Delivery of Mesenchymal Stem Cells from Gelatin–Alginate Hydrogels to Stomach Lumen for Treatment of Gastroparesis
Abstract
1. Introduction
2. Materials and Methods
2.1. Materials and Procedure for Fabrication of the Alginate–Gelatin Hydrogel
2.2. Characterization and Analysis of the Hydrogel
- (a)
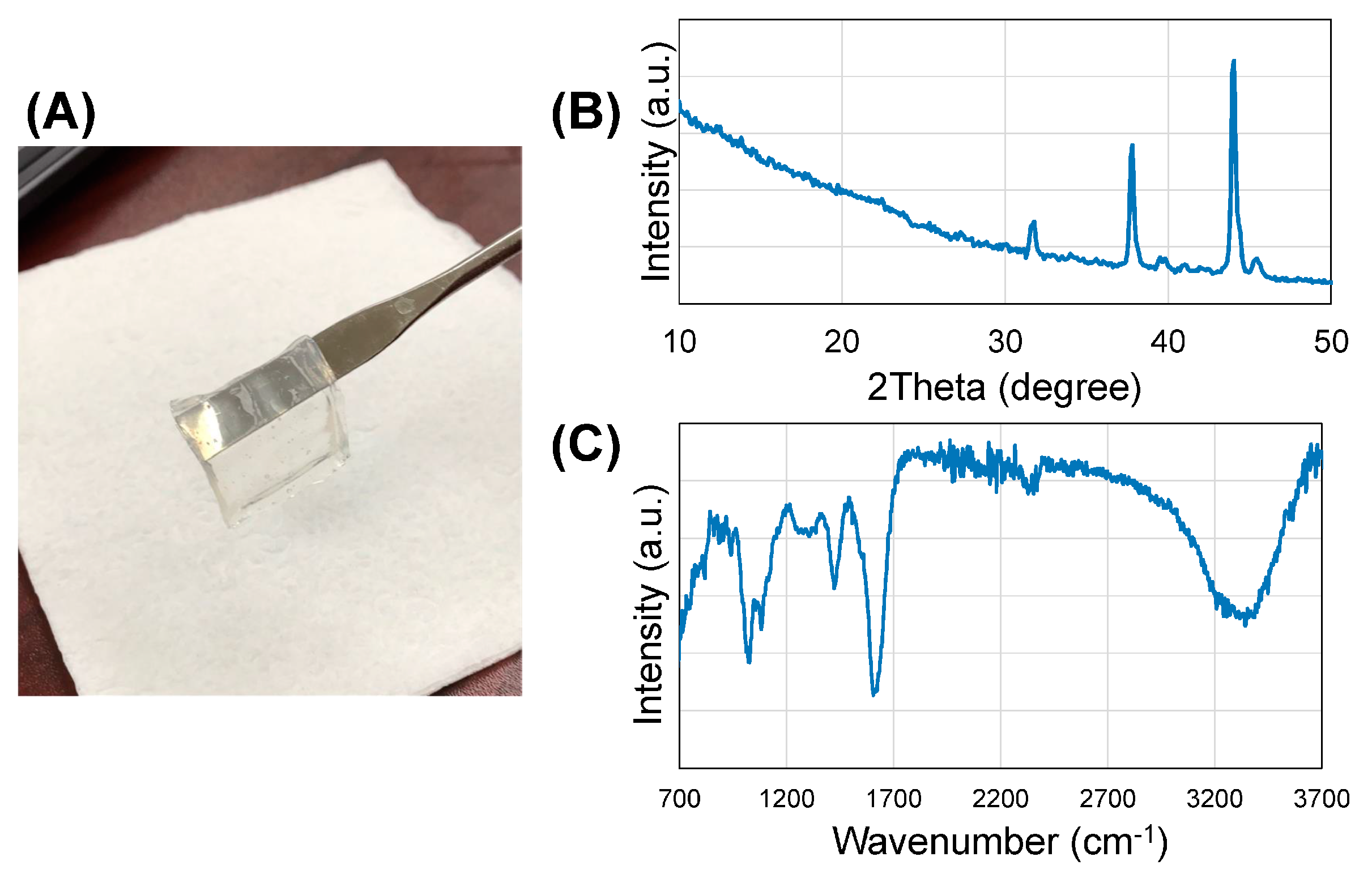
- X-ray diffraction (XRD) analysis—for the phase analysis using XRD, the gel samples were frozen and lyophilized prior to XRD (D8 Discover, Bruker’s diffractometer, Karlsruhe, Germany). XRD was carried out at 40 kV voltage and 40 mA current with CuKα wavelength (1.54056 Å) and 2θ ranges from 10° to 50° at a scanning rate of 3°/min with a step size of 0.1°.
- (b)
- Fourier-transform infrared spectroscopy (FTIR)—FTIR was used to reveal information about the molecular structure of the crosslinked gel sheet. Attenuated total reflectance (ATR)–FTIR spectra of a representative gel sample were acquired using a Perkin-Elmer, Spectrum 100, Universal ATR Sampling Accessory within the range of 700–3700 cm−1 in transmittance mode. Spectral manipulations were performed using the spectral analysis software GRAMS/32 (Galactic Industries Corp., Salem, NH, USA). External-reflection FTIR was recorded on a Specac grazing angle accessory using an s-polarized beam at an angle of incidence of 40° and a mercury cadmium telluride (MCT/A) detector. A piranha-treated silicon wafer was used as the background.
- (c)
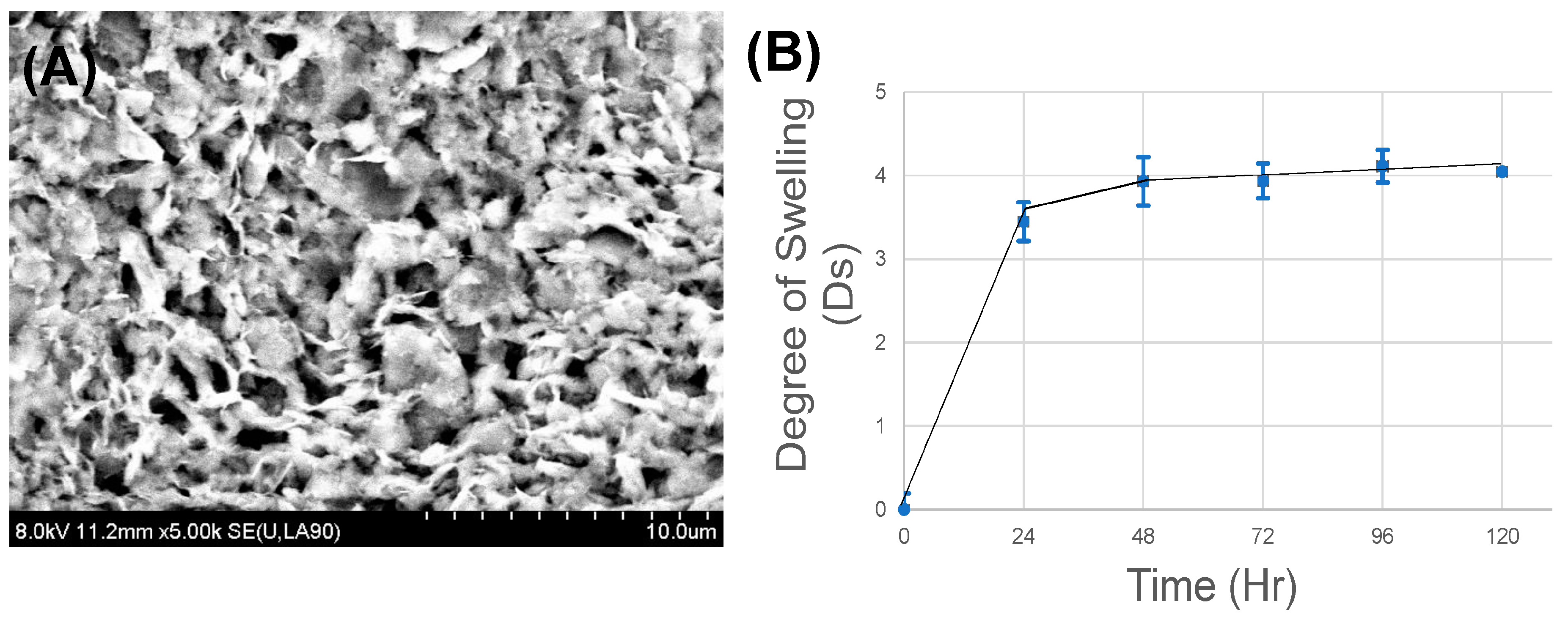
- Scanning electron microscopy (SEM)—SEM was operated in secondary electron mode for the analysis of the morphology of the gel samples, as done before [25]. Samples were visualized using SEM (S-4800, Hitachi, Japan) at voltages of 8 kV. Prior to SEM, to minimize charging during observation, samples were coated using graphite spray (Electron Microscopy Sciences, Hatfield, PA, USA).
- (d)
- Swelling and degradation—to account for the hydration parameters of the alginate–gelatin gels leading to swelling, gels were allowed to swell to equilibrium for 5 days in Simulated Gastric Fluid (Ricca Chemical, Arlington, TX, USA) at 25 °C, to identify the time point when the weight of the gels was found to be constant, or the final swelling degree was attained [25]. Disc-shaped punch-out samples (8-mm biopsy punch) were lyophilized to reveal their dry weight (W0), prior to being exposed to the aqueous media. The gels were then allowed to swell, during which time they were taken out at regular intervals of 1 day, the excess surface liquid was absorbed using blotting paper and the gels were weighed (Wt). The swelling ratio (Ds), or the degree of swelling, was calculated using (1), where Ds was the degree of swelling, and W0 and Wt were the weights of the samples in the dry and swollen states, respectively.Ds = (Wt − W0)/W0
- (e)
- Mechanical testing—all mechanical testing and analysis was done using an ElectroForce 5100 Biodynamics Test Instrument from ElectroForce Systems (Bose Corporation, Framingham, MA, USA). For the mechanical testing, it was absolutely necessary to use gels that exhibited smooth surfaces, after being cast and crosslinked. For making samples, dog-bone-shaped gels were cut using a mold placed on the alginate–gelatin hydrogels and carefully mounted between pressure grips, as done before [25]. Mounted specimens had an estimated cross-sectional area of 5 mm and a gauge length of 15 mm. They were maintained in CaCl2 to prevent aging of the hydrogels. The mechanical properties of the hydrogels were evaluated by measuring stress–strain curves via uniaxial compression at the rate of 1 mm min−1 until they were completely fractured. The elastic modulus of each sample was calculated from the slope of the stress–strain linear curves generated. Data are expressed as the mean ± standard deviation.
2.3. Cell Culture and Proliferation
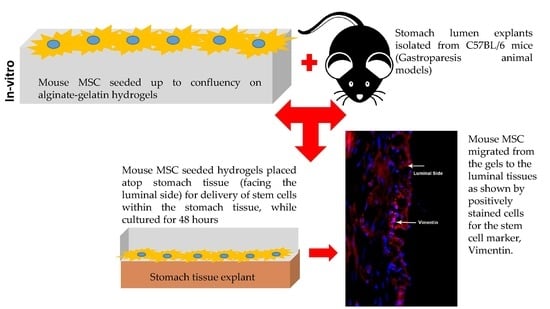
2.4. In-Vitro Transplants of Cell–Gel Constructs Atop Stomach Tissue
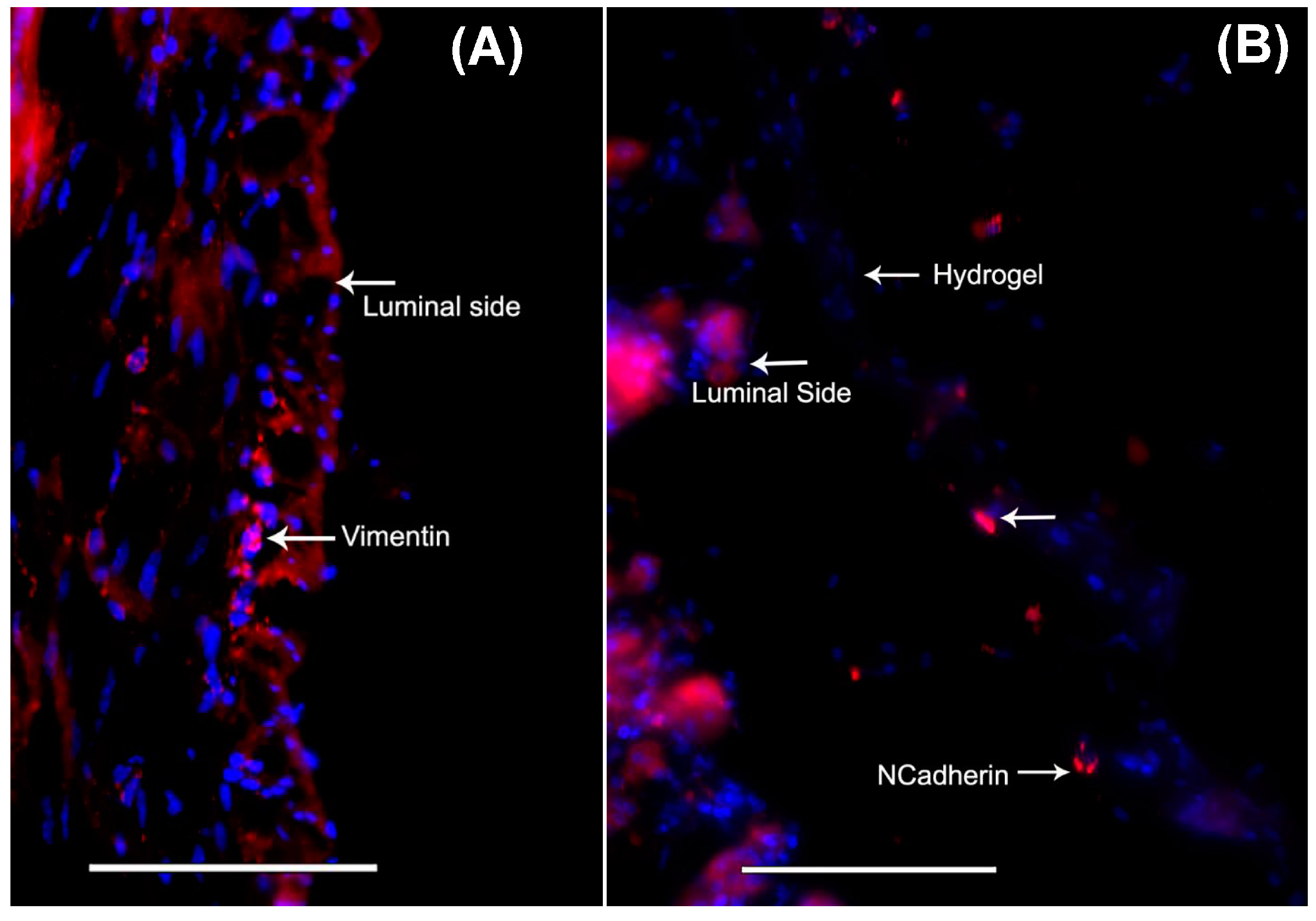
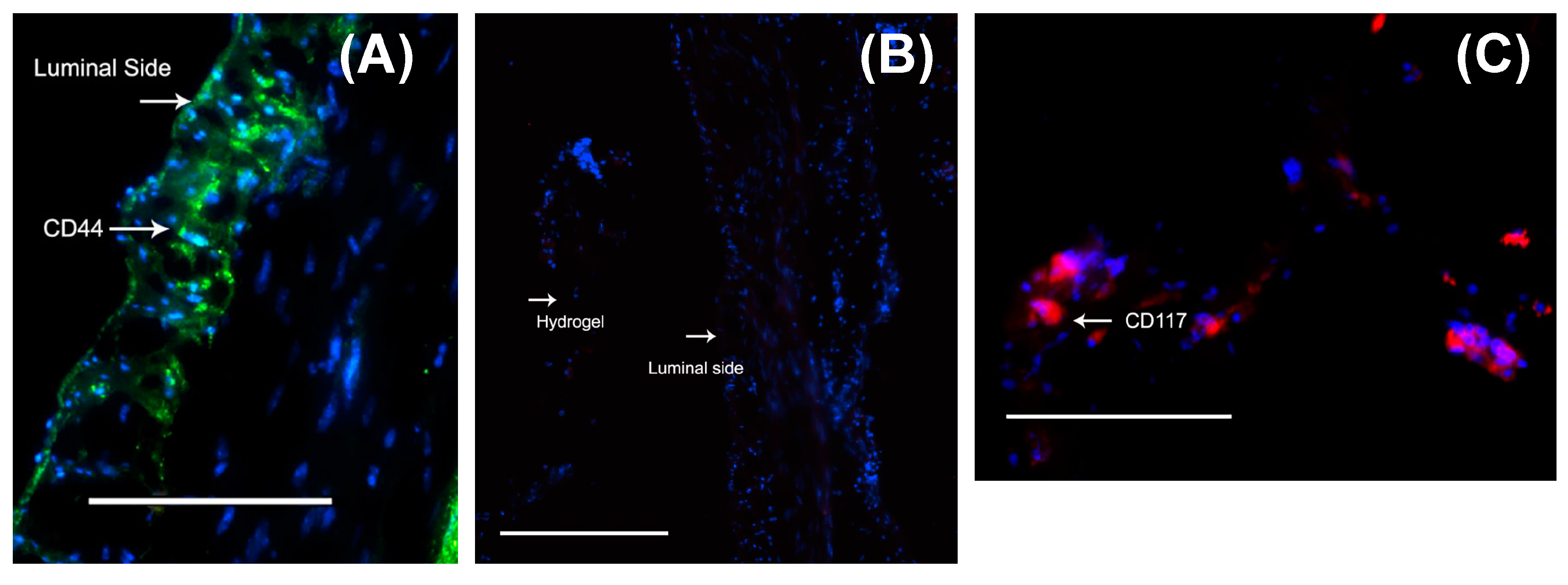
2.5. Probing the Migration of MSCs from Gels into Tissue (Immunocytochemistry)
3. Results
3.1. Phase Identification and Chemical Characterization
3.2. Microstructure Imaging
3.3. In-Vitro Stability
3.4. Mechanical Stiffness
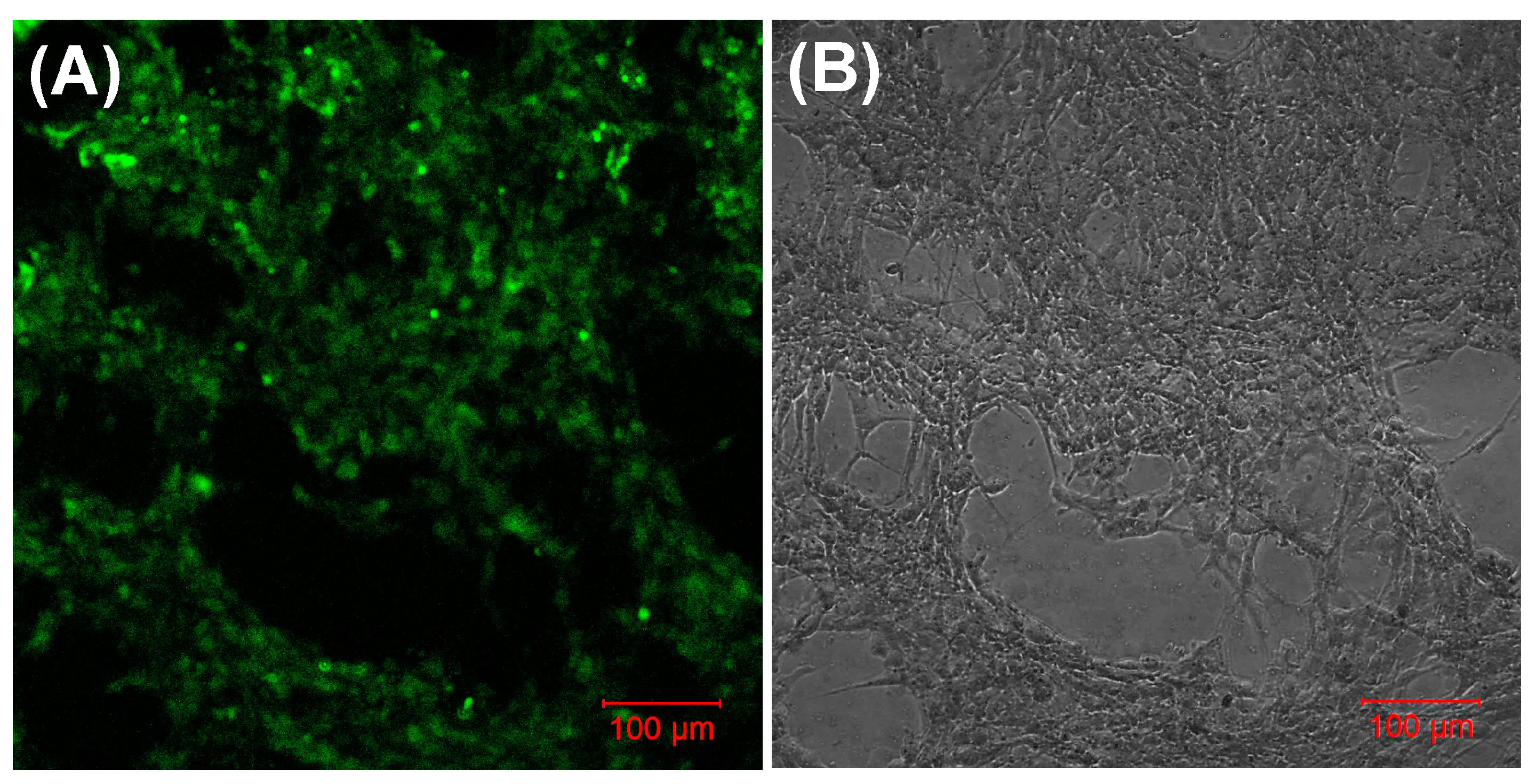
3.5. Biocompatibility
3.6. Delivery of Mouse MSCs from Gels to Stomach Tissue
4. Discussion
5. Conclusions
Supplementary Materials
Acknowledgments
Author Contributions
Conflicts of Interest
References
- Mohammad, B.; Richard, W.M. Is Interstitial Cells of Cajal-opathy Present in Gastroparesis? J. Neurogastroenterol. Motil. 2015, 21, 486–493. [Google Scholar]
- Maemura, T.; Shin, M.; Kinoshita, M. Tissue engineering of the stomach. J. Surg. Res. 2013, 183, 285–295. [Google Scholar] [CrossRef] [PubMed]
- Zheng, H.; He, Y.; Tong, J.; Sun, L.; Yang, D.; Li, H.; Ao, N.; Jin, X.; Zhang, Q. Is gastrointestinal dysfunction induced by gastric cancer peritoneal metastasis relevant to impairment of interstitial cells of Cajal? Clin. Exp. Metastasis 2011, 28, 291–299. [Google Scholar] [CrossRef] [PubMed]
- Lu, H.-L.; Huang, X.; Wu, Y.-S.; Zhang, C.-M.; Meng, X.-M.; Liu, D.-H.; Kim, Y.-c.; Xu, W.-X. Gastric nNOS reduction accompanied by natriuretic peptides signaling pathway upregulation in diabetic mice. World J. Gastroenterol. 2014, 20, 4626–4635. [Google Scholar] [CrossRef] [PubMed]
- Maemura, T.; Shin, M.; Ishii, O.; Mochizuki, H.; Vacanti, J.P. Initial assessment of a tissue engineered stomach derived from syngeneic donors in a rat model. Asaio J. 2004, 50, 468–472. [Google Scholar] [CrossRef] [PubMed]
- Araki, M.; Tao, H.; Sato, T.; Nakajima, N.; Hyon, S.H.; Nagayasu, T.; Nakamura, T. Development of a new tissue-engineered sheet for reconstruction of the stomach. Artif. Organs 2009, 33, 818–826. [Google Scholar] [CrossRef] [PubMed]
- Hori, Y.; Nakamura, T.; Kimura, D.; Kaino, K.; Kurokawa, Y.; Satomi, S.; Shimizu, Y. Functional Analysis of the Tissue-Engineered Stomach Wall. Artif. Organs 2002, 26, 868–872. [Google Scholar] [CrossRef] [PubMed]
- Dave, M.; Hayashi, Y.; Gajdos, G.B.; Smyrk, T.C.; Svingen, P.A.; Kvasha, S.M.; Lorincz, A.; Dong, H.; Faubion, W.A.; Ordog, T. Stem cells for murine interstitial cells of Cajal suppress cellular immunity and colitis via prostaglandin E(2) secretion. Gastroenterology 2015, 148, 978–990. [Google Scholar] [CrossRef] [PubMed]
- Zhou, Y.; Wang, Y.; Olson, J.; Yang, J.; Besner, G.E. Heparin-binding EGF-like growth factor promotes neuronal nitric oxide synthase expression and protects the enteric nervous system after necrotizing enterocolitis. Pediatr. Res. 2017, 82, 490–500. [Google Scholar] [CrossRef] [PubMed]
- Yang, J.; Watkins, D.; Chen, C.L.; Bhushan, B.; Zhou, Y.; Besner, G.E. Heparin-binding epidermal growth factor-like growth factor and mesenchymal stem cells act synergistically to prevent experimental necrotizing enterocolitis. J. Am. Coll. Surg. 2012, 215, 534–545. [Google Scholar] [CrossRef] [PubMed]
- Orlic, D. Stem cell repair in ischemic heart disease: An experimental model. Int. J. Hematol. 2002, 76 (Suppl. 1), 144–145. [Google Scholar] [CrossRef] [PubMed]
- Streutker, C.; Huizinga, J.; Driman, D.; Riddell, R. Interstitial cells of Cajal in health and disease. Part I: Normal ICC structure and function with associated motility disorders. Histopathology 2007, 50, 176–189. [Google Scholar] [CrossRef] [PubMed]
- Torihashi, S.; Ward, S.M.; Sanders, K.M. Development of c-Kit-positive cells and the onset of electrical rhythmicity in murine small intestine. Gastroenterology 1997, 112, 144–155. [Google Scholar] [CrossRef]
- Brittan, M.; Wright, N.A. Gastrointestinal stem cells. J. Pathol. 2002, 197, 492–509. [Google Scholar] [CrossRef] [PubMed]
- Lorincz, A.; Redelman, D.; Horváth, V.J.; Bardsley, M.R.; Chen, H.; Ördög, T. Progenitors of interstitial cells of cajal in the postnatal murine stomach. Gastroenterology 2008, 134, 1083–1093. [Google Scholar] [CrossRef] [PubMed]
- Park, K.S. Is this the era of interstitial cells of cajal transplantation? J. Neurogastroenterol. Motil. 2014, 20, 281–283. [Google Scholar] [CrossRef] [PubMed]
- Stzepourginski, I.; Nigro, G.; Jacob, J.-M.; Dulauroy, S.; Sansonetti, P.J.; Eberl, G.; Peduto, L. CD34+ mesenchymal cells are a major component of the intestinal stem cells niche at homeostasis and after injury. Proc. Natl. Acad. Sci. USA 2017, 114, E506–E513. [Google Scholar] [CrossRef] [PubMed]
- Brittan, M.; Wright, N. The gastrointestinal stem cell. Cell Prolif. 2004, 37, 35–53. [Google Scholar] [CrossRef] [PubMed]
- Lundorff, P.; Donnez, J.; Korell, M.; Audebert, A.J.M.; Block, K.; diZerega, G.S. Clinical evaluation of a viscoelastic gel for reduction of adhesions following gynaecological surgery by laparoscopy in Europe. Hum. Reprod. 2005, 20, 514–520. [Google Scholar] [CrossRef] [PubMed]
- Utech, S.; Prodanovic, R.; Mao, A.S.; Ostafe, R.; Mooney, D.J.; Weitz, D.A. Microfluidic generation of monodisperse, structurally homogeneous alginate microgels for cell encapsulation and 3D cell culture. Adv. Healthc. Mater. 2015, 4, 1628–1633. [Google Scholar] [CrossRef] [PubMed]
- Wang, K.; Nune, K.C.; Misra, R.D. The functional response of alginate-gelatin-nanocrystalline cellulose injectable hydrogels toward delivery of cells and bioactive molecules. Acta Biomater. 2016, 36, 143–151. [Google Scholar] [CrossRef] [PubMed]
- Hernandez, I.; Kumar, A.; Joddar, B. A Bioactive Hydrogel and 3D Printed Polycaprolactone System for Bone Tissue Engineering. Gels 2017, 3, 26. [Google Scholar] [CrossRef] [PubMed]
- Kuijpers, A.J.; Engbers, G.H.; Krijgsveld, J.; Zaat, S.A.; Dankert, J.; Feijen, J. Cross-linking and characterisation of gelatin matrices for biomedical applications. J. Biomater. Sci. Polym. Ed. 2000, 11, 225–243. [Google Scholar] [CrossRef] [PubMed]
- Kuijpers, A.J.; Engbers, G.H.M.; Feijen, J.; De Smedt, S.C.; Meyvis, T.K.L.; Demeester, J.; Krijgsveld, J.; Zaat, S.A.J.; Dankert, J. Characterization of the Network Structure of Carbodiimide Cross-Linked Gelatin Gels. Macromolecules 1999, 32, 3325–3333. [Google Scholar] [CrossRef]
- Joddar, B.; Garcia, E.; Casas, A.; Stewart, C.M. Development of functionalized multi-walled carbon-nanotube-based alginate hydrogels for enabling biomimetic technologies. Sci. Rep. 2016, 6, 32456. [Google Scholar] [CrossRef] [PubMed]
- Cai, L.; Lu, J.; Sheen, V.; Wang, S. Optimal Poly(l-lysine) Grafting Density in Hydrogels for Promoting Neural Progenitor Cell Functions. Biomacromolecules 2012, 13, 1663–1674. [Google Scholar] [CrossRef] [PubMed]
- Begum, J.; Day, W.; Henderson, C.; Purewal, S.; Cerveira, J.; Summers, H.; Rees, P.; Davies, D.; Filby, A. A method for evaluating the use of fluorescent dyes to track proliferation in cell lines by dye dilution. Cytometry A 2013, 83, 1085–1095. [Google Scholar] [CrossRef] [PubMed]
- Vadstrup, K.; Galsgaard, E.D.; Gerwien, J.; Vester-Andersen, M.K.; Pedersen, J.S.; Rasmussen, J.; Neermark, S.; Kiszka-Kanowitz, M.; Jensen, T.; Bendtsen, F. Validation and Optimization of an Ex Vivo Assay of Intestinal Mucosal Biopsies in Crohn’s Disease: Reflects Inflammation and Drug Effects. PLoS ONE 2016, 11, e0155335. [Google Scholar] [CrossRef] [PubMed]
- Qi, Y.; Jiang, M.; Cui, Y.-L.; Zhao, L.; Zhou, X. Synthesis of Quercetin Loaded Nanoparticles Based on Alginate for Pb(II) Adsorption in Aqueous Solution. Nanoscale Res. Lett. 2015, 10, 408. [Google Scholar] [CrossRef] [PubMed]
- Rajesh, R.; Dominic Ravichandran, Y. Development of a new carbon nanotube–alginate–hydroxyapatite tricomponent composite scaffold for application in bone tissue engineering. Int. J. Nanomed. 2015, 10 (Suppl. 1), 7–15. [Google Scholar]
- Jalaja, K.; Naskar, D.; Kundu, S.C.; James, N.R. Fabrication of cationized gelatin nanofibers by electrospinning for tissue regeneration. RSC Adv. 2015, 5, 89521–89530. [Google Scholar]
- Sarker, B.; Papageorgiou, D.G.; Silva, R.; Zehnder, T.; Gul-E-Noor, F.; Bertmer, M.; Kaschta, J.; Chrissafis, K.; Detsch, R.; Boccaccini, A.R. Fabrication of alginate-gelatin crosslinked hydrogel microcapsules and evaluation of the microstructure and physico-chemical properties. J. Mater. Chem. B 2014, 2, 1470–1482. [Google Scholar] [CrossRef]
- Rydén, L.; Omar, O.; Johansson, A.; Jimbo, R.; Palmquist, A.; Thomsen, P. Inflammatory cell response to ultra-thin amorphous and crystalline hydroxyapatite surfaces. J. Mater. Sci. Mater. Med. 2016, 28, 9. [Google Scholar] [CrossRef] [PubMed]
- Jiang, C.; Wang, Z.; Zhang, X.; Zhu, X.; Nie, J.; Ma, G. Crosslinked polyelectrolyte complex fiber membrane based on chitosan-sodium alginate by freeze-drying. RSC Adv. 2014, 4, 41551–41560. [Google Scholar] [CrossRef]
- Zhuang, C.; Tao, F.; Cui, Y. Anti-degradation gelatin films crosslinked by active ester based on cellulose. RSC Adv. 2015, 5, 52183–52193. [Google Scholar] [CrossRef]
- Banerjee, A.; Arha, M.; Choudhary, S.; Ashton, R.S.; Bhatia, S.R.; Schaffer, D.V.; Kane, R.S. The Influence of Hydrogel Modulus on the Proliferation and Differentiation of Encapsulated Neural Stem Cells. Biomaterials 2009, 30, 4695–4699. [Google Scholar] [CrossRef] [PubMed]
- Stowers, R.S.; Allen, S.C.; Suggs, L.J. Dynamic phototuning of 3D hydrogel stiffness. Proc. Natl. Acad. Sci. USA 2015, 112, 1953–1958. [Google Scholar] [CrossRef] [PubMed]
- Huebsch, N.; Lippens, E.; Lee, K.; Mehta, M.; Koshy, S.T.; Darnell, M.C.; Desai, R.; Madl, C.M.; Xu, M.; Zhao, X.; et al. Matrix Elasticity of Void-Forming Hydrogels Controls Transplanted Stem Cell-Mediated Bone Formation. Nat. Mater. 2015, 14, 1269–1277. [Google Scholar] [CrossRef] [PubMed]
- Hadden, W.J.; Young, J.L.; Holle, A.W.; McFetridge, M.L.; Kim, D.Y.; Wijesinghe, P.; Taylor-Weiner, H.; Wen, J.H.; Lee, A.R.; Bieback, K. Stem cell migration and mechanotransduction on linear stiffness gradient hydrogels. Proc. Natl. Acad. Sci. USA 2017, 114, 5647–5652. [Google Scholar] [CrossRef] [PubMed]
- Lim, Y.J.; Deo, D.; Singh, T.P.; Jones, D.B.; De, S. In Situ Measurement and Modeling of Biomechanical Response of Human Cadaveric Soft Tissues for Physics-Based Surgical Simulation. Surg. Endosc. 2009, 23, 1298–1307. [Google Scholar] [CrossRef] [PubMed]
- Lin, X.; Wu, L.; Zhang, Z.; Yang, R.; Guan, Q.; Hou, X.; Wu, Q. MiR-335-5p Promotes Chondrogenesis in Mouse Mesenchymal Stem Cells and Is Regulated Through Two Positive Feedback Loops. J. Bone Miner. Res. 2014, 29, 1575–1585. [Google Scholar] [CrossRef] [PubMed]
- Wu, Z.; Su, X.; Xu, Y.; Kong, B.; Sun, W.; Mi, S. Bioprinting three-dimensional cell-laden tissue constructs with controllable degradation. Sci. Rep. 2016, 6, 24474. [Google Scholar] [CrossRef] [PubMed]
- Kim, Y.-S.; Yi, B.-R.; Kim, N.-H.; Choi, K.-C. Role of the epithelial–mesenchymal transition and its effects on embryonic stem cells. Exp. Mol. Med. 2014, 46, e108. [Google Scholar] [CrossRef] [PubMed]
- Li, B.; Zheng, Y.W.; Sano, Y.; Taniguchi, H. Evidence for mesenchymal-epithelial transition associated with mouse hepatic stem cell differentiation. PLoS ONE 2011, 6, e17092. [Google Scholar] [CrossRef] [PubMed]
- Speer, A.L.; Sala, F.G.; Matthews, J.A.; Grikscheit, T.C. Murine tissue-engineered stomach demonstrates epithelial differentiation. J. Surg. Res. 2011, 171, 6–14. [Google Scholar] [CrossRef] [PubMed]
- Sharma, P.; Bolten, Z.T.; Wagner, D.R.; Hsieh, A.H. Deformability of Human Mesenchymal Stem Cells Is Dependent on Vimentin Intermediate Filaments. Ann. Biomed. Eng. 2017, 45, 1365–1374. [Google Scholar] [CrossRef] [PubMed]
- Le Blanc, K.; Rasmusson, I.; Sundberg, B.; Götherström, C.; Hassan, M.; Uzunel, M.; Ringdén, O. Treatment of severe acute graft-versus-host disease with third party haploidentical mesenchymal stem cells. Lancet 2004, 363, 1439–1441. [Google Scholar] [CrossRef]
- Ortiz-Hidalgo, C.; de Leon Bojorge, B.; Albores-Saavedra, J. Stromal tumor of the gallbladder with phenotype of interstitial cells of Cajal: A previously unrecognized neoplasm. Am. J. Surg. Pathol. 2000, 24, 1420–1423. [Google Scholar] [CrossRef] [PubMed]
- Robinson, T.L.; Sircar, K.; Hewlett, B.R.; Chorneyko, K.; Riddell, R.H.; Huizinga, J.D. Gastrointestinal stromal tumors may originate from a subset of CD34-positive interstitial cells of Cajal. Am. J. Surg. Pathol. 2000, 156, 1157–1163. [Google Scholar] [CrossRef]
- Bashashati, M.; McCallum, R.W. Motility: Is ‘ICC-opathy’ present in gastroparesis-like syndrome? Nat. Rev. Gastroenterol. Hepatol. 2015, 12, 375–376. [Google Scholar] [CrossRef] [PubMed]
- Yun, H.-Y.; Sung, R.; Kim, Y.C.; Choi, W.; Kim, H.S.; Kim, H.; Lee, G.J.; You, R.Y.; Park, S.-M.; Yun, S.J.; et al. Regional Distribution of Interstitial Cells of Cajal (ICC) in Human Stomach. Korean J. Physiol. Pharmacol. 2010, 14, 317–324. [Google Scholar] [CrossRef] [PubMed]
- Workman, M.J.; Mahe, M.M.; Trisno, S.; Poling, H.M.; Watson, C.L.; Sundaram, N.; Chang, C.-F.; Schiesser, J.; Aubert, P.; Stanley, E.G.; et al. Engineered human pluripotent-stem-cell-derived intestinal tissues with a functional enteric nervous system. Nat. Med. 2017, 23, 49–59. [Google Scholar] [CrossRef] [PubMed]
- Monsel, A.; Zhu, Y.G.; Gennai, S.; Hao, Q.; Liu, J.; Lee, J.W. Cell-based therapy for acute organ injury: Preclinical evidence and ongoing clinical trials using mesenchymal stem cells. Anesthesiology 2014, 121, 1099–1121. [Google Scholar] [CrossRef] [PubMed]
- Nakatsu, H.; Ueno, T.; Oga, A.; Nakao, M.; Nishimura, T.; Kobayashi, S.; Oka, M. Influence of mesenchymal stem cells on stomach tissue engineering using small intestinal submucosa. J. Tissue Eng. Regen. Med. 2015, 9, 296–304. [Google Scholar] [CrossRef] [PubMed]
- Engevik, A.C.; Feng, R.; Choi, E.; White, S.; Bertaux-Skeirik, N.; Li, J.; Mahe, M.M.; Aihara, E.; Yang, L.; DiPasquale, B.; et al. The Development of Spasmolytic Polypeptide/TFF2-Expressing Metaplasia (SPEM) During Gastric Repair Is Absent in the Aged Stomach. Cell. Mol. Gastroenterol. Hepatol. 2016, 2, 605–624. [Google Scholar] [CrossRef] [PubMed]
- Yanagida, H.; Yanase, H.; Sanders, K.M.; Ward, S.M. Intestinal surgical resection disrupts electrical rhythmicity, neural responses, and interstitial cell networks. Gastroenterology 2004, 127, 1748–1759. [Google Scholar] [CrossRef] [PubMed]
- Jiang, Y.; Jahagirdar, B.N.; Reinhardt, R.L.; Schwartz, R.E.; Keene, C.D.; Ortiz-Gonzalez, X.R.; Reyes, M.; Lenvik, T.; Lund, T.; Blackstad, M. Pluripotency of mesenchymal stem cells derived from adult marrow. Nature 2002, 418, 41–49. [Google Scholar] [CrossRef] [PubMed]
- McCann, C.J.; Hwang, S.-J.; Bayguinov, Y.; Colletti, E.J.; Sanders, K.M.; Ward, S.M. Establishment of pacemaker activity in tissues allotransplanted with interstitial cells of Cajal. Neurogastroenterol.Motil. 2013, 25, e418–e428. [Google Scholar] [CrossRef] [PubMed]
- Joddar, B.; Kumar, S.A.; Kumar, A. A Contact-Based Method for Differentiation of Human Mesenchymal Stem Cells into an Endothelial Cell-Phenotype. Cell Biochem. Biophys. 2017, 1–9. [Google Scholar] [CrossRef] [PubMed]
- Sun, J.; Tan, H. Alginate-Based Biomaterials for Regenerative Medicine Applications. Materials 2013, 6, 1285. [Google Scholar] [CrossRef] [PubMed]
- Boskey, A.; Maresca, M.; Ullrich, W.; Doty, S.; Butler, W.; Prince, C. Osteopontin-hydroxyapatite interactions in vitro: Inhibition of hydroxyapatite formation and growth in a gelatin-gel. Bone Miner. 1993, 22, 147–159. [Google Scholar] [CrossRef]
- Balakrishnan, B.; Joshi, N.; Jayakrishnan, A.; Banerjee, R. Self-crosslinked oxidized alginate/gelatin hydrogel as injectable, adhesive biomimetic scaffolds for cartilage regeneration. Acta Biomater. 2014, 10, 3650–3663. [Google Scholar] [CrossRef] [PubMed]
- Khademhosseini, A.; Langer, R. Microengineered hydrogels for tissue engineering. Biomaterials 2007, 28, 5087–5092. [Google Scholar] [CrossRef] [PubMed]
- Jgamadze, D.; Liu, L.; Vogler, S.; Chu, L.-Y.; Pautot, S. Thermoswitching microgel carriers improve neuronal cell growth and cell release for cell transplantation. Tissue Eng. C 2014, 21, 65–76. [Google Scholar] [CrossRef] [PubMed]

© 2018 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (http://creativecommons.org/licenses/by/4.0/).
Share and Cite
Joddar, B.; Tasnim, N.; Thakur, V.; Kumar, A.; McCallum, R.W.; Chattopadhyay, M. Delivery of Mesenchymal Stem Cells from Gelatin–Alginate Hydrogels to Stomach Lumen for Treatment of Gastroparesis. Bioengineering 2018, 5, 12. https://doi.org/10.3390/bioengineering5010012
Joddar B, Tasnim N, Thakur V, Kumar A, McCallum RW, Chattopadhyay M. Delivery of Mesenchymal Stem Cells from Gelatin–Alginate Hydrogels to Stomach Lumen for Treatment of Gastroparesis. Bioengineering. 2018; 5(1):12. https://doi.org/10.3390/bioengineering5010012
Chicago/Turabian StyleJoddar, Binata, Nishat Tasnim, Vikram Thakur, Alok Kumar, Richard W. McCallum, and Munmun Chattopadhyay. 2018. "Delivery of Mesenchymal Stem Cells from Gelatin–Alginate Hydrogels to Stomach Lumen for Treatment of Gastroparesis" Bioengineering 5, no. 1: 12. https://doi.org/10.3390/bioengineering5010012
APA StyleJoddar, B., Tasnim, N., Thakur, V., Kumar, A., McCallum, R. W., & Chattopadhyay, M. (2018). Delivery of Mesenchymal Stem Cells from Gelatin–Alginate Hydrogels to Stomach Lumen for Treatment of Gastroparesis. Bioengineering, 5(1), 12. https://doi.org/10.3390/bioengineering5010012