Similarity Gait Networks with XAI for Parkinson’s Disease Classification: A Pilot Study
Abstract
1. Introduction
Related Works
2. Materials and Methods
2.1. Subjects and Clinical Evaluation
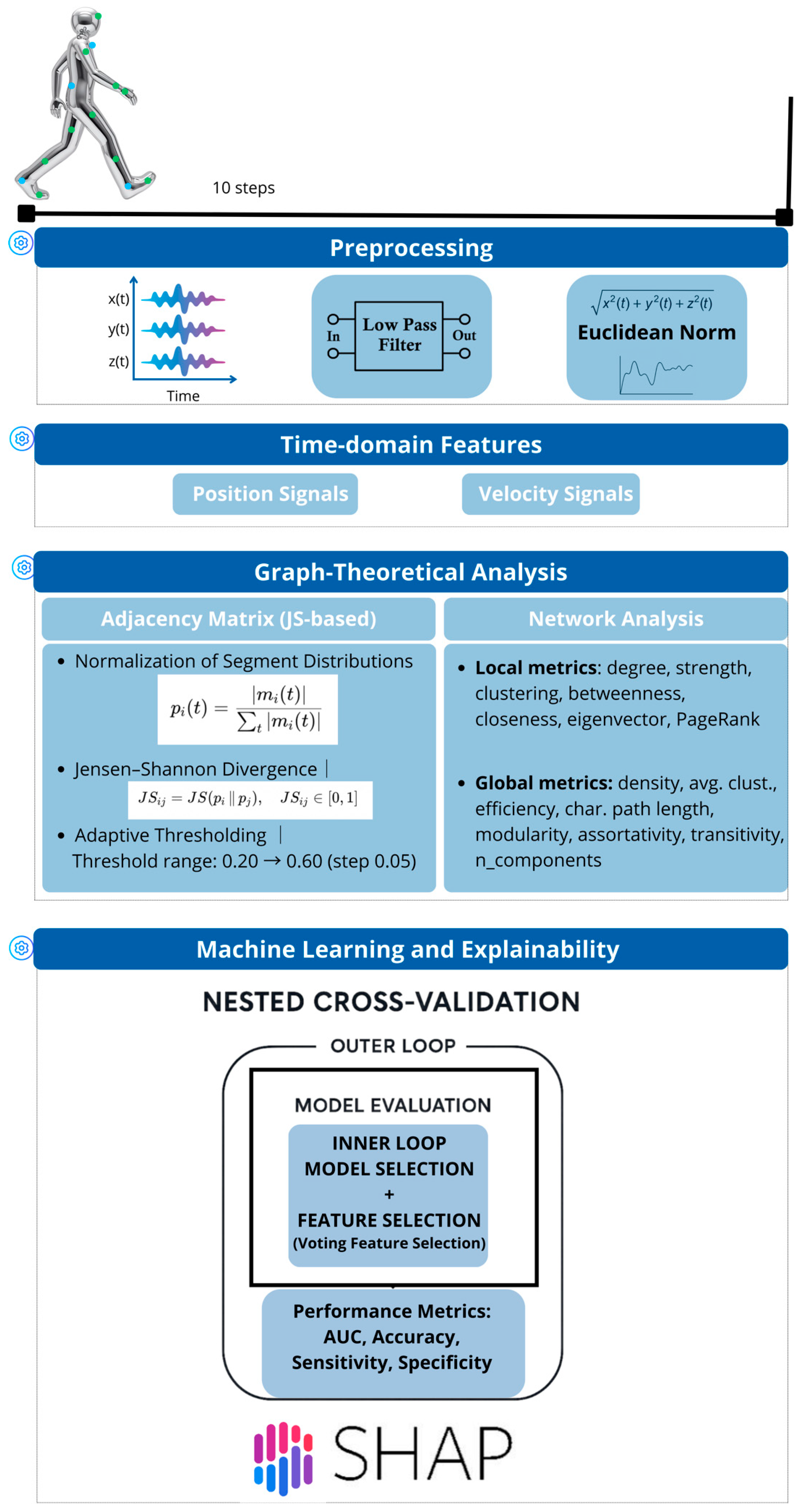
2.2. Data Acquisition and Preprocessing
2.3. Graph-Theoretical Feature Construction
2.4. Machine Learning Models
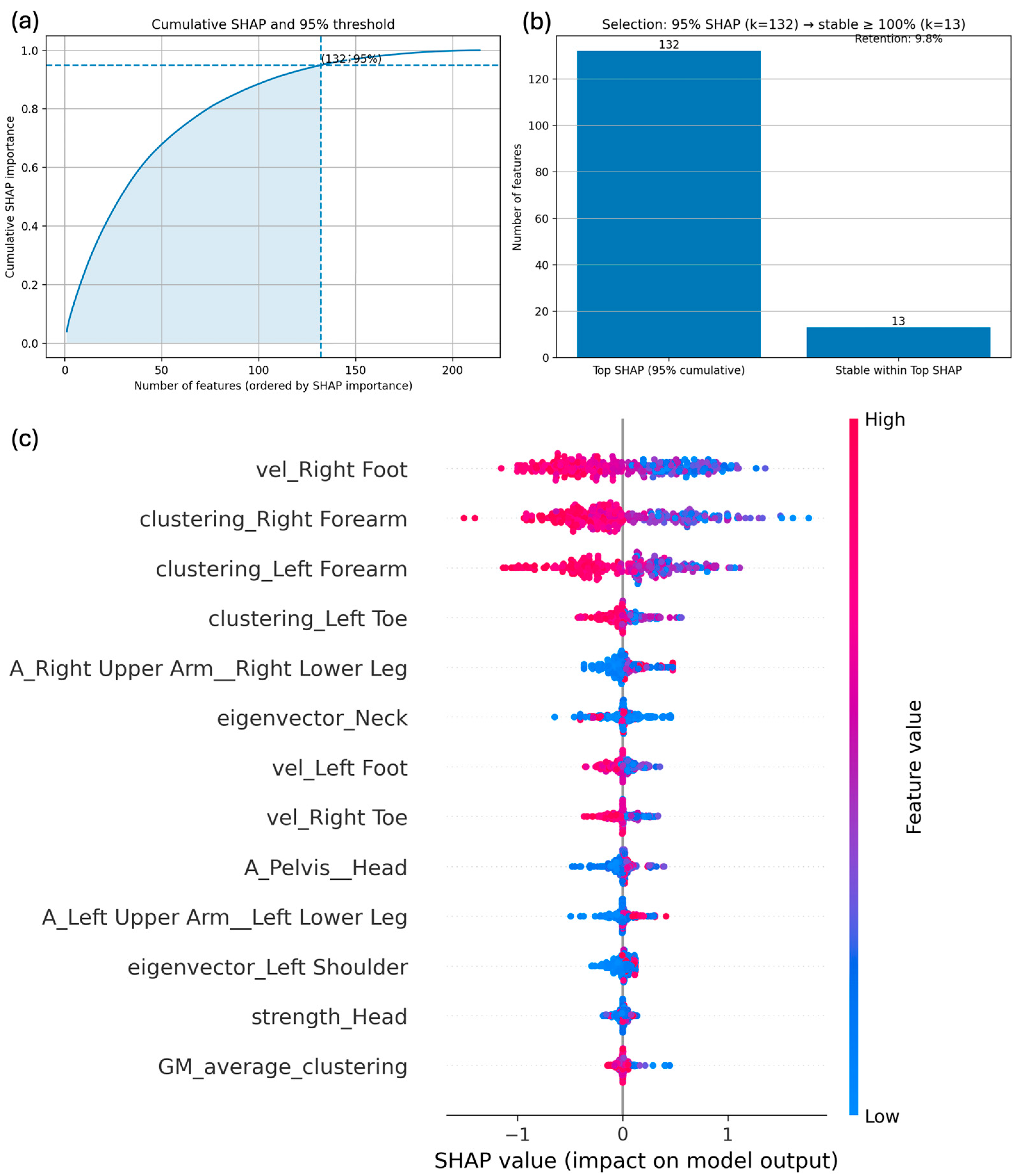
2.5. Explainability and Stability Analysis with SHAP
2.6. Statistical Analysis
3. Results
3.1. Demographic and Clinical Features
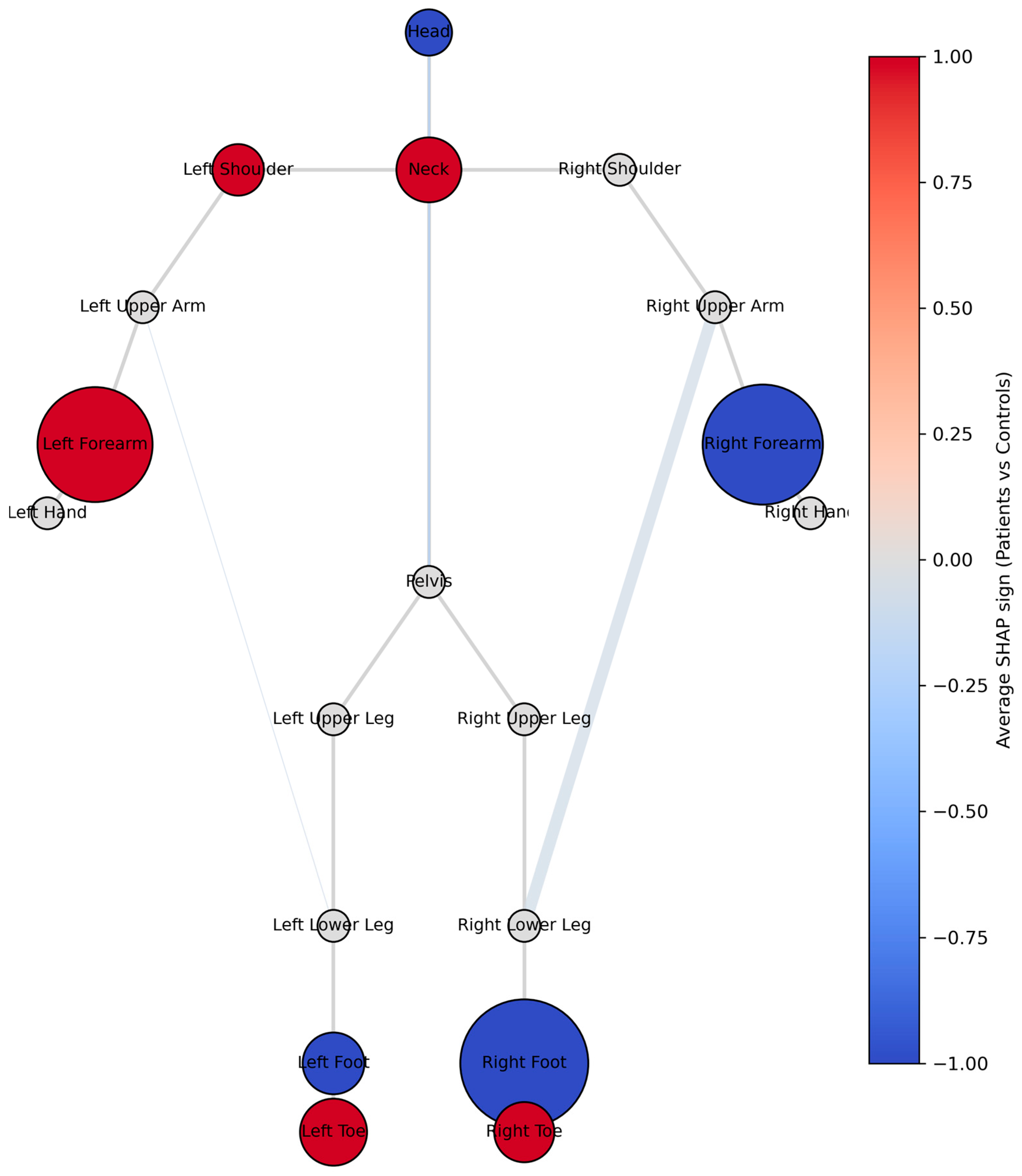
3.2. Machine Learning Models and Explainability
3.3. Correlation
4. Discussion
4.1. Main Findings
4.2. Clinical Implications
4.3. Strengths and Limitations
4.4. Future Research
5. Conclusions
Supplementary Materials
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Data Availability Statement
Conflicts of Interest
References
- van Rooden, S.M.; Visser, M.; Verbaan, D.; Marinus, J.; van Hilten, J.J. Motor patterns in Parkinson’s disease: A data-driven approach. Mov. Disord. 2009, 24, 1042–1047. [Google Scholar] [CrossRef]
- Bologna, M.; Espay, A.J.; Fasano, A.; Paparella, G.; Hallett, M.; Berardelli, A. Redefining Bradykinesia. Mov. Disord. 2023, 38, 551–557. [Google Scholar] [CrossRef]
- Kehnemouyi, Y.M.; Coleman, T.P.; Tass, P.A. Emerging wearable technologies for multisystem monitoring and treatment of Parkinson’s disease: A narrative review. Front. Netw. Physiol. 2024, 4, 1354211. [Google Scholar] [CrossRef] [PubMed]
- Brognara, L.; Mazzotti, A.; Zielli, S.O.; Arceri, A.; Artioli, E.; Traina, F.; Faldini, C. Wearable Technology Applications and Methods to Assess Clinical Outcomes in Foot and Ankle Disorders: Achievements and Perspectives. Sensors 2024, 24, 7059. [Google Scholar] [CrossRef]
- Caballol, N.; Bayés, À.; Prats, A.; Martín-Baranera, M.; Quispe, P. Feasibility of a wearable inertial sensor to assess motor complications and treatment in Parkinson’s disease. PLoS ONE 2023, 18, e0279910. [Google Scholar] [CrossRef] [PubMed]
- Calomino, C.; Gramigna, V.; Bianco, M.G.; Cristofaro, A.; Quattrone, A.; Quattrone, A. A Quantitative Kinematic Evaluation of Postural Response in Parkinson’s Disease Subtypes. In Proceedings of the 2023 International Workshop on Biomedical Applications, Technologies and Sensors (BATS), Catanzaro, Italy, 28–29 September 2023; pp. 74–78. [Google Scholar] [CrossRef]
- Dietz, V.; Zijlstra, W.; Prokop, T.; Berger, W. Leg muscle activation during gait in Parkinson’s disease: Adaptation and interlimb coordination. Electroencephalogr. Clin. Neurophysiol./Electromyogr. Mot. Control 1995, 97, 408–415. [Google Scholar] [CrossRef] [PubMed]
- Allen, N.E.; Canning, C.G.; Sherrington, C.; Fung, V.S.C. Bradykinesia, muscle weakness and reduced muscle power in Parkinson’s disease. Mov. Disord. 2009, 24, 1344–1351. [Google Scholar] [CrossRef]
- Rovini, E.; Maremmani, C.; Cavallo, F. How Wearable Sensors Can Support Parkinson’s Disease Diagnosis and Treatment: A Systematic Review. Front. Neurosci. 2017, 11, 555. [Google Scholar] [CrossRef]
- Bassett, D.S.; Sporns, O. Network neuroscience. Nat. Neurosci. 2017, 20, 353–364. [Google Scholar] [CrossRef]
- Lodin, J.; Jelínek, M.; Sameš, M.; Vachata, P. Quantitative Gait Analysis of Patients with Severe Symptomatic Spinal Stenosis Utilizing the Gait Profile Score: An Observational Clinical Study. Sensors 2022, 22, 1633. [Google Scholar] [CrossRef]
- Calomino, C.; Quattrone, A.; Bianco, M.G.; Nisticò, R.; Buonocore, J.; Crasà, M.; Vaccaro, M.G.; Sarica, A.; Quattrone, A. Combined cortical thickness and blink reflex recovery cycle to differentiate essential tremor with and without resting tremor. Front. Neurol. 2024, 15, 1372262. [Google Scholar] [CrossRef]
- Bianco, M.G.; Caligiuri, M.E.; Calomino, C.; Bonacci, M.C.; Aquila, V.; Buonocore, J.; Augimeri, A.; Sarica, A.; Vaccaro, M.G.; Quattrone, A.; et al. Volumetric Assessment and Graph Theoretical Analysis of Thalamic Nuclei in Essential Tremor. Brain Behav. 2025, 15, e70346. [Google Scholar] [CrossRef]
- Zhang, Y.; Nie, L. Human motion similarity evaluation based on deep metric learning. Sci. Rep. 2024, 14, 30908. [Google Scholar] [CrossRef]
- Ali, L.; Leung, M.-F.; Khan, M.A.; Nour, R.; Imrana, Y.; Vasilakos, A.V. ChiGa-Net: A genetically optimized neural network with refined deeply extracted features using statistical score for trustworthy Parkinson’s disease detection. Neurocomputing 2025, 624, 129450. [Google Scholar] [CrossRef]
- He, J.; Zhuang, L.; Chaurasia, A.; Najafi, A. Investigating the Application of Stress Wave Factors in Machine Learning for Delamination Location Prediction in a Composite Laminate. In AIAA SCITECH 2024 Forum; American Institute of Aeronautics and Astronautics: Reston, Virginia, 2024. [Google Scholar] [CrossRef]
- Mahajan, H.; Banerjee, S. A machine learning framework for guided wave-based damage detection of rail head using surface-bonded piezo-electric wafer transducers. Mach. Learn. Appl. 2022, 7, 100216. [Google Scholar] [CrossRef]
- Kovar, L.; Gleicher, M.; Pighin, F. Motion Graphs. In Seminal Graphics Papers: Pushing the Boundaries; ACM: New York, NY, USA, 2023; Volume 2, pp. 723–732. [Google Scholar] [CrossRef]
- Jalata, I.K.; Truong, T.-D.; Allen, J.L.; Seo, H.-S.; Luu, K. Movement Analysis for Neurological and Musculoskeletal Disorders Using Graph Convolutional Neural Network. Future Internet 2021, 13, 194. [Google Scholar] [CrossRef]
- Naro, A.; Calabrò, R.S.; La Rosa, G.; Andronaco, V.A.; Billeri, L.; Lauria, P.; Bramanti, A.; Bramanti, P. Toward understanding the neurophysiological basis of peripersonal space: An EEG study on healthy individuals. PLoS ONE 2019, 14, e0218675. [Google Scholar] [CrossRef] [PubMed]
- Rashnu, A.; Salimi-Badr, A.; Rashnu, A.; Salimi-Badr, A. Integrative Deep Learning Framework for Parkinson’s Disease Early Detection using Gait Cycle Data Measured by Wearable Sensors: A CNN-GRU-GNN Approach. arXiv 2024, arXiv:2404.15335. [Google Scholar]
- Postuma, R.B.; Berg, D.; Stern, M.; Poewe, W.; Olanow, C.W.; Oertel, W.; Obeso, J.; Marek, K.; Litvan, I.; Lang, A.E.; et al. MDS clinical diagnostic criteria for Parkinson’s disease. Mov. Disord. 2015, 30, 1591–1601. [Google Scholar] [CrossRef]
- Goetz, C.G.; Tilley, B.C.; Shaftman, S.R.; Stebbins, G.T.; Fahn, S.; Martinez-Martin, P.; Poewe, W.; Sampaio, C.; Stern, M.B.; Dodel, R.; et al. Movement Disorder Society-sponsored revision of the Unified Parkinson’s Disease Rating Scale (MDS-UPDRS): Scale presentation and clinimetric testing results. Mov. Disord. 2008, 23, 2129–2170. [Google Scholar] [CrossRef]
- Movella (Xsens Technologies). Xsens MVN Animate. 2020. Available online: https://www.movella.com/motion-capture/xsens-mvn-animate (accessed on 1 December 2025).
- Li, P.; Yan, H.; Lu, X. A Siamese neural network for learning the similarity metrics of linear features. Int. J. Geogr. Inf. Sci. 2023, 37, 684–711. [Google Scholar] [CrossRef]
- Duong, T. ks: Kernel Density Estimation and Kernel Discriminant Analysis for Multivariate Data in R. J. Stat. Softw. 2007, 21, 1–16. [Google Scholar] [CrossRef]
- Fornito, A.; Zalesky, A.; Breakspear, M. The connectomics of brain disorders. Nat. Rev. Neurosci. 2015, 16, 159–172. [Google Scholar] [CrossRef] [PubMed]
- Fornito, A.; Zalesky, A.; Bullmore, E.T. Fundamentals of Brain Network Analysis; Academic Press: Cambridge, MA, USA, 2016; ISBN 978-0-12-407908. [Google Scholar] [CrossRef]
- Del Din, S.; Godfrey, A.; Mazzà, C.; Lord, S.; Rochester, L. Free-living monitoring of Parkinson’s disease: Lessons from the field. Mov. Disord. 2016, 31, 1293–1313. [Google Scholar] [CrossRef]
- Herman, T.; Weiss, A.; Brozgol, M.; Giladi, N.; Hausdorff, J.M. Gait and balance in Parkinson’s disease subtypes: Objective measures and classification considerations. J. Neurol. 2014, 261, 2401–2410. [Google Scholar] [CrossRef]
- Patel, S.; Lorincz, K.; Hughes, R.; Huggins, N.; Growdon, J.; Standaert, D.; Akay, M.; Dy, J.; Welsh, M.; Bonato, P. Monitoring Motor Fluctuations in Patients with Parkinson’s Disease Using Wearable Sensors. IEEE Trans. Inf. Technol. Biomed. 2009, 13, 864–873. [Google Scholar] [CrossRef] [PubMed]
- Gong, L.; Wang, J. Complexity analysis of electroencephalogram signal based on Jensen-Shannon divergence. In Proceedings of the 2013 6th International Conference on Biomedical Engineering and Informatics, Barcelona, Spain, 11–14 February 2013; pp. 219–223. [Google Scholar] [CrossRef]
- Shih, P.J.; Saadat, H.; Parameswaran, S.; Gamaarachchi, H. Efficient real-time selective genome sequencing on resource-constrained devices. Gigascience 2022, 12, giad046. [Google Scholar] [CrossRef]
- Faes, L.; Porta, A.; Nollo, G.; Javorka, M. Information Decomposition in Multivariate Systems: Definitions, Implementation and Application to Cardiovascular Networks. Entropy 2016, 19, 5. [Google Scholar] [CrossRef]
- Plotnik, M.; Giladi, N.; Hausdorff, J.M. Bilateral coordination of walking and freezing of gait in Parkinson’s disease. Eur. J. Neurosci. 2008, 27, 1999–2006. [Google Scholar] [CrossRef]
- Mirelman, A.; Bonato, P.; Camicioli, R.; Ellis, T.D.; Giladi, N.; Hamilton, J.L.; Hass, C.J.; Hausdorff, J.M.; Pelosin, E.; Almeida, Q.J.; et al. Gait impairments in Parkinson’s disease. Lancet Neurol. 2019, 18, 697–708. [Google Scholar] [CrossRef]
- Sreenivasan, K.; Bonato, P.; Camicioli, R.; Ellis, T.D.; Giladi, N.; Hamilton, J.L.; Hass, C.J.; Hausdorff, J.M.; Pelosin, E.; Almeida, Q.J. Topological reorganization of functional hubs in patients with Parkinson’s disease with freezing of gait. J. Neuroimaging 2023, 33, 547–557. [Google Scholar] [CrossRef] [PubMed]
- Suzuki, M.; Mitoma, H.; Yoneyama, M. Quantitative Analysis of Motor Status in Parkinson’s Disease Using Wearable Devices: From Methodological Considerations to Problems in Clinical Applications. Park. Dis. 2017, 2017, 6139716. [Google Scholar] [CrossRef] [PubMed]
- Verschueren, S.M.P.; Swinnen, S.P.; Dom, R.; De Weerdt, W. Interlimb coordination in patients with Parkinson’s disease: Motor learning deficits and the importance of augmented information feedback. ExBrain Res. 1997, 113, 497–508. [Google Scholar] [CrossRef]
- Trabassi, D.; Serrao, M.; Varrecchia, T.; Ranavolo, A.; Coppola, G.; De Icco, R.; Tassorelli, C.; Castiglia, S.F. Machine Learning Approach to Support the Detection of Parkinson’s Disease in IMU-Based Gait Analysis. Sensors 2022, 22, 3700. [Google Scholar] [CrossRef]
- Cubo, E.; Martín, P.M.; Martin-Gonzalez, J.A.; Rodríguez-Blázquez, C.; Kulisevsky, J. Motor laterality asymmetry and nonmotor symptoms in Parkinson’s disease. Mov. Disord. 2010, 25, 70–75. [Google Scholar] [CrossRef]
- Wu, T.; Hallett, M. The cerebellum in Parkinson’s disease. Brain 2013, 136, 696–709. [Google Scholar] [CrossRef]
- Park, H.; Shin, S.; Youm, C.; Cheon, S.-M. Deep learning-based detection of affected body parts in Parkinson’s disease and freezing of gait using time-series imaging. Sci. Rep. 2024, 14, 23732. [Google Scholar] [CrossRef]
- Salarian, A.; Russmann, H.; Wider, C.; Burkhard, P.R.; Vingerhoets, F.J.G.; Aminian, K. Quantification of Tremor and Bradykinesia in Parkinson’s Disease Using a Novel Ambulatory Monitoring System. IEEE Trans. Biomed. Eng. 2007, 54, 313–322. [Google Scholar] [CrossRef] [PubMed]
- Chicco, D.; Jurman, G. The advantages of the Matthews correlation coefficient (MCC) over F1 score and accuracy in binary classification evaluation. BMC Genom. 2020, 21, 6. [Google Scholar] [CrossRef]
| Data | PD (N = 51) | HC (N = 53) | p-Value |
|---|---|---|---|
| Gender, (M/F) | 37/14 | 19/34 | <0.001 a |
| Age at examination, ys b | 69.6 ± 8.67 | 51.3 ± 17.03 | <0.001 c |
| Disease onset b | 62.0 ± 8.96 | - | - |
| Disease duration, ys b | 7.6 ± 4.49 | - | - |
| MDS UPDRS TOTAL b | 29.9 ± 19.0 | - | - |
| MDS UPDRS-III b | 17.0 ± 11.0 | - | - |
| H-Y score b | 1.54 ± 0.60 | - | - |
| Bradykinesia_tot_left | 2.91 ± 3.39 | - | - |
| Bradykinesia_tot_right | 3.08 ± 3.22 | - | - |
| Rigidity_tot_left | 0.94 ± 0.94 | - | - |
| Rigidity_tot_right | 1.13 ± 1.19 | - | - |
| Tremor_tot_left | 0.61 ± 1.37 | - | - |
| Tremor_tot_right | 0.74 ± 1.15 | - | - |
| Total_left | 4.46 ± 4.80 | - | - |
| Total_right | 4.95 ± 4.62 | - | - |
Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2026 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license.
Share and Cite
Bianco, M.G.; Calomino, C.; Crasà, M.; Cristofaro, A.; Sgrò, G.; Novellino, F.; Pullano, S.A.; Islam, S.K.; Buonocore, J.; Quattrone, A.; et al. Similarity Gait Networks with XAI for Parkinson’s Disease Classification: A Pilot Study. Bioengineering 2026, 13, 151. https://doi.org/10.3390/bioengineering13020151
Bianco MG, Calomino C, Crasà M, Cristofaro A, Sgrò G, Novellino F, Pullano SA, Islam SK, Buonocore J, Quattrone A, et al. Similarity Gait Networks with XAI for Parkinson’s Disease Classification: A Pilot Study. Bioengineering. 2026; 13(2):151. https://doi.org/10.3390/bioengineering13020151
Chicago/Turabian StyleBianco, Maria Giovanna, Camilla Calomino, Marianna Crasà, Alessia Cristofaro, Giulia Sgrò, Fabiana Novellino, Salvatore Andrea Pullano, Syed Kamrul Islam, Jolanda Buonocore, Aldo Quattrone, and et al. 2026. "Similarity Gait Networks with XAI for Parkinson’s Disease Classification: A Pilot Study" Bioengineering 13, no. 2: 151. https://doi.org/10.3390/bioengineering13020151
APA StyleBianco, M. G., Calomino, C., Crasà, M., Cristofaro, A., Sgrò, G., Novellino, F., Pullano, S. A., Islam, S. K., Buonocore, J., Quattrone, A., Quattrone, A., & Nisticò, R. (2026). Similarity Gait Networks with XAI for Parkinson’s Disease Classification: A Pilot Study. Bioengineering, 13(2), 151. https://doi.org/10.3390/bioengineering13020151









