A Standardized Collagen-Based Scaffold Improves Human Hepatocyte Shipment and Allows Metabolic Studies over 10 Days
Abstract
1. Introduction
2. Materials and Methods
2.1. Scaffold Manufacturing
2.2. Physical Characterization of Scaffolds
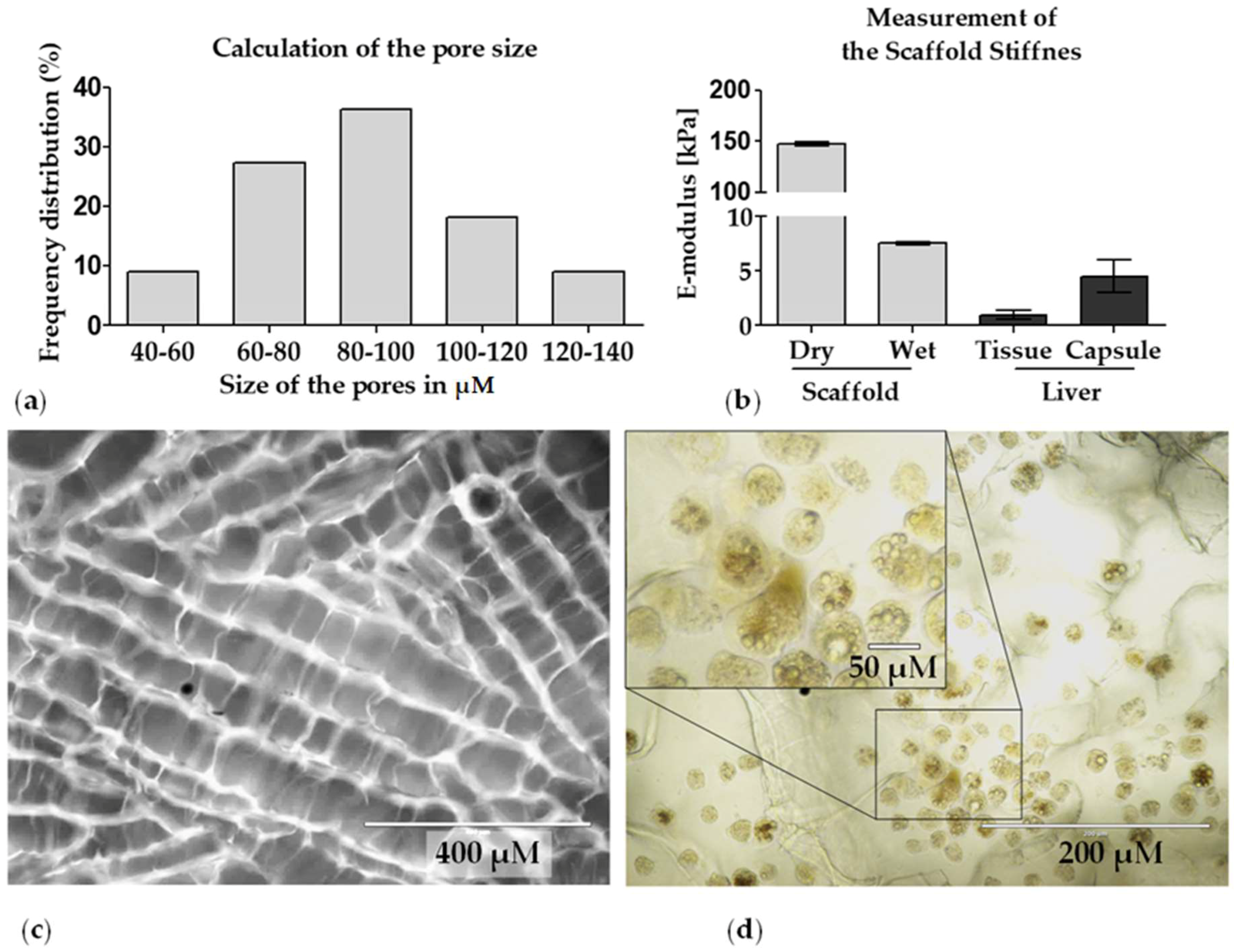
2.2.1. Pore Size
2.2.2. Porosity
2.2.3. Permeability
2.2.4. Water-Uptake and Swelling Ratio
2.2.5. Matrix Stiffness
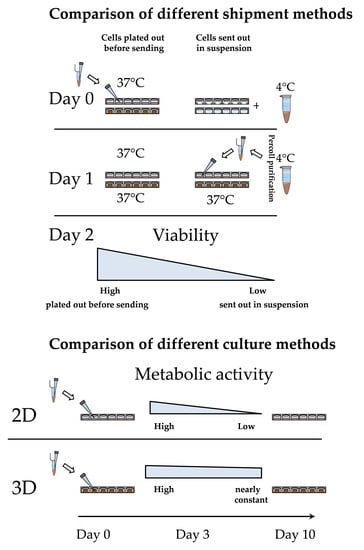
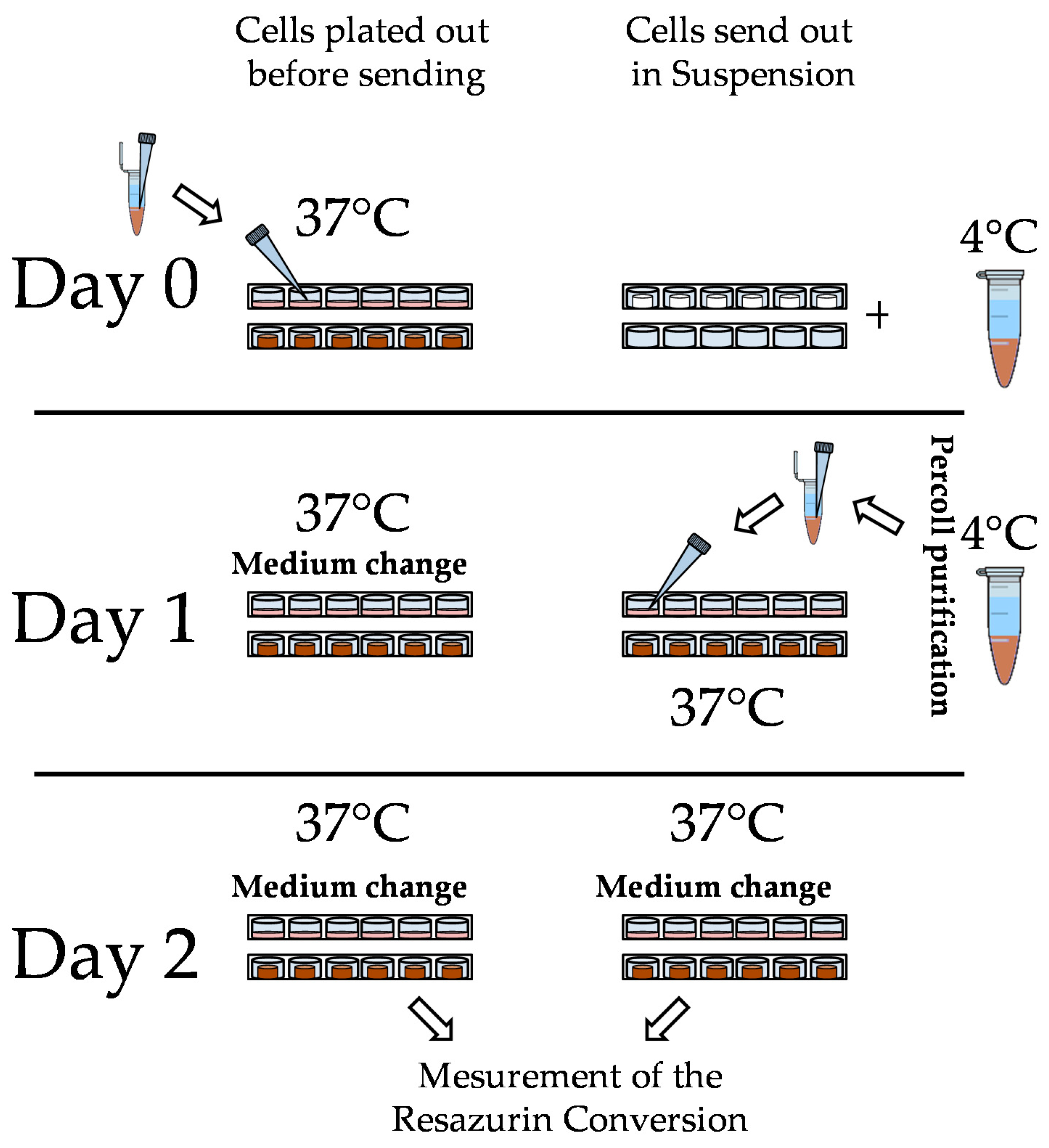
2.3. Hepatocyte Isolation, Shipment, and Culture
2.4. Functional Testing
2.4.1. Urea Measurement
2.4.2. Albumin ELISA
2.4.3. CYP Activity Measurement
2.4.4. Resazurin Conversion
2.4.5. Statistic
3. Results
3.1. Characterization of the Optimaix-3D Collagen Scaffold
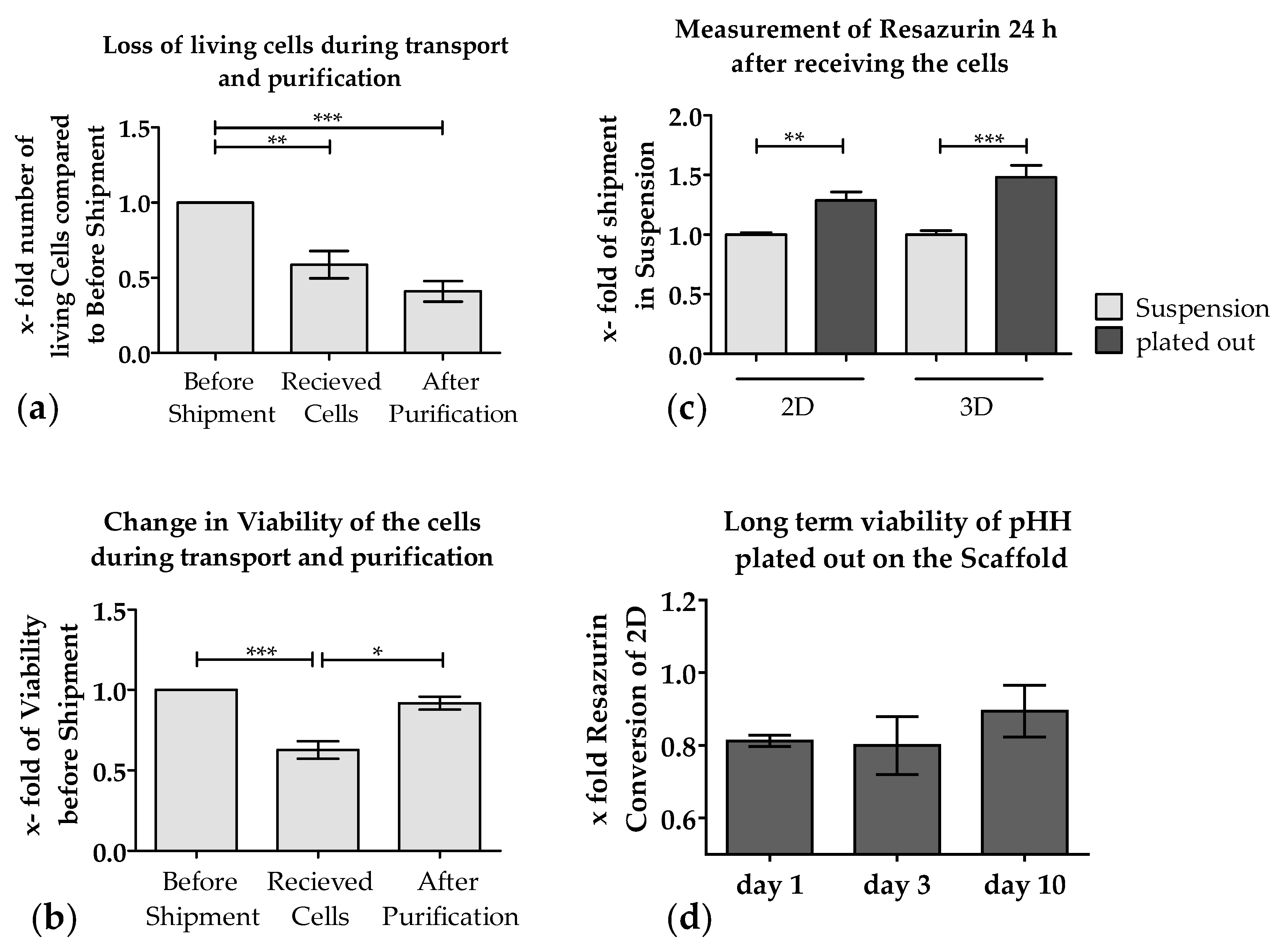
3.2. Loss of PHHs During Transport Is Significantly Reduced When Shipped on Optimaix-3D Scaffold
3.3. Metabolic Function of PHHs on the Optimaix-3D Collagen Scaffold
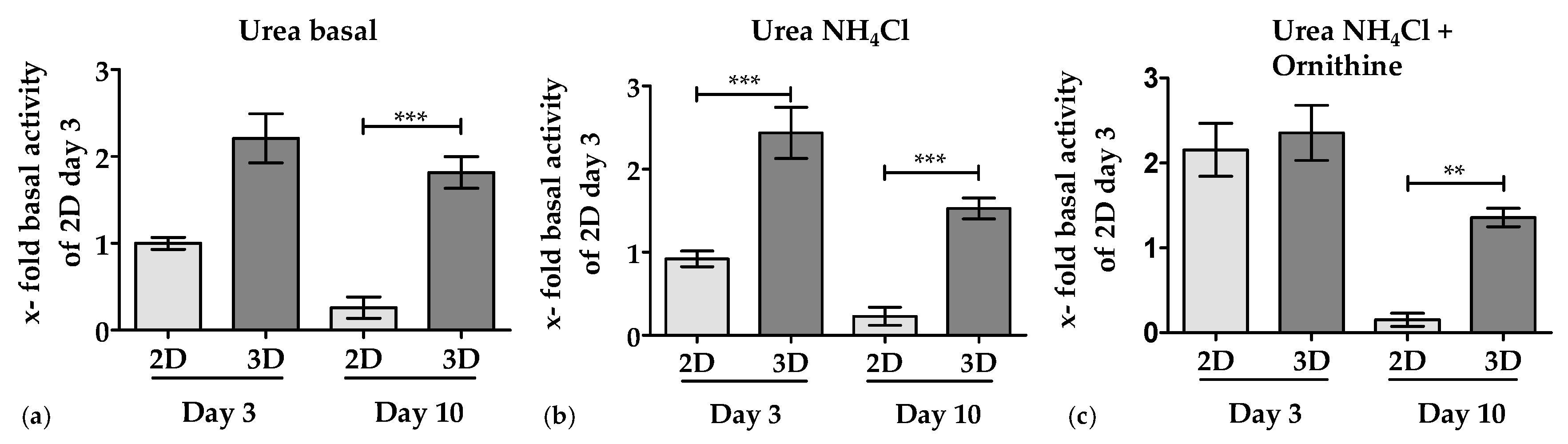
3.3.1. 3D Environment by the Optimaix-3D Scaffolds Supports Urea Production in PHHs
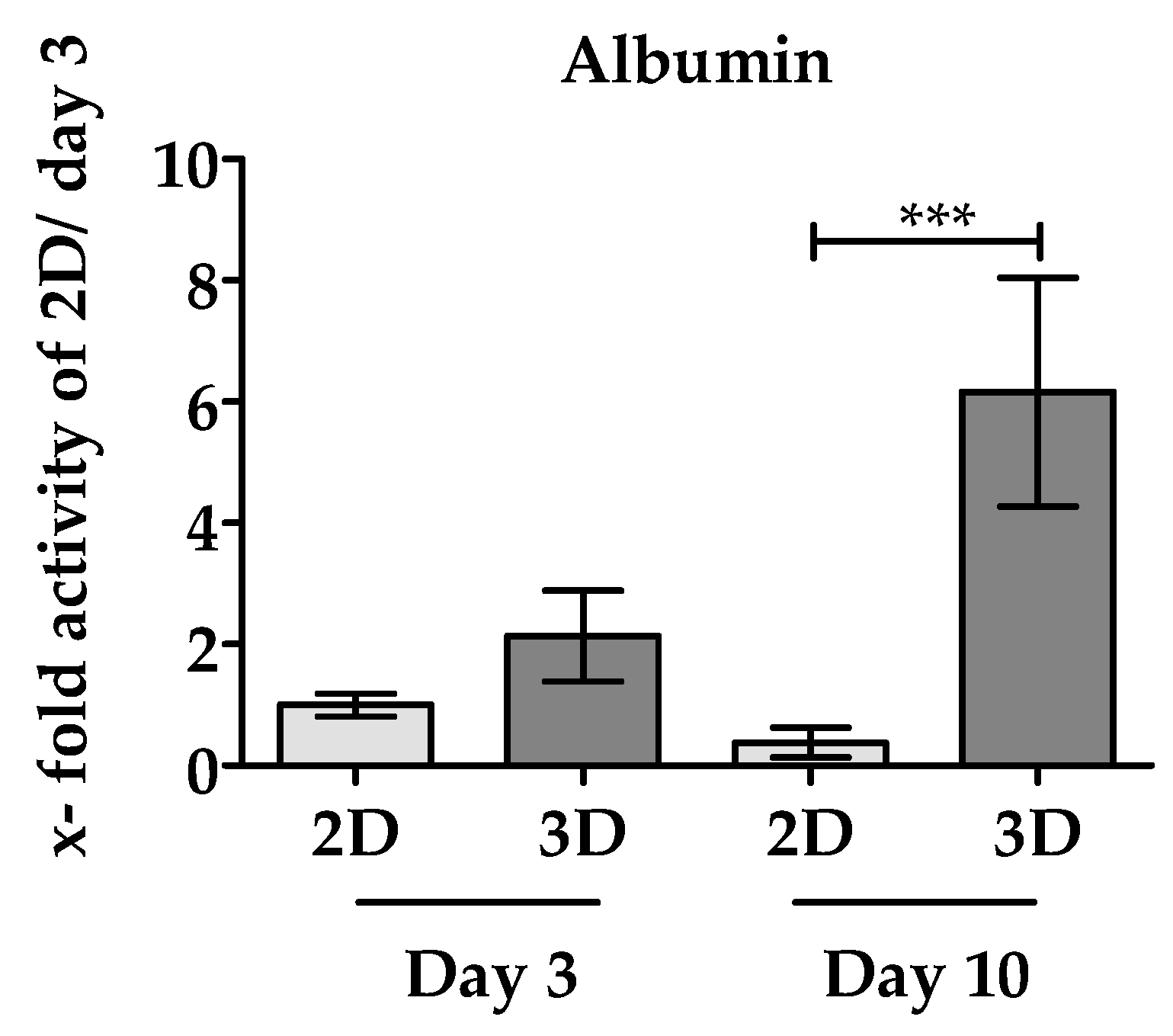
3.3.2. 3D Environment of the Optimaix-3D Scaffolds Favors Albumin Synthesis in PHHs
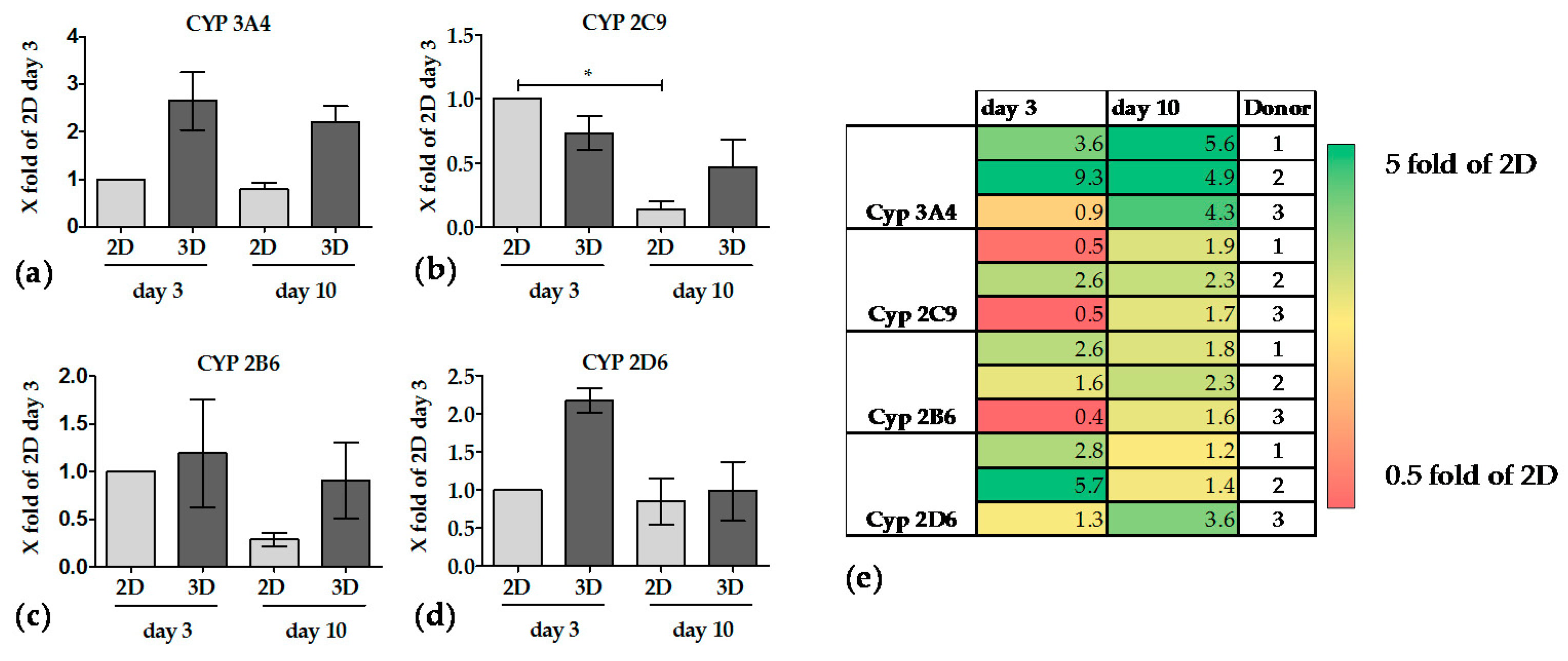
3.3.3. The Activity of CYP Enzymes was Constant in 3D over 10 Days
4. Discussion
5. Conclusions
Author Contributions
Funding
Conflicts of Interest
Abbreviations
| PHH | Primary human hepatocytes |
| NIH | National Institutes of Health |
| RGD | Arginylglycylaspartic acid |
| CYP | CYP450 monooxygenase |
| ECM | Extracellular matrix |
| EDC | 1-ethyl-3-(3-dimethylaminopropyl)carbodiimide hydrochloride |
References
- Kaplowitz, N. Idiosyncratic drug hepatotoxicity. Nat. Rev. Drug Discov. 2005, 4, 489–499. [Google Scholar] [CrossRef] [PubMed]
- Olson, H.; Betton, G.; Robinson, D.; Thomas, K.; Monro, A.; Kolaja, G.; Lilly, P.; Sanders, J.; Sipes, G.; Bracken, W.; et al. Concordance of the toxicity of pharmaceuticals in humans and in animals. Regul. Toxicol. Pharmacol. 2000, 32, 56–67. [Google Scholar] [CrossRef] [PubMed]
- Ballet, F. Hepatotoxicity in drug development: Detection, significance and solutions. J. Hepatol. 1997, 26 (Suppl. 2), 26–36. [Google Scholar] [CrossRef]
- Lewis, D.F.; Ioannides, C.; Parke, D.V. Cytochromes P450 and species differences in xenobiotic metabolism and activation of carcinogen. Environ. Health Perspect. 1998, 106, 633–641. [Google Scholar] [CrossRef] [PubMed]
- Paul, S.M.; Mytelka, D.S.; Dunwiddie, C.T.; Persinger, C.C.; Munos, B.H.; Lindborg, S.R.; Schacht, A.L. How to improve R&D productivity: The pharmaceutical industry’s grand challenge. Nat. Rev. Drug Discov. 2010, 9, 203–214. [Google Scholar] [PubMed]
- Soldatow, V.Y.; LeCluyse, E.L.; Griffith, L.G.; Rusyn, I. In vitro models for liver toxicity testing. Toxicol. Res. 2013, 2, 23–39. [Google Scholar] [CrossRef] [PubMed]
- Ramachandran, S.D.; Vivarès, A.; Klieber, S.; Hewitt, N.J.; Muenst, B.; Heinz, S.; Walles, H.; Braspenning, J. Applicability of second-generation upcyte® human hepatocytes for use in CYP inhibition and induction studies. Pharmacol. Res. Perspect. 2015, 3, e00161. [Google Scholar] [CrossRef] [PubMed]
- Kafert-Kasting, S.; Alexandrova, K.; Barthold, M.; Laube, B.; Friedrich, G.; Arseniev, L.; Hengstler, J.G. Enzyme induction in cryopreserved human hepatocyte cultures. Toxicology 2006, 220, 117–125. [Google Scholar] [CrossRef] [PubMed]
- Knobeloch, D.; Ehnert, S.; Schyschka, L.; Büchler, P.; Schoenberg, M.; Kleeff, J.; Thasler, W.E.; Nussler, N.C.; Godoy, P.; Hengstler, J.; et al. Human hepatocytes: Isolation, culture, and quality procedures. In Human Cell Culture Protocols; Mitry, R.R., Hughes, R.D., Eds.; Humana Press: Totowa, NJ, USA, 2012; pp. 99–120. [Google Scholar]
- Lin, J.; Schyschka, L.; Mühl-Benninghaus, R.; Neumann, J.; Hao, L.; Nussler, N.; Dooley, S.; Liu, L.; Stöckle, U.; Nussler, A.K.; et al. Comparative analysis of phase I and II enzyme activities in 5 hepatic cell lines identifies Huh-7 and HCC-T cells with the highest potential to study drug metabolism. Arch. Toxicol. 2012, 86, 87–95. [Google Scholar] [CrossRef] [PubMed]
- Seeliger, C.; Culmes, M.; Schyschka, L.; Yan, X.; Damm, G.; Wang, Z.; Kleeff, J.; Thasler, W.E.; Hengstler, J.; Stöckle, U. Decrease of global methylation improves significantly hepatic differentiation of Ad-MSCs: Possible future application for urea detoxification. Cell Transplant. 2013, 22, 119–131. [Google Scholar] [CrossRef] [PubMed]
- Godoy, P.; Schmidt-Heck, W.; Natarajan, K.; Lucendo-Villarin, B.; Szkolnicka, D.; Asplund, A.; Bjorquist, P.; Widera, A.; Stoeber, R.; Campos, G.; et al. Gene networks and transcription factor motifs defining the differentiation of stem cells into hepatocyte-like cells. J. Hepatol. 2015, 63, 934–942. [Google Scholar] [CrossRef] [PubMed]
- Zeilinger, K.; Freyer, N.; Damm, G.; Seehofer, D.; Knöspel, F. Cell sources for in vitro human liver cell culture models. Exp. Biol. Med. 2016, 241, 1684–1698. [Google Scholar] [CrossRef] [PubMed]
- Stéphenne, X.; Najimi, M.; Sokal, E.M. Hepatocyte cryopreservation: Is it time to change the strategy? World J. Gastroenterol. 2010, 16, 1–14. [Google Scholar] [PubMed]
- Godoy, P.; Hewitt, N.; Albrecht, U.; Andersen, M.; Ansari, N.; Bhattacharya, S.; Bode, J.; Bolleyn, J.; Borner, C.; Böttger, J.; et al. Recent advances in 2D and 3D in vitro systems using primary hepatocytes, alternative hepatocyte sources and non-parenchymal liver cells and their use in investigating mechanisms of hepatotoxicity, cell signaling and ADME. Arch. Toxicol. 2013, 87, 1315–1530. [Google Scholar] [CrossRef] [PubMed]
- Ostrowska, A.; Gu, K.; Bode, D.C.; Van Buskirk, R.G. Hypothermic storage of isolated human hepatocytes: A comparison between university of wisconsin solution and a hypothermosol platform. Arch. Toxicol. 2009, 83, 493–502. [Google Scholar] [CrossRef] [PubMed]
- Godoy, P.; Hengstler, J.G.; Ilkavets, I.; Meyer, C.; Bachmann, A.; Muller, A.; Tuschl, G.; Mueller, S.O.; Dooley, S. Extracellular matrix modulates sensitivity of hepatocytes to fibroblastoid dedifferentiation and transforming growth factor beta-induced apoptosis. Hepatology 2009, 49, 2031–2043. [Google Scholar] [CrossRef] [PubMed]
- Bachmann, A.; Moll, M.; Gottwald, E.; Nies, C.; Zantl, R.; Wagner, H.; Burkhardt, B.; Sanchez, J.J.; Ladurner, R.; Thasler, W.; et al. 3D cultivation techniques for primary human hepatocytes. Microarrays 2015, 4, 64–83. [Google Scholar] [CrossRef] [PubMed]
- O’Brien, F.J. Biomaterials & scaffolds for tissue engineering. Mater. Today 2011, 14, 88–95. [Google Scholar]
- Ranucci, C.S.; Kumar, A.; Batra, S.P.; Moghe, P.V. Control of hepatocyte function on collagen foams: Sizing matrix pores toward selective induction of 2-D and 3-D cellular morphogenesis. Biomaterials 2000, 21, 783–793. [Google Scholar] [CrossRef]
- Kumari, J.; Karande, A.A.; Kumar, A. Combined effect of cryogel matrix and temperature-reversible soluble–insoluble polymer for the development of in vitro human liver tissue. ACS Appl. Mater. Interfaces 2016, 8, 264–277. [Google Scholar] [CrossRef] [PubMed]
- Wells, R.G. The role of matrix stiffness in regulating cell behavior. Hepatology 2008, 47, 1394–1400. [Google Scholar] [CrossRef] [PubMed]
- Mueller, S.; Sandrin, L. Liver stiffness: A novel parameter for the diagnosis of liver disease. Hepat. Med. Evid. Res. 2010, 2, 49–67. [Google Scholar] [CrossRef]
- Wei, G.; Wang, J.; Lv, Q.; Liu, M.; Xu, H.; Zhang, H.; Jin, L.; Yu, J.; Wang, X. Three-dimensional coculture of primary hepatocytes and stellate cells in silk scaffold improves hepatic morphology and functionality in vitro. J. Biomed. Mater. Res. Part A 2018, 106, 2171–2180. [Google Scholar] [CrossRef] [PubMed]
- Yan, S.; Wei, J.; Liu, Y.; Zhang, H.; Chen, J.; Li, X. Hepatocyte spheroid culture on fibrous scaffolds with grafted functional ligands as an in vitro model for predicting drug metabolism and hepatotoxicity. Acta Biomater. 2015, 28, 138–148. [Google Scholar] [CrossRef] [PubMed]
- Rajendran, D.; Hussain, A.; Yip, D.; Parekh, A.; Shrirao, A.; Cho, C.H. Long-term liver-specific functions of hepatocytes in electrospun chitosan nanofiber scaffolds coated with fibronectin. J. Biomed. Mater. Res. Part A 2017, 105, 2119–2128. [Google Scholar] [CrossRef] [PubMed]
- Heschel, I.; Rau, G. Method for Producing Porous Structures. U.S. Patent No. 6,447,701 B1, 10 September 2002. [Google Scholar]
- Shimizu, K.; Ito, A.; Honda, H. Enhanced cell-seeding into 3D porous scaffolds by use of magnetite nanoparticles. J. Biomed. Mater. Res. Part B Appl. Biomater. 2005, 77, 265–272. [Google Scholar] [CrossRef] [PubMed]
- Fan, J.; Jia, X.; Huang, Y.; Fu, B.M.; Fan, Y. Greater scaffold permeability promotes growth of osteoblastic cells in a perfused bioreactor. J. Tissue Eng. Regen. Med. 2015, 9, E210–E218. [Google Scholar] [CrossRef] [PubMed]
- Chung, E.J.; Ju, H.W.; Park, H.J.; Park, C.H. Three-layered scaffolds for artificial esophagus using poly(ε-caprolactone) nanofibers and silk fibroin: An experimental study in a rat model. J. Biomed. Mater. Res. Part A 2015, 103, 2057–2065. [Google Scholar] [CrossRef] [PubMed]
- Tamjid, E.; Simchi, A.; Dunlop, J.W.C.; Fratzl, P.; Bagheri, R.; Vossoughi, M. Tissue growth into three-dimensional composite scaffolds with controlled micro-features and nanotopographical surfaces. J. Biomed. Mater. Res. Part A 2013, 101, 2796–2807. [Google Scholar] [CrossRef] [PubMed]
- Lee, S.M.L.; Schelcher, C.; Demmel, M.; Hauner, M.; Thasler, W.E. Isolation of human hepatocytes by a two-step collagenase perfusion procedure. J. Vis. Exp. 2013, 79, 50615. [Google Scholar] [CrossRef] [PubMed]
- Pfeiffer, E.; Kegel, V.; Zeilinger, K.; Hengstler, J.G.; Nussler, A.K.; Seehofer, D.; Damm, G. Featured article: Isolation, characterization, and cultivation of human hepatocytes and non-parenchymal liver cells. Exp. Biol. Med. 2015, 240, 645–656. [Google Scholar] [CrossRef] [PubMed]
- Thasler, W.E.; Weiss, T.S.; Schillhorn, K.; Stoll, P.-T.; Irrgang, B.; Jauch, K.-W. Charitable state-controlled foundation human tissue and cell research: Ethic and legal aspects in the supply of surgically removed human tissue for research in the academic and commercial sector in Germany. Cell Tissue Bank. 2003, 4, 49–56. [Google Scholar] [CrossRef] [PubMed]
- Jurgens, W.J.; Kroeze, R.J.; Bank, R.A.; Ritt, M.J.; Helder, M.N. Rapid attachment of adipose stromal cells on resorbable polymeric scaffolds facilitates the one-step surgical procedure for cartilage and bone tissue engineering purposes. J. Orthop. Res. 2011, 29, 853–860. [Google Scholar] [CrossRef] [PubMed]
- Zanger, U.M.; Schwab, M. Cytochrome p450 enzymes in drug metabolism: Regulation of gene expression, enzyme activities, and impact of genetic variation. Pharmacol. Ther. 2013, 138, 103–141. [Google Scholar] [CrossRef] [PubMed]
- Hoffmann, S.A.; Müller-Vieira, U.; Biemel, K.; Knobeloch, D.; Heydel, S.; Lübberstedt, M.; Nüssler, A.K.; Andersson Tommy, B.; Gerlach, J.C.; Zeilinger, K. Analysis of drug metabolism activities in a miniaturized liver cell bioreactor for use in pharmacological studies. Biotechnol. Bioeng. 2012, 109, 3172–3181. [Google Scholar] [CrossRef] [PubMed]
- Ehnert, S.; Falldorf, K.; Fentz, A.-K.; Ziegler, P.; Schröter, S.; Freude, T.; Ochs, B.G.; Stacke, C.; Ronniger, M.; Sachtleben, J.; et al. Primary human osteoblasts with reduced alkaline phosphatase and matrix mineralization baseline capacity are responsive to extremely low frequency pulsed electromagnetic field exposure—Clinical implication possible. Bone Rep. 2015, 3, 48–56. [Google Scholar] [CrossRef] [PubMed]
- Loh, Q.L.; Choong, C. Three-dimensional scaffolds for tissue engineering applications: Role of porosity and pore size. Tissue Eng. Part B Rev. 2013, 19, 485–502. [Google Scholar] [CrossRef] [PubMed]
- Hengstler, J.G.; Utesch, D.; Steinberg, P.; Platt, K.L.; Diener, B.; Ringel, M.; Swales, N.; Fischer, T.; Biefang, K.; Gerl, M. Cryopreserved primary hepatocytes as a constantly available in vitro model for the evaluation of human and animal drug metabolism and enzyme induction. Drug Metab. Rev. 2000, 32, 81–118. [Google Scholar] [CrossRef] [PubMed]
- Duret, C.; Moreno, D.; Balasiddaiah, A.; Roux, S.; Briolotti, P.; Raulet, E.; Herrero, A.; Ramet, H.; Biron-Andreani, C.; Gerbal-Chaloin, S.; et al. Cold preservation of human adult hepatocytes for liver cell therapy. Cell Transplant. 2015, 24, 2541–2555. [Google Scholar] [CrossRef] [PubMed]
- Arias, I.M.; Wolkoff, A.W.; Boyer, J.L.; Shafritz, D.A.; Fausto, N.; Alter, H.J.; Cohen, D.E. The Liver: Biology and Pathobiology; John Wiley & Sons: Hoboken, NJ, USA, 2011. [Google Scholar]
- Zhang, H.; Gao, N.; Tian, X.; Liu, T.; Fang, Y.; Zhou, J.; Wen, Q.; Xu, B.; Qi, B.; Gao, J.; et al. Content and activity of human liver microsomal protein and prediction of individual hepatic clearance in vivo. Sci. Rep. 2015, 5, 17671. [Google Scholar] [CrossRef] [PubMed]
- Pless-Petig, G.; Singer, B.B.; Rauen, U. Cold storage of rat hepatocyte suspensions for one week in a customized cold storage solution—Preservation of cell attachment and metabolism. PLoS ONE 2012, 7, e40444. [Google Scholar] [CrossRef] [PubMed]
- Schyschka, L.; Sanchez, J.J.; Wang, Z.; Burkhardt, B.; Muller-Vieira, U.; Zeilinger, K.; Bachmann, A.; Nadalin, S.; Damm, G.; Nussler, A.K. Hepatic 3D cultures but not 2D cultures preserve specific transporter activity for acetaminophen-induced hepatotoxicity. Arch. Toxicol. 2013, 87, 1581–1593. [Google Scholar] [CrossRef] [PubMed]
- Schoof, H.; Apel, J.; Heschel, I.; Rau, G. Control of pore structure and size in freeze-dried collagen sponges. J. Biomed. Mater. Res. 2001, 58, 352–357. [Google Scholar] [CrossRef] [PubMed]
- Baiocchini, A.; Montaldo, C.; Conigliaro, A.; Grimaldi, A.; Correani, V.; Mura, F.; Ciccosanti, F.; Rotiroti, N.; Brenna, A.; Montalbano, M.; et al. Extracellular matrix molecular remodeling in human liver fibrosis evolution. PLoS ONE 2016, 11, e0151736. [Google Scholar] [CrossRef] [PubMed]
- Lee, C.H.; Singla, A.; Lee, Y. Biomedical applications of collagen. Int. J. Pharm. 2001, 221, 1–22. [Google Scholar] [CrossRef]
- Khan, R.; Khan, M.H. Use of collagen as a biomaterial: An update. J. Indian Soc. Periodontol. 2013, 17, 539–542. [Google Scholar] [CrossRef] [PubMed]
- Martinez-Hernandez, A.; Amenta, P.S. The hepatic extracellular matrix. Virchows Arch. A Pathol. Anat. Histopathol. 1993, 423, 77–84. [Google Scholar] [CrossRef] [PubMed]
- Yin, M.; Talwalkar, J.A.; Glaser, K.J.; Manduca, A.; Grimm, R.C.; Rossman, P.J.; Fidler, J.L.; Ehman, R.L. A preliminary assessment of hepatic fibrosis with magnetic resonance elastography. Clin. Gastroenterol. Hepatol. 2007, 5, 1207–1213.e2. [Google Scholar] [CrossRef] [PubMed]
- Fung, J.; Lee, C.-K.; Chan, M.; Seto, W.-K.; Wong, D.K.-H.; Lai, C.-L.; Yuen, M.-F. Defining normal liver stiffness range in a normal healthy chinese population without liver disease. PLoS ONE 2013, 8, e85067. [Google Scholar] [CrossRef] [PubMed]
- Theile, D.; Haefeli, W.E.; Seitz, H.K.; Millonig, G.; Weiss, J.; Mueller, S. Association of liver stiffness with hepatic expression of pharmacokinetically important genes in alcoholic liver disease. Alcohol. Clin. Exp. Res. 2013, 37 (Suppl. 1), E17–E22. [Google Scholar] [CrossRef] [PubMed]
- Damania, A.; Kumar, A.; Teotia, A.K.; Kimura, H.; Kamihira, M.; Ijima, H.; Sarin, S.K.; Kumar, A. Decellularized liver matrix-modified cryogel scaffolds as potential hepatocyte carriers in bioartificial liver support systems and implantable liver constructs. ACS Appl. Mater. Interfaces 2018, 10, 114–126. [Google Scholar] [CrossRef] [PubMed]
- Amirikia, M.; Shariatzadeh, S.M.A.; Jorsaraei, S.G.A.; Soleimani-Mehranjani, M. Impact of pre-incubation time of silk fibroin scaffolds in culture medium on cell proliferation and attachment. Tissue Cell 2017, 49, 657–663. [Google Scholar] [CrossRef] [PubMed]
- Galperin, A.; Long, T.J.; Ratner, B.D. Degradable, thermo-sensitive poly(N-isopropyl acrylamide)-based scaffolds with controlled porosity for tissue engineering applications. Biomacromolecules 2010, 11, 2583–2592. [Google Scholar] [CrossRef] [PubMed]
- Yamada, M.; Sugaya, S.; Naganuma, Y.; Seki, M. Microfluidic synthesis of chemically and physically anisotropic hydrogel microfibers for guided cell growth and networking. Soft Matter 2012, 8, 3122–3130. [Google Scholar] [CrossRef]
- Berendsen, T.A.; Izamis, M.L.; Xu, H.; Liu, Q.; Hertl, M.; Berthiaume, F.; Yarmush, M.L.; Uygun, K. Hepatocyte viability and atp content decrease linearly over time during conventional cold storage of rat liver grafts. Transplant. Proc. 2011, 43, 1484–1488. [Google Scholar] [CrossRef] [PubMed]
- Rauen, U.; Polzar, B.; Stephan, H.; Mannherz, H.G.; Groot, H.D. Cold-induced apoptosis in cultured hepatocytes and liver endothelial cells: Mediation by reactive oxygen species. FASEB J. 1999, 13, 155–168. [Google Scholar] [CrossRef] [PubMed]
- Kim, Y.B.; Kim, G. Rapid-prototyped collagen scaffolds reinforced with PCL/β-TCP nanofibres to obtain high cell seeding efficiency and enhanced mechanical properties for bone tissue regeneration. J. Mater. Chem. 2012, 22, 16880–16889. [Google Scholar] [CrossRef]
- Thevenot, P.; Nair, A.; Dey, J.; Yang, J.; Tang, L. Method to analyze three-dimensional cell distribution and infiltration in degradable scaffolds. Tissue Eng. Part C Methods 2008, 14, 319–331. [Google Scholar] [CrossRef] [PubMed]
- Kurashina, Y.; Takemura, K.; Miyata, S.; Komotori, J.; Koyama, T. Effective cell collection method using collagenase and ultrasonic vibration. Biomicrofluidics 2014, 8, 054118. [Google Scholar] [CrossRef] [PubMed]
- Watford, M. The urea cycle: Teaching intermediary metabolism in a physiological setting. Biochem. Mol. Biol. Educ. 2006, 31, 289–297. [Google Scholar] [CrossRef]
- Liu, H.; Yu, Y.; Glorioso, J.; Mao, S.; Rodysil, B.; Amiot, B.P.; Rinaldo, P.; Nyberg, S.L. Cold storage of rat hepatocyte spheroids. Cell Transplant. 2014, 23, 819–830. [Google Scholar] [CrossRef] [PubMed]
- Schleicher, J.; Tokarski, C.; Marbach, E.; Matz-Soja, M.; Zellmer, S.; Gebhardt, R.; Schuster, S. Zonation of hepatic fatty acid metabolism—The diversity of its regulation and the benefit of modeling. Biochim. Biophys. Acta 2015, 1851, 641–656. [Google Scholar] [CrossRef] [PubMed]
- Bell, C.C.; Dankers, A.C.A.; Lauschke, V.M.; Sison-Young, R.; Jenkins, R.; Rowe, C.; Goldring, C.E.; Park, K.; Regan, S.L.; Walker, T.; et al. Comparison of hepatic 2D sandwich cultures and 3D spheroids for long-term toxicity applications: A multicenter study. Toxicol. Sci. 2018, 162, 655–666. [Google Scholar] [CrossRef] [PubMed]
- Siltanen, C.; Diakatou, M.; Lowen, J.; Haque, A.; Rahimian, A.; Stybayeva, G.; Revzin, A. One step fabrication of hydrogel microcapsules with hollow core for assembly and cultivation of hepatocyte spheroids. Acta Biomater. 2017, 50, 428–436. [Google Scholar] [CrossRef] [PubMed]
- Rothschild, D.E.; Srinivasan, T.; Aponte-Santiago, L.A.; Shen, X.; Allen, I.C. The ex vivo culture and pattern recognition receptor stimulation of mouse intestinal organoids. J. Vis. Exp. 2016, 111, 54033. [Google Scholar] [CrossRef] [PubMed]
- Fey, S.J.; Wrzesinski, K. Determination of drug toxicity using 3D spheroids constructed from an immortal human hepatocyte cell line. Toxicol. Sci. 2012, 127, 403–411. [Google Scholar] [CrossRef] [PubMed]
- Bell, C.C.; Hendriks, D.F.G.; Moro, S.M.L.; Ellis, E.; Walsh, J.; Renblom, A.; Fredriksson Puigvert, L.; Dankers, A.C.A.; Jacobs, F.; Snoeys, J.; et al. Characterization of primary human hepatocyte spheroids as a model system for drug-induced liver injury, liver function and disease. Sci. Rep. 2016, 6, 25187. [Google Scholar] [CrossRef] [PubMed]
- Ullah, I.; Kim, Y.; Lim, M.; Oh, K.B.; Hwang, S.; Shin, Y.; Kim, Y.; Im, G.-S.; Hur, T.-Y.; Ock, S.A. In vitro 3-D culture demonstrates incompetence in improving maintenance ability of primary hepatocytes. Anim. Cells Syst. 2017, 21, 332–340. [Google Scholar] [CrossRef]
| Substrate | Isoenzyme | Incubation Time in h | Concentration | Reaction |
|---|---|---|---|---|
| Bupropion | CYP 2B6 | 1 | 100 μM | Bupropion-hydroxylation |
| Diclofenac | CYP 2C9 | 1 | 9 μM | Diclofenac-4′-hydroxylation |
| Testosterone | CYP 3A4 | 1 | 50 μM | testosterone-6β-hydroxylation |
| Bufuralol | CYP 2D6 | 2 | 9 μM | Bufuralol-1-hydroxylation |
| Optimaix-3D Scaffold | Pore Size (Mean Diameter/μm) | Porosity (%) | Permeability (μm2) | Water-Uptake (%) | Swelling-Ratio (%) |
|---|---|---|---|---|---|
| Average | 88.9 | 96.3 | 54.5 | 97.1 | 3386.6 |
| Standard Deviation | 21.1 | 0.3 | 4.0 | 0.1 | 127.2 |
| Amount of Living Cells before Shipment (in mio) | Viability before Shipment | Amount of Living Cells after Percoll Purification (in mio) | Viability after Percoll | |
|---|---|---|---|---|
| Donor 1 | 119 | 80% | 54 | 76% |
| Donor 2 | 111 | 85% | 43 | 74% |
| Donor 3 | 78 | 83% | 25 | 80% |
| Average | 103 | 82% | 40 | 77% |
| Shipment Method | Survival of the Cells after Shipment | Adherence Time before Shipment | Advantages | Disadvantages | Ref. |
|---|---|---|---|---|---|
| Suspension | Low | No | Cells can be plated out by the recipient on demand | Cell loss during shipment, Reduced cell attachment metabolic capacity in culture | [14,44,59] |
| Cryopreserved hepatocytes | Low | No | Cells can be used anytime, anywhere | Massive cell loss during freezing/thawing, Low metabolic activity | [14,40] |
| 2D culture | High | 4 h | Cells can be plated in the different well-plate formats | Cell shipment requires a large volume. Risk of contamination | [15,17] |
| Optimaix-3D Collagen Scaffold | High | 2 h | Seeding large cell numbers in a low volume | Cell detachment difficult | This work |
| Cells maintain metabolic functions over 10 days |
© 2018 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (http://creativecommons.org/licenses/by/4.0/).
Share and Cite
Ruoß, M.; Häussling, V.; Schügner, F.; Olde Damink, L.H.H.; Lee, S.M.L.; Ge, L.; Ehnert, S.; Nussler, A.K. A Standardized Collagen-Based Scaffold Improves Human Hepatocyte Shipment and Allows Metabolic Studies over 10 Days. Bioengineering 2018, 5, 86. https://doi.org/10.3390/bioengineering5040086
Ruoß M, Häussling V, Schügner F, Olde Damink LHH, Lee SML, Ge L, Ehnert S, Nussler AK. A Standardized Collagen-Based Scaffold Improves Human Hepatocyte Shipment and Allows Metabolic Studies over 10 Days. Bioengineering. 2018; 5(4):86. https://doi.org/10.3390/bioengineering5040086
Chicago/Turabian StyleRuoß, Marc, Victor Häussling, Frank Schügner, Leon H. H. Olde Damink, Serene M. L. Lee, Liming Ge, Sabrina Ehnert, and Andreas K. Nussler. 2018. "A Standardized Collagen-Based Scaffold Improves Human Hepatocyte Shipment and Allows Metabolic Studies over 10 Days" Bioengineering 5, no. 4: 86. https://doi.org/10.3390/bioengineering5040086
APA StyleRuoß, M., Häussling, V., Schügner, F., Olde Damink, L. H. H., Lee, S. M. L., Ge, L., Ehnert, S., & Nussler, A. K. (2018). A Standardized Collagen-Based Scaffold Improves Human Hepatocyte Shipment and Allows Metabolic Studies over 10 Days. Bioengineering, 5(4), 86. https://doi.org/10.3390/bioengineering5040086