Decellularized Human Umbilical Artery Used as Nerve Conduit
Abstract
:1. Introduction
2. Materials and Methods
2.1. Collection and Isolation of Human Umbilical Arteries
2.2. Decellularization of Human Umbilical Arteries
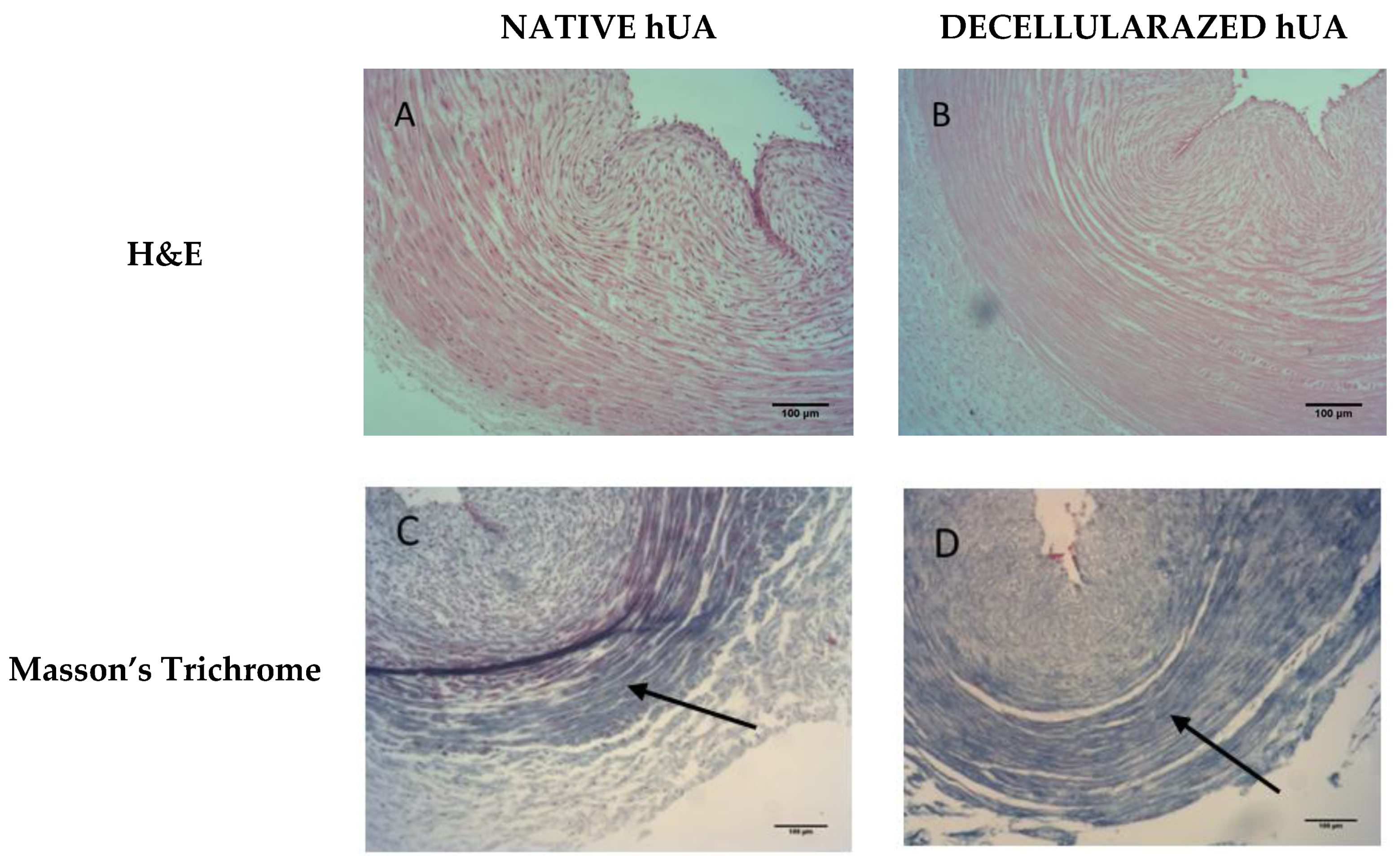
2.3. Histological Evaluation of Decellularized Arteries
2.4. Evaluation of Toxicity of Decellularized Umbilical Arteries
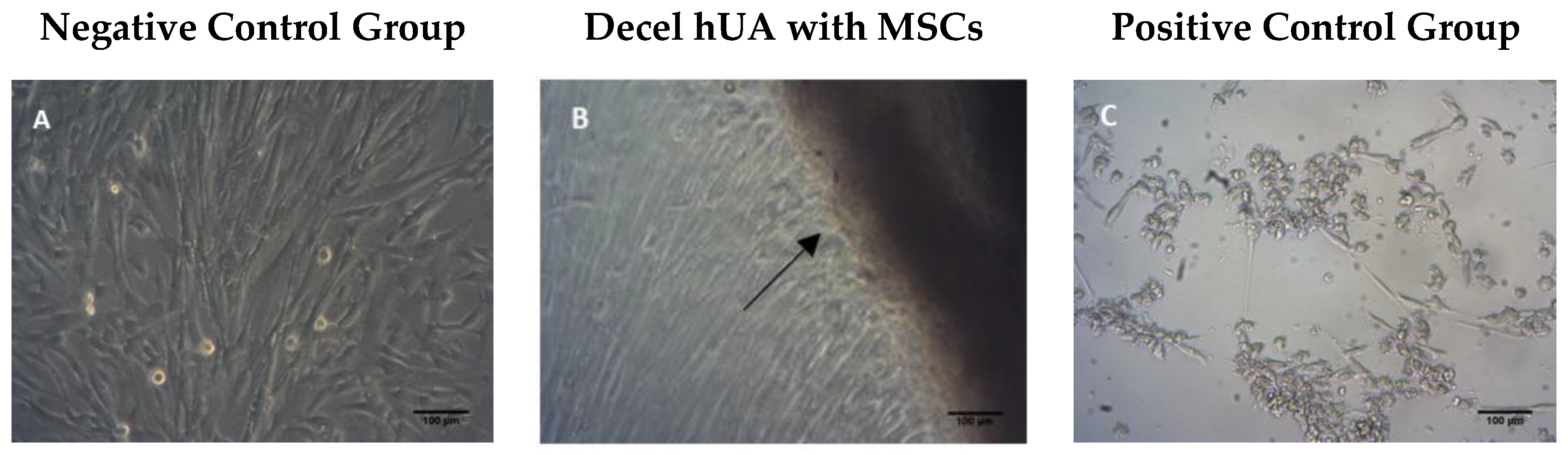
2.4.1. Contact Cytotoxicity Assay
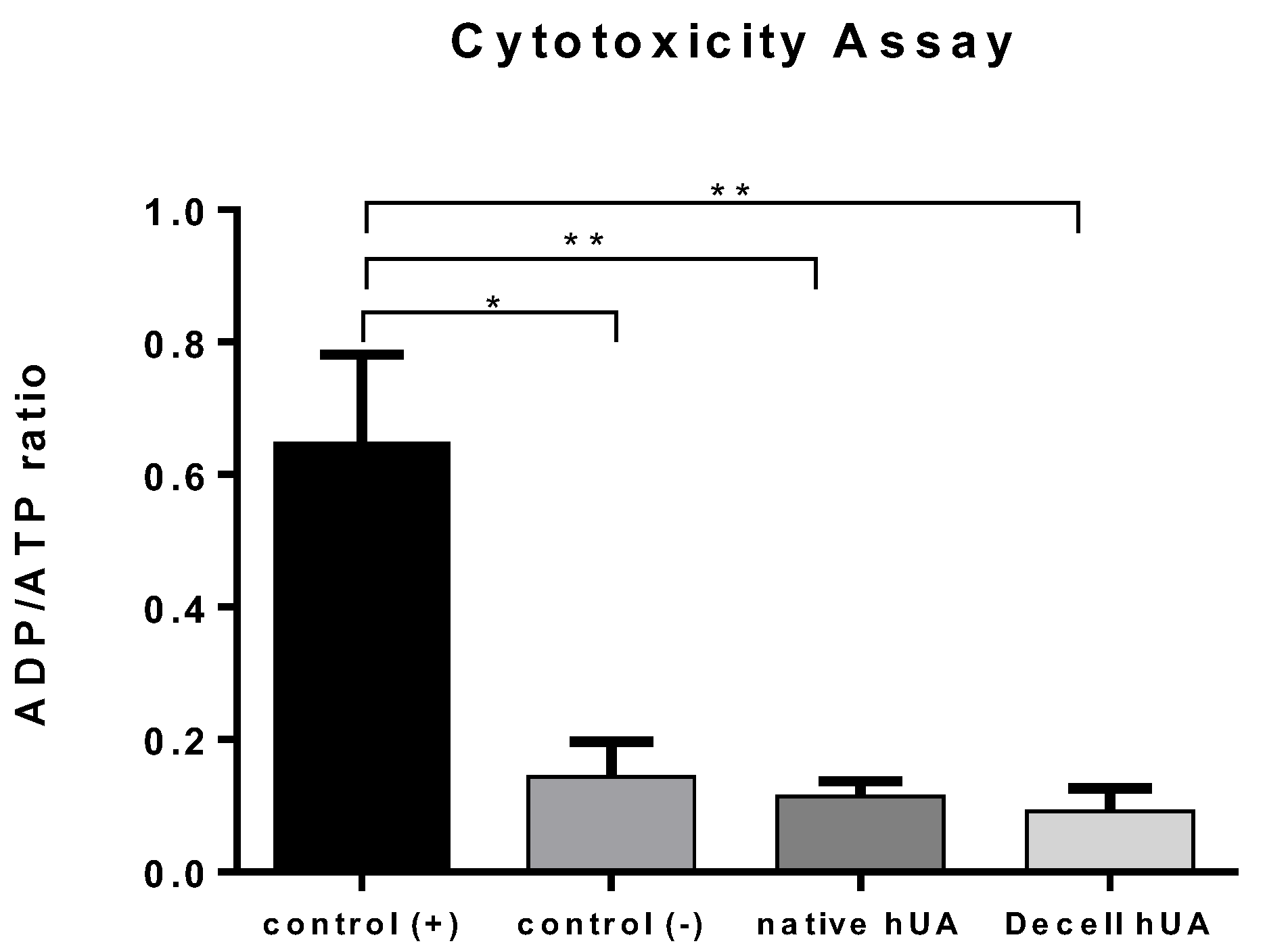
2.4.2. ADP/ATP Ratio Assay
2.5. Animals
2.6. Surgical Procedure
2.7. Sciatic Functional Index (SFI)
2.8. Nerve Graft Harvested Tissue
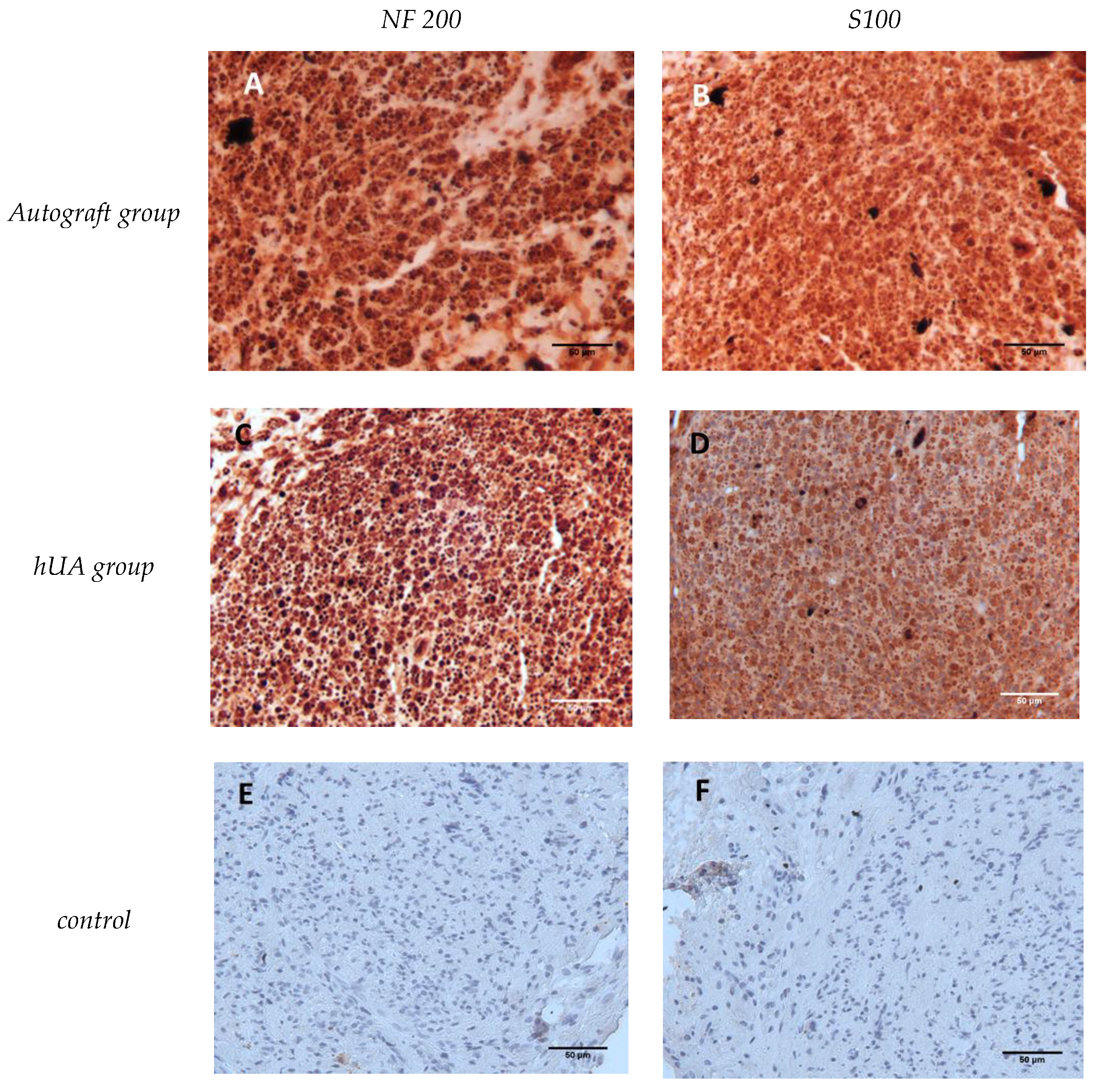
2.9. Nerve Immunohistochemistry
2.10. Morphometric Analysis of Nerve
2.11. Gastrocnemius Muscle Histology and Muscle Weight Ratio
2.12. Statistical Analysis
3. Results
3.1. Histological Analysis
3.2. Cytotoxicity Tolerance of the Decellularized hUA
3.3. Macroscopic Examination of the Experimental Sciatic Nerve In Situ
3.4. Motor Function Assessment
3.5. Immunohistochemical Detection of Neurofilaments and Schwann Cells
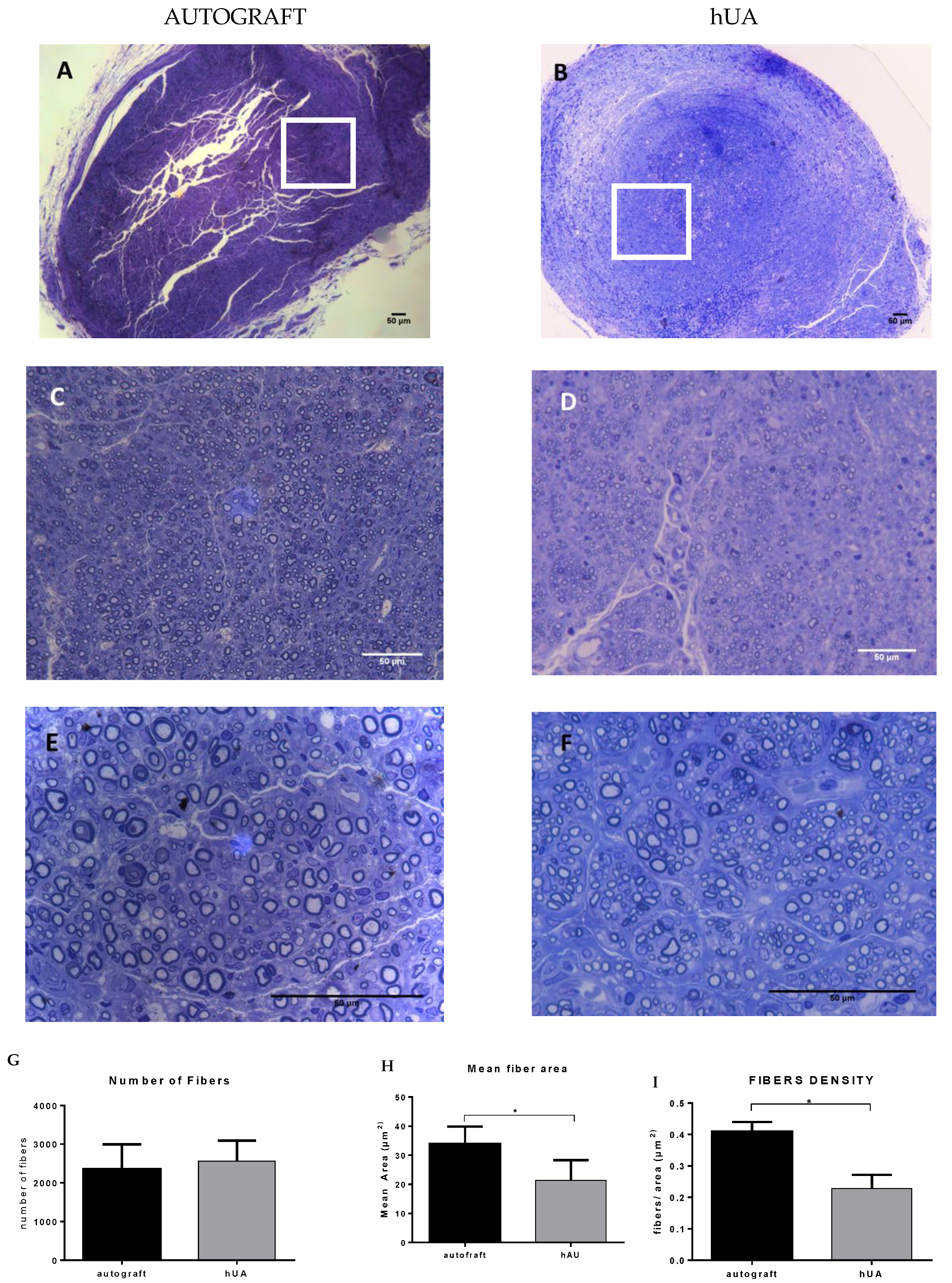
3.6. Morphometric Analysis
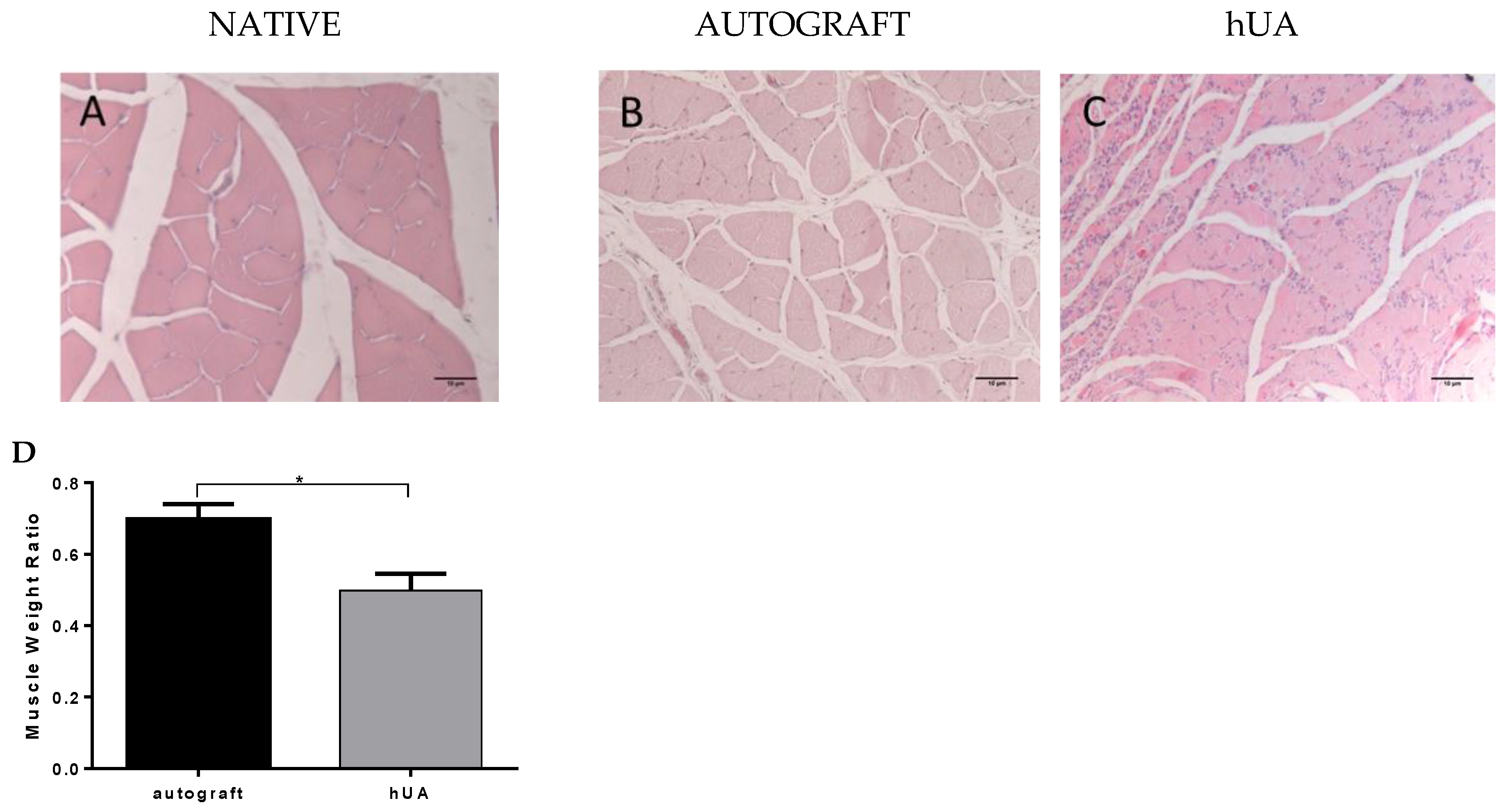
3.7. Gastrocnemius Muscle Histology and Muscle Weight Ratio
4. Discussion
5. Conclusions
Author Contributions
Funding
Conflicts of Interest
References
- Robinson, L.R. Traumatic injury to peripheral nerves. Muscle Nerve 2000, 23, 863–873. [Google Scholar] [CrossRef]
- Taylor, C.A.; Braza, D. The incidence of peripheral nerve injury in extremity trauma. Am. J. Phys. Med. Rehabil. 2008, 8, 381–385. [Google Scholar] [CrossRef] [PubMed]
- Asplund, M.; Nilsson, M. Incidence of traumatic peripheral nerve injuries and amputations in Sweden between 1998 and 2006. Neuroepidemiology 2009, 32, 217–228. [Google Scholar] [CrossRef] [PubMed]
- Ciardelli, G.; Chiono, V. Materials for peripheral nerve regeneration. Macromol. Biosci. 2006, 6, 13–26. [Google Scholar] [CrossRef] [PubMed]
- Alluin, O.; Wittmann, C. Functional recovery after peripheral nerve injury and implantation of a collagen guide. Biometarials 2009, 30, 363–373. [Google Scholar] [CrossRef] [PubMed]
- Daly, W.; Yao, L.A. Biomaterial approach to peripheral nerve regeneration: Bringing the peripheral nerve gap and enhancing functional recovery. J. R. Soc. Interface 2012, 9, 202–221. [Google Scholar] [CrossRef] [PubMed]
- Ansselin, A.D.; Davey, D.F. Axonal regeneration through peripheral nerve grafts: The effect of proximal-distal orientation. Microsurgery 1988, 9, 103–113. [Google Scholar] [CrossRef] [PubMed]
- Nichols, C.M.; Brenner, M.J. Effects of motor versus sensory nerve grafts on peripheral nerve regeneration. Exp. Neurol. 2004, 190, 347–355. [Google Scholar] [CrossRef] [PubMed]
- Konofaos, P.; Ver Halen, J.P. Nerve repair by means of tubulization: Past, present, future. J. Reconstr. Microsurg. 2013, 29, 149–164. [Google Scholar] [CrossRef] [PubMed]
- Crapo, P.M.; Gilbert, T.W. An overview of tissue and whole organ decellularization Processes. Biomaterials 2011, 32, 3233–3243. [Google Scholar] [CrossRef] [PubMed]
- Bellamkonda, R.V. Peripheral nerve regeneration: An opinion of channels scaffolds and anisotropy. Biometarials 2006, 27, 3515–3518. [Google Scholar] [CrossRef] [PubMed]
- Jiang, X.; Lim, S.H. Current applications and future perspectives of artificial nerve conduit. Exp. Neurol. 2010, 223, 86–101. [Google Scholar] [CrossRef] [PubMed]
- Philips, C.; Cornelissen, M. Evaluation methods as quality control in the generation of decellularized peripheral nerve allografts. J. Neural. Eng. 2018, 15, 021003. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Gu, X.; Ding, F. Construction of tissue engineered nerve grafts and their application in peripheral nerve regeneration. Prog. Neurobiol. 2011, 93, 204–230. [Google Scholar] [CrossRef] [PubMed]
- Karabekmez, F.E.; Duymaz, A. Early clinical outcomes with the use of decellularized nerve allograft for repair of sensory defects within the hand. HAND 2009, 4, 245–249. [Google Scholar] [CrossRef] [PubMed]
- Ahmad, I.; Alhtar, M.S. Use of vein conduit and isolated nerve graft in peripheral nerve repair: A comparative study. Plast. Surg. Int. 2014, 27, 587968. [Google Scholar] [CrossRef] [PubMed]
- Mallis, P.; Gontika, I. Evaluation of decellularization in umbilical cord artery. Transplant. Proc. 2014, 46, 3232–3239. [Google Scholar] [CrossRef] [PubMed]
- Liao, I.C.; Wan, H. Preclinical evaluations of acellular biological conduits for peripheral nerve regeneration. J. Tissue Eng. 2013, 28. [Google Scholar] [CrossRef] [PubMed]
- Jongbae, C.; Jun, H.K. Decellularized sciatic nerve matrix as a biodegradable conduit for peripheral nerve regeneration. Neural. Regen. Res. 2018, 13, 1796–1803. [Google Scholar] [CrossRef]
- Valiyaveettil, M.; Achur, R.N. Characterization of chondroitin sulfate and dermatan sulfate proteoglycans of extracellular matrices of human umbilical cord blood vessels and Wharton’s jelly. Glycoconj. J. 2004, 21, 361–375. [Google Scholar] [CrossRef] [PubMed]
- Bain, J.R.; Mackinnon, S.E. Functional evaluation of complete sciatic, peroneal tibial nerve lesions in the rat. Plast. Reconstr. Surg. 1989, 83, 129–138. [Google Scholar] [CrossRef] [PubMed]
- Rafijah, G.; Bowen, A.J. The effects of adjuvant fibrin sealant on the surgical repair of segmental nerve defects in an animal model. J. Hand Surg. Am. 2013, 38, 847–855. [Google Scholar] [CrossRef] [PubMed]
- Gilbert, T.W.; Sellaro, T.L. Decellularization of tissues and organs. Biomaterials 2006, 27, 3675–3683. [Google Scholar] [CrossRef] [PubMed]
- Sun, F.; Zhou, K. Combined use of decellularized allogeneic artery conduits with autologous transdifferentiated adipose-derived stem cells for facial nerve regeneration in rats. Biomaterials 2011, 23, 8118–8128. [Google Scholar] [CrossRef] [PubMed]
- Wakimura, Y.; Wang, W. An experimental study to bridge a nerve gap with a decellularized allogeneic nerve. Plast. Reconstr. Surg. 2015, 136, 319–327. [Google Scholar] [CrossRef] [PubMed]
- Barcelos, A.S.; Rodriges, A.C. Inside out vein graft and inside out artery graft in rat sciatic nerve repair. Microsurgery 2003, 23, 66–71. [Google Scholar] [CrossRef] [PubMed]
- Thanos, P.K.; Okajima, S. Ultrastructure and cellular biology of nerve regeneration. J. Reconstr. Microsurg. 1998, 14, 423–436. [Google Scholar] [CrossRef] [PubMed]
- Armstrong, S.J.; Wiberg, M. ECM molecules mediate both Schwann cell proliferation and activation to enhance neurite outgrowth. Tissue Eng. 2007, 13, 2863–2870. [Google Scholar] [CrossRef] [PubMed]
- Zu, S.; Ge, J. A synthetic oxygen carrier olfactory ensheathing cell composition system for the promotion of sciatic nerve regeneration. Biometerials 2014, 35, 1450–1461. [Google Scholar] [CrossRef]
- Jiang, X.; Lim, S.H. Current application and future perspectives of artificial conduits. Exp. Neurol. 2010, 223, 86–101. [Google Scholar] [CrossRef] [PubMed]
- Zheng, C.; Zhu, Q.C. Improved peripheral nerve regeneration using acellular nerve allografts loaded with platelet-rich plasma. Tissue Eng. Part A 2014, 20, 3228–3240. [Google Scholar] [CrossRef] [PubMed]
- Kim, J.Y.; Jeon, W.J. An iside out vein graft filled with platelet-rich plasma for repair of a short sciatic nerve defect in rats. Neural Reg Res. 2014, 9, 1351–1357. [Google Scholar] [CrossRef]
- Sabongi, R.G.; De Rizzo, L.A. Nerve regeneration: Is there an alternative to nervous graft? J. Reconstr. Microsurg. 2014, 30, 607–616. [Google Scholar] [CrossRef] [PubMed]
- Sarker, M.D.; Saman, N. Regeneration of peripheral nerves by nerve guidance conduits: Influence of design, biopolymers, cells, growth factors, and physical stimuli. Prog. Neurobiol. 2018, in press.. [Google Scholar] [CrossRef] [PubMed]
- Komiyama, T.; Nakao, Y. Novel technique for peripheral nerve reconstruction in the absence of an artificial conduit. J. Neurosci. Methods 2004, 134, 133–140. [Google Scholar] [CrossRef] [PubMed]
- Yu, W.; Zhao, W. Sciatic nerve regeneration in rats by a promising electrospun collagen/poly(ε-caprolactone) nerve conduit with tailored degradation rate. BMC Neurosci. 2011, 12, 68. [Google Scholar] [CrossRef] [PubMed]
- Bodine, S.C.; Latres, E. Identification of ubiquitin ligases required for skeletal muscle atrophy. Science 2001, 294, 1704–1708. [Google Scholar] [CrossRef] [PubMed]

| Pre-Operative | 4 Weeks | 12 Weeks | |
|---|---|---|---|
| Autograft group | −10.07 ± 3,38 | −87.36 ± 6,27 | −51.35 ± 7.84 |
| hUA group | −8.26 ± 5,1 | −80.89 ± 9,22 | −70.56 ± 15.38 |
© 2018 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (http://creativecommons.org/licenses/by/4.0/).
Share and Cite
Gontika, I.; Katsimpoulas, M.; Antoniou, E.; Kostakis, A.; Stavropoulos-Giokas, C.; Michalopoulos, E. Decellularized Human Umbilical Artery Used as Nerve Conduit. Bioengineering 2018, 5, 100. https://doi.org/10.3390/bioengineering5040100
Gontika I, Katsimpoulas M, Antoniou E, Kostakis A, Stavropoulos-Giokas C, Michalopoulos E. Decellularized Human Umbilical Artery Used as Nerve Conduit. Bioengineering. 2018; 5(4):100. https://doi.org/10.3390/bioengineering5040100
Chicago/Turabian StyleGontika, Ioanna, Michalis Katsimpoulas, Efstathios Antoniou, Alkiviadis Kostakis, Catherine Stavropoulos-Giokas, and Efstathios Michalopoulos. 2018. "Decellularized Human Umbilical Artery Used as Nerve Conduit" Bioengineering 5, no. 4: 100. https://doi.org/10.3390/bioengineering5040100





