A Three-Compartment Pharmacokinetic Model to Predict the Interstitial Concentration of Talaporfin Sodium in the Myocardium for Photodynamic Therapy: A Method Combining Measured Fluorescence and Analysis of the Compartmental Origin of the Fluorescence
Abstract
:1. Introduction
2. Materials and Methods
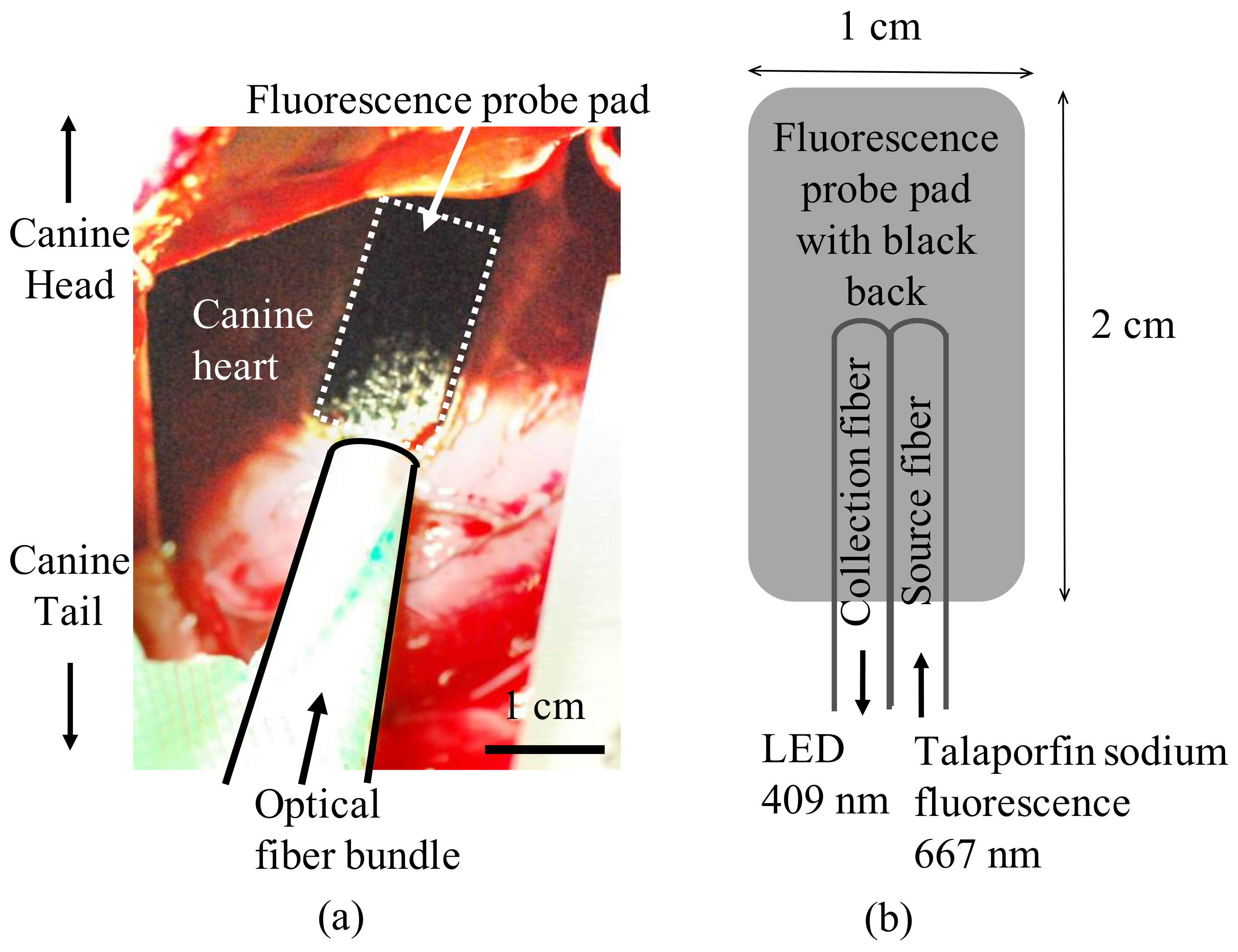
2.1. Measurement of Plasma Concentration and Myocardial Fluorescence of Talaporfin Sodium in Canines
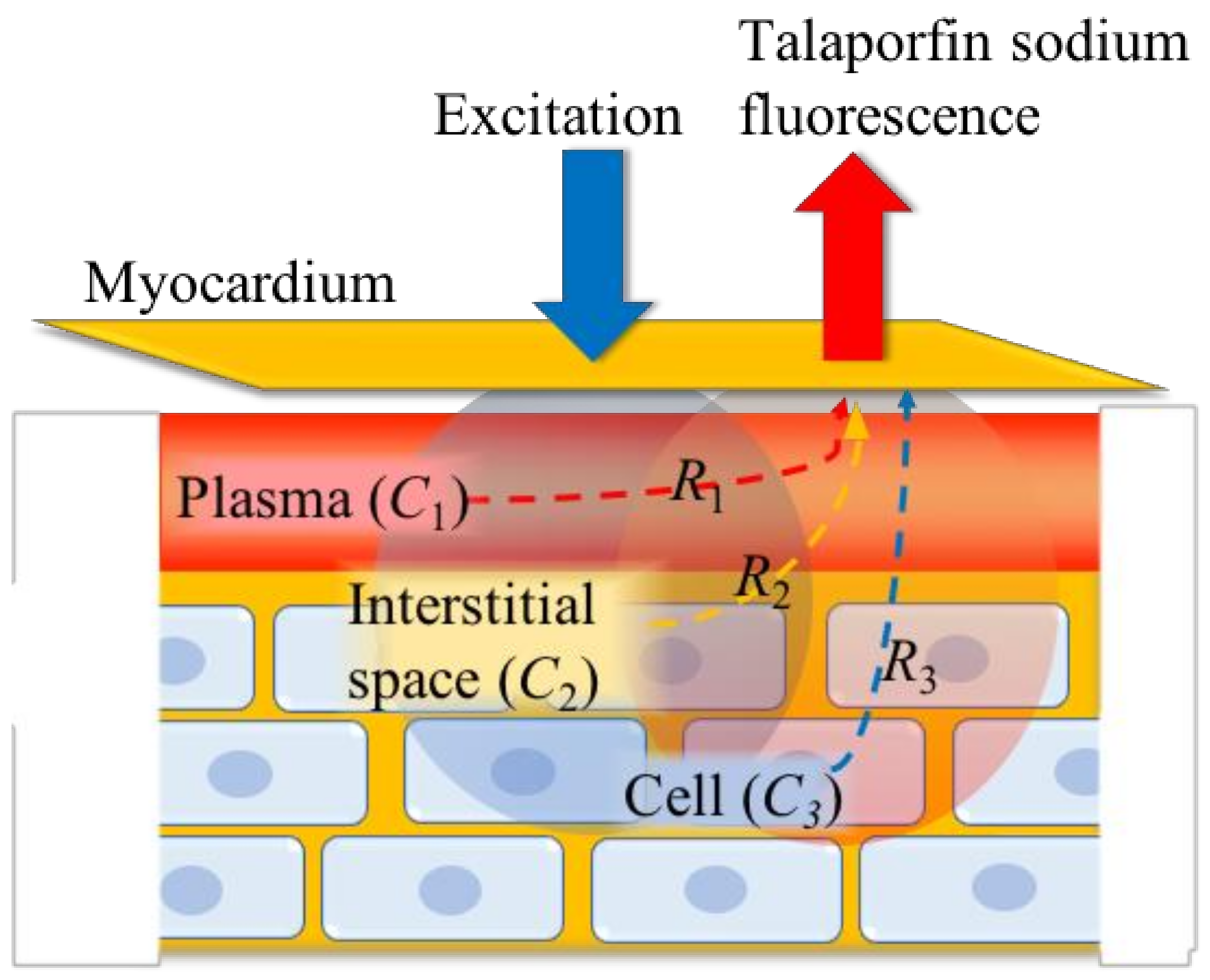
2.2. Determination of the Components of Talaparfin Sodium Myocardial Fluorescence Based on Histological Examinations
2.3. Determination of the Compartment Volumes in the Three-Compartment Model
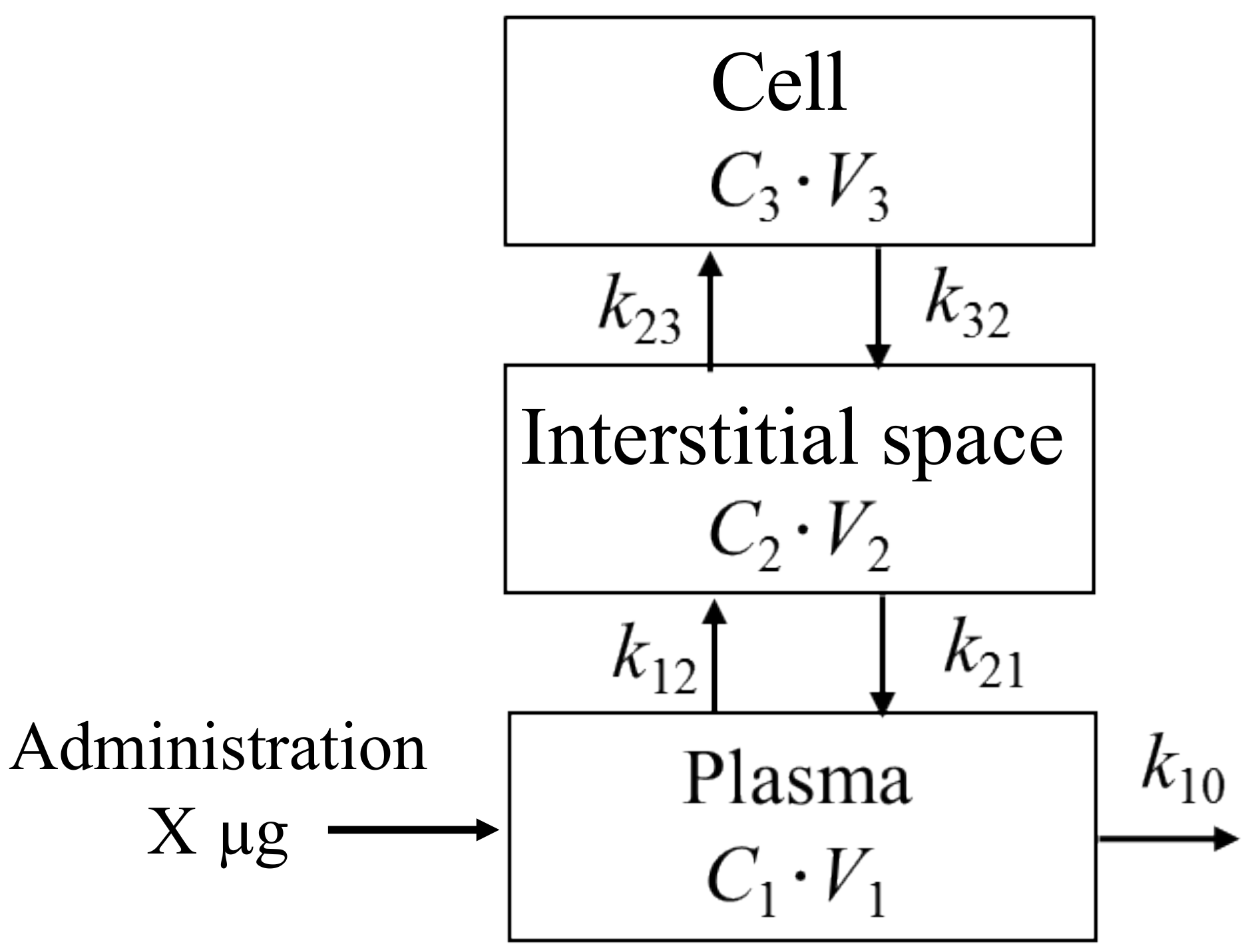
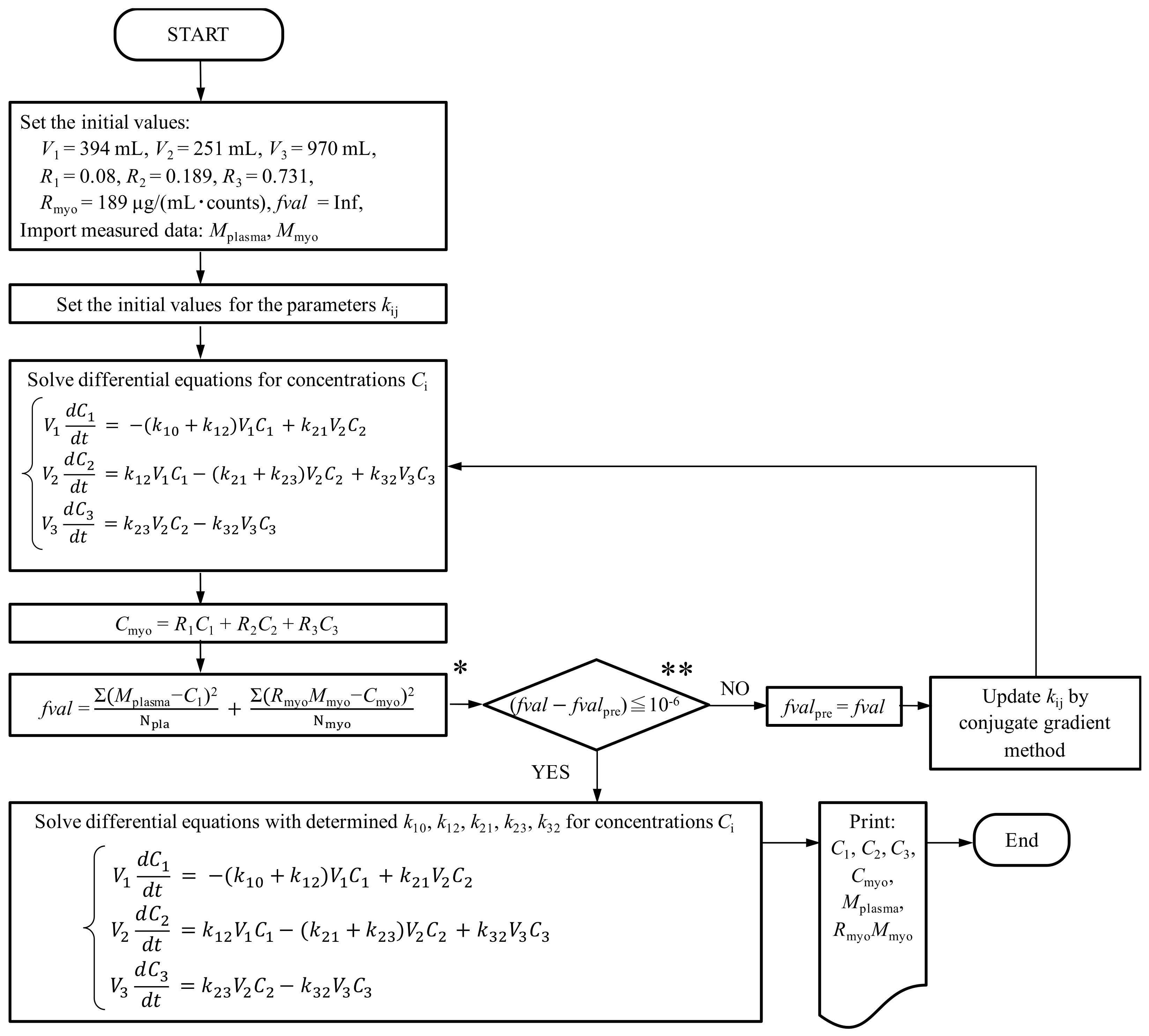
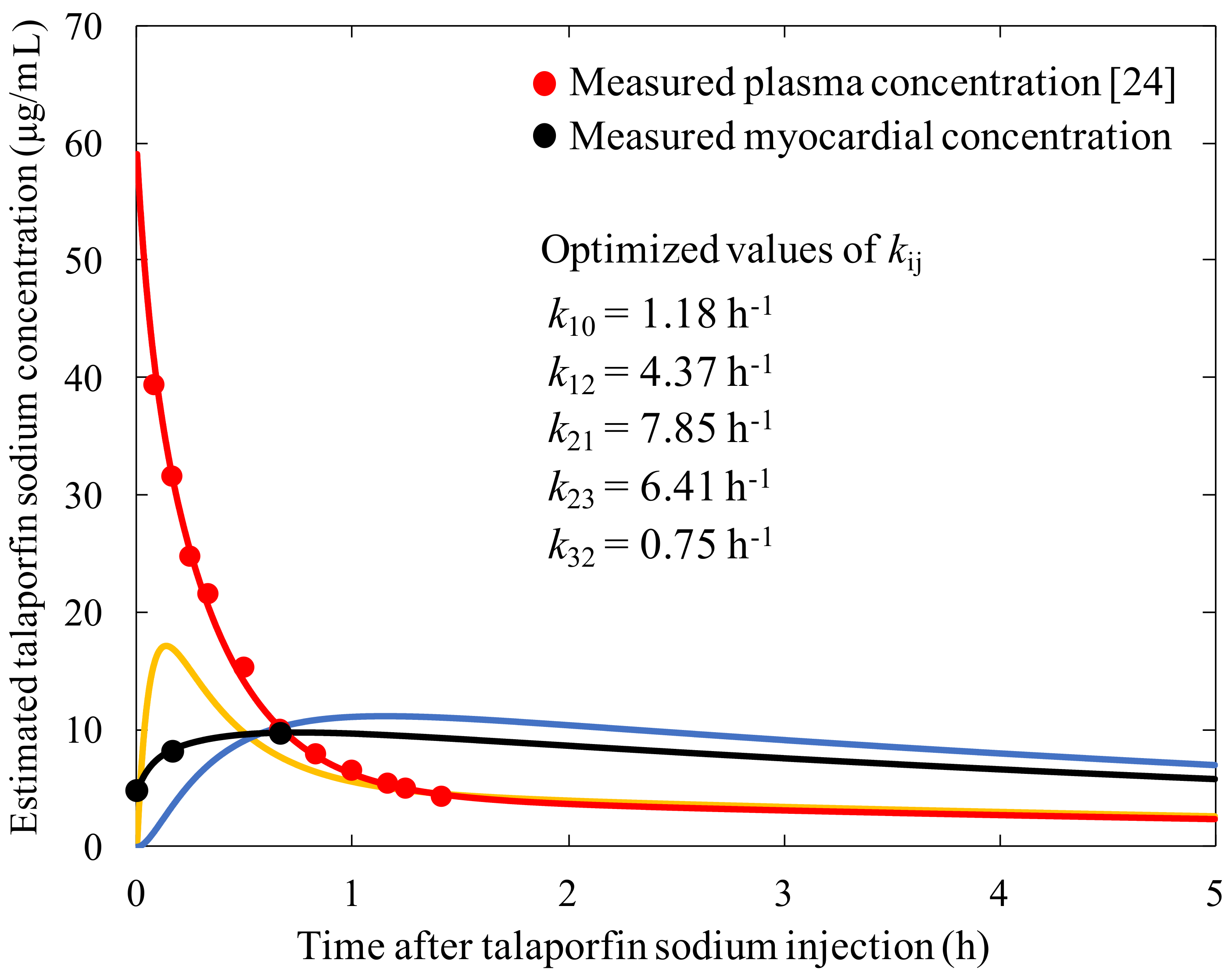
2.4. Three-Compartment Mathematical Modeling of Pharmacokinetics
3. Results
3.1. Determination of Volume Ratios (R1, R2, and R3) and Compartment Volumes (V1, V2, and V3)
3.2. Construction of the Three-Compartment Model Using the Measured Plasma Concentration and Myocardial Fluorescence
4. Discussion
4.1. Methodology of the Constructed Three-Compartment Model for Talaporfin Sodium Using the Myocardial Fluorescence Time History and Volume Ratios Measured from Histological Examinations
4.2. Application of the Three-Compartment Model
4.3. Limitations
5. Conclusions
Author Contributions
Funding
Acknowledgments
Conflicts of Interest
Appendix A: How to Obtain a Conversion Constant (Rmyo)
References
- Ito, A.; Hosokawa, S.; Miyoshi, S.; Soejima, K.; Ogawa, S.; Arai, T. The myocardial electrical blockade induced by photosensitization reaction. IEEE Trans. Biomed. Eng. 2010, 57, 488–495. [Google Scholar] [CrossRef] [PubMed]
- Kimura, T.; Takatsuki, S.; Miyoshi, S.; Fukumoto, K.; Takahashi, M.; Ogawa, E.; Ito, A.; Arai, T.; Ogawa, S.; Fukuda, K. Non-thermal cardiac catheter ablation using photodynamic therapy. Circ. Arrhythm. Electrophysiol. 2013, 6, 1025–1031. [Google Scholar] [CrossRef]
- Kimura, T.; Takatsuki, S.; Miyoshi, S.; Takahashi, M.; Ogawa, E.; Katsumata, Y.; Nishiyama, T.; Nishiyama, N.; Tanimoto, Y.; Aizawa, Y.; et al. Optimal conditions for cardiac catheter ablation using photodynamic therapy. Europace 2015, 17, 1309–1315. [Google Scholar] [CrossRef] [Green Version]
- Kimura, T.; Takatsuki, S.; Miyoshi, S.; Takahashi, M.; Ogawa, E.; Nakajima, K.; Kashimura, S.; Katsumata, Y.; Nishiyama, T.; Nishiyama, N.; et al. Electrical superior vena cava isolation using photodynamic therapy in a canine model. Europace 2016, 18, 294–300. [Google Scholar] [CrossRef]
- Kato, H.; Furukawa, K.; Sato, M.; Okunaka, T.; Kusunoki, Y.; Kawahara, M.; Fukuoka, M.; Miyazawa, T.; Yana, T.; Matsui, K.; et al. Phase II clinical study of photodynamic therapy using mono-L-aspartyl chlorin e6 and diode laser for early superficial squamous cell carcinoma of the lung. Lung Cancer 2003, 42, 103–111. [Google Scholar] [CrossRef]
- Ito, A.; Kimura, T.; Miyoshi, S.; Ogawa, S.; Arai, T. Photosensitization reaction-induced acute electrophysiological cell response of rat myocardial cells in short loading periods of talaporfin sodium or porfimer sodium. Photochem. Photobiol. 2011, 87, 199–207. [Google Scholar] [CrossRef]
- Hamblin, M.R.; Hasan, T. Photodynamic therapy: A new antimicrobial approach to infectious disease? Photochem. Photobiol. Sci. 2004, 3, 436–450. [Google Scholar] [CrossRef] [PubMed]
- Berg, K.; Nordstrand, S.; Selbo, P.K.; Tran, D.T.T.; Petersen, E.A.; Høgset, A. Disulfonated tetraphenyl chlorin (TPCS 2a), a novel photosensitizer developed for clinical utilization of photochemical internalization. Photochem. Photobiol. Sci. 2011, 10, 1637–1651. [Google Scholar] [CrossRef]
- Lee, C.C.; Pogue, B.W.; O’Hara, J.A.; Wilmot, C.M.; Strawbridge, R.R.; Burke, G.C.; Hoopes, P.J. Spatial heterogeneity and temporal kinetics of photosensitizer (AlPcS 2) concentration in murine tumors RIF-1 and MTG-B. Photochem. Photobiol. Sci. 2003, 2, 145–150. [Google Scholar] [CrossRef]
- Frazier, D.L.; Barnhill, M.A.; Vodinh, T.; Legendre, A.M.; Overholt, B.F. Comparative pharmacokinetics of the photosensitizer tin-etiopurpurin in dogs and rats. J. Vet. Pharmacol. Ther. 1992, 15, 275–281. [Google Scholar] [CrossRef] [PubMed]
- Chen, J.C.; Chang, S.M.; Hsu, F.Y.; Wang, H.E.; Liu, R.S. MicroPET-based pharmacokinetic analysis of the radiolabeled boron compound [18F] FBPA-F in rats with F98 glioma. Appl. Radiat. Isot. 2004, 61, 887–891. [Google Scholar] [CrossRef] [PubMed]
- Kondo, K.; Miyoshi, T.; Fujino, H.; Takizewa, H.; Imai, S.; Kobayashi, N.; Kenzaki, K.; Sakiyama, S.; Tangoku, A. Photodynamic therapy using a second generation photosensitizer, Talaporfin. Photodiag. Photodyn. 2007, 4, 269–274. [Google Scholar] [CrossRef]
- Walker, D.K. Pharmacokinetics and metabolism of sildenafil in mouse, rat, rabbit, dog and man. Xenobiotica 1999, 29, 297–310. [Google Scholar] [CrossRef] [PubMed]
- Kosa, T.; Maruyama, T.; Otagiri, M. Species differences of serum albumins: I. Drug binding sites Pharm. Res. 1997, 14, 1607–1612. [Google Scholar] [CrossRef]
- Ogawa, E.; Arai, T. Development of a practical animal model of photodynamic therapy using a high concentration of extracellular talaporfin sodium in interstitial fluid: Influence of albumin animal species on myocardial cell photocytotoxicity in vitro. Lasers Med. Sci. 2017, 32, 1–5. [Google Scholar] [CrossRef]
- Takahashi, M.; Arai, T. Fluorescence sensing system by Soret-band LED light excitation for estimating relative talaporfin sodium concentration in skin. Photodiagn. Photodyn. Ther. 2014, 11, 586–594. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Rivas, F.; Cobb, F.R.; Bache, R.J.; Greenfield, J.C. Relationship between blood flow to ischemic regions and extent of myocardial infarction. Serial measurement of blood flow to ischemic regions in dogs. Circ. Res. 1976, 38, 439–447. [Google Scholar] [CrossRef]
- Gartner, L.P.; Hiatt, J.L. Color Textbook of Histology, 3rd ed.; Elsevier Saunders: Amsterdam, The Netherlands, 2006; p. 149. [Google Scholar]
- Wollert, K.C.; Taga, T.; Saito, M.; Narazaki, M.; Kishimoto, T.; Glembotski, C.C.; Vernallis, A.B.; Heath, J.K.; Pennica, D.; Wood, W.I.; et al. Cardiotrophin-1 activates a distinct form of cardiac muscle cell hypertrophy Assembly of sarcomeric units in series VIA gp130/leukemia inhibitory factor receptor-dependent pathways. J. Biol. Chem. 1996, 271, 9535–9545. [Google Scholar] [CrossRef] [PubMed]
- Ueki, A. Biochemical Data Book I, 1st ed.; Tokyo Kagaku Dojin: Tokyo, Japan, 1979; p. 1636. (In Japanese) [Google Scholar]
- Laine, G.A.; Allen, S.J. Left ventricular myocardial edema. Lymph flow, interstitial fibrosis, and cardiac function. Circ. Res. 1991, 68, 1713–1721. [Google Scholar] [CrossRef] [PubMed]
- Best, C.H.; Taylor, N.B. The Physiological Basis of Medical Practice; A Text in Applied Physiology, 6th ed.; The Williams and Wilkins Company: Baltimore, PA, USA, 1955; p. 604. [Google Scholar]
- Radziuk, J.; Norwich, K.H.; Vranic, M. Experimental validation of measurements of glucose turnover in nonsteady state. Am. Physiol. Soc. 1978, 234, E84. [Google Scholar] [CrossRef]
- Uno, Y.; Ogawa, E.; Arai, T. 3-compartment pharmacokinetic model for estimation of talaporfin sodium concentration in interstitial space. Trans. Jpn. Soc. Med. Biol. Eng. 2017, 55, 550–551. [Google Scholar] [CrossRef]
- Kessel, D. Pharmacokinetics of N-aspartyl chlorin e6 in cancer patients. J. Photochem. Photobiol. B Biol. 1997, 39, 81–83. [Google Scholar] [CrossRef]
- Dougherty, T.J.; Gomer, C.J.; Henderson, B.W.; Jori, G.; Kessel, D.; Korbelik, M.; Moan, J.; Peng, Q. Photodynamic therapy. J. Natl. Cancer Inst. 1998, 90, 889–905. [Google Scholar] [CrossRef]
- Mimura, S.; Narahara, H.; Otani, T.; Okuda, S. Progress of photodynamic therapy in gastric cancer. Diagn. Ther. Endosc. 1999, 5, 175–182. [Google Scholar] [CrossRef]
- Frisoli, J.K.; Tudor, E.G.; Flotte, T.J.; Hasan, T.; Deutsch, T.F.; Schomacker, K.T. Pharmacokinetics of a fluorescent drug using laser-induced fluorescence. Cancer Res. 1993, 53, 5954–5961. [Google Scholar] [PubMed]
© 2018 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (http://creativecommons.org/licenses/by/4.0/).
Share and Cite
Uno, Y.; Ogawa, E.; Aiyoshi, E.; Arai, T. A Three-Compartment Pharmacokinetic Model to Predict the Interstitial Concentration of Talaporfin Sodium in the Myocardium for Photodynamic Therapy: A Method Combining Measured Fluorescence and Analysis of the Compartmental Origin of the Fluorescence. Bioengineering 2019, 6, 1. https://doi.org/10.3390/bioengineering6010001
Uno Y, Ogawa E, Aiyoshi E, Arai T. A Three-Compartment Pharmacokinetic Model to Predict the Interstitial Concentration of Talaporfin Sodium in the Myocardium for Photodynamic Therapy: A Method Combining Measured Fluorescence and Analysis of the Compartmental Origin of the Fluorescence. Bioengineering. 2019; 6(1):1. https://doi.org/10.3390/bioengineering6010001
Chicago/Turabian StyleUno, Yuko, Emiyu Ogawa, Eitaro Aiyoshi, and Tsunenori Arai. 2019. "A Three-Compartment Pharmacokinetic Model to Predict the Interstitial Concentration of Talaporfin Sodium in the Myocardium for Photodynamic Therapy: A Method Combining Measured Fluorescence and Analysis of the Compartmental Origin of the Fluorescence" Bioengineering 6, no. 1: 1. https://doi.org/10.3390/bioengineering6010001



