Tracking Lysosome Migration within Chinese Hamster Ovary (CHO) Cells Following Exposure to Nanosecond Pulsed Electric Fields
Abstract
1. Introduction
2. Materials and Methods
2.1. Cell Culture Procedures
2.2. Nanosecond Pulse Generator and Exposure Setup
2.3. Confocal Fluorescence Imaging
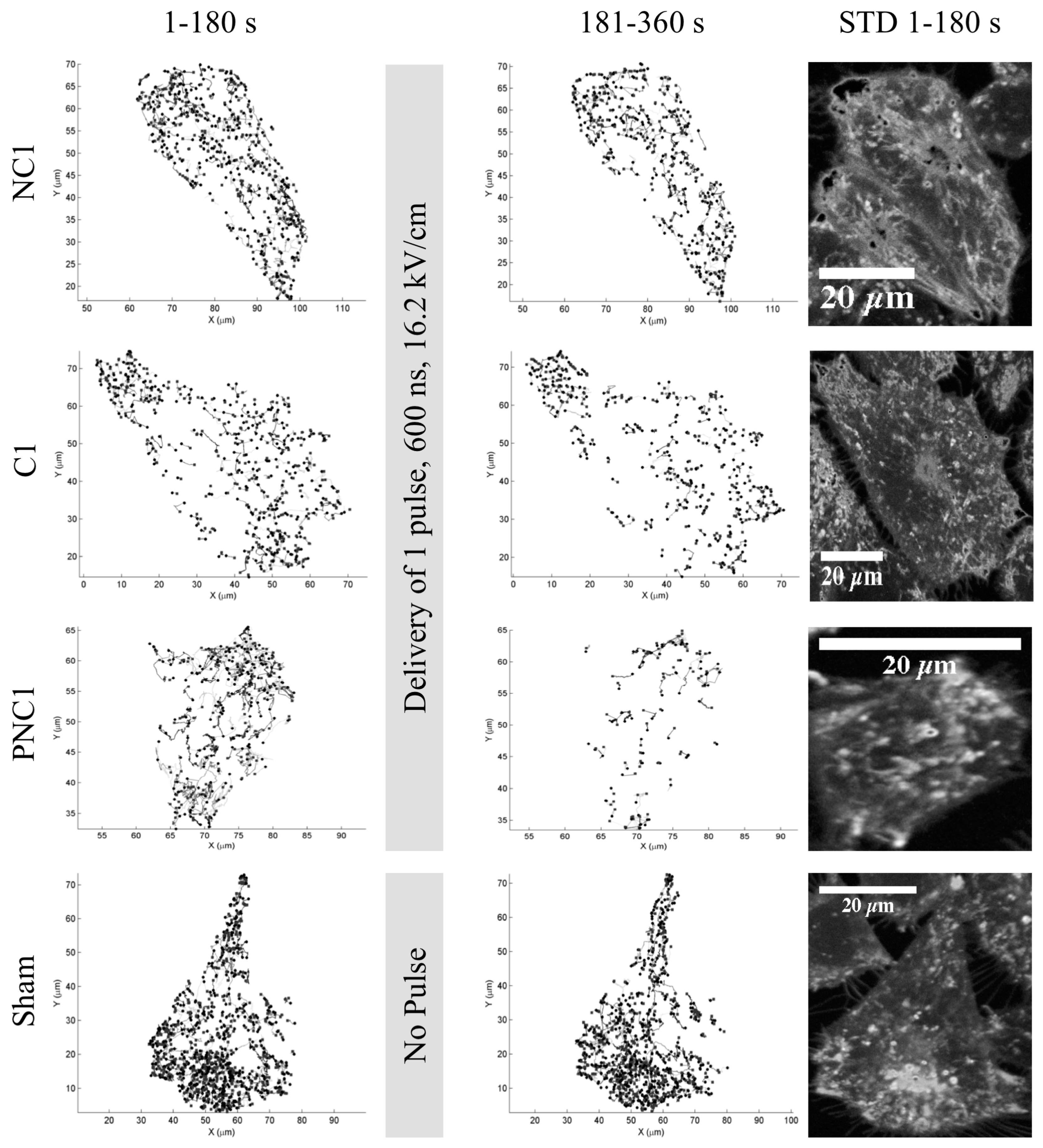
2.4. Image Processing and Lysosome Tracking
2.5. Data Analysis
3. Results
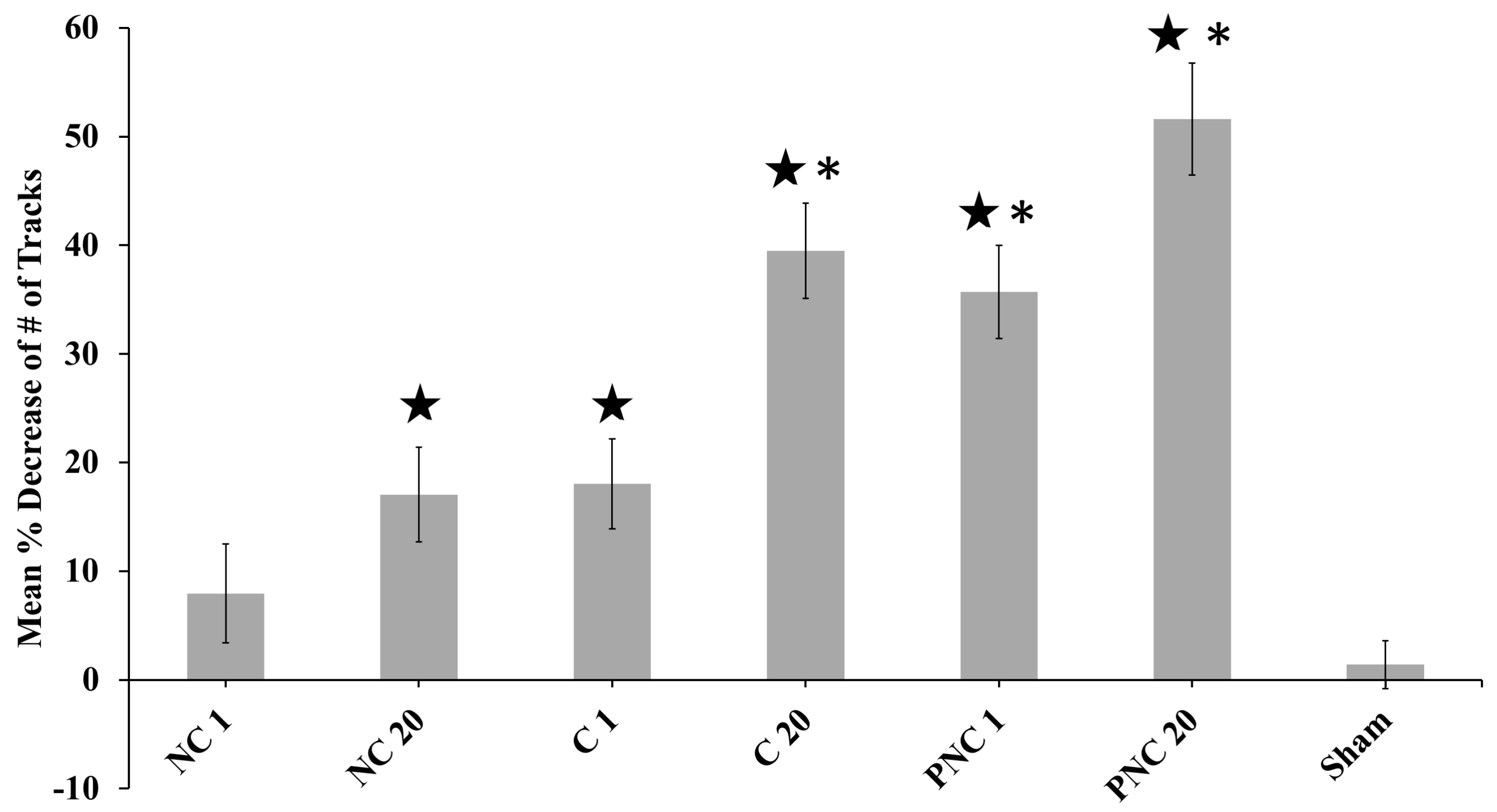
3.1. The Number of Lysosome Tracks Decreases Following nsPEF Exposure
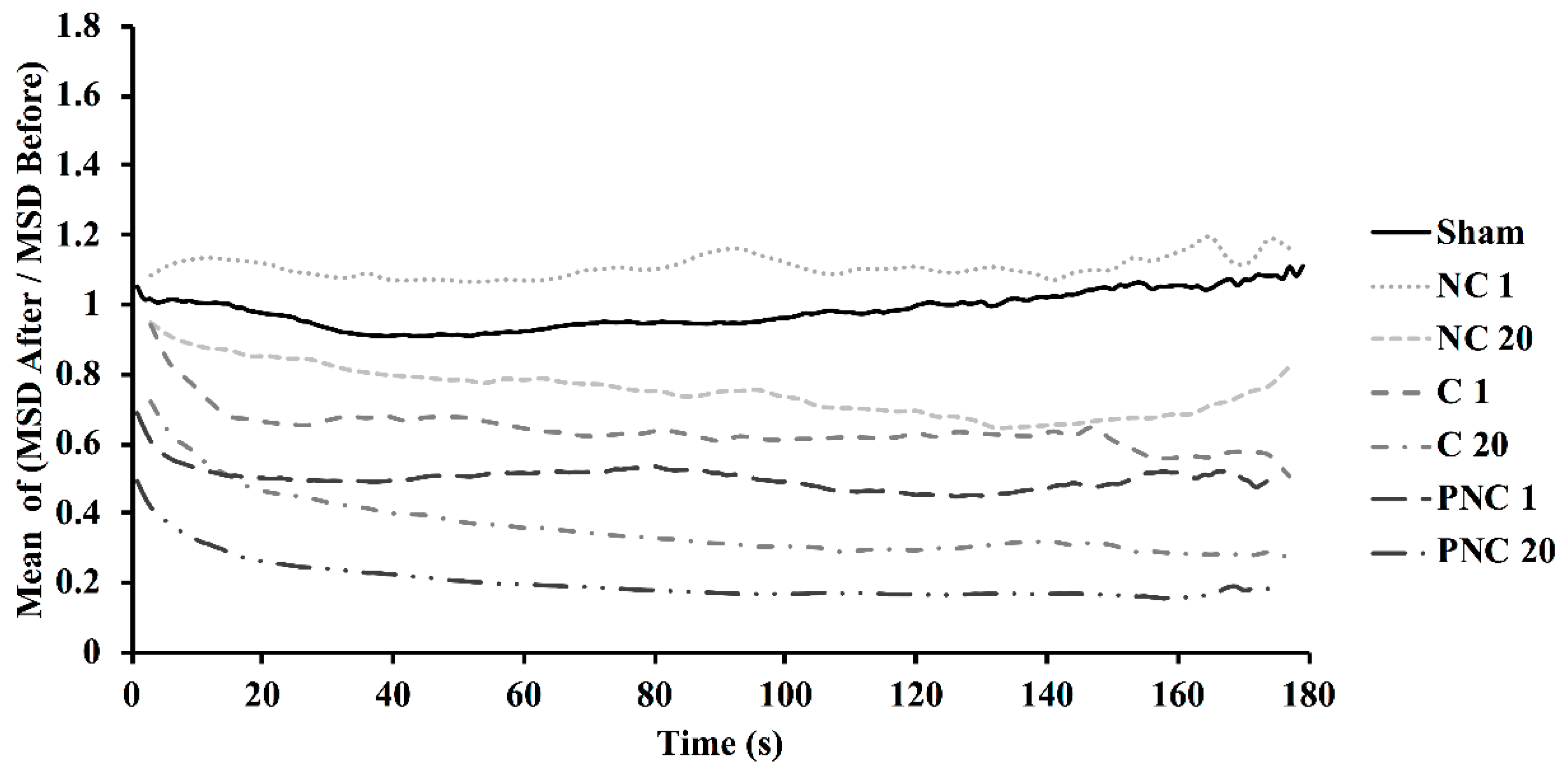
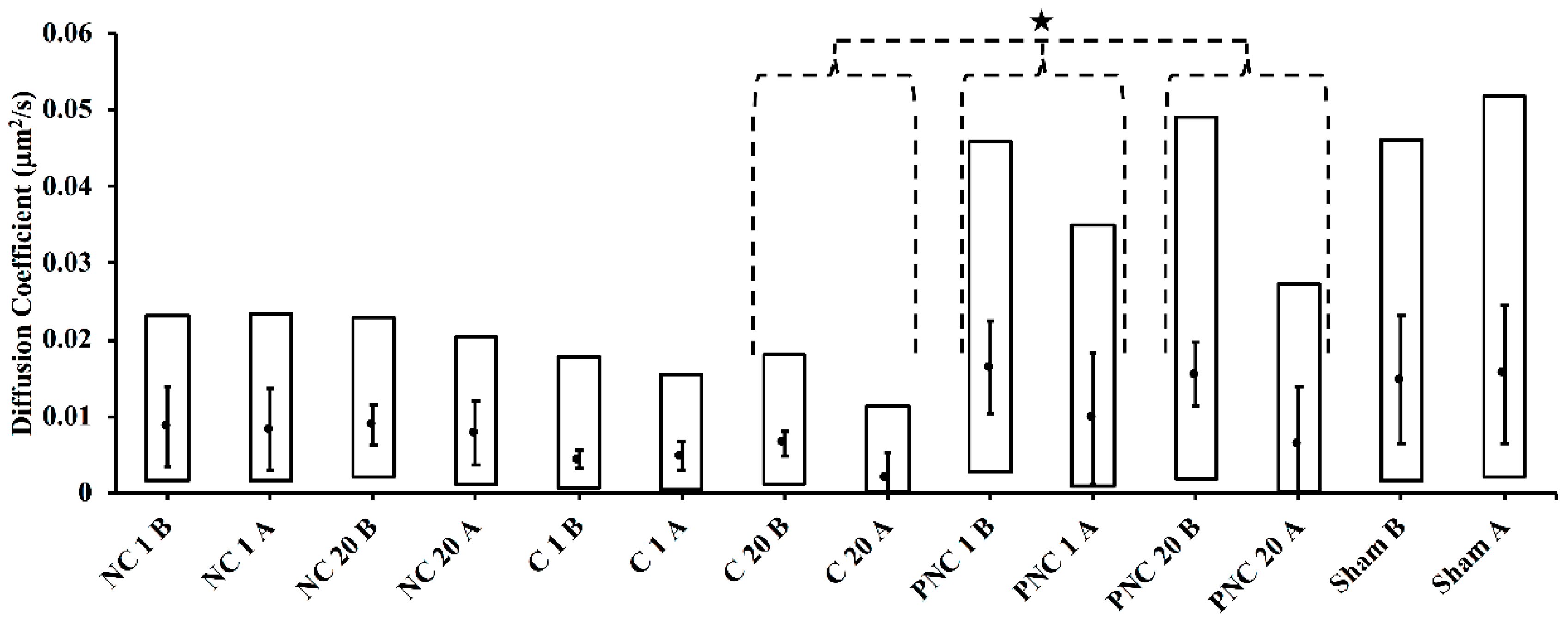
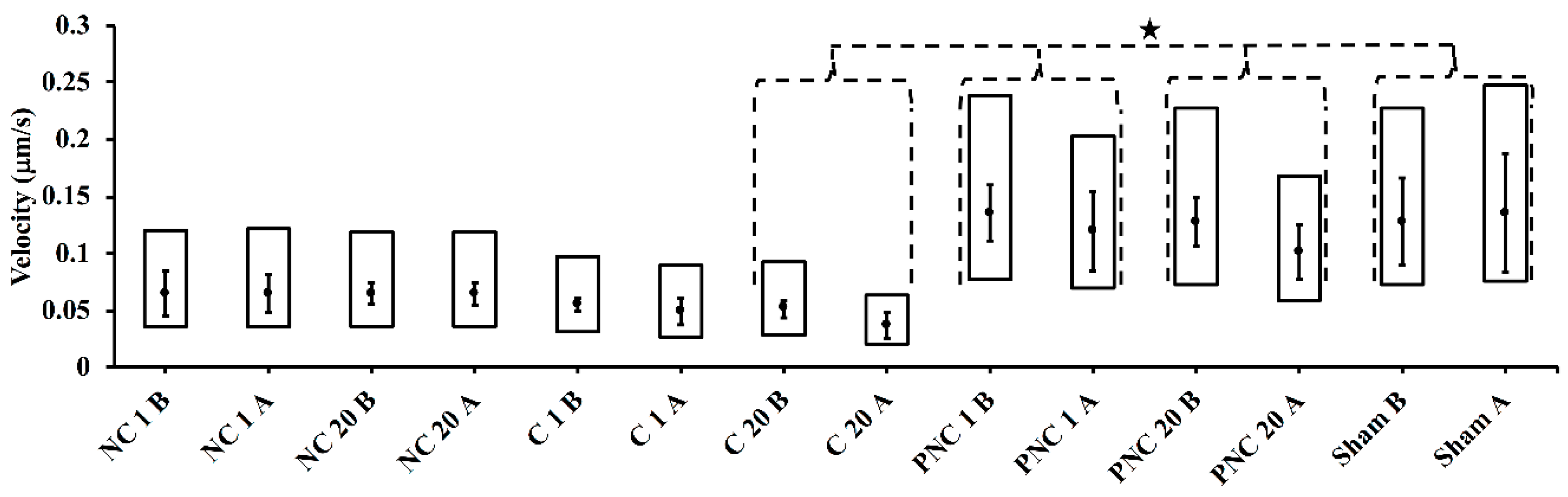
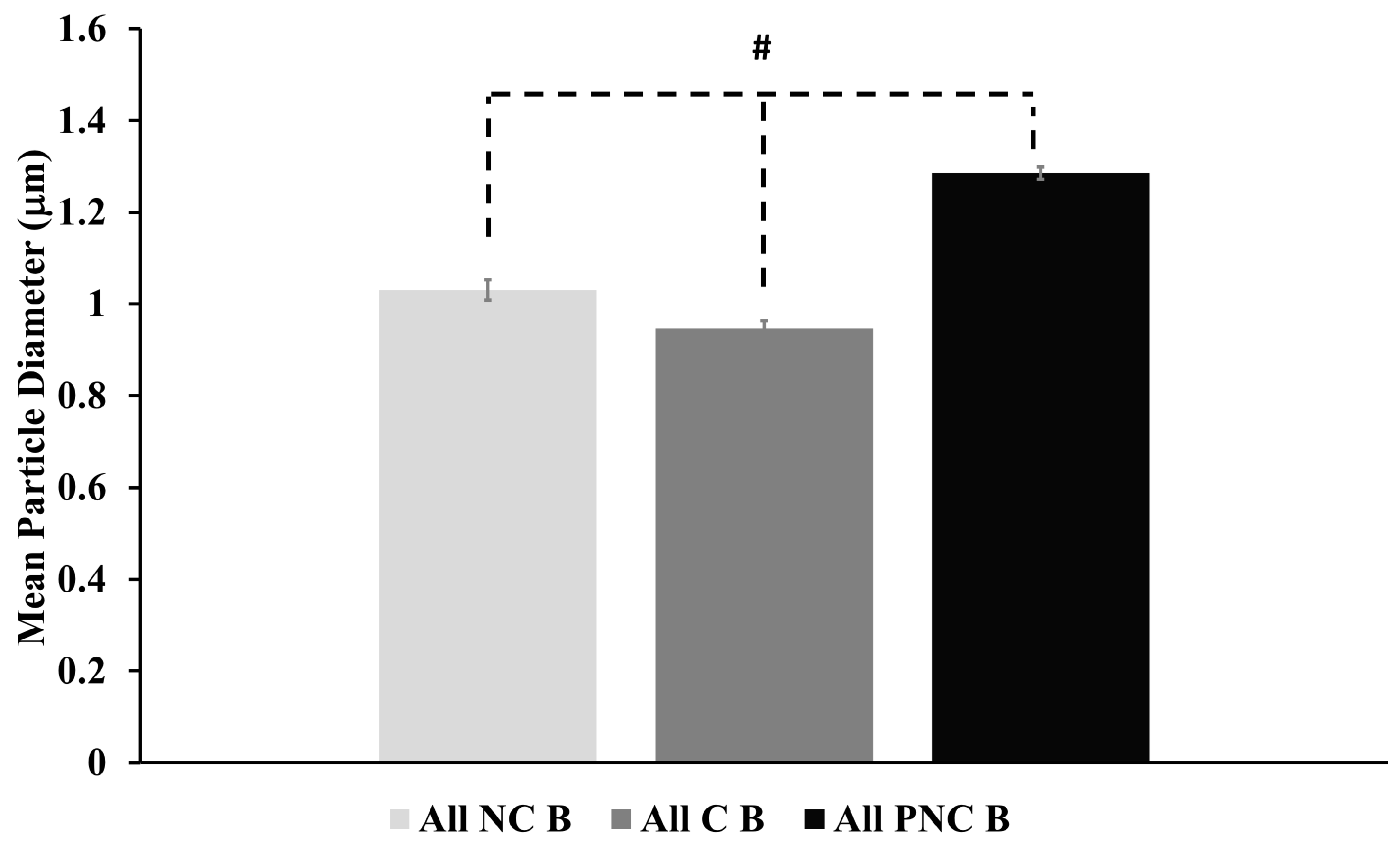
3.2. Exposure to nsPEF Decreases MSD, Diffusion Coefficients, and Velocities of Lysosome Translocation
4. Discussion
Supplementary Materials
Author Contributions
Funding
Acknowledgments
Conflicts of Interest
References
- Tokman, M.; Lee, J.H.; Levine, Z.A.; Ho, M.C.; Colvin, M.E.; Vernier, P.T. Electric field-driven water dipoles: Nanoscale architecture of electroporation. PLoS ONE 2013, 8. [Google Scholar] [CrossRef] [PubMed]
- Schoenbach, K.H.; Hargrave, B.; Joshi, R.P.; Kolb, J.F.; Nuccitelli, R.; Osgood, C.; Pakhomov, A.; Stacey, M.; Swanson, R.J.; White, J.A.; et al. Bioelectric effects of intense nanosecond pulses. IEEE Trans. Dielectr. Electr. Insul. 2007, 14, 1088–1109. [Google Scholar] [CrossRef]
- Napotnik, T.B.; Rebersek, M.; Vernier, P.T.; Mali, B.; Miklavcic, D. Effects of high voltage nanosecond electric pulses on eukaryotic cells (in vitro): A systematic review. Bioelectrochemistry 2016, 110, 1–12. [Google Scholar] [CrossRef] [PubMed]
- Moen, E.K.; Ibey, B.L.; Beier, H.T.; Armani, A.M. Quantifying pulsed electric field-induced membrane nanoporation in single cells. Biochim. Biophys. Acta 2016, 1858, 2795–2803. [Google Scholar] [CrossRef] [PubMed]
- Pakhomov, A.G.; Kolb, J.F.; White, J.A.; Joshi, R.P.; Xiao, S.; Schoenbach, K.H. Long-lasting plasma membrane permeabilization in mammalian cells by nanosecond pulsed electric field (nsPEF). Bioelectromagnetics 2007, 28, 655–663. [Google Scholar] [CrossRef] [PubMed]
- McNeil, P.L. Repairing a torn cell surface: Make way, lysosomes to the rescue. J. Cell Sci. 2002, 115, 873–879. [Google Scholar] [PubMed]
- Qian, H.; Sheetz, M.P.; Elson, E.L. Single-particle tracking—Analysis of diffusion and flow in 2-dimensional systems. Biophys. J. 1991, 60, 910–921. [Google Scholar] [CrossRef]
- Gamper, N.; Stockand, J.D.; Shapiro, M.S. The use of Chinese hamster ovary (CHO) cells in the study of ion channels. J. Pharmacol. Toxicol. Methods 2005, 51, 177–185. [Google Scholar] [CrossRef] [PubMed]
- Nesin, O.M.; Pakhomova, O.N.; Xiao, S.; Pakhomov, A.G. Manipulation of cell volume and membrane pore comparison following single cell permeabilization with 60-and 600-ns electric pulses. Biochim. Biophys. Acta 2011, 1808, 792–801. [Google Scholar] [CrossRef] [PubMed]
- Ibey, B.L.; Mixon, D.G.; Payne, J.A.; Bowman, A.; Sickendick, K.; Wilmink, G.J.; Roach, W.P.; Pakhomov, A.G. Plasma membrane permeabilization by trains of ultrashort electric pulses. Bioelectrochemistry 2010, 79, 114–121. [Google Scholar] [CrossRef] [PubMed]
- Thompson, G.L.; Roth, C.C.; Dalzell, D.R.; Kuipers, M.; Ibey, B.L. Calcium influx affects intracellular transport and membrane repair following nanosecond pulsed electric field exposure. J. Biomed. Opt. 2014, 19. [Google Scholar] [CrossRef] [PubMed]
- Roth, C.C.; Tolstykh, G.P.; Payne, J.A.; Kuipers, M.A.; Thompson, G.L.; DeSilva, M.N.; Ibey, B.L. Nanosecond pulsed electric field thresholds for nanopore formation in neural cells. J. Biomed. Opt. 2013, 18. [Google Scholar] [CrossRef] [PubMed]
- Reddy, A.; Caler, E.V.; Andrews, N.W. Plasma membrane repair is mediated by Ca2+-regulated exocytosis of lysosomes. Cell 2001, 106, 157–169. [Google Scholar] [CrossRef]
- Tinevez, J.Y.; Perry, N.; Schindelin, J.; Hoopes, G.M.; Reynolds, G.D.; Laplantine, E.; Bednarek, S.Y.; Shorte, S.L.; Eliceiri, K.W. TrackMate: An open and extensible platform for single-particle tracking. Methods 2017, 115, 80–90. [Google Scholar] [CrossRef] [PubMed]
- Tarantino, N.; Tinevez, J.Y.; Crowell, E.F.; Boisson, B.; Henriques, R.; Mhlanga, M.; Agou, F.; Israel, A.; Laplantine, E. TNF and IL-1 exhibit distinct ubiquitin requirements for inducing NEMO-IKK supramolecular structures. J. Cell Biol. 2014, 204, 231–245. [Google Scholar] [CrossRef] [PubMed]
- Saxton, M.J. Single-particle tracking: Models of directed transport. Biophys. J. 1994, 67, 2110–2119. [Google Scholar] [CrossRef]
- Zaiontz, C. Real Statistics Using Excel. Available online: www.real-statistics.com (accessed on 16 August 2018).
- Katrukha, E.A.; Mikhaylova, M.; van Brakel, H.X.; Henegouwen, P.M.V.E.; Akhmanova, A.; Hoogenraad, C.C.; Kapitein, L.C. Probing cytoskeletal modulation of passive and active intracellular dynamics using nanobody-functionalized quantum dots. Nat. Commun. 2017, 8. [Google Scholar] [CrossRef] [PubMed]
- Grady, M.E.; Parrish, E.; Caporizzo, M.A.; Seeger, S.C.; Composto, R.J.; Eckmann, D.M. Intracellular nanoparticle dynamics affected by cytoskeletal integrity. Soft Matter 2017, 13, 1873–1880. [Google Scholar] [CrossRef] [PubMed]
- Mittasch, M.; Gross, P.; Nestler, M.; Fritsch, A.W.; Iserman, C.; Kar, M.; Munder, M.; Voigt, A.; Alberti, S.; Grill, S.W.; et al. Non-invasive perturbations of intracellular flow reveal physical principles of cell organization. Nat. Cell Biol. 2018, 20, 344. [Google Scholar] [CrossRef] [PubMed]
- Balint, S.; Vilanova, I.V.; Alvarez, A.S.; Lakadamyali, M. Correlative live-cell and superresolution microscopy reveals cargo transport dynamics at microtubule intersections. Proc. Natl. Acad. Sci. USA 2013, 110, 3375–3380. [Google Scholar] [CrossRef] [PubMed]
- Semenov, I.; Xiao, S.; Pakhomov, A.G. Primary pathways of intracellular Ca2+ mobilization by nanosecond pulsed electric field. Biochim. Biophys. Acta 2013, 1828, 981–989. [Google Scholar] [CrossRef] [PubMed]
- Bandyopadhyay, D.; Cyphersmith, A.; Zapata, J.A.; Kim, Y.J.; Payne, C.K. Lysosome transport as a function of lysosome diameter. PLoS ONE 2014, 9. [Google Scholar] [CrossRef] [PubMed]
- Deville, S.; Penjweini, R.; Smisdom, N.; Notelaers, K.; Nelissen, I.; Hooyberghs, J.; Ameloot, M. Intracellular dynamics and fate of polystyrene nanoparticles in A549 Lung epithelial cells monitored by image (cross-) correlation spectroscopy and single particle tracking. Biochim. Biophys. Acta Mol. Cell Res. 2015, 1853, 2411–2419. [Google Scholar] [CrossRef] [PubMed]
- Cordonnier, M.N.; Dauzonne, D.; Louvard, D.; Coudrier, E. Actin filaments and myosin I alpha cooperate with microtubules for the movement of lysosomes. Mol. Biol. Cell 2001, 12, 4013–4029. [Google Scholar] [CrossRef] [PubMed]
- Chenouard, N.; Smal, I.; de Chaumont, F.; Maska, M.; Sbalzarini, I.F.; Gong, Y.H.; Cardinale, J.; Carthel, C.; Coraluppi, S.; Winter, M.; et al. Objective comparison of particle tracking methods. Nat. Methods 2014, 11, 281. [Google Scholar] [CrossRef] [PubMed]
- Carr, L.; Bardet, S.M.; Burke, R.C.; Arnaud-Cormos, D.; Leveque, P.; O’Connor, R.P. Calcium-independent disruption of microtubule dynamics by nanosecond pulsed electric fields in U87 human glioblastoma cells. Sci. Rep. 2017, 7. [Google Scholar] [CrossRef] [PubMed]
- Weaver, J.C.; Smith, K.C.; Esser, A.T.; Son, R.S.; Gowrishankar, T.R. A brief overview of electroporation pulse strength-duration space: A region where additional intracellular effects are expected. Bioelectrochemistry 2012, 87, 236–243. [Google Scholar] [CrossRef] [PubMed]
- Su, Q.P.; Du, W.Q.; Ji, Q.H.; Xue, B.X.; Jiang, D.; Zhu, Y.Y.; Lou, J.Z.; Yu, L.; Sun, Y.J. Vesicle size regulates nanotube formation in the cell. Sci. Rep. 2016, 6. [Google Scholar] [CrossRef] [PubMed]
- Korolchuk, V.I.; Saiki, S.; Lichtenberg, M.; Siddiqi, F.H.; Roberts, E.A.; Imarisio, S.; Jahreiss, L.; Sarkar, S.; Futter, M.; Menzies, F.M.; et al. Lysosomal positioning coordinates cellular nutrient responses. Nat. Cell Biol. 2011, 13, 453. [Google Scholar] [CrossRef] [PubMed]
- Ullery, J.C.; Tarango, M.; Roth, C.C.; Ibey, B.L. Activation of autophagy in response to nanosecond pulsed electric field exposure. Biochem. Biophys. Res. Commun. 2015, 458, 411–417. [Google Scholar] [CrossRef] [PubMed]
- Ivey, J.W.; Latouche, E.L.; Sano, M.B.; Rossmeisl, J.H.; Davalos, R.V.; Verbridge, S.S. Targeted cellular ablation based on the morphology of malignant cells. Sci. Rep. 2015, 5. [Google Scholar] [CrossRef] [PubMed]
- Tolstykh, G.P.; Thompson, G.L.; Beier, H.T.; Steelman, Z.A.; Ibey, B.L. nsPEF-induced PIP2 depletion, PLC activity and actin cytoskeletal cortex remodeling are responsible for post-exposure cellular swelling and blebbing. Biochem. Biophys. Rep. 2017, 9, 36–41. [Google Scholar] [CrossRef] [PubMed]

© 2018 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (http://creativecommons.org/licenses/by/4.0/).
Share and Cite
Thompson, G.L.; Beier, H.T.; Ibey, B.L. Tracking Lysosome Migration within Chinese Hamster Ovary (CHO) Cells Following Exposure to Nanosecond Pulsed Electric Fields. Bioengineering 2018, 5, 103. https://doi.org/10.3390/bioengineering5040103
Thompson GL, Beier HT, Ibey BL. Tracking Lysosome Migration within Chinese Hamster Ovary (CHO) Cells Following Exposure to Nanosecond Pulsed Electric Fields. Bioengineering. 2018; 5(4):103. https://doi.org/10.3390/bioengineering5040103
Chicago/Turabian StyleThompson, Gary L., Hope T. Beier, and Bennett L. Ibey. 2018. "Tracking Lysosome Migration within Chinese Hamster Ovary (CHO) Cells Following Exposure to Nanosecond Pulsed Electric Fields" Bioengineering 5, no. 4: 103. https://doi.org/10.3390/bioengineering5040103
APA StyleThompson, G. L., Beier, H. T., & Ibey, B. L. (2018). Tracking Lysosome Migration within Chinese Hamster Ovary (CHO) Cells Following Exposure to Nanosecond Pulsed Electric Fields. Bioengineering, 5(4), 103. https://doi.org/10.3390/bioengineering5040103





