Conceptual Design of Micro-Bioreactors and Organ-on-Chips for Studies of Cell Cultures
Abstract
1. Introduction
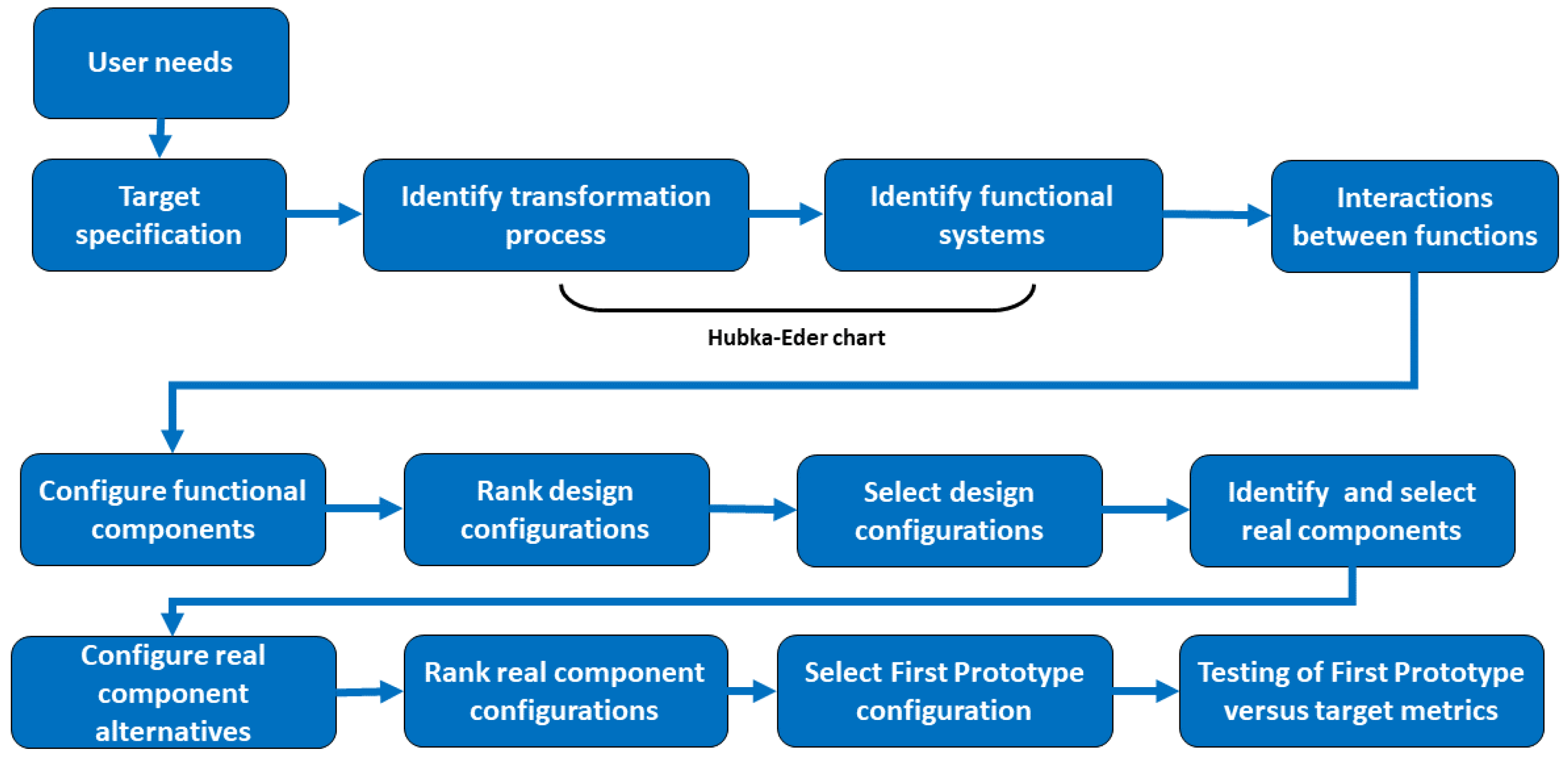
2. Conceptual Design Methodology
2.1. Design Objectives, User Needs and Specification of Target Values for Design
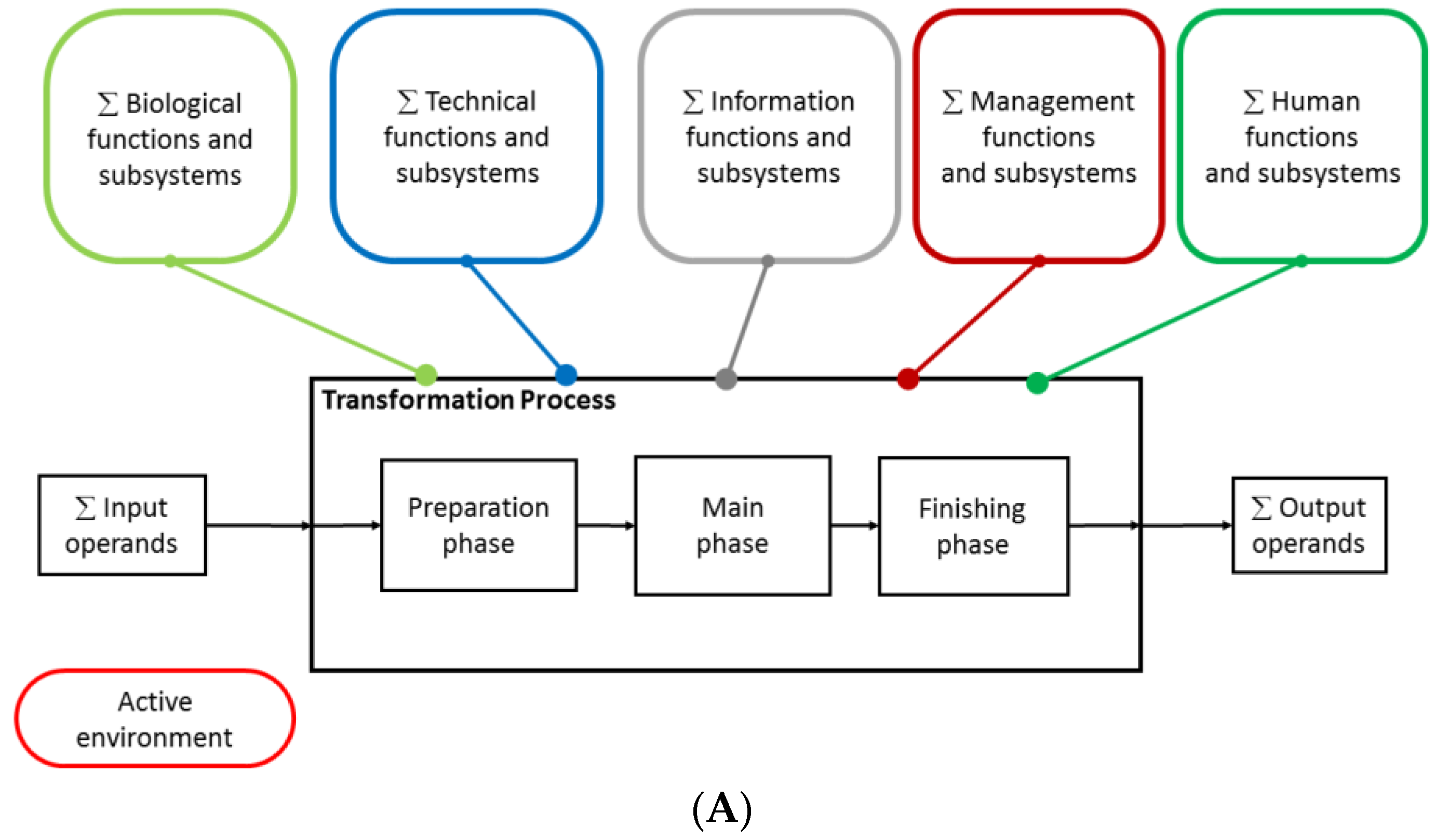
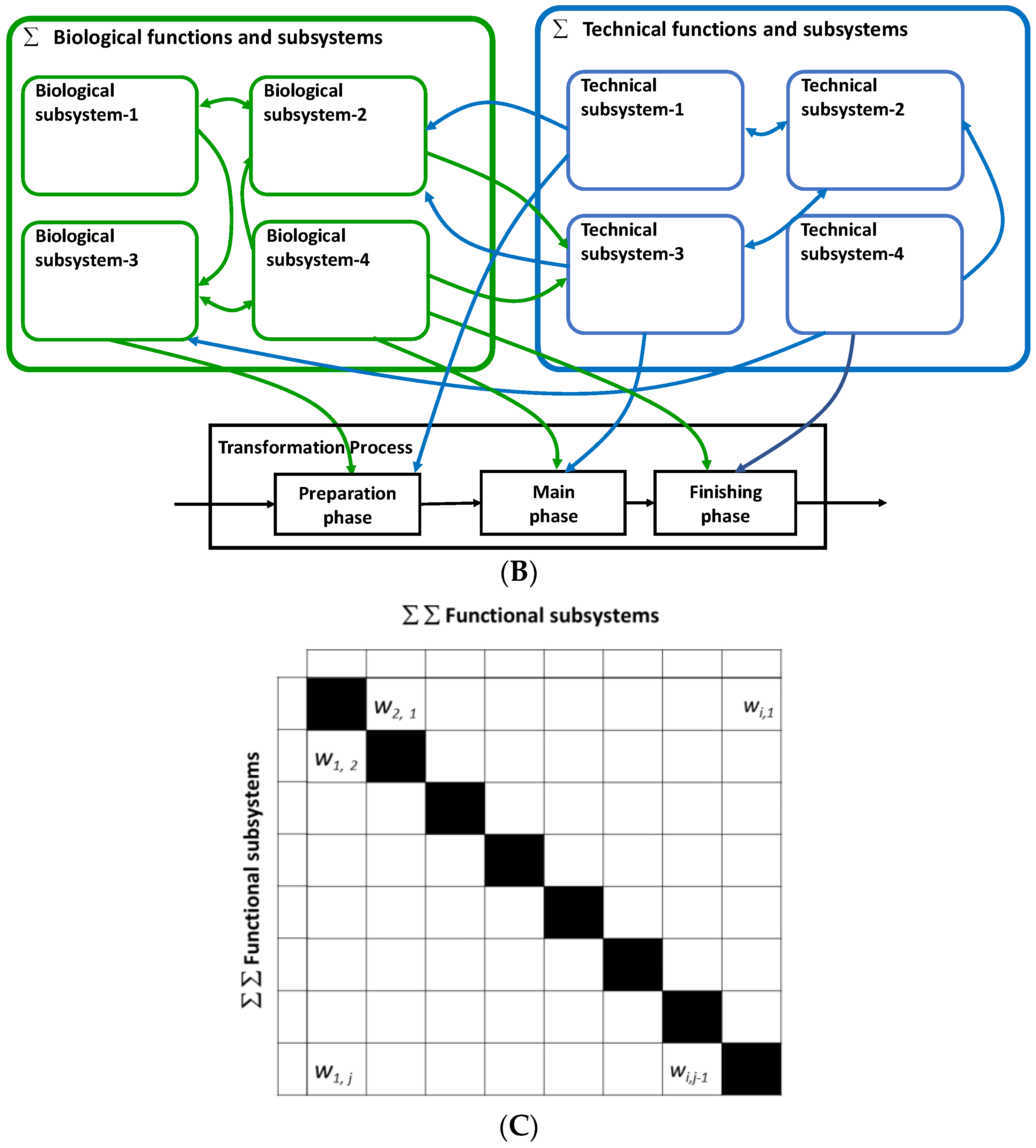
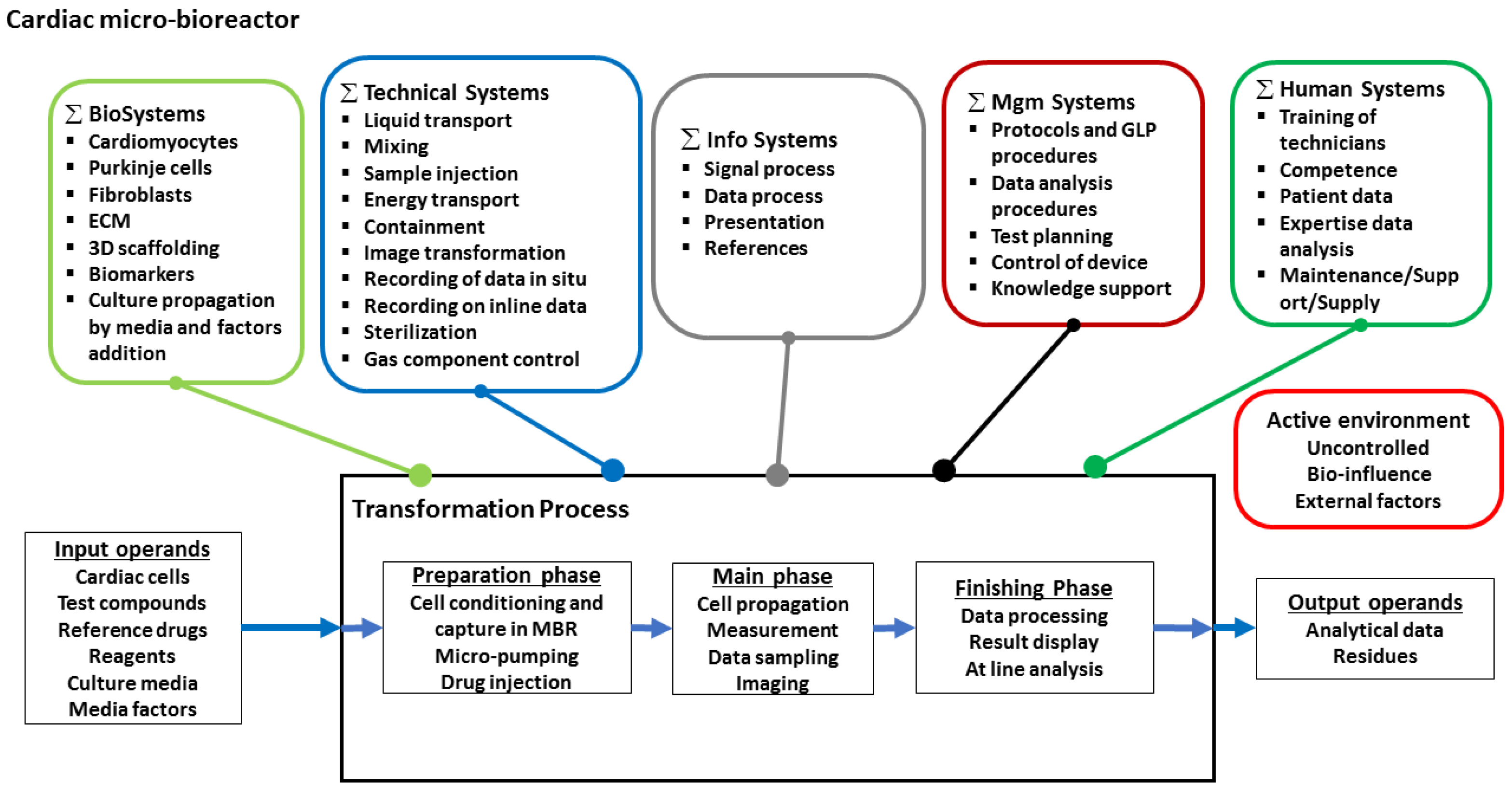
2.2. Mapping of the Functional Systems of the Transformation Process and Assessing Their Interactions
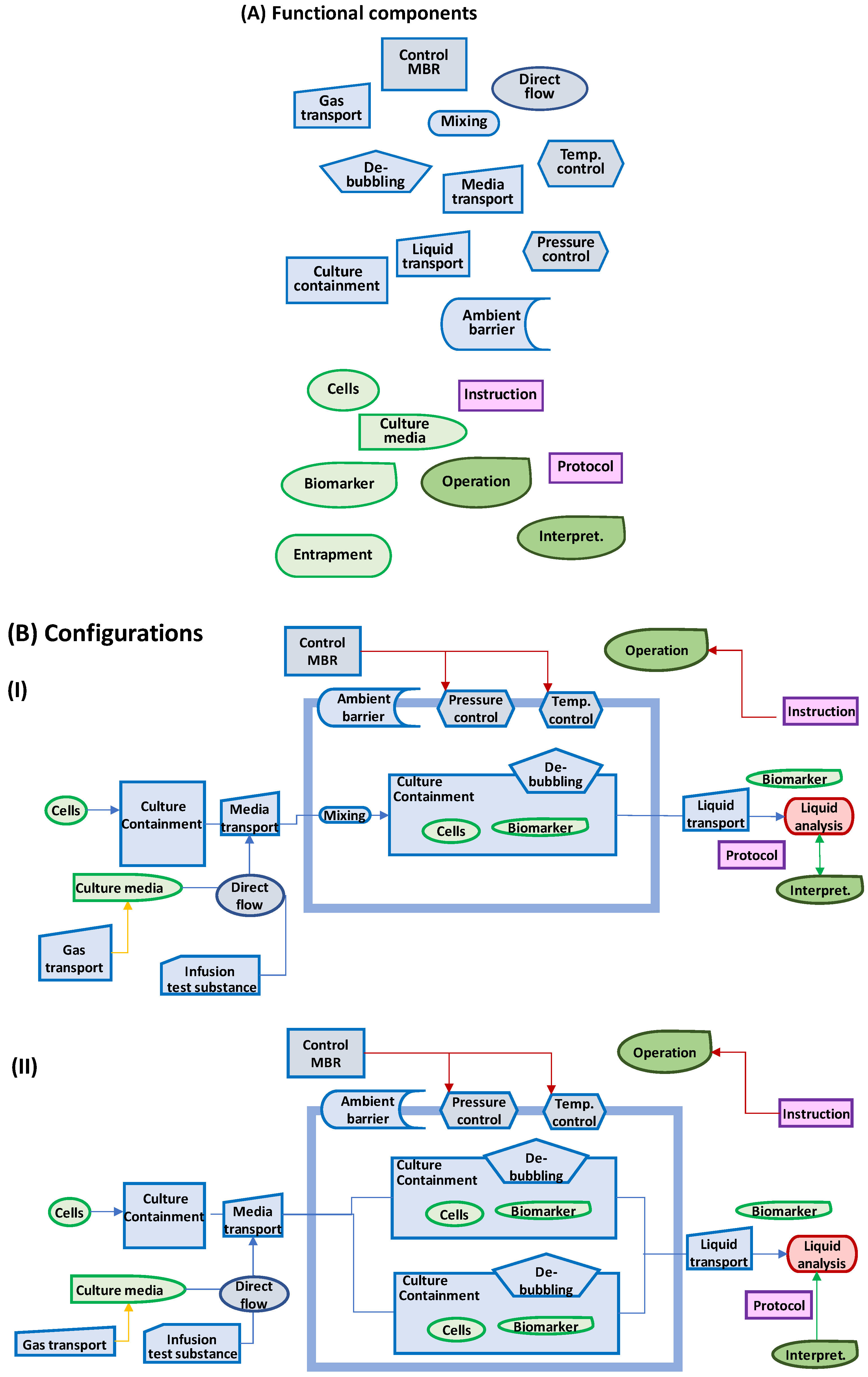
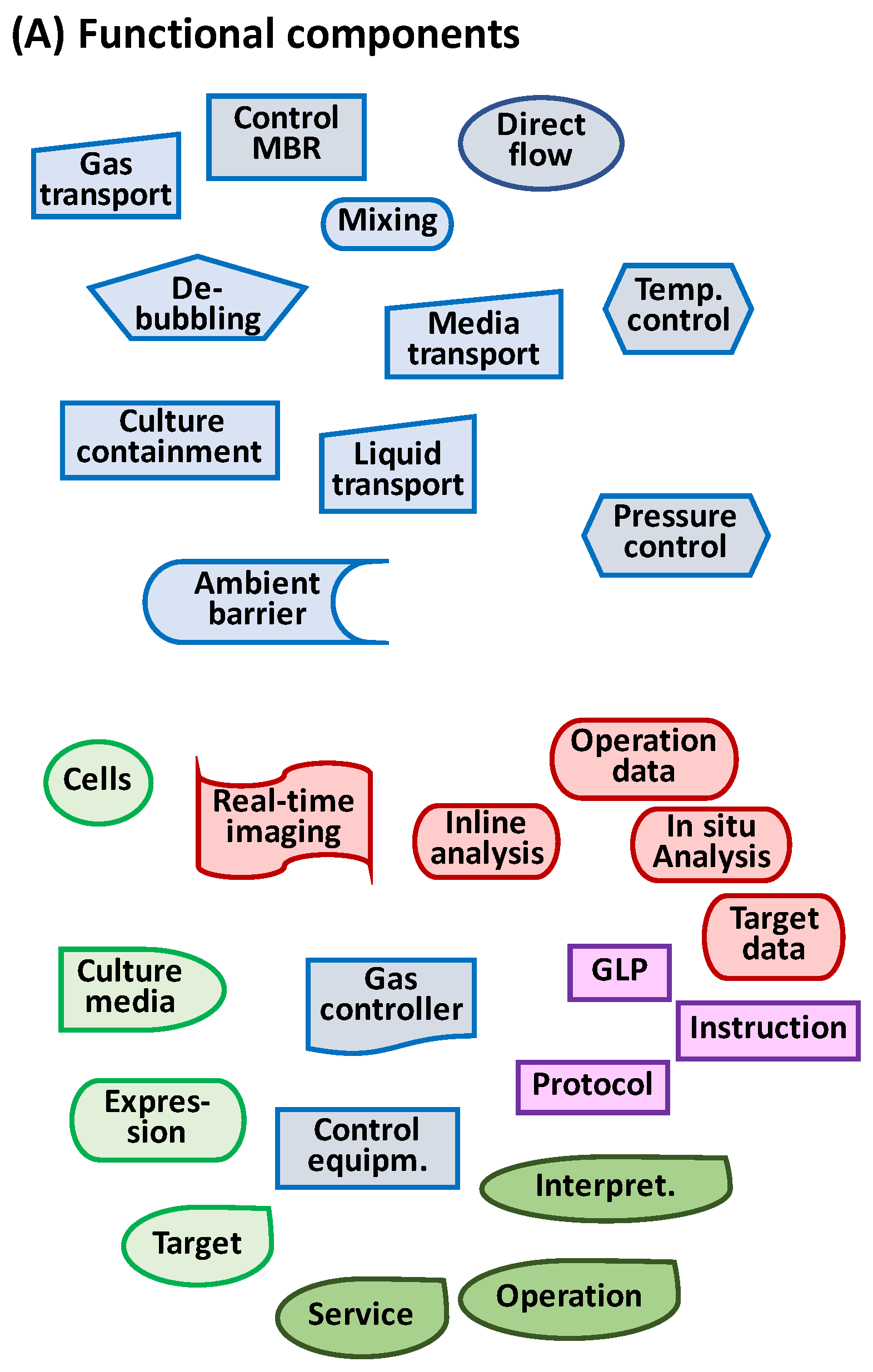
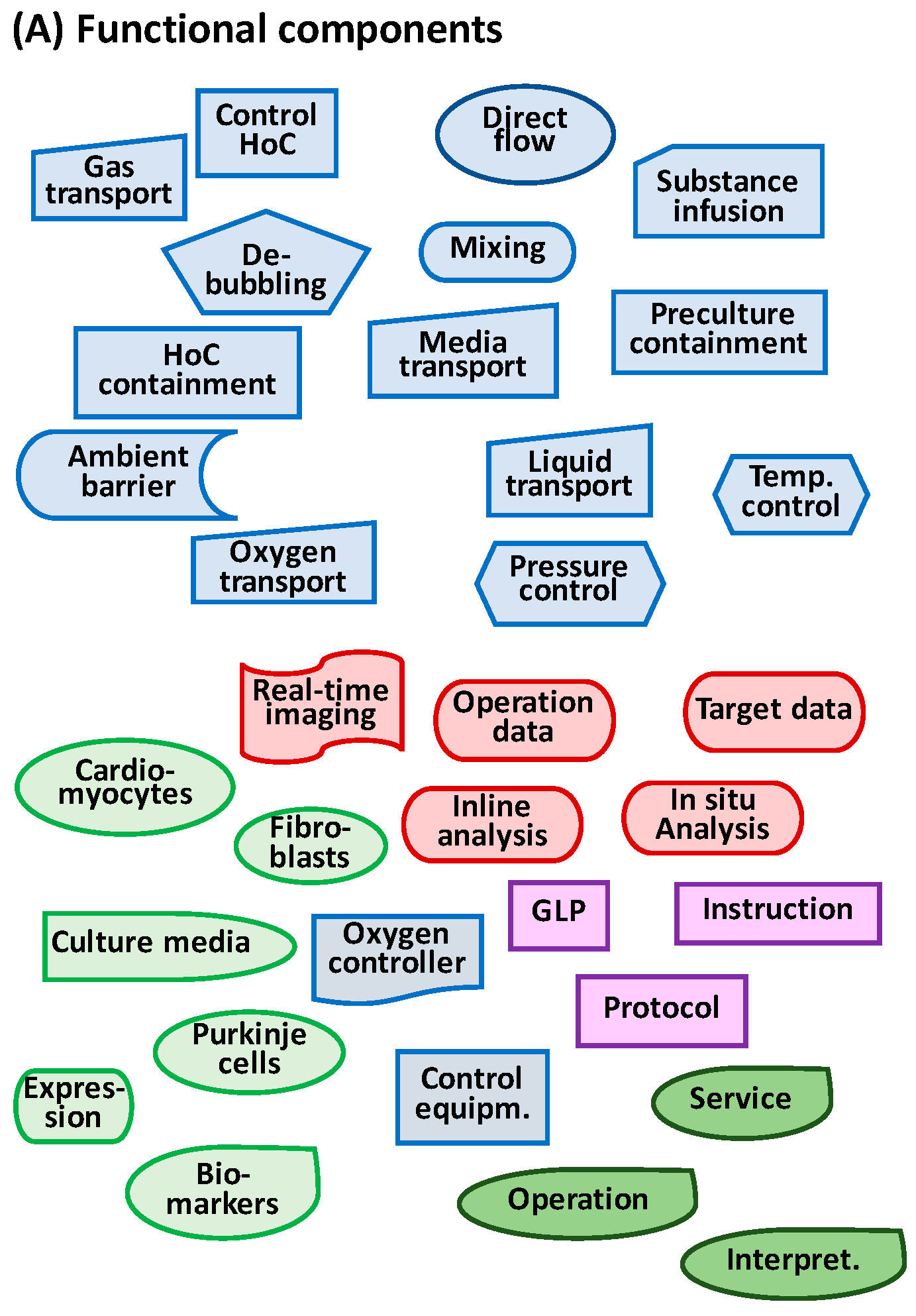
2.3. Key Functional Components
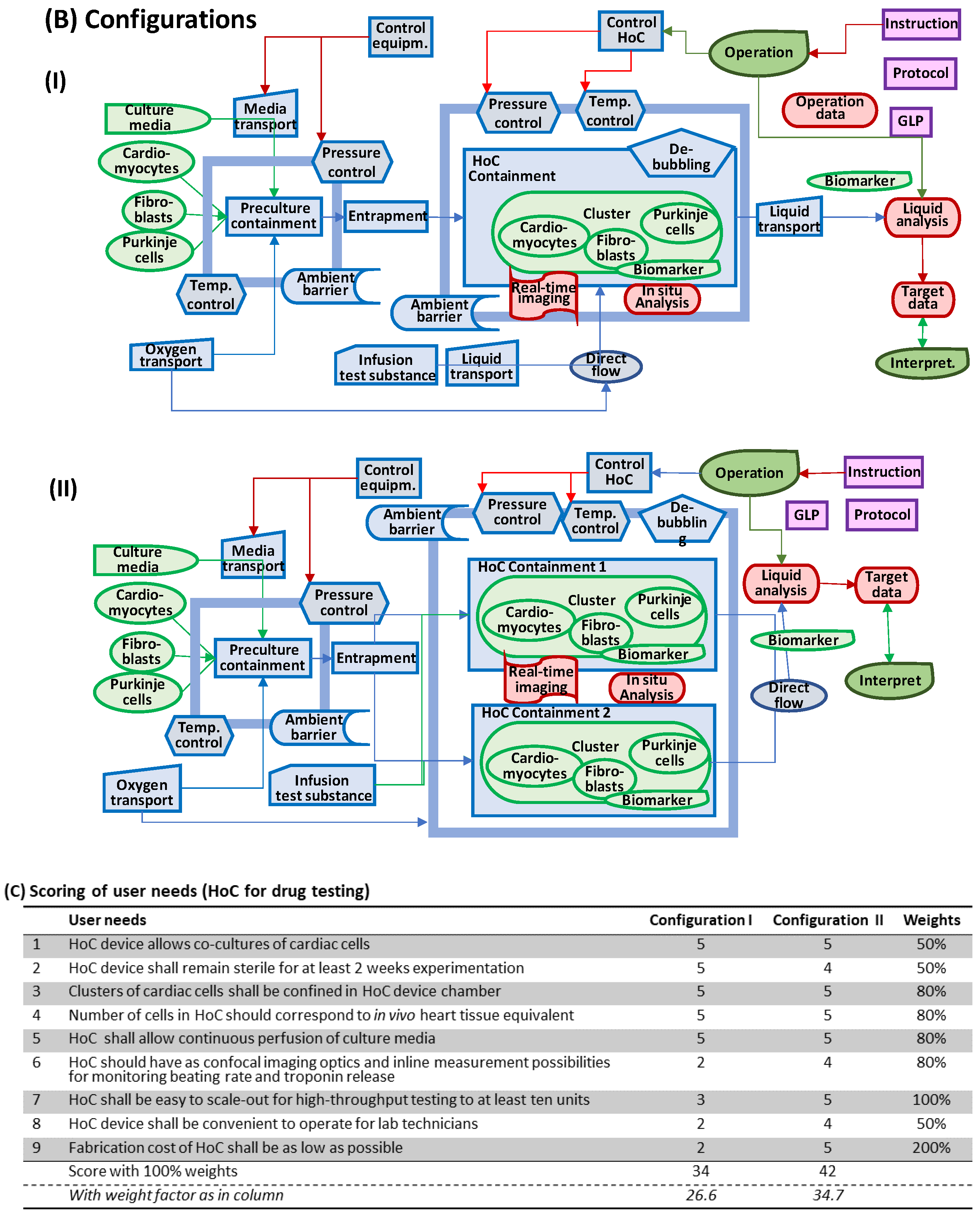
2.4. Assessment of Configuration Alternatives
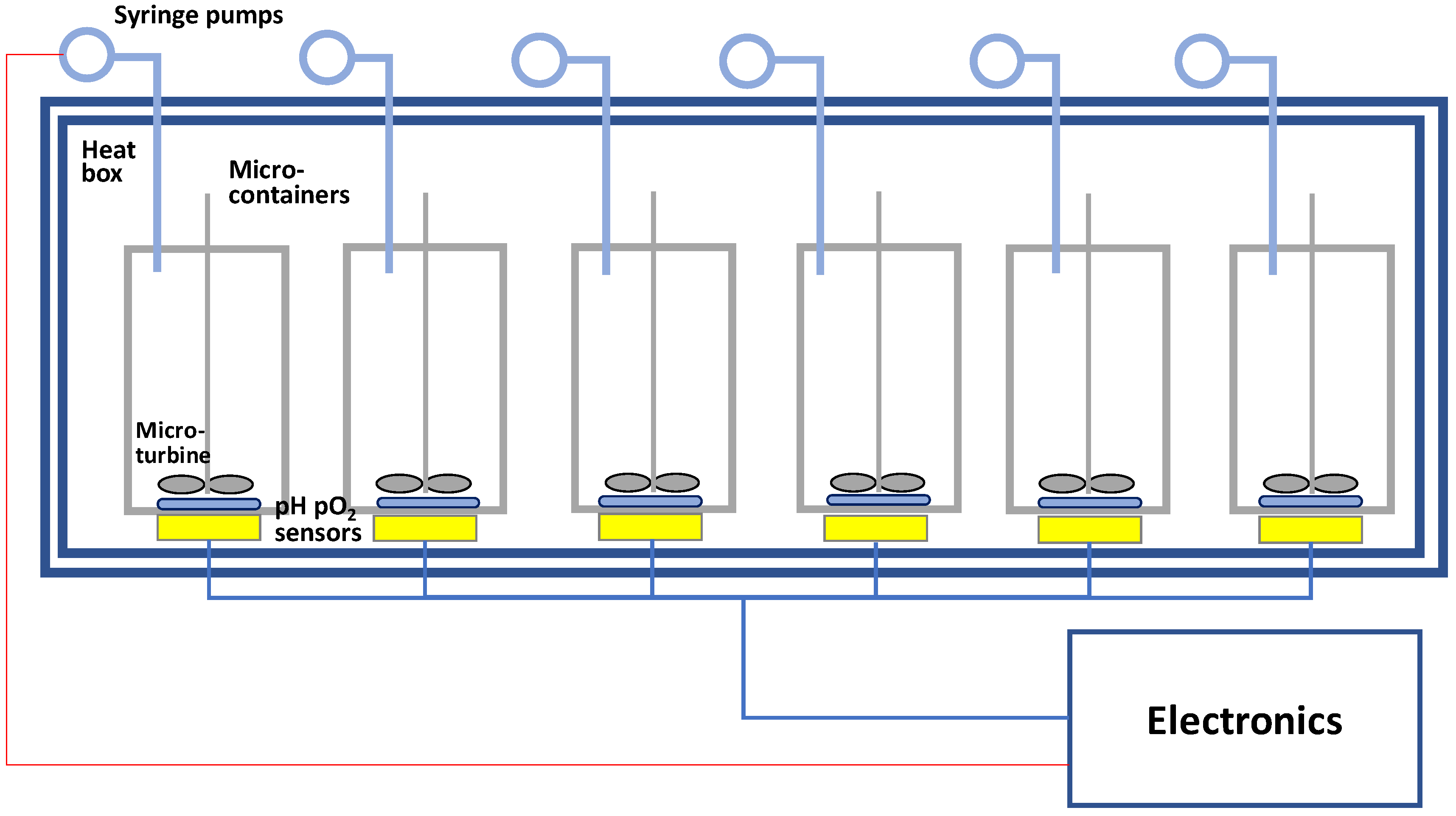
3. A Micro-Bioreactor for Process Development of Monoclonal Antibody Production
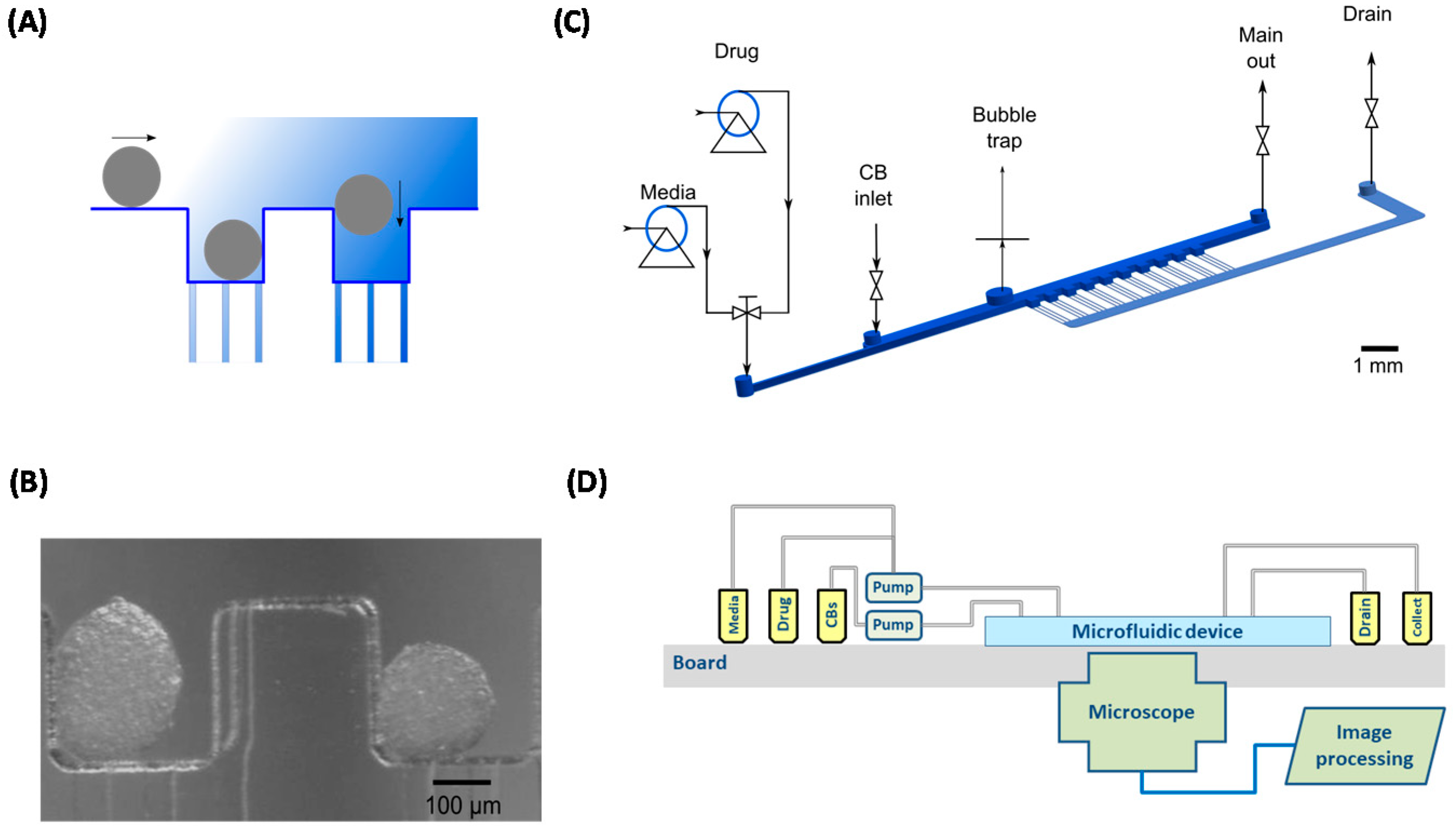
4. A Heart-on-a-Chip Micro-Bioreactor for Assessment of Drug Safety and Efficacy
5. Conclusions and Outlook
Supplementary Materials
Funding
Acknowledgments
Conflicts of Interest
References
- Bhatia, S.N.; Ingber, D.E. Microfluidic organs-on-chips. Nat. Biotechnol. 2014, 32, 760–772. [Google Scholar] [CrossRef] [PubMed]
- Lattermann, C.; Buchs, J. Design and operation of microbioreactor systems for screening and process development. In Bioreactors: Design, Operation and Novel Applications; Mandenius, C.F., Ed.; Wiley-VCH: Weinheim, Germany, 2016; pp. 35–78. ISBN 978-3-527-33768-2. [Google Scholar]
- Huh, D.; Matthews, B.D.; Mammoto, A.; Montoya-Zavala, M.; Hsin, H.Y.; Ingber, D.E. Reconstituting organ-level lung functions on a chip. Science 2010, 328, 1662–1668. [Google Scholar] [CrossRef] [PubMed]
- Hegab, M.H.; ElMekawy, A.; Stakenborg, T. Review of microfluidic microbioreactor technology for high-throughput submerged microbiology cultivation. Biomicrofluidics 2013, 7, 021502. [Google Scholar] [CrossRef] [PubMed]
- Van Duinen, V.; Treetsch, J.; Joore, J.; Vulto, P.; Hankemeier, T. Microfluidic 3D cell culture: From tools to tissue models. Curr. Opin. Biotechnol. 2015, 35, 118–126. [Google Scholar] [CrossRef] [PubMed]
- Sun, W.; Chen, Y.-Q.; Luo, G.-A.; Zhang, M.; Zhang, H.-Y.; Wang, Y.-R.; Hu, P. Organ-on-chips and its applications. Chin. J. Anal. Chem. 2016, 44, 533–541. [Google Scholar] [CrossRef]
- Selden, C.; Fuller, B. Role of bioreactor technology in tissue engineering for clinical use and therapeutic target design. Bioengineering 2018, 5, 32. [Google Scholar] [CrossRef] [PubMed]
- Neuzi, P.; Giselbrecht, S.; Lange, K.; Huang, T.J.; Manz, A. Revisiting lab-on-a-chip technology for drug discovery. Nat. Rev. Drug Discov. 2012, 11, 620–632. [Google Scholar] [CrossRef] [PubMed]
- Esch, E.W.; Bahinski, A.; Huh, D. Organs-on-chips at the frontiers of drug discovery. Nat. Rev. Drug Discov. 2015, 14, 248–260. [Google Scholar] [CrossRef] [PubMed]
- Skardal, A.; Shupe, T.; Atala, A. Organoid-on-a-chip and body-on-a-chip systems for drug screening and disease modelling. Drug Discov. Today 2016, 21, 1399–1411. [Google Scholar] [CrossRef] [PubMed]
- Hemmerich, J.; Noack, S.; Wiechert, W.; Oldiges, M. Microbioreactor systems for accelerated bioprocess development. Biotechnol. J. 2018, 13, 1700141. [Google Scholar] [CrossRef] [PubMed]
- Schapper, D.; Zainal Alam, M.N.H.; Szita, N.; Eliasson Lantz, A.; Gernaey, K.V. Application of microbioreactors in fermentation process development: A review. Anal. Bioanal. Chem. 2009, 395, 679–695. [Google Scholar] [CrossRef] [PubMed]
- Betts, J.I.; Baganz, F. Miniature bioreactors, current practice and future opportunities. Microb. Cell Fact. 2006, 5, 21. [Google Scholar] [CrossRef] [PubMed][Green Version]
- Duertz, W.A. Microtiter plates as mini-bioreactors: Miniaturization of fermentation methods. Trends Microbiol. 2007, 15, 469–475. [Google Scholar] [CrossRef] [PubMed]
- Van Noort, D. Bioreactors on a chip. In Bioreactors: Design, Operation and Novel Applications; Mandenius, C.F., Ed.; Wiley-VCH: Weinheim, Germany, 2016; pp. 77–112. ISBN 978-3-527-33768-2. [Google Scholar]
- Zanzotto, A.; Boccazzi, P.; Lessard, P.; Sinskey, A.J.; Jensen, K.F. Membrane-areated microbioreactor for high-throughput bioprocessing. Biotechnol. Bioeng. 2004, 87, 243–254. [Google Scholar] [CrossRef] [PubMed]
- Zhang, Z.; Perozziello, G.; Boccazzi, P.; Geschke, O.; Sinskey, A.J.; Jensen, K.F. Microbioreactor for bioprocess development. J. Assoc. Lab. Autom. 2007, 12, 143–151. [Google Scholar] [CrossRef]
- Rameez, S.; Mostafa, S.S.; Miller, C.; Shukla, A.A. High-throughput miniaturized bioreactors for cell culture process, development: Reproducibility, scalability, and control. Biotechnol. Prog. 2014, 30, 718–727. [Google Scholar] [CrossRef] [PubMed]
- ambr® 250. Available online: https://www.sartorius.com/sartorius/en/EUR/products/bioreactors-fermentors/single-use/ambr-250 (accessed on 1 July 2018).
- BioLector. Available online: https://www.m2p-labs.com/bioreactors/ (accessed on 1 July 2018).
- Khetani, S.R.; Bhatia, S.N. Microscale culture of human liver cells for drug development. Nat. Biotechnol. 2008, 26, 120–126. [Google Scholar] [CrossRef] [PubMed]
- Polini, A.; Prodanov, L.; Bhise, N.S.; Manoharan, V.; Dokmeci, M.R.; Khademhosseini, A. Organs on a chip a new tool for drug discovery. Exp. Opin. Drug Discov. 2014, 9, 335–352. [Google Scholar] [CrossRef] [PubMed]
- Freyer, N.; Greuel, S.; Knöspel, F.; Gerstmann, F.; Storoch, L.; Damm, G.; Seehofer, D.; Foster Harris, J.; Iyer, R.; Schubert, F.; et al. Microscale 3D liver construct for hepatotoxicity testing in a perfused human in vitro culture platform. Bioengineering 2018, 5, 24. [Google Scholar] [CrossRef] [PubMed]
- Wrzesinski, K.; Fey, S.J. Metabolic reprogramming and the recovery of physiological functionality in 3D cultures in micro-bioreactors. Bioengineering 2018, 5, 22. [Google Scholar] [CrossRef] [PubMed]
- Mandenius, C.F.; Andersson, T.B.; Alves, P.M.; Batzl-Hartmann, C.; Björquist, P.; Carrondo, M.J.T.; Chesne, C.; Coecke, S.; Edsbagge, J.; Fredriksson, J.M.; et al. Towards preclinical predictive drug testing for metabolism and hepatotoxicity by in vitro models derived from human embryonic stem cells: A report on the Vitrocellomics EU-project. Altern. Lab. Anim. 2011, 39, 147–171. [Google Scholar] [PubMed]
- Tajsoleiman, T.; Abdekhodaie, M.J.; Gernaey, K.V.; Krühne, U. Efficient computational design of a cartilage cell regeneration. Bioengineering 2018, 5, 33. [Google Scholar] [CrossRef] [PubMed]
- Seo, J.; Huh, D. A human blinking eye-on-a-chip. In Proceedings of the 18th International Conference on Miniaturized Systems for Chemistry and Life Sciences, San Antonio, TX, USA, 26–30 October 2014. [Google Scholar]
- Rountree, C.M.; Raghunathan, A.; Troy, J.B.; Saggere, L. Prototype chemical synapse chip for spatially patterned neurotransmitter stimulation of the retina ex vivo. Microsyst. Nanoeng. 2017, 3, 17052. [Google Scholar] [CrossRef]
- Puleo, C.M.; McIntosh Ambrose, W.; Takezawa, T.; Elisseeff, J.; Wang, T.H. Integration and application of vitrified collagen in multi-layered microfluidic devices for corneal micro-tissue culture. Lab Chip 2009, 9, 3221–3227. [Google Scholar] [CrossRef] [PubMed]
- Materne, E.M.; Maschmeyer, I.; Lorenz, A.K.; Horland, R.; Schimek, K.M.; Busek, M.; Sonntag, F.; Lauster, R.; Marx, U. The multi-organ chip—A microfluidic platform for long-term multi-tissue coculture. J. Visual. Exp. 2015, 98, e52526. [Google Scholar] [CrossRef] [PubMed]
- Bauer, S.; Huldt, C.W.; Kanebratt, K.P.; Durieux, I.; Gunne, D.; Andersson, S.; Ewart, L.; Haynes, W.G.; Maschmeyer, I.; Winter, A.; et al. Functional coupling of human pancreatic islets and liver spheroids on-a-chip: Towards a novel human ex vivo type 2 diabetes model. Sci. Rep. 2017, 7, 14620. [Google Scholar] [CrossRef] [PubMed]
- Toh, Y.-C.; Raja, A.; Yu, H.; van Noort, D. A 3D microfluidic model to recapitulate cancer cell migration and invasion. Bioengineering 2018, 5, 29. [Google Scholar] [CrossRef] [PubMed]
- Kühlbach, C.; da Luz, S.; Mueller, M.M.; Baganz, F.; Volker, C.; Hass, V.C. A microfluidic system for investigation of tumor cell extravasation. Bioengineering 2018, 5, 40. [Google Scholar] [CrossRef] [PubMed]
- Gernaey, K.V.; Baganz, F.; Franco-Lara, E.; Kensy, F.; Krühne, U.; Luebberstedt, M.; Marx, U.; Palmquist, E.; Schmid, A.; Schubert, F.; et al. Monitoring and control of microbioreactors: An expert opinion on development needs. Biotechnol. J. 2012, 7, 1308–1314. [Google Scholar] [CrossRef] [PubMed]
- Maoz, B.M.; Herland, A.; Henry, O.Y.F.; Leineweber, W.D.; Yadid, M.; Doyle, J.; Mannix, R.; Kujala, W.D.; FitzGerald, E.A.; Parker, K.K.; et al. Organs-on-Chips with combined multi-electrode array and transepithelial electrical resistance measurement capabilities. Lab Chip 2017, 17, 2294–2302. [Google Scholar] [CrossRef] [PubMed]
- Zhang, Y.S.; Aleman, J.; Shin, S.R.; Kilic, T.; Kim, D.; Shaegh, S.A.; Massa, S.; Riahi, R.; Chae, S.; Hu, N.; et al. Multi-sensor integrated organs-on-chips platform for automated and continual in situ monitoring of organoid behaviors. Proc. Natl. Acad. Sci. USA 2017, 114, E2293–E2302. [Google Scholar] [CrossRef] [PubMed]
- Fernandez, A.C.; Halder, J.M.; Nestl, B.M.; Hauer, B.; Gernaey, K.V.; Krühne, U. Biocatalyst screening with a twist: Application of oxygen sensors integrated in microchannels for screening whole cell biocatalyst variants. Bioengineering 2018, 5, 30. [Google Scholar] [CrossRef] [PubMed]
- Hubka, V.; Eder, E.W. Design Science: Introduction to the Needs, Scope and Organization of Engineering Design Knowledge, 1st ed.; Springer: Berlin, Germany, 1996. [Google Scholar]
- Pahl, G.; Beitz, W.; Feldhusen, J.; Grote, K. Engineering Design: A Systematic Approach, 3rd ed.; Springer: Berlin, Germany, 2007. [Google Scholar]
- Ulrich, K.T.; Eppinger, S.D. Product Design and Development, 3rd ed.; McGraw-Hill: New York, NY, USA, 2007. [Google Scholar]
- Derelöv, M.; Jonas Detterfelt, J.; Mats Björkman, M.; Mandenius, C.-F. Engineering design methodology for bio-mechatronic products. Biotechnol. Prog. 2008, 24, 232–244. [Google Scholar] [CrossRef] [PubMed]
- Mandenius, C.-F.; Björkman, M. Mechatronics design principles for biotechnology product development. Trends Biotechnol. 2010, 28, 230–236. [Google Scholar] [CrossRef] [PubMed]
- Mandenius, C.F.; Björkman, M. Scale-up of bioreactors using biomechantronic design methodology. Biotechnol. J. 2012, 7, 1026–1039. [Google Scholar] [CrossRef] [PubMed]
- Mandenius, C.-F. Biomechatronics for designing bioprocess monitoring and control systems: Application to stem cell production. J. Biotechnol. 2012, 162, 430–440. [Google Scholar] [CrossRef] [PubMed]
- Mandenius, C.F. Design of monitoring and sensor systems for bioprocesses using biomechatronic principles. Chem. Eng. Technol. 2012, 35, 1412–1420. [Google Scholar] [CrossRef]
- Gerlach, I.; Hass, V.C.; Mandenius, C.F. Conceptual design of an operating training simulator for a bio-ethanol plant. Processes 2015, 3, 664–683. [Google Scholar] [CrossRef]
- Christoffersson, J.; van Noort, D.; Mandenius, C.-F. Developing organ-on-a-chip concepts using bio-mechatronic design methodology. Biofabrication 2017, 9, 025023. [Google Scholar] [CrossRef] [PubMed]
- Mandenius, C.-F.; Björkman, M. Biomechatronic Design in Biotechnology—A Methodology for Development of Biotechnological Products, 1st ed.; Wiley & Sons: New York, NY, USA, 2011. [Google Scholar]
- Yetisen, A.K.; Volpatti, L.R.; Coskun, A.F.; Cho, S.; Kamrani, E.; Butt, H.; Khademhosseinidfgh, A.; Yun, S.H. Entrepreneurship. Lab Chip 2015, 15, 3638–3660. [Google Scholar] [CrossRef] [PubMed]
- Junaid, A.; Mashaghi, A.; Henkemeier, T.; Vulto, P. An end-user perspective on Organ-on-a-chip: Assays and usability aspects. Curr. Opin. Biomed. Eng. 2017, 1, 15–22. [Google Scholar] [CrossRef]
- Nienow, A.W.; Rielly, C.D.; Brosnan, K.; Bargh, N.; Lee, K.; Coopman, K.; Hewitt, C.J. The physical characterisation of a microscale parallel bioreactor platform with an industrial CHO cell line expressing an IgG4. Biochem. Eng. J. 2013, 76, 25–36. [Google Scholar] [CrossRef]
- Janakiraman, V.; Kwiatkowski, C.; Kshirsagar, R.; Ryll, T.; Huang, Y.M. Application of high-throughput mini-bioreactor system for systematic scale-down modeling, process characterization, and control strategy development. Biotechnol. Prog. 2015, 31, 623–632. [Google Scholar] [CrossRef] [PubMed]
- Hsu, W.T.; Aulakh, R.P.S.; Traul, D.L.; Yuk, I.H. Advanced microscale bioreactor system: A representative scale-down model for bench-top bioreactors. Cytotechnology 2012, 64, 667–678. [Google Scholar] [CrossRef] [PubMed]
- Zhang, Z.; Boccazzi, P.; Choi, H.G.; Perozziello, G.; Sinskey, A.J.; Jensen, K.F. Microchemostat, a microbial continuous culture in a polymer-based, instrumented microbioreactor. Lab Chip 2006, 6, 906–913. [Google Scholar] [CrossRef] [PubMed]
- Wurm, F.M. Production of recombinant protein therapeutics in cultivated mammalian cells. Nat. Biotechnol. 2004, 22, 1393–1398. [Google Scholar] [CrossRef] [PubMed]
- Huang, Y.M.; Hu, W.W.; Rustandi, E.; Chang, K.; Yusuf-Makagianser, H.; Ryll, T. Maximizing productivity of CHO cell-based fed-batch culture using chemically defined media conditions and typical manufacturing equipment. Biotechnol. Prog. 2010, 26, 1400–1410. [Google Scholar] [CrossRef] [PubMed]
- Griffiths, J.B. Mammalian cell culture reactors, scale-up. In Encyclopedia of Industrial Biotechnology; Flickinger, M.C., Ed.; John Wiley & Sons: Hoboken, NJ, USA, 2010; Volume 5, pp. 3228–3241. [Google Scholar]
- Nienow, A.W. Reactor engineering in large scale animal cell culture. Cytotechnology 2006, 50, 9–33. [Google Scholar] [CrossRef] [PubMed]
- Becker, E.; Florin, L.; Pfizenmaier, K.; Kaufmann, H. An XBP-1 dependent bottle-neck in production of IgG subtype antibodies in chemically defined serum-free Chinese hamster ovary (CHO) fed-batch processes. J. Biotechnol. 2008, 135, 217–223. [Google Scholar] [CrossRef] [PubMed]
- Xu, P.; Clark, C.; Ryder, T.; Sparks, C.; Zhou, J.; Wang, M.; Russell, R.; Scott, C. Characterization of TAP Ambr 250 disposable bioreactors, as a reliable scale-down model for biologics process development. Biotechnol. Prog. 2017, 33, 478–789. [Google Scholar] [CrossRef] [PubMed]
- Kommenhoek, E.E.; van Leeuwen, M.; Gardeniers, H.; van Gulik, W.M.; van den Berg, A.; Li, X.; Ottens, M.; van der Wielen, L.A.M.; Heijnen, J.J. Lab-scale fermentation tests of microchip with integrated electrochemical sensors for pH, temperature, dissolved oxygen and viable biomass concentration. Biotechnol. Bioeng. 2008, 99, 884–892. [Google Scholar] [CrossRef] [PubMed]
- Thuenauer, R.; Juhasz, K.; Mayr, R.; Fruhwirth, T.; Lipp, A.M.; Balogi, Z.; Sonnleitner, A. A PDMS-based biochip with integrated sub-micrometre position control for TIRF microscopy of the apical cell membrane. Lab Chip 2011, 11, 3064–3071. [Google Scholar] [CrossRef] [PubMed]
- Mandenius, C.F. Bioreactors: Design, Operation and Novel Applications; Mandenius, C.F., Ed.; Wiley-VCH: Weinheim, Germany, 2016; ISBN 978-3-527-33768-2. [Google Scholar]
- LabSmith. Available online: http://labsmith.com/ (accessed on 1 July 2018).
- Bengtsson, K.; Christoffersson, J.; Mandenius, C.F.; Robinson, N.D. A clip-on electroosmotic pump for oscillating flow in microfluidic cell culture devices. Microfluid. Nanofluid. 2018, 22, 27. [Google Scholar] [CrossRef]
- Joeris, K.; Frerichs, J.G.; Konstantinov, K.; Scheper, T. In-situ microscopy: Online process monitoring of mammalian cell cultures. Cytotechnology 2002, 38, 129–134. [Google Scholar] [CrossRef] [PubMed]
- Noll, T.; Biselli, M. Dielectric spectroscopy in the cultivation of suspended and immobilized hybridoma cells. J. Biotechnol. 1998, 63, 187–198. [Google Scholar] [CrossRef]
- Tanaka, Y.; Sato, K.; Shimizu, T.; Yamoto, M.; Okano, T.; Kitamori, T. A micro-spherical heart pump powered by cultured cardiomyocytes. Lab Chip 2007, 7, 207–212. [Google Scholar] [CrossRef] [PubMed]
- Grosberg, A.; Alford, P.W.; McCain, M.L.; Parker, K.K. Ensembles of engineered cardiac tissues for physiological and pharmacological study: Heart on a chip. Lab Chip 2011, 11, 4165–4173. [Google Scholar] [CrossRef] [PubMed]
- Martewicz, S.; Michielin, F.; Serena, E.; Zambon, A.; Mongillo, M.; Elvassore, N. Reversible alteration of calcium dynamics in cardiomyocytes during acute hypoxia transient in a microfluidic platform. Integr. Biol. 2012, 4, 153–164. [Google Scholar] [CrossRef] [PubMed]
- Agarwal, A.; Goss, J.A.; Cho, A.; McCain, M.L.; Parker, K.K. Microfluidic heart on a chip for higher throughput pharmacological studies. Lab Chip 2013, 13, 3599–3608. [Google Scholar] [CrossRef] [PubMed]
- Annabi, N.; Selimovic, S.; Acevedo Cox, J.P.; Ribas, J.; Afshar Bakooshli, M.; Heintze, D.; Weiss, A.S.; Cropek, D.; Khademhosseini, A. Hydrogel-coated microfluidic channels for cardiomyocyte culture. Lab Chip 2013, 13, 3569–3577. [Google Scholar] [CrossRef] [PubMed]
- Ren, L.; Liu, W.; Wang, Y.; Wang, J.C.; Tu, Q.; Xu, J.; Liu, R.; Shen, S.F.; Wang, J. Investigation of Hypoxia-Induced Myocardial Injury Dynamics in a Tissue Interface Mimicking Microfluidic Device. Anal. Chem. 2013, 85, 235–244. [Google Scholar] [CrossRef] [PubMed]
- Kaneko, T.; Nomura, F.; Hamada, T.; Abe, Y.; Takamori, H.; Sakakura, T.; Takasuna, K.; Sanbuissho, A.; Hyllner, J.; Sartipy, P.; et al. On-chip in vitro cell-network pre-clinical cardiac toxicity using spatiotemporal human cardiomyocyte measurement on a chip. Sci Rep. 2014, 4, 4670. [Google Scholar] [CrossRef] [PubMed]
- Marsano, A.; Conficconi, C.; Lemme, M.; Occhetta, P.; Gaudiello, E.; Votta, E.; Cerino, G.; Redaelli, A.; Rasponi, M. Beating heart on a chip: A novel microfluidic platform to generate functional 3D cardiac microtissues. Lab Chip 2016, 16, 599–610. [Google Scholar] [CrossRef] [PubMed]
- Zhang, Y.S.; Arneri, A.; Bersini, S.; Shin, S.R.; Zhu, K.; Goli-Malekabadi, Z.; Aleman, J.; Colosi, C.; Busignani, F.; Dell’Erba, V.; et al. Bioprinting 3D microfibrous scaffolds for engineering endothelialized myocardium and heart-on-a-chip. Biomaterials 2016, 110, 45–59. [Google Scholar] [CrossRef] [PubMed]
- Zweigerdt, R.; Gruh, I.; Martin, U. Your heart on a chip: IPSC-based modeling of Barth-syndrome-associated cardiomyopathy. Cell Stem Cell 2014, 15, 9–11. [Google Scholar] [CrossRef] [PubMed]
- Jastrzebska, E.; Tomecka, E.; Jesion, I. Heart-on-a-chip based on stem cell biology. Biosens. Bioelectron. 2016, 75, 67–81. [Google Scholar] [CrossRef] [PubMed]
- Bergström, G.; Christoffersson, J.; Zweigerdt, R.; Schwanke, K.; Mandenius, C.F. Stem cell derived cardiac bodies in a microfluidic device for toxicity testing by beating frequency imaging. Lab Chip 2015, 15, 3242–3249. [Google Scholar] [CrossRef] [PubMed]
- iCell® Cardiomyocytes. Available online: https://cellulardynamics.com/products-services/icell-products/icell-cardiomyocytes/ (accessed on 1 July 2018).
- Morrison, M.; Klein, C.; Clemann, N.; Collier, D.A.; Hardy, J.; Heiβerer, B.; Cader, M.Z.; Graf, M.; Kaye, J. StemBANCC: Governing access to material and data in a large stem cell research consortium. Stem Cell Rev. Rep. 2015, 11, 681–687. [Google Scholar] [CrossRef] [PubMed]
- Mandenius, C.F.; Steel, D.; Noor, F.; Meyer, T.; Heinzle, E.; Asp, J.; Arain, S.; Kraushaar, U.; Bremer, S.; Class, R.; et al. Cardiotoxicity testing using pluripotent stem cell derived human cardiomyocytes and state-of-the-art bioanalytics: A review. J. Appl. Toxicol. 2011, 31, 191–205. [Google Scholar] [CrossRef] [PubMed]
- Andersson, H.; Steel, D.; Asp, J.; Dahlenborg, K.; Jonsson, M.; Kågedal, B.; Jeppsson, A.; Lindahl, A.; Sartipy, P.; Mandenius, C.-F. Assaying cardiac biomarkers for toxicity testing using biosensing and cardiomyocytes derived from human embryonic stem cells. J. Biotechnol. 2010, 150, 175–181. [Google Scholar] [CrossRef] [PubMed]
- Christoffersson, J.; Meier, F.; Kempf, H.; Schwanke, K.; Coffee, M.; Beilmann, M.; Zweigerdt, R.; Mandenius, C.F. A cardiac cell outgrowth assay for evaluating drug compounds using a cardiac spheroid-on-a-chip device. Bioengineering 2018, 5, 36. [Google Scholar] [CrossRef] [PubMed]
- Bergström, G.; Nilsson, K.; Robinson, N.; Mandenius, C.F. Macroporous microcarriers for introducing cells in a microfluidic chip. Lab Chip 2014, 14, 3502–3504. [Google Scholar] [CrossRef] [PubMed]
- PerkinElmer. Available online: www.perkinelmer.com/HighContent/Screening (accessed on 1 July 2018).
- Mimetas. Available online: https://mimetas.com/page/products (accessed on 1 July 2018).
- Estlack, Z.; Bennet, D.; Reid, T.; Kim, J. Microengineered biomimetic ocular models for ophthalmological drug development. Lab Chip 2017, 17, 1539–1551. [Google Scholar] [CrossRef] [PubMed]
- Chan, Y.K.; Sy, K.H.; Wong, C.Y.; Man, P.K.; Wong, D.; Shum, H.C. In vitro modeling of emulsification of silicone oil as intraocular tamponade using micro-engineered Eye-on-a-Chip. Investig. Ophthalmol. Vis. Sci. 2015, 56, 3314–3319. [Google Scholar] [CrossRef] [PubMed]
- Dodson, K.H.; Echevarria, F.D.; Li, D.; Sappington, R.M.; Edd, J.F. Retina-on-a-chip: A microfluidic platform for point access signaling studies. Biomed. Microdevices 2015, 17, 114. [Google Scholar] [CrossRef] [PubMed]
- Seo, J.; Byun, W.Y.; Frank, A.; Massaro-Giordano, M.; Lee, V.; Bunya, V.Y.; Huh, D. Human blinking eye-on-a-chip. Investig. Ophthalmol. Vis. Sci. 2016, 57, 3872. [Google Scholar]
- Grause, S.; Hsu, K.H.; Shafor, C.; Dixon, P.; Powell, K.C.; Chauhan, A. Mechanistic modelling of ophthalmic drug delivery to the anterior chamber by eye drops and contact lenses. Adv. Colloid Interface Sci. 2016, 233, 139–154. [Google Scholar]
- Darkins, C.L.; Mandenius, C.F. Design of large-scale manufacturing of induced pluripotent stem cell derived cardiomyocytes. Chem. Eng. Res. Des. 2014, 92, 1142–1152. [Google Scholar] [CrossRef]
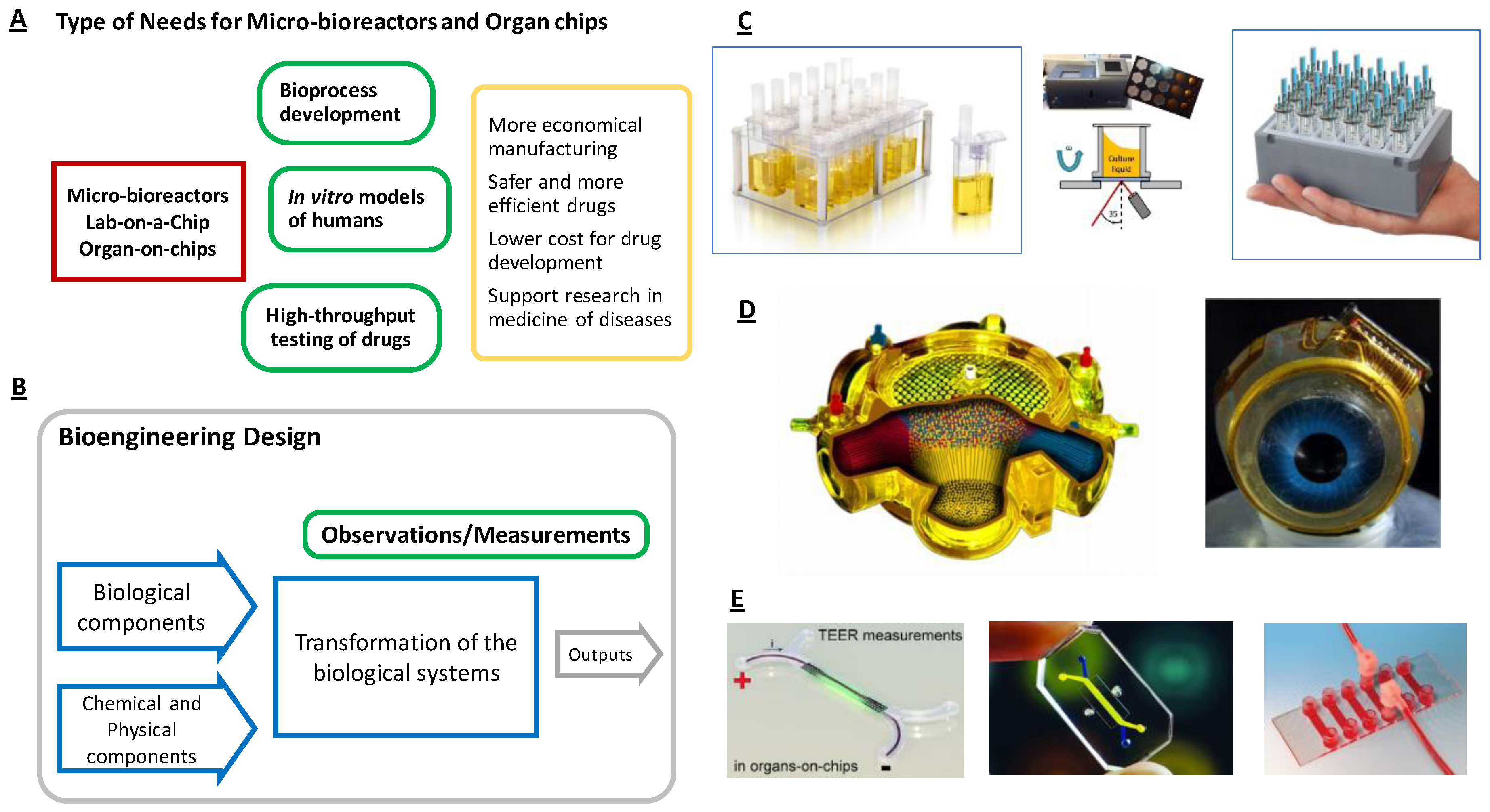


| Users’ Needs and Requirements | Rationales or Examples | Priority |
|---|---|---|
| Maintain human organ cells with number of a corresponding human organ equivalents | Lower number of cells in a reactor unit would not show relevant data | 10 |
| Cells shall maintain the same functionality in vitro as they do in vivo | In most assays, the functionality is the target end-point to be observed | 10 |
| A multi-cellular system should be recapitulated in the MBR/OoC | A human organ or tissue is in vivo, like if it interacts with adjacent cells | 9 |
| Excreted metabolites shall be analysed in situ or at line with sensitive analytical means | Amounts of analytes produced in the MBR are minutes due to scale | 9 |
| Cell densities equivalent to an industrial production system | In-process development should have cell concentrations on a large scale | 8 |
| Material properties of MBR should not interfere with the biological transformation | Some materials are toxic, absorb drugs, or affect gradients of gases | 8 |
| Microfluidic conditions in the device should not harm the cells capacity | Shear force in the micro-reactor shall not change cells functional behaviour | 7 |
| The MBR/OoC shall be operable with stable performance over extended time periods | Short-term acute effects (<1 day) are of lesser value than chronic (>14 days) | 7 |
| Compounds should be exposed to cells or cell organoids in a relevant way | Diffusion, shear, and gradients in MBR should reproduce in vivo perfusion | 6 |
| Allow controlled addition of media factors | The exposure of factors | 2 |
| Gradients in the MBR of O2, CO2, pressure, and temperature should be in vivo-like | Variations in gradients are known to influence cellular response | 1 |
| User Needs | Target Metrics (Examples) | Specification (Examples) |
|---|---|---|
| MBR shall have multi-cellular systems | At least three cell types | Hepatocytes, Kupfer cells, fibroblast |
| Cells shall have in vivo-like functionality | Cells per MBR unit | 50,000–75,000 cells |
| Cells as in human organ equivalent | Cells in equivalent | 25,000 cells |
| Extracellular matrix | Hydrogel type | Matrigel or RGD-PEG |
| Flow of nutrients | Shear force number | |
| Measurement be extended time periods | Days | >10 days |
| Controlled addition of growth factors | Pump rates | |
| Microscope In situ inspection | Microscopic resolution | ±10 nm |
| Sampling of effluent fluid | Number sampling ports | 3 |
| Oxygen transfer | Dissolved oxygen tension | More than 10% |
| Oxygen permeability of device material | mg O2 per mL and hour | |
| Material properties of device | Porosity | 25–30% |
| Recycling of media | Recycling ratio | 1–2 |
| Design Alternatives User Needs | Alternative 1 | Alternative 2 | Alternative 3 | Alternative 4 |
|---|---|---|---|---|
| Allow co-culture of cells | •• | ••• | •• | • |
| Cells have in vivo-like functionality | •• | ••• | • | • |
| Number of cells in device as in an in vivo equivalent | • | •• | •• | ••• |
| Extracellular matrix possible to mimic | ••• | ••• | ••• | ••• |
| Continuous flow of nutrients | •• | ••• | •• | ••• |
| Measurement periods up to 3 weeks | - | • | ••• | - |
| Exposure of test compounds to cells | ••• | ••• | ••• | ••• |
| Allow controlled addition of growth factors | ••• | ••• | - | •• |
| In situ inspection with confocal microscope without interference | ••• | ••• | - | - |
| Sampling of effluent fluid | ••• | ••• | ••• | ••• |
| Oxygen transfer through device | • | • | ••• | ••• |
| Liquid permeability of device | •• | •• | •• | - |
| Device shall allow recycling of media or exposed compounds | ••• | ••• | ••• | ••• |
| Material properties of device not interfering | • | • | • | •• |
| Recycling of outlet flow | ••• | ••• | - | • |
| Total score of ranking | 32 | 37 | 28 | 28 |
| User Needs | Target Metrics | Specification |
|---|---|---|
| Biological functions | ||
| Mammalian cells shall be used | Cell type | CHO, HEK cells |
| Concentration range of cell culture | Cell/mL | 10,000–10,000,000 |
| Expression of extracellular protein | Proteins expressed | IgGs |
| Same culture media shall promote both growth and expression | Type of media to be used | Serum-free medium |
| Culture time | Days | 7–14 days |
| Technical functions | ||
| Gentle well-distributed mixing | Shaken or stirred | Shaken |
| In situ inspection with confocal microscope without interference | Yes/No | Yes |
| Sampling of effluent fluid | Offline/inline | Offline |
| Oxygen transfer | kLa value for OTR | >100 h−1 |
| Permeability of device | Oxygen permeability (%) | <1% |
| Material properties of device | Surface hydrophobicity (angle) | 10 degree |
| Information functions | ||
| Online information about physical conditions in the MBR | Sensor types | Temp., pH, pO2 |
| Offline information about content of culture media | Analytes analyzed offline | All monomers in culture media |
| Offline information about IgG forms | Analytes analyzed offline | IgG forms |
| Low fabrication cost | Percentage of the sales price | >10% |
| User Needs | Target Metrics | Specification |
|---|---|---|
| Biological needs | ||
| Co-culture of cardiomyocytes/fibroblasts | Number other cell types than CM | 2–4 other cell types |
| Cardiomyocyte assemblies beating | Beats per minutes (bpm) | 30–100 |
| cardiac cells clustered in aggregate | Number of cells per aggregate | 500–1000 |
| Sufficient cells in HoC to generate measurable signals | Cardiac cells per HoC chamber | 500,000–1,000,000 |
| Extracellular matrix created inside MBR | Type of biomaterials | PEG, Matrigel |
| Technical needs | ||
| Shear force on cells corresponds in vivo of liquid media (nutrients, test solutions) | Distribution of flow rates in HoC Psi/cm | ±10% |
| Thermostable condition for cells in HoC | Temperature range inside HoC | 35–38 °C |
| Sampling ports for HoC effluent fluid | No. of ports and where | in: 2–3, out: 1 |
| Oxygen transfer to cardiac bodies | Dissolved oxygen tension in aggregates | above 5% |
| Non-toxic fabrication materials of MBR | Type of materials | Plastics, metal |
| Sterile conditions | Sterility time | 2 weeks |
| Information needs | Methods; performance | Confocal microscopy Magnification ×50 |
| In situ non-destructive inspection of cells | ||
| In situ observation of biomarkers | ||
| Measurement acquisition online | Methods; performance | HCI |
| Inline monitoring of excreted substances | Methods; performance | MS, immunosensor |
| Product and manufacturing requirements | ||
| Production cost per device/10,000 per year | EUR/unit | 2–4 EUR |
| Consumable cost per assay | Range EUR/assay | 1–5 EUR |
| Technician training time | Days | 3 days |
© 2018 by the author. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (http://creativecommons.org/licenses/by/4.0/).
Share and Cite
Mandenius, C.-F. Conceptual Design of Micro-Bioreactors and Organ-on-Chips for Studies of Cell Cultures. Bioengineering 2018, 5, 56. https://doi.org/10.3390/bioengineering5030056
Mandenius C-F. Conceptual Design of Micro-Bioreactors and Organ-on-Chips for Studies of Cell Cultures. Bioengineering. 2018; 5(3):56. https://doi.org/10.3390/bioengineering5030056
Chicago/Turabian StyleMandenius, Carl-Fredrik. 2018. "Conceptual Design of Micro-Bioreactors and Organ-on-Chips for Studies of Cell Cultures" Bioengineering 5, no. 3: 56. https://doi.org/10.3390/bioengineering5030056
APA StyleMandenius, C.-F. (2018). Conceptual Design of Micro-Bioreactors and Organ-on-Chips for Studies of Cell Cultures. Bioengineering, 5(3), 56. https://doi.org/10.3390/bioengineering5030056




