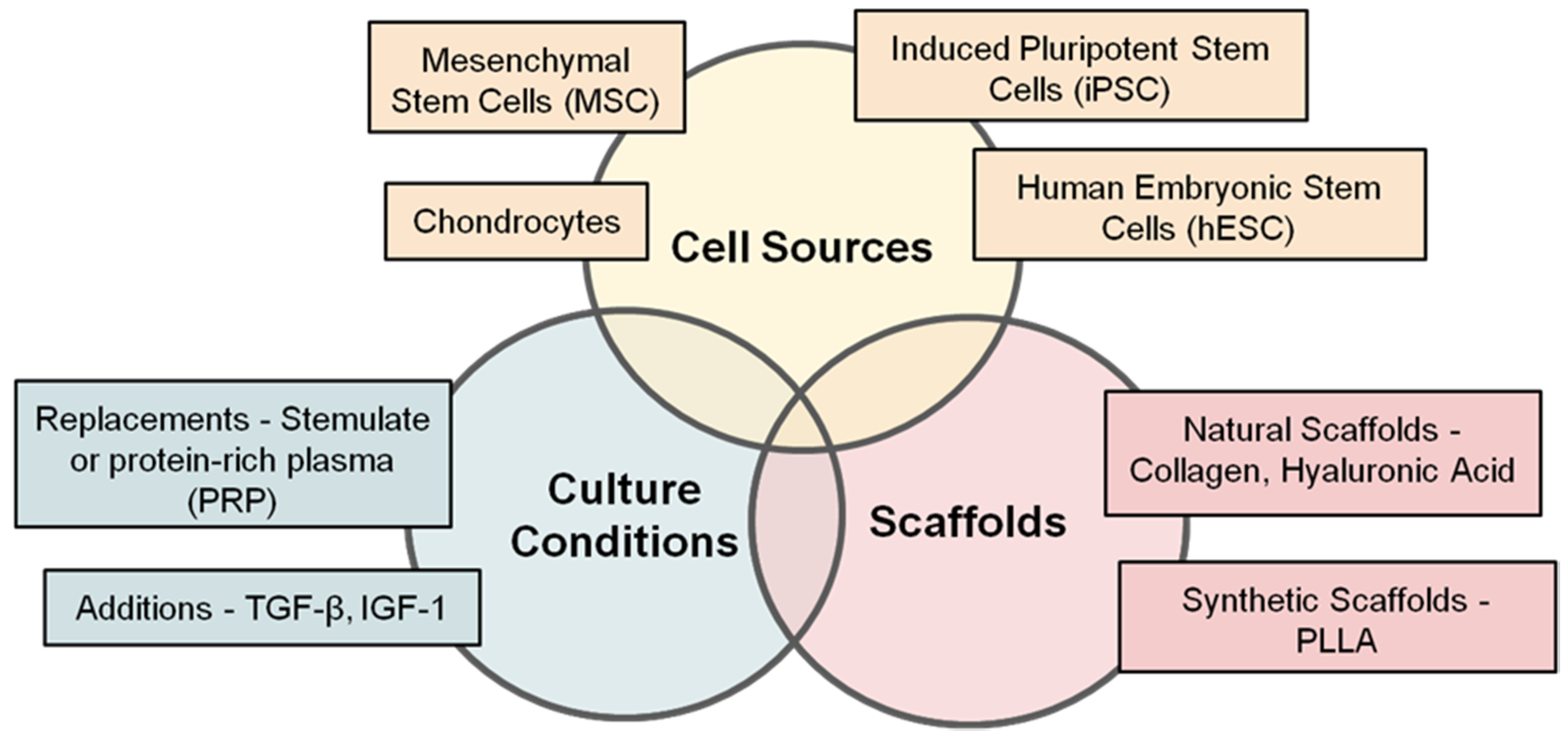
6. Where Are We with Respect to Tissue Sources and Cell Types for Cartilage Cell Therapy?
6.1. Adult Nasal Chondrocytes
6.2. Human Embryonic Stem Cells (hESCs)
6.3. Inducible Pluripotent Stem Cells (iPSCs)
6.4. Bone Marrow-Derived Mesenchymal Stem Cells (BM-MSCs)
6.5. Adipose-Derived Stem Cells (hADSCs)
6.6. Allogenic Chondrocytes
7. Where Are We with Respect to Natural and Synthetic Scaffolds for Cartilage Cell Therapy?
8. Where Are We with Respect to Growth Factors and Supplements for Cartilage Cell Therapy?
9. Conclusions
Author Contributions
Funding
Acknowledgments
Conflicts of Interest
References
- Poole, C.A.; Flint, M.H.; Beaumont, B.W. Chondrons in cartilage: Ultrastructural analysis of the pericellular microenvironment in adult human articular cartilages. J. Orthop. Res. 1987, 5, 509–522. [Google Scholar] [CrossRef] [PubMed]
- Wang, Q.G.; El Haj, A.J.; Kuiper, N.J. Glycosaminoglycans in the pericellular matrix of chondrons and chondrocytes. J. Anat. 2008, 213, 266–273. [Google Scholar] [CrossRef] [PubMed]
- Wang, Q.G.; Nguyen, B.; Thomas, C.R.; Zhang, Z.; El Haj, A.J.; Kuiper, N.J. Molecular profiling of single cells in response to mechanical force: Comparison of chondrocytes, chondrons and encapsulated chondrocytes. Biomaterials 2009, 31, 1619–1625. [Google Scholar] [CrossRef] [PubMed]
- Wilusz, R.E.; Sanchez-Adams, J.; Guilak, F. The structure and function of the pericellular matrix of articular cartilage. Matrix Biol. 2014, 39, 25–32. [Google Scholar] [CrossRef]
- Zhang, Z. Chondrons and the pericellular matrix of chondrocytes. Tissue Eng. Part B Rev. 2014, 21, 267–277. [Google Scholar] [CrossRef] [PubMed]
- Poole, A.R.; Kojima, T.; Yasuda, T.; Mwale, F.; Kobayashi, M.; Laverty, S. Composition and structure of articular cartilage: A template for tissue repair. Clin. Orthop. Relat. Res. 2001, 391, S26–S33. [Google Scholar] [CrossRef]
- Carballo, C.B.; Nakagawa, Y.; Sekiya, I.; Rodeo, S.A. Basic science of articular cartilage. Clin. Sports Med. 2017, 36, 413–425. [Google Scholar] [CrossRef] [PubMed]
- Antons, J.; Marascio, M.G.M.; Nohava, J.; Martin, R.; Applegate, L.A.; Bourban, P.E.; Pioletti, D.P. Zone-dependent mechanical properties of human articular cartilage obtained by indentation measurements. J. Mater. Sci.: Mater. Med. 2018, 29, 57. [Google Scholar] [CrossRef]
- Bayliss, M.T. Proteoglycan structure and metabolism during maturation and ageing of human articular cartilage. Biochem. Soc. Trans. 1990, 18, 799–802. [Google Scholar] [CrossRef] [PubMed]
- Kuiper, N.; Sharma, A. A detailed quantitative outcome measure of glycosaminoglycans in human articular cartilage for cell therapy and tissue engineering strategies. Osteoarthr. Cartil. 2015, 23, 2233–2241. [Google Scholar] [CrossRef]
- Lindahl, A.; Brittberg, M.; Peterson, L. Cartilage repair with chondrocytes: clinical and cellular aspects. In Proceedings of the Novartis Foundation Symposium, Chichester, NY, USA, 22 July 2003; pp. 175–185. [Google Scholar]
- Waters, N.P.; Stoker, A.M.; Carson, W.L.; Pfeiffer, F.M.; Cook, J.L. Biomarkers affected by impact velocity and maximum strain of cartilage during injury. J. Biomech. 2014, 47, 3185–3195. [Google Scholar] [CrossRef] [PubMed]
- Martel-Pelletier, J.; Barr, A.J.; Cicuttini, F.M.; Conaghan, P.G.; Cooper, C.; Goldring, M.B.; Goldring, S.R.; Jones, G.; Teichtahl, A.J.; Pelletier, J.P. Osteoarthritis. Nat. Rev. Dis. Primers. 2016, 2, 16072. [Google Scholar] [CrossRef] [PubMed]
- Jungmann, P.M.; Salzmann, G.M.; Schmal, H.; Pestka, J.M.; Südkamp, N.P.; Niemeyer, P. Autologous chondrocyte implantation for treatment of cartilage defects of the knee: What predicts the need for reintervention? Am. J. Sports Med. 2012, 40, 58–67. [Google Scholar] [CrossRef] [PubMed]
- Solheim, E.; Hegna, J.; Inderhaug, E. Early determinants of long-term clinical outcome after cartilage repair surgery in the knee. J. Orthop. 2018, 15, 222–225. [Google Scholar] [CrossRef] [PubMed]
- Jackson, R.W.; Dieterichs, C. The results of arthroscopic lavage and debridement of osteoarthritic knees based on the severity of degeneration. Arthrosc. J. Arthrosc. Relat. Surg. 2003, 19, 13–20. [Google Scholar] [CrossRef] [PubMed]
- Steadman, J.; Rodkey, W.; Singleton, S.; Briggs, K. Microfracture technique for full-thickness chondral defects: Technique and clinical results. Oper. Tech. Orthop. 1997, 7, 300–304. [Google Scholar] [CrossRef]
- Steadman, J.; Briggs, K.; Rodrigo, J.; Kocher, M.; Gill, T.; Rodkey, W. Outcomes of microfracture for traumatic chondral defects of the knee: Average 11-year follow-up. Arthrosc. J. Arthrosc. Relat. Surg. 2003, 19, 477–484. [Google Scholar] [CrossRef]
- Gross, A.E.; Shasha, N.; Aubin, P. Long-term followup of the use of fresh osteochondral allografts for posttraumatic knee defects. Clin. Orthop. Relat. Res. 2005, 435, 79–87. [Google Scholar] [CrossRef]
- Hangody, L.; Vásárhelyi, G.; Hangody, L.R.; Sükösd, Z.; Tibay, G.; Bartha, L.; Bodó, G. Autologous osteochondral grafting—technique and long-term results. Injury 2008, 39, 32–39. [Google Scholar] [CrossRef] [PubMed]
- Brinkman, J.M.; Lobenhoffer, P.; Agneskirchner, J.D.; Staubli, A.E.; Wymenga, A.B.; Van Heerwaarden, R.J. Osteotomies around the knee: patient selection, stability of fixation and bone healing in high tibial osteotomies. J. Bone Jt. Surg. 2008, 90, 1548–1557. [Google Scholar] [CrossRef]
- Coventry, M.B.; Ilstrup, D.M.; Wallrichs, S.L. Proximal tibial osteotomy. A critical long-term study of eighty-seven cases. J. Bone Jt. Surg. 1993, 75, 196–201. [Google Scholar] [CrossRef]
- Brittberg, M.; Lindahl, A.; Nilsson, A.; Ohlsson, C.; Isaksson, O.; Peterson, L. Treatment of deep cartilage defects in the knee with autologous chondrocyte transplantation. New Engl. J. Med. 1994, 331, 889–895. [Google Scholar] [CrossRef]
- Minas, T. A Primer in Cartilage Repair and Joint Preservation of the Knee, 1st ed.; Elsevier Health Sciences: Philadelphia, PA, USA, 2011; ISBN 978-1-4160-6654-5. [Google Scholar]
- Gille, J.; Behrens, P.; Schulz, A.; Oheim, R.; Kienast, B. Matrix-associated autologous chondrocyte implantation: A clinical follow-up at 15 years. Cartilage 2016, 7, 309–315. [Google Scholar] [CrossRef]
- Grande, D.; Pitman, M.; Peterson, L.; Menche, D.; Klein, M. The repair of experimentally produced defects in rabbit articular cartilage by autologous chondrocyte transplantation. J. Orthop. Res. 1989, 7, 208–218. [Google Scholar] [CrossRef] [PubMed]
- Haddo, O.; Mahroof, S.; Higgs, D.; David, L.; Pringle, J.; Bayliss, M.; Cannon, S.; Briggs, T. The use of chondrogide membrane in autologous chondrocyte implantation. The Knee 2004, 11, 51–55. [Google Scholar] [CrossRef]
- Wood, J.J.; Malek, M.A.; Frassica, F.J.; Polder, J.A.; Mohan, A.K.; Bloom, E.T.; Braun, M.M.; Coté, T.R. Autologous cultured chondrocytes: Adverse events reported to the United States Food and Drug Administration. J. Bone Jt. Surg. 2006, 88, 503–507. [Google Scholar] [CrossRef]
- Brittberg, M. Autologous chondrocyte implantation-technique and long-term follow-up. Injury 2008, 39, 40–49. [Google Scholar] [CrossRef] [PubMed]
- Takahashi, T.; Tins, B.; McCall, I.W.; Richardson, J.B.; Takagi, K.; Ashton, K. MR appearance of autologous chondrocyte implantation in the knee: Correlation with the knee features and clinical outcome. Skelet. Radiol. 2006, 35, 16–26. [Google Scholar] [CrossRef] [PubMed]
- Van Osch, G.J.; Brittberg, M.; Dennis, J.E.; Bastiaansen-Jenniskens, Y.M.; Erben, R.G.; Konttinen, Y.T.; Luyten, F.P. Cartilage repair: past and future–lessons for regenerative medicine. J. Cell. Mol. Med. 2009, 13, 792–810. [Google Scholar] [CrossRef]
- Ogura, T.; Mosier, B.; Bryant, T.; Minas, T. A 20-year follow-up after first-generation autologous chondrocyte implantation. Am. J. Sports Med. 2017, 45, 2751–2761. [Google Scholar] [CrossRef]
- De Bie, C. Genzyme: 15 years of cell and gene therapy research. Regen. Med. 2007, 2, 95–97. [Google Scholar] [CrossRef] [PubMed]
- Roberts, S.; McCall, I.W.; Darby, A.J.; Menage, J.; Evans, H.; Harrison, P.E.; Richardson, J.B. Autologous chondrocyte implantation for cartilage repair: monitoring its success by magnetic resonance imaging and histology. Arthritis Res. Ther. 2002, 5, R60–73. [Google Scholar] [CrossRef] [PubMed]
- McCarthy, H.S.; McCall, I.W.; Williams, J.M.; Mennan, C.; Dugard, M.N.; Richardson, J.B.; Roberts, S. Magnetic resonance imaging parameters at 1-year correlate with clinical outcomes up to 17 years after autologous chondrocyte implantation. Orthop. J. Sports Med. 2018, 6, 2325967118788280. [Google Scholar] [CrossRef] [PubMed]
- Roberts, S.; Menage, J.; Sandell, L.J.; Evans, E.H.; Richardson, J.B. Immunohistochemical study of collagen types I and II and procollagen IIA in human cartilage repair tissue following autologous chondrocyte implantation. The Knee 2009, 16, 398–404. [Google Scholar] [CrossRef] [PubMed]
- Sharma, A.; Wood, L.D.; Richardson, J.B.; Roberts, S.; Kuiper, N.J. Glycosaminoglycan profiles of repair tissue formed following autologous chondrocyte implantation differ from control cartilage. Arthritis Res. Ther. 2007, 9, R79. [Google Scholar] [CrossRef] [PubMed]
- Sharma, A.; Rees, D.; Roberts, S.; Kuiper, N.J. A case study: Glycosaminoglycan profiles of autologous chondrocyte implantation (ACI) tissue improve as the tissue matures. The Knee 2017, 24, 149–157. [Google Scholar] [CrossRef] [PubMed]
- Niemeyer, P.; Pestka, J.M.; Kreuz, P.C.; Erggelet, C.; Schmal, H.; Suedkamp, N.P.; Steinwachs, M. Characteristic complications after autologous chondrocyte implantation for cartilage defects of the knee joint. Am. J. Sports Med. 2008, 36, 2091–2099. [Google Scholar] [CrossRef]
- McCarthy, H.S.; Roberts, S. A histological comparison of the repair tissue formed when using either Chondrogide® or periosteum during autologous chondrocyte implantation. Osteoarthr. Cartil. 2013, 21, 2048–2057. [Google Scholar] [CrossRef]
- Niemeyer, P.; Porichis, S.; Steinwachs, M.; Erggelet, C.; Kreuz, P.; Schmal, H.; Uhl, M.; Ghanem, N.; Südkamp, N.; Salzmann, G. Long-term outcomes after first-generation autologous chondrocyte implantation for cartilage defects of the knee. Am. J. Sports Med. 2014, 42, 150–157. [Google Scholar] [CrossRef] [PubMed]
- Bartlett, W.; Gooding, C.; J Carrington, R.; Skinner, J.; R Briggs, T.; Bentley, G. Autologous chondrocyte implantation at the knee using a bilayer collagen membrane with bone graft: a preliminary report. J. Bone Jt. Surg. 2005, 87, 330–332. [Google Scholar] [CrossRef]
- Basad, E.; Wissing, F.; Fehrenbach, P.; Rickert, M.; Steinmeyer, J.; Ishaque, B. Matrix-induced autologous chondrocyte implantation (MACI) in the knee: Clinical outcomes and challenges. Knee Surg. Sports Traumatol. Arthrosc. 2015, 23, 3729–3735. [Google Scholar] [CrossRef]
- Filardo, G.; Kon, E.; Andriolo, L.; Di Matteo, B.; Balboni, F.; Marcacci, M. Clinical profiling in cartilage regeneration: Prognostic factors for midterm results of matrix-assisted autologous chondrocyte transplantation. Am. J. Sports Med. 2014, 42, 898–905. [Google Scholar] [CrossRef] [PubMed]
- Benya, P.D.; Shaffer, J.D. Dedifferentiated chondrocytes re-express the differentiated collagen phenotype when cultured in agarose gels. Cell. 1982, 30, 215–224. [Google Scholar] [CrossRef]
- Larson, C.M.; Kelley, S.S.; Blackwood, A.D.; Banes, A.J.; Lee, G.M. Retention of the native chondrocyte pericellular matrix results in significantly improved matrix production. Matrix Biol. 2002, 2, 349–359. [Google Scholar] [CrossRef]
- Vonk, L.; de Windt, T.; Kragten, A.; Beekhuizen, M.; Mastbergen, S.; Dhert, W.; Lafeber, F.; Creemers, L.; Saris, D.B. Enhanced cell-induced articular cartilage regeneration by chondrons; the influence of joint damage and harvest site. Osteoarthr. Cartil. 2014, 22, 1910–1917. [Google Scholar] [CrossRef] [PubMed]
- Becher, C.; Laute, V.; Fickert, S.; Zinser, W.; Niemeyer, P.; John, T.; Diehl, P.; Kolombe, T.; Siebold, R.; Fay, J. Safety of three different product doses in autologous chondrocyte implantation: Results of a prospective, randomised, controlled trial. J. Orthop. Surg. Res. 2017, 12, 71. [Google Scholar] [CrossRef] [PubMed]
- Kafienah, W.; Jakob, M.; Démarteau, O.; Frazer, A.; Barker, M.; Martin, I.; Hollander, A. Three-dimensional tissue engineering of hyaline cartilage: comparison of adult nasal and articular chondrocytes. Tissue Eng. 2002, 8, 817–826. [Google Scholar] [CrossRef]
- Shafiee, A.; Kabiri, M.; Langroudi, L.; Soleimani, M.; Ai, J. Evaluation and comparison of the in vitro characteristics and chondrogenic capacity of four adult stem/progenitor cells for cartilage cell-based repair. J. Biomed. Mater. Res. Part A 2016, 104, 600–610. [Google Scholar] [CrossRef]
- Mumme, M.; Barbero, A.; Miot, S.; Wixmerten, A.; Feliciano, S.; Wolf, F.; Asnaghi, A.M.; Baumhoer, D.; Bieri, O.; Kretzschmar, M.; Pagenstert, G. Nasal chondrocyte-based engineered autologous cartilage tissue for repair of articular cartilage defects: an observational first-in-human trial. The Lancet 2016, 388, 1985–1994. [Google Scholar] [CrossRef]
- Thomson, J.A.; Itskovitz-Eldor, J.; Shapiro, S.S.; Waknitz, M.A.; Swiergiel, J.J.; Marshall, V.S.; Jones, J.M. Embryonic stem cell lines derived from human blastocysts. Science 1998, 282, 1145–1147. [Google Scholar] [CrossRef] [PubMed]
- Cheng, A.; Kapacee, Z.; Peng, J.; Lu, S.; Lucas, R.J.; Hardingham, T.E.; Kimber, S.J. Cartilage repair using human embryonic stem cell-derived chondroprogenitors. Stem Cells Transl. Med. 2014, 3, 1287–1294. [Google Scholar] [CrossRef] [PubMed]
- Oldershaw, R.A.; Baxter, M.A.; Lowe, E.T.; Bates, N.; Grady, L.M.; Soncin, F.; Brison, D.R.; Hardingham, T.E.; Kimber, S.J. Directed differentiation of human embryonic stem cells toward chondrocytes. Nat Biotechnol. 2010, 28, 1187–1194. [Google Scholar] [CrossRef]
- Olee, T.; Grogan, S.; Lotz, M.; Colwell, C.; D’Lima, D.; Snyder, E. Repair of cartilage defects in arthritic tissue with differentiated human embryonic stem cells. Tissue Eng. Part A 2014, 20, 683–692. [Google Scholar] [CrossRef] [PubMed]
- Cheng, A.; Cain, S.A.; Tian, P.; Baldwin, A.K.; Uppanan, P.; Kielty, C.M.; Kimber, S.J. Recombinant extracellular matrix protein fragments support human embryonic stem cell chondrogenesis. Tissue Eng. Part A 2018, 24, 968–978. [Google Scholar] [CrossRef] [PubMed]
- Takahashi, K.; Yamanaka, S. Induction of pluripotent stem cells from mouse embryonic and adult fibroblast cultures by defined factors. Cell 2006, 126, 663–676. [Google Scholar] [CrossRef] [PubMed]
- Lee, J.; Smeriglio, P.; Chu, C.R.; Bhutani, N. Human iPSC-derived chondrocytes mimic juvenile chondrocyte function for the dual advantage of increased proliferation and resistance to IL-1β. Stem Cell Res. Ther. 2017, 8, 244. [Google Scholar] [CrossRef]
- Stelcer, E.; Kulcenty, K.; Rucinski, M.; Jopek, K.; Richter, M.; Trzeciak, T.; Suchorska, W. Forced differentiation in vitro leads to stress-induced activation of DNA damage response in hiPSC-derived chondrocyte-like cells. PLoS ONE 2018, 13, e0198079. [Google Scholar] [CrossRef]
- Ko, J.; Kim, K.; Park, S.; Im, G. In vitro chondrogenesis and in vivo repair of osteochondral defect with human induced pluripotent stem cells. Biomaterials 2014, 35, 3571–3581. [Google Scholar] [CrossRef]
- Dominici, M.; Le Blanc, K.; Mueller, I.; Slaper-Cortenbach, I.; Marini, F.; Krause, D.; Deans, R.; Keating, A.; Prockop, D.; Horwitz, E. Minimal criteria for defining multipotent mesenchymal stromal cells. The International Society for Cellular Therapy position statement. Cytotherapy 2006, 8, 315–317. [Google Scholar] [CrossRef]
- Jo, C.; Lee, Y.; Shin, W.; Kim, H.; Chai, J.; Jeong, E.; Kim, J.; Shim, H.; Shin, J.; Shin, I.; Ra, J. Intra-articular injection of mesenchymal stem cells for the treatment of osteoarthritis of the knee: A proof-of-concept clinical trial. Stem Cells 2014, 32, 1254–1266. [Google Scholar] [CrossRef]
- Vega, A.; Martín-Ferrero, M.; Del Canto, F.; Alberca, M.; García, V.; Munar, A.; Orozco, L.; Soler, R.; Fuertes, J.; Huguet, M.; Sánchez, A. Treatment of knee osteoarthritis with allogeneic bone marrow mesenchymal stem cells: a randomized controlled trial. Transplantation 2015, 99, 1681–1690. [Google Scholar] [CrossRef] [PubMed]
- Wakitani, S.; Goto, T.; Pineda, S.J.; Young, R.G.; Mansour, J.M.; Caplan, A.I.; Goldberg, V.M. Mesenchymal cell-based repair of large, full-thickness defects of articular cartilage. J. Bone Jt. Surg. Am. 1994, 76, 579–592. [Google Scholar] [CrossRef]
- Wakitani, S.; Okabe, T.; Horibe, S.; Mitsuoka, T.; Saito, M.; Koyama, T. Safety of autologous bone marrow-derived mesenchymal stem cell transplantation for cartilage repair in 41 patients with 45 joints followed for up to 11 years and 5 months. J. Tissue Eng. Regen. Med. 2011, 5, 146–150. [Google Scholar] [CrossRef] [PubMed]
- Nejadnik, H.; Hui, J.H.; Feng Choong, E.P.; Tai, B.C.; Lee, E.H. Autologous bone marrow–derived mesenchymal stem cells versus autologous chondrocyte implantation: an observational cohort study. Am. J. Sports Med. 2010, 38, 1110–1116. [Google Scholar] [CrossRef] [PubMed]
- Akgun, I.; Unlu, M.; Erdal, O.; Ogut, T.; Erturk, M.; Ovali, E.; Kentarci, F.; Caliskan, G.; Akgun, Y. Matrix-induced autologous mesenchymal stem cell implantation versus matrix-induced autologous chondrocyte implantation in the treatment of chondral defects of the knee: A 2-year randomized study. Arch. Orthop. Trauma Surg. 2015, 135, 251–263. [Google Scholar] [CrossRef] [PubMed]
- Amann, E.; Wolff, P.; Breel, E.; van Griensven, M.; Balmayor, E. Hyaluronic acid facilitates chondrogenesis and matrix deposition of human adipose derived mesenchymal stem cells and human chondrocytes co-cultures. Acta Biomater. 2017, 52, 130–140. [Google Scholar] [CrossRef]
- Bekkers, J.E.; Tsuchida, A.I.; van Rijen, M.H.; Vonk, L.A.; Dhertm, W.J.; Creemers, L.B.; Saris, D.B. Single-stage cell-based cartilage regeneration using a combination of chondrons and mesenchymal stromal cells: comparison with microfracture. Am. J. Sports Med. 2013, 41, 2158–2166. [Google Scholar] [CrossRef]
- de Windt, T.; Vonk, L.; Slaper-Cortenbach, I.; van den Broek, M.; Nizak, R.; van Rijen, M.; de Weger, R.; Dhert, W.; Saris, D. Allogeneic mesenchymal stem cells stimulate cartilage regeneration and are safe for single-stage cartilage repair in humans upon mixture with recycled autologous chondrons. Stem Cells 2017, 35, 256–264. [Google Scholar] [CrossRef]
- de Windt, T.S.; Vonk, L.A.; Slaper-Cortenbach, I.C.M.; Nizak, R.; van Rijen, M.H.P.; Saris, D.B.F. Allogeneic MSCs and Recycled Autologous Chondrons Mixed in a One-Stage Cartilage Cell Transplantion: A First-in-Man Trial in 35 Patients. Stem Cells 2017, 35, 1984–1993. [Google Scholar] [CrossRef]
- Lai, J.H.; Kajiyama, G.; Smith, R.L.; Maloney, W.; Yang, F. Stem cells catalyze cartilage formation by neonatal articular chondrocytes in 3D biomimetic hydrogels. Sci. Rep. 2013, 3, 3553. [Google Scholar] [CrossRef]
- Owida, H.; De Las Heras Ruiz, T.; Dhillon, A.; Yang, Y.; Kuiper, N.J. Co-culture of chondrons and mesenchymal stromal cells reduces the loss of collagen VI and improves extracellular matrix production. Histochem. Cell Biol. 2017, 148, 625–638. [Google Scholar] [CrossRef] [PubMed]
- de Windt, T.; Saris, D.; Slaper-Cortenbach, I.; van Rijen, M.; Gawlitta, D.; Creemers, L.; de Weger, R.; Dhert, W.; Vonk, L. Direct cell–cell contact with chondrocytes is a key mechanism in multipotent mesenchymal stromal cell-mediated chondrogenesis. Tissue Eng. Part A 2015, 21, 2536–2547. [Google Scholar] [CrossRef] [PubMed]
- Wu, L.; Leijten, J.C.; Georgi, N.; Post, J.N.; van Blitterswijk, C.A.; Karperien, M. Trophic effects of mesenchymal stem cells increase chondrocyte proliferation and matrix formation. Tissue Eng Part A 2011, 17, 425–436. [Google Scholar] [CrossRef] [PubMed]
- Wu, L.; Prins, H.J.; Helder, M.N.; van Blitterswijk, C.A.; Karperien, M. Trophic effects of mesenchymal stem cells in chondrocyte co-cultures are independent of culture conditions and cell sources. Tissue Eng Part A 2012, 18, 1542–1551. [Google Scholar] [CrossRef] [PubMed]
- Lai, R.C.; Arslan, F.; Lee, M.M.; Sze, N.S.; Choo, A.; Chen, T.S.; Salto-Tellez, M.; Timmers, L.; Lee, C.N.; El Oakley, R.M.; et al. Exosome secreted by MSC reduces myocardial ischemia/reperfusion injury. Stem Cell Res. 2010, 4, 214–222. [Google Scholar] [CrossRef] [PubMed]
- Zhang, S.; Chu, W.C.; Lai, R.C.; Lim, S.K.; Hui, J.H.P.; Toh, W.S. Exosomes derived from human embryonic mesenchymal stem cells promote osteochondral regeneration. Osteoarthr. Cartil. 2016, 24, 2135–2140. [Google Scholar] [CrossRef]
- Zhang, B.; Yin, Y.; Lai, R.C.; Tan, S.S.; Choo, A.B.; Lim, S.K. Mesenchymal stem cells secrete immunologically active exosomes. Stem Cells Dev. 2014, 23, 1233. [Google Scholar] [CrossRef]
- D’Andrea, F.; De Francesco, F.; Ferraro, G.A.; Desiderio, V.; Tirino, V.; De Rosa, A.; Papaccio, G. Large-scale production of human adipose tissue from stem cells: A new tool for regenerative medicine and tissue banking. Tissue Eng. Part C Methods. 2008, 14, 233–242. [Google Scholar] [CrossRef]
- Garcia, J.; Mennan, C.; McCarthy, H.S.; Roberts, S.; Richardson, J.B.; Wright, K.T. Chondrogenic potency analyses of donor-matched chondrocytes and mesenchymal stem cells derived from bone marrow, infrapatellar fat pad, and subcutaneous fat. Stem Cells Int. 2016, 2016, 6969726. [Google Scholar] [CrossRef]
- Onofrillo, C.; Duchi, S.; O’Connell, C.D.; Blanchard, R.; O’Connor, J.; Scott, M.; Wallace, G.G.; Choong, P.F.M.; Di Bella, C. Biofabrication of human articular cartilage: a path towards the development of a clinical treatment. Biofabrication. 2018, 10, 045006. [Google Scholar] [CrossRef]
- Song, Y.; Du, H.; Dai, C.; Zhang, L.; Li, S.; Hunter, D.J.; Lu, L.; Bao, C. Human adipose-derived mesenchymal stem cells for osteoarthritis: a pilot study with long-term follow-up and repeated injections. Regen Med. 2018, 13, 295–307. [Google Scholar] [CrossRef] [PubMed]
- Jones, I.A.; Wilson, M.; Togashi, R.; Han, B.; Mircheff, A.K.; Thomas Vangsness, C. A randomized, controlled study to evaluate the efficacy of intra-articular, autologous adipose tissue injections for the treatment of mild-to-moderate knee osteoarthritis compared to hyaluronic acid: a study protocol. BMC Musculoskelet Disord. 2018, 19, 383. [Google Scholar] [CrossRef] [PubMed]
- Mortazavi, F.; Shafaei, H.; Rad, J.S.; Rushangar, L.; Montaceri, A.; Jamshidi, M. High quality of infant chondrocytes in comparison with adult chondrocytes for cartilage tissue engineering. World J. Plast. Surg. 2017, 6, 183–189. [Google Scholar]
- Yuan, T.; Zhang, L.; Li, K.; Fan, H.; Fan, Y.; Liang, J.; Zhang, X. Collagen hydrogel as an immunomodulatory scaffold in cartilage tissue engineering. J. Biomed. Mater. Res. Part B Appl. Biomater. 2014, 102, 337–344. [Google Scholar] [CrossRef] [PubMed]
- Zak, L.; Albrecht, C.; Wondrasch, B.; Widhalm, H.; Vekszler, G.; Trattnig, S.; Marlovits, S.; Aldrian, S. Results 2 years after matrix-associated autologous chondrocyte transplantation using the Novocart 3D scaffold: An analysis of clinical and radiological data. Am. J. Sports Med. 2014, 42, 1618–1627. [Google Scholar] [CrossRef] [PubMed]
- Niethammer, T.; Pietschmann, M.; Horng, A.; Roßbach, B.; Ficklscherer, A.; Jansson, V.; Müller, P. Graft hypertrophy of matrix-based autologous chondrocyte implantation: a two-year follow-up study of NOVOCART 3D implantation in the knee. Knee Surg. Sports Traumatol. Arthrosc. 2014, 22, 1329–1336. [Google Scholar] [CrossRef]
- Aigner, J.; Tegeler, J.; Hutzler, P.; Campoccia, D.; Pavesio, A.; Hammer, C.; Kastenbauer, E.; Naumann, A. Cartilage tissue engineering with novel nonwoven structured biomaterial based on hyaluronic acid benzyl ester. J. Biomed. Mater. Res. 1998, 42, 172–181. [Google Scholar] [CrossRef]
- Zhu, M.; Feng, Q.; Sun, Y.; Li, G.; Bian, L. Effect of cartilaginous matrix components on the chondrogenesis and hypertrophy of mesenchymal stem cells in hyaluronic acid hydrogels. J. Biomed. Mater. Res. Part B Appl. Biomater. 2017, 105, 2292–2300. [Google Scholar] [CrossRef] [PubMed]
- Bauer, C.; Berger, M.; Baumgartner, R.R.; Höller, S.; Zwickl, H.; Niculescu-Morzsa, E.; Halbwirth, F.; Nehrer, S. A novel cross-linked hyaluronic acid porous scaffold for cartilage repair: an in vitro study with osteoarthritic chondrocytes. Cartilage 2016, 7, 265–273. [Google Scholar] [CrossRef]
- Shui, W.; Yin, L.; Luo, J.; Li, R.; Zhang, W.; Zhang, J.; Huang, W.; Hu, N.; Liang, X.; Deng, Z.; et al. Characterization of chondrocyte scaffold carriers for cell-based gene therapy in articular cartilage repair. J. Biomed. Mater. Res. Part A 2013, 101, 3542–3550. [Google Scholar] [CrossRef] [PubMed]
- Li, Z.; Liu, P.; Yang, T.; Sun, Y.; You, Q.; Li, J.; Wang, Z.; Han, B. Composite poly(l -lactic-acid)/silk fibroin scaffold prepared by electrospinning promotes chondrogenesis for cartilage tissue engineering. J. Biomed. Appl. 2016, 30, 1552–1565. [Google Scholar] [CrossRef] [PubMed]
- Conoscenti, G.; Schneider, T.; Stoelzel, K.; Pavia, F.; Brucato, V.; Goegele, C.; La Carrubba, V.; Schulze-Tanzil, G. PLLA scaffolds produced by thermally induced phase separation (TIPS) allow human chondrocyte growth and extracellular matrix formation dependent on pore size. Mater. Sci. Eng. C 2017, 80, 449–459. [Google Scholar] [CrossRef] [PubMed]
- Zhang, X.; Wu, Y.; Pan, Z.; Sun, H.; Wang, J.; Yu, D.; Zhu, S.; Dai, J.; Chen, Y.; Tian, N.; Heng, B. The effects of lactate and acid on articular chondrocytes function: Implications for polymeric cartilage scaffold design. Acta Biomater. 2016, 42, 329–340. [Google Scholar] [CrossRef] [PubMed]
- Chu, J.; Zeng, S.; Gao, L.; Groth, T.; Li, Z.; Kong, J.; Zhao, M.; Li, L. Poly (l-lactic acid) porous scaffold-supported alginate hydrogel with improved mechanical properties and biocompatibility. Int. J. Artif. Organs 2016, 39, 435–443. [Google Scholar] [CrossRef] [PubMed]
- Sykes, J.; Kuiper, J.; Richardson, J.; Roberts, S.; Wright, K.; Kuiper, N. Impact of human platelet lysate on the expansion and chondrogenic capacity of cultured human chondrocytes for cartilage cell therapy. Osteoarthr. Cartil. 2018, 26, S103. [Google Scholar] [CrossRef]
- Jeyakumar, V.; Niculescu-Morzsa, E.; Bauer, C.; Lacza, Z.; Nehrer, S. Platelet-rich plasma supports proliferation and redifferentiation of chondrocytes during in vitro expansion. Front. Bioeng. Biotechnol. 2017, 5, 75. [Google Scholar] [CrossRef] [PubMed]
- Moussa, M.; Lajeunesse, D.; Hilal, G.; El Atat, O.; Haykal, G.; Serhal, R.; Chalhoub, A.; Khalil, C.; Alaaeddine, N. Platelet rich plasma (PRP) induces chondroprotection via increasing autophagy, anti-inflammatory markers, and decreasing apoptosis in human osteoarthritic cartilage. Exp. Cell Res. 2017, 352, 146–156. [Google Scholar] [CrossRef] [PubMed]
- Francioli, S.E.; Martin, I.; Sie, C.P.; Hagg, R.; Tommasini, R.; Candrian, C.; Heberer, M.; Barbero, A. Growth factors for clinical-scale expansion of human articular chondrocytes: relevance for automated bioreactor systems. Tissue Eng. 2007, 13, 1227–1234. [Google Scholar] [CrossRef] [PubMed]
- Patel, J.M.; Saleh, K.S.; Burdick, J.A.; Mauck, R.L. Bioactive factors for cartilage repair and regeneration: improving delivery, retention, and activity. Acta Biomater. 2019. [Google Scholar] [CrossRef]
- Jakobsen, R.; Østrup, E.; Zhang, X.; Mikkelsen, T.; Brinchmann, J. Analysis of the effects of five factors relevant to in vitro chondrogenesis of human mesenchymal stem cells using factorial design and high throughput mRNA-profiling. PLoS ONE 2014, 9, e96615. [Google Scholar] [CrossRef]
- Murphy, M.; Huey, D.; Hu, J.; Athanasiou, K. TGF-β1, GDF-5, and BMP-2 stimulation induces chondrogenesis in expanded human articular chondrocytes and marrow-derived stromal cells. Stem Cells 2015, 33, 762–773. [Google Scholar] [CrossRef]
- Mullen, L.; Best, S.; Ghose, S.; Wardale, J.; Rushton, N.; Cameron, R. Bioactive IGF-1 release from collagen–GAG scaffold to enhance cartilage repair in vitro. J. Mater. Sci. Mater. Med. 2015, 26, 2. [Google Scholar] [CrossRef]
- Frisch, J.; Orth, P.; Rey-Rico, A.; Venkatesan, J.; Schmitt, G.; Madry, H.; Kohn, D.; Cucchiarini, M. Peripheral blood aspirates overexpressing IGF-I via rAAV gene transfer undergo enhanced chondrogenic differentiation processes. J. Cell. Mol. Med. 2017, 21, 2748–2758. [Google Scholar] [CrossRef]
- Ortved, K.; Begum, L.; Mohammed, H.; Nixon, A. Implantation of rAAV5-IGF-I transduced autologous chondrocytes improves cartilage repair in full-thickness defects in the equine model. Mol. Ther. 2015, 23, 363–373. [Google Scholar] [CrossRef] [PubMed]
- Griffin, D.; Ortved, K.; Nixon, A.; Bonassar, L. Mechanical properties and structure-function relationships in articular cartilage repaired using IGF-I gene-enhanced chondrocytes. J. Orthop. Res. 2016, 34, 149–153. [Google Scholar] [CrossRef] [PubMed]
- Rothdiener, M.; Uynuk-Ool, T.; Südkamp, N.; Aurich, M.; Grodzinsky, A.J.; Kurz, B.; Rolauffs, B. Human osteoarthritic chondrons outnumber patient-and joint-matched chondrocytes in hydrogel culture—Future application in autologous cell-based OA cartilage repair? J. Tissue Eng. Regen. Med. 2018, 12, e1206–e1220. [Google Scholar] [CrossRef] [PubMed]
- Jinek, M.; Chylinski, K.; Fonfara, I.; Hauer, M.; Doudna, J.A.; Charpentier, E. A programmable dual-RNA–guided DNA endonuclease in adaptive bacterial immunity. Science 2012, 337, 816–821. [Google Scholar] [CrossRef] [PubMed]
- Seidl, C.; Fulga, T.; Murphy, C. CRISPR-Cas9 targeting of MMP13 in human chondrocytes leads to significantly reduced levels of the metalloproteinase and enhanced type II collagen accumulation. Osteoarthr. Cartil. 2018, 27, 140–147. [Google Scholar] [CrossRef] [PubMed]
- Kang, X.; Caparas, C.; Soh, B.; Fan, Y. Addressing challenges in the clinical applications associated with CRISPR/Cas9 technology and ethical questions to prevent its misuse. Protein and Cell 2017, 8, 791–795. [Google Scholar] [CrossRef]
| Treatment | Procedure | Pros | Cons | References |
|---|---|---|---|---|
| Lavage | Washing of the affected joint to clear away cartilage tissue and debris. | Minimally invasive arthroscopic approach, immediate weight bearing. | Only beneficial for acute/early OA, does not encourage repair or regeneration. | [16] |
| Microfracture | Drilling at the injury site to encourage cell migration to undertake natural repair mechanisms. | Minimally invasive arthroscopic approach, no need to harvest patient tissue. | Only used for lesions <2.5 cm2, encourages formation of inferior fibrocartilage, weight bearing limited for six to eight weeks. | [17,18] |
| Osteochondral allograft transfer | Transfer of a cartilage allograft (sourced from a cadaver or tissue bank) into the patient at the site of injury. | No risk of donor site morbidity and repairs large lesions. | Allogenic tissue (problems with graft availability, cell viability, disease, and immune responses), requires arthrotomy procedure, weight bearing limited for eight weeks. | [19] |
| Osteochondral autograft transfer (mosaicplasty) | Transfer of a cartilage ‘plug’ from a lower bearing area to the site of injury of the same patient. | Arthroscopic or small arthrotomy approach, aims to produce native hyaline cartilage. | Requires harvesting of healthy cartilage tissue from alternative joint, cannot treat large lesions, problems with tissue integration. | [20] |
| Osteotomy | Surgical reshaping of the affected joint to remove pressure from the area of cartilage injury. | Delays the need for joint replacement, allows a return to high-impact activity. | Invasive procedure, weight bearing limited for six weeks. | [21,22] |
© 2019 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (http://creativecommons.org/licenses/by/4.0/).
Share and Cite
Davies, R.L.; Kuiper, N.J. Regenerative Medicine: A Review of the Evolution of Autologous Chondrocyte Implantation (ACI) Therapy. Bioengineering 2019, 6, 22. https://doi.org/10.3390/bioengineering6010022
Davies RL, Kuiper NJ. Regenerative Medicine: A Review of the Evolution of Autologous Chondrocyte Implantation (ACI) Therapy. Bioengineering. 2019; 6(1):22. https://doi.org/10.3390/bioengineering6010022
Chicago/Turabian StyleDavies, Rebecca L, and Nicola J Kuiper. 2019. "Regenerative Medicine: A Review of the Evolution of Autologous Chondrocyte Implantation (ACI) Therapy" Bioengineering 6, no. 1: 22. https://doi.org/10.3390/bioengineering6010022
APA StyleDavies, R. L., & Kuiper, N. J. (2019). Regenerative Medicine: A Review of the Evolution of Autologous Chondrocyte Implantation (ACI) Therapy. Bioengineering, 6(1), 22. https://doi.org/10.3390/bioengineering6010022






