Physicochemical and Mechanical Characterization of Two Self-Curing Composite Resins for Direct Provisional Prostheses
Abstract
1. Introduction
- 2,2-bis-[4-(2-hydroxy-3-methacryloxypropoxy) phenyl] propane (Bis-GMA)
- Bisphenol A glycerolate dimethacrylate), bisphenol A ethoxylate dimethacrylate (Bis-EMA)
- 1,6-bis-bis-(methacryloxy-2-ethoxyamino)-2,4,4-trimethylhexane, urethane dimethacrylate monomer (UDMA)
- Triethylene glycol dimethacrylate (TEGDMA)
2. Materials and Methods
2.1. Flexural Strength Determination
2.2. Scanning Electron Microscopy (SEM)
2.3. Surface Roughness
2.4. Contact Angle Determination
2.5. Microhardness Test
2.6. Water Absroption
2.7. Wear Resistance Study
2.8. Scratch Resistance Evaluation
- (a)
- Study of the impression made by the indenter using optical microscopy: from the impression recorded on the sample surface, the width is measured using image processing.
- (b)
- The determination of the scratch channel width was carried out using optical microscopy techniques and subsequent image analysis of the acquired micrographs. For image capture, the Olympus GX51 camera (Tokyo, Japan) with a “JVC F-1030” digital camera and OmniMet Enterprise analysis software (Bühler Technologies GmbH, Ratingen, Germany) was used for this part of the study.
2.9. Density Determination
2.10. Statistical Study
3. Results
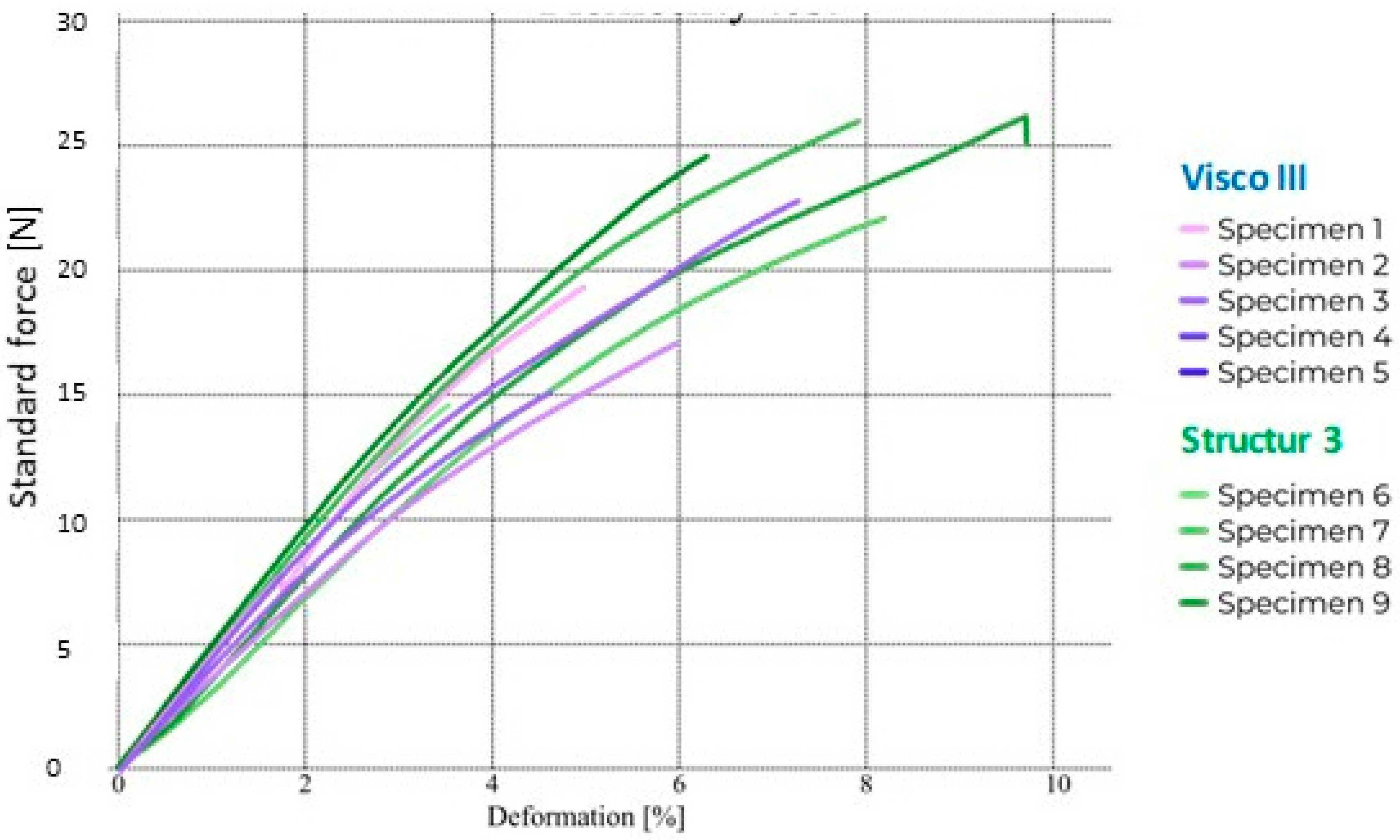
3.1. Flexural Strength
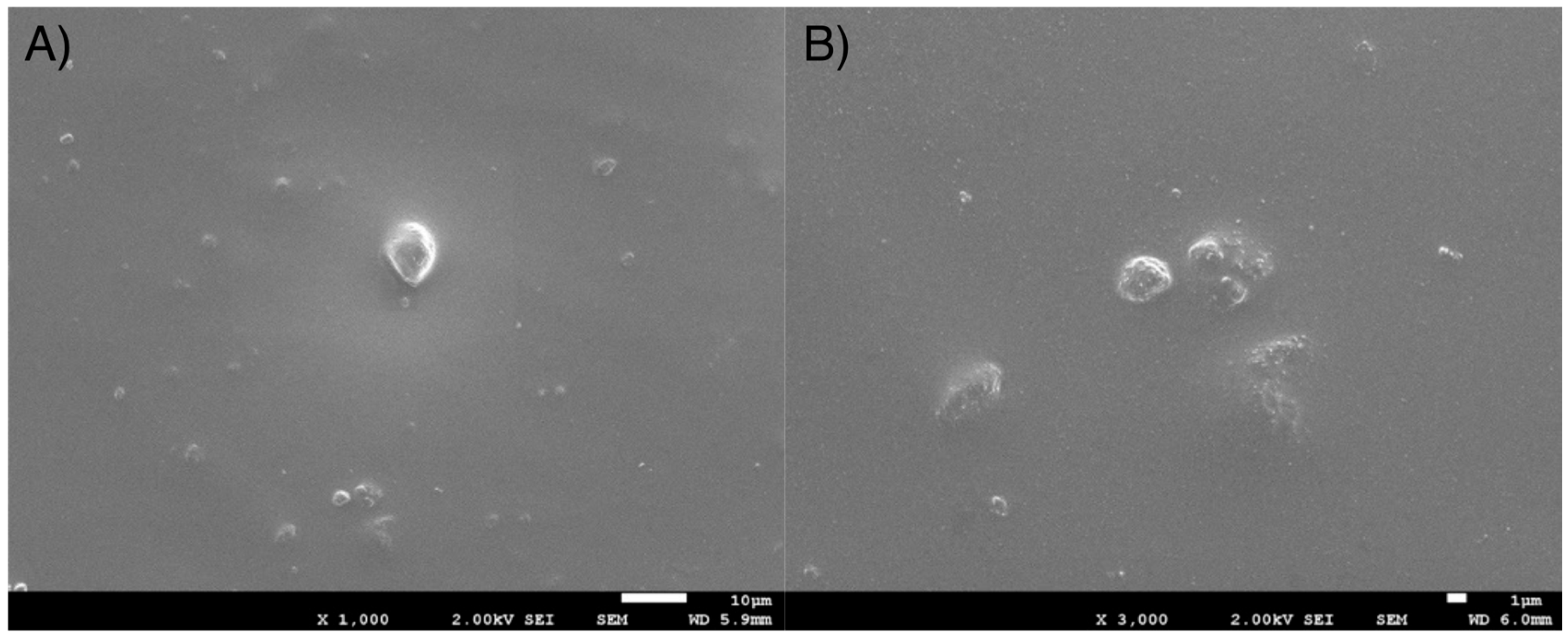
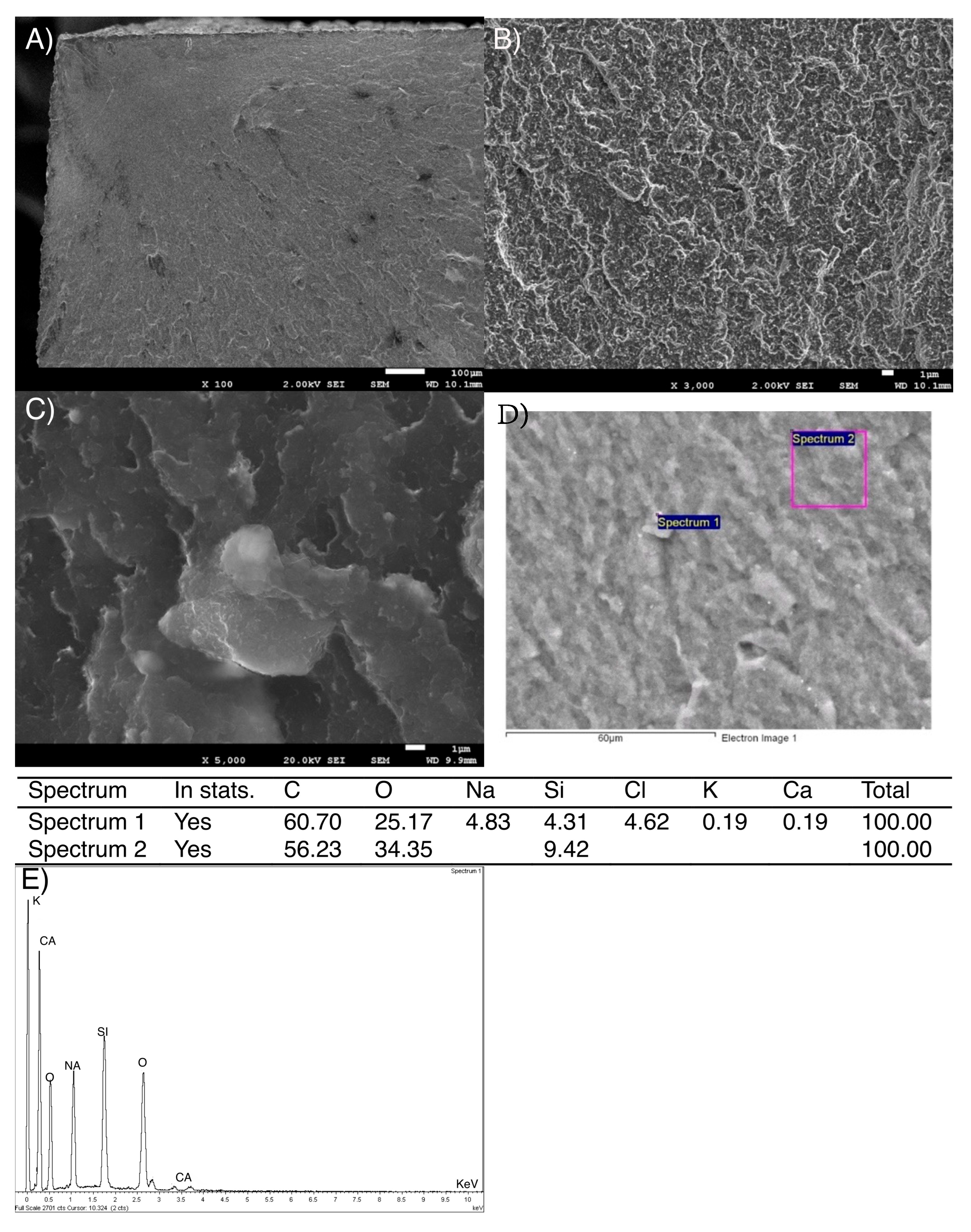
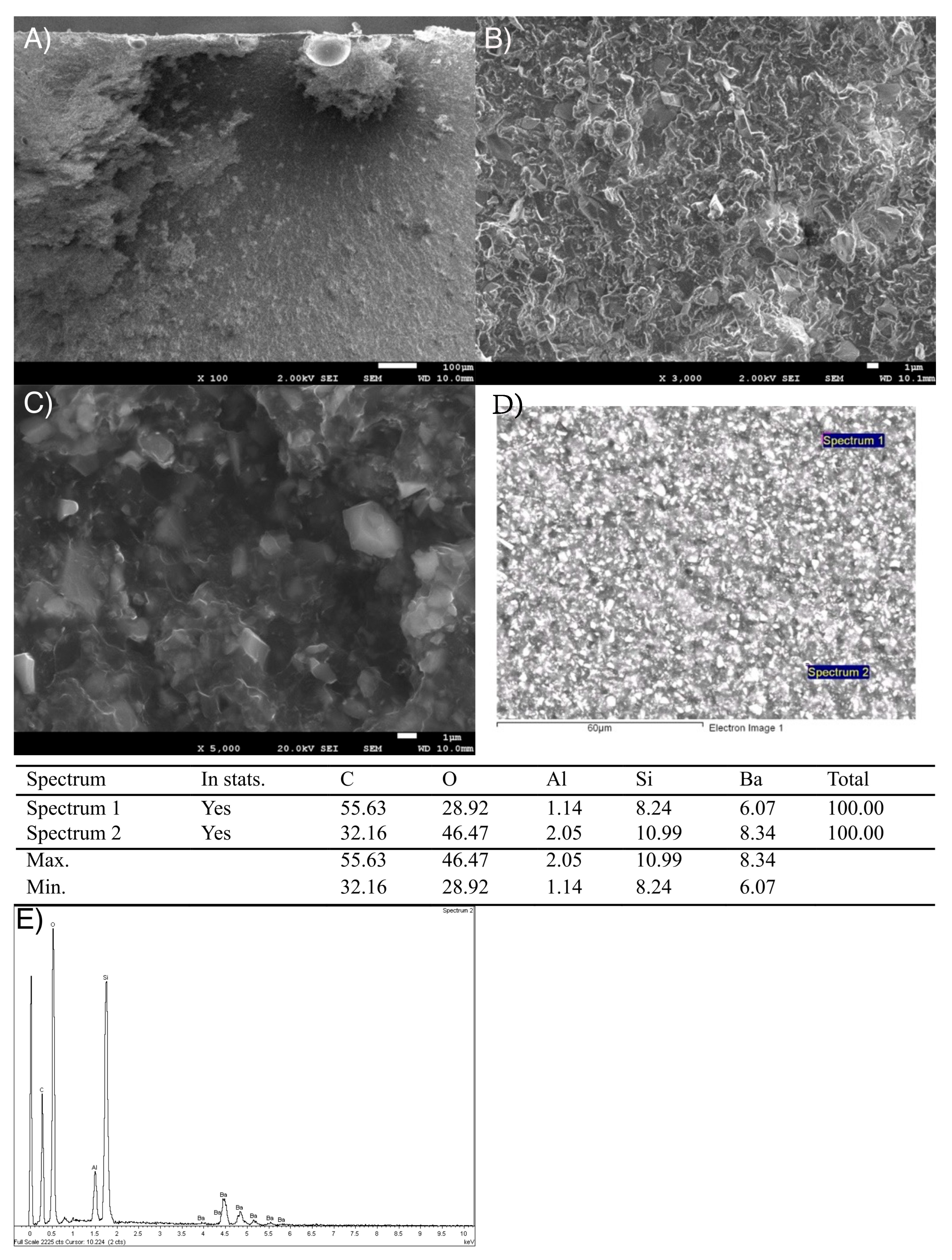
3.2. Scanning Electron Microscopy (SEM) Study
3.2.1. Dental Composite Surfaces Study and X-Ray Spectroscopy (EDS)
3.2.2. Fractographic Study by SEM
3.3. Contact Angle Measurement
3.4. Density
3.5. Microhardness Determination
3.6. Water Absorption
3.7. Wear Resistance Study
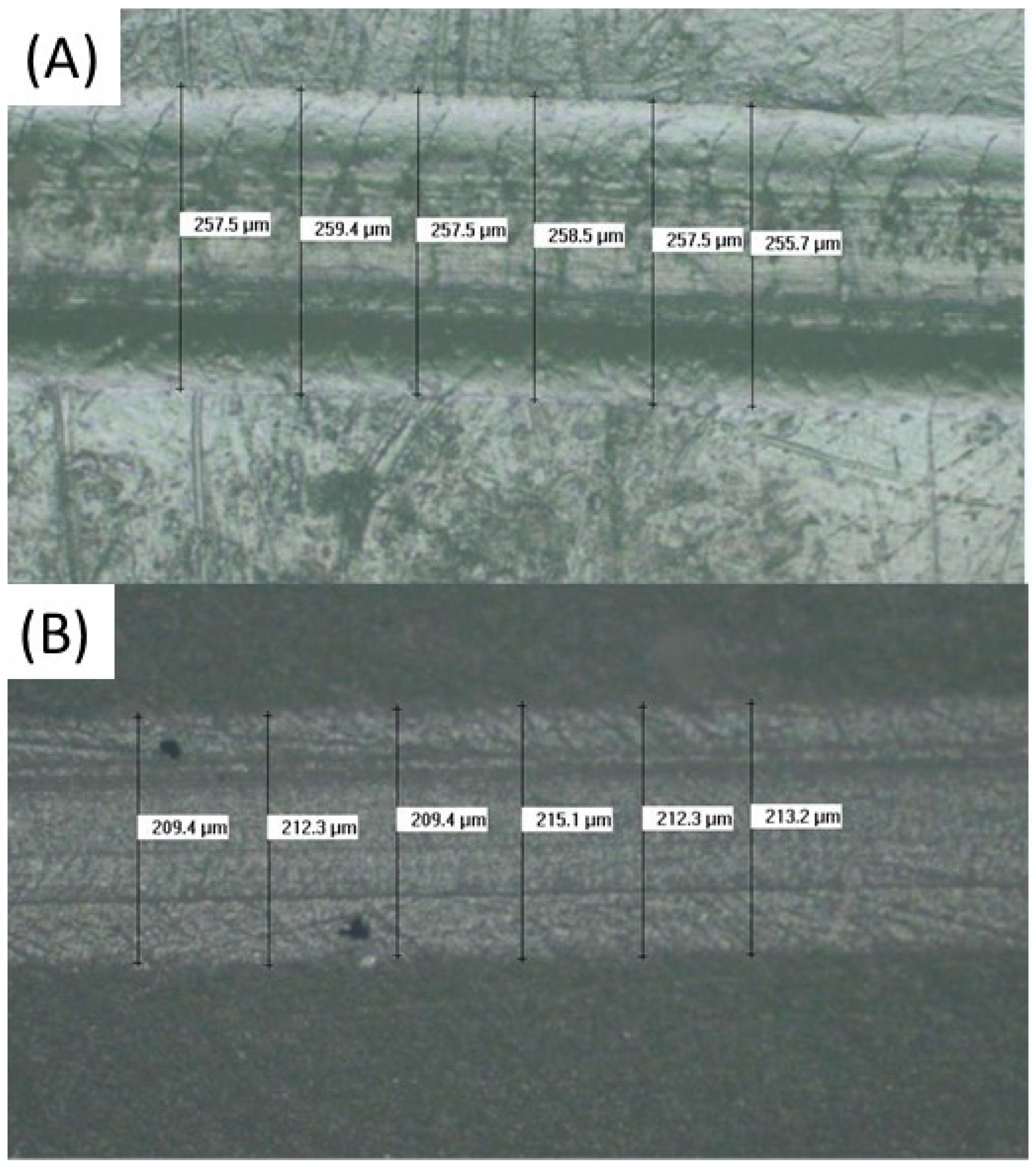
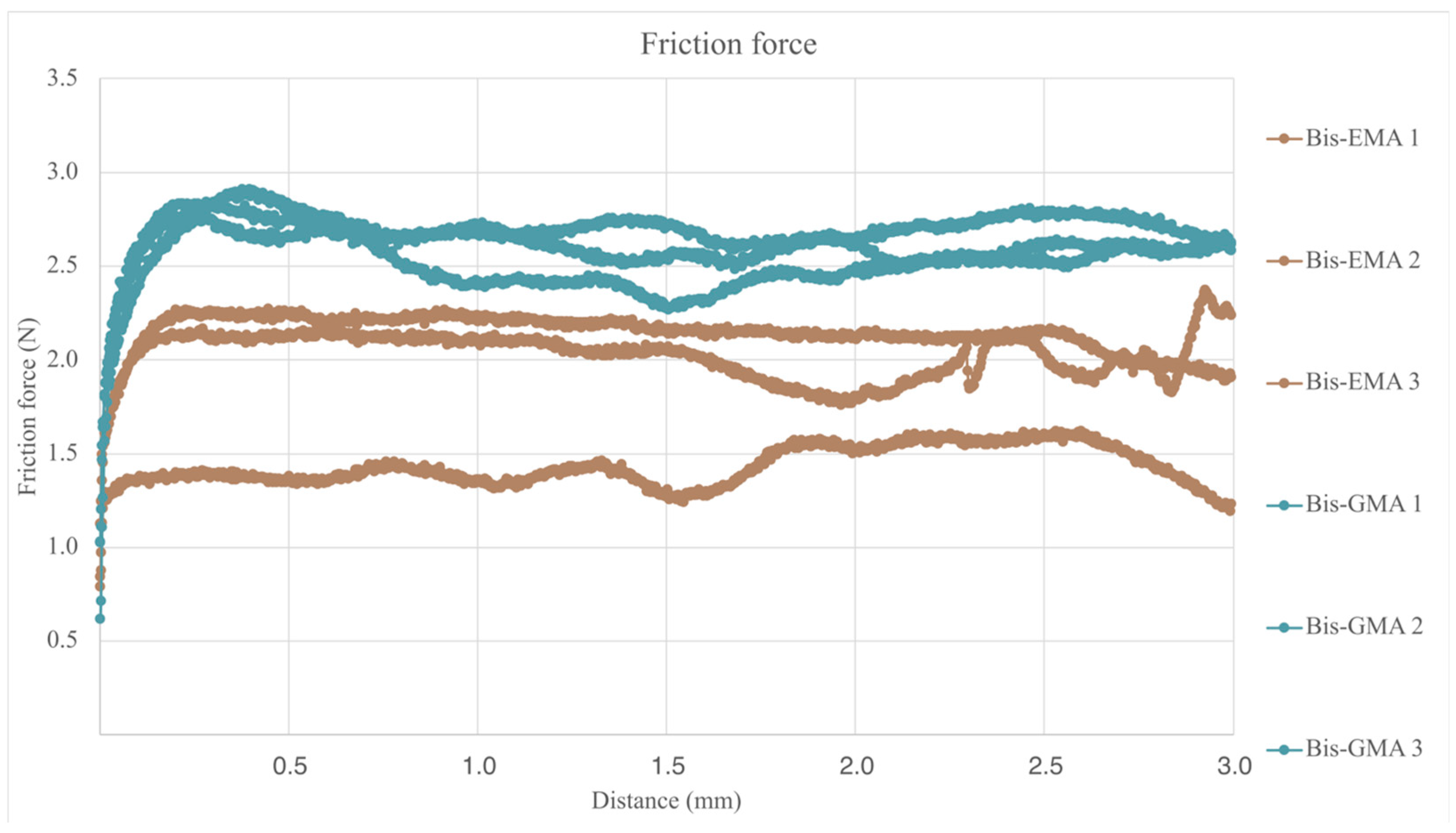
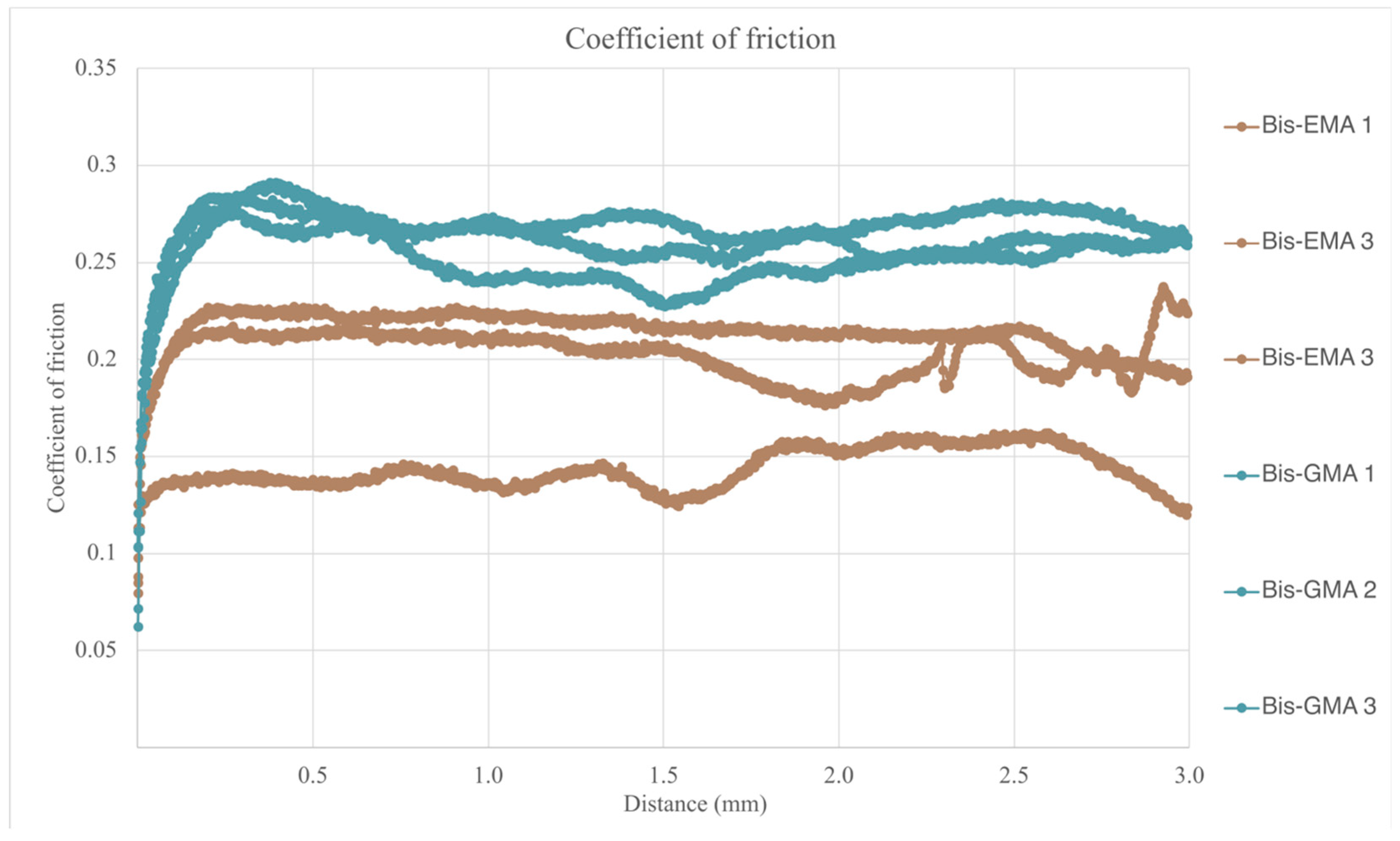
3.8. Scratch Resistance Evaluation
4. Discussion
4.1. Mechanical Properties
4.2. Physical Chemical Properties
4.3. Surface Properties and Wear Resistance
5. Conclusions
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Data Availability Statement
Conflicts of Interest
References
- Brånemark, P.I.; Adell, R.; Breine, U.; Hansson, B.O.; Lindström, J.; Ohlsson, A. Intra-osseous anchorage of dental prostheses: I. Experimental studies. Scand. J. Plast. Reconstr. Surg. 1969, 3, 81–100. [Google Scholar] [CrossRef]
- Luthardt, R.G.; Stößel, M.; Hinz, M.; Vollandt, R. Clinical performance and periodontal outcome of temporary crowns and fixed partial dentures: A randomized clinical trial. J. Prosthet. Dent. 2000, 83, 32–39. [Google Scholar] [CrossRef]
- Vahidi, F. The Provisional Restoration. Dent. Clin. N. Am. 1987, 31, 363–381. [Google Scholar]
- Lowe, R.A. The art and science of provisionalization. Int. J. Periodontics Restor. Dent. 1987, 7, 64–73. [Google Scholar]
- Baldissara, P.; Comin, G.; Martone, F.; Scotti, R. Comparative study of the marginal microleakage of six cements in fixed provisional crowns. J. Prosthet. Dent. 1998, 80, 417–422. [Google Scholar] [CrossRef]
- Zinner, I.D.; Trachtenberg, D.I.; Miller, R.D. Provisional Restorations in Fixed Partial Prosthodontics. Dent. Clin. N. Am. 1989, 33, 355–377. [Google Scholar] [PubMed]
- Peutzfeldt, A. Resin composites in dentistry: The monomer systems. Eur. J. Oral Sci. 1997, 105, 97–116. [Google Scholar] [CrossRef] [PubMed]
- Bowen, R.L. Dental Filling Material Comprising Vinyl Silane Treated Fused Silica and a Binder Consisting of the Reaction Product of Bis Phenol and Glycidyl Acrylate. US3066112A. 1962. Available online: https://patents.google.com/patent/US3066112A/en (accessed on 12 June 2024).
- Pratap, B.; Gupta, R.K. Department of Mechanical Engineering, Manipal University Jaipur, Rajasthan, India. Evaluation of Physical Properties of Silica filled Resin Based Dental Composites. Int. J. Eng. Adv. Technol. 2019, 8, 5047–5049. [Google Scholar]
- Durner, J.; Schrickel, K.; Watts, D.C.; Ilie, N. Determination of homologous distributions of bisEMA dimethacrylates in bulk-fill resin-composites by GC-MS. Dent. Mater. 2015, 31, 473–480. [Google Scholar] [PubMed]
- Katayama, Y.; Ohashi, K.; Iwasaki, T.; Kameyama, Y.; Wada, Y.; Miyake, K.; Tanimoto, Y.; Nihei, T. A study on the characteristics of resin composites for provisional restorations. Dent. Mater. J. 2022, 41, 256–265. [Google Scholar] [CrossRef]
- Pascual, B.; Gurruchaga, M.; Ginebra, M.P.; Gil, F.J.; Planell, J.A.; Goñi, I. Influence of the modification of P/L ratio on a new formulation of acrylic bone cement. Biomaterials 1999, 20, 465–474. [Google Scholar] [CrossRef]
- Vázquez, B.; Ginebra, M.P.; Gil, F.J.; Planell, J.A.; López Bravo, A.; San Román, J. Radiopaque acrylic cements prepared with a new acrylic derivative of iodo-quinoline. Biomaterials 1999, 20, 2047–2053. [Google Scholar] [CrossRef]
- ISO4049-2019; Dentistry—Polymer-Based Restorative Materials. International Standard Organization: Geneva, Switzerland, 2019.
- Pantea, M.; Ciocoiu, R.C.; Greabu, M.; Ripszky Totan, A.; Imre, M.; Țâncu, A.M.C.; Sfeatcu, R.; Spînu, T.C.; Ilinca, R.; Petre, A.E. Compressive and Flexural Strength of 3D-Printed and Conventional Resins Designated for Interim Fixed Dental Prostheses: An In Vitro Comparison. Materials 2022, 15, 3075. [Google Scholar] [CrossRef]
- ASTM ASTMG99 Standard Test Method for Wear Testing with a Pin-on-Disk Apparatus (1999). Wear 2011, 5, 1–5.
- Randolph, L.D.; Palin, W.M.; Leloup, G.; Leprince, J.G. Filler characteristics of modern dental resin composites and their influence on physico-mechanical properties. Dent. Mater. 2016, 32, 1586–1599. [Google Scholar] [CrossRef]
- Gajewski, V.E.S.; Pfeifer, C.S.; Fróes-Salgado, N.R.G.; Boaro, L.C.; Braga, R.R. Monomers used in resin composites: Degree of conversion, mechanical properties and water sorption/solubility. Braz. Dent. J. 2012, 23, 508–514. [Google Scholar] [CrossRef] [PubMed]
- Asmussen, E.; Peutzfeldt, A. Influence of UEDMA, BisGMA, and TEGDMA on selected mechanical properties of experimental resin composites-Buscar con Google. Dent. Mater. 1998, 14, 51–56. [Google Scholar] [PubMed]
- Gonçalves, F.; Kawano, Y.; Pfeifer, C.; Stansbury, J.W.; Braga, R.R. Influence of BisGMA, TEGDMA, and BisEMA contents on viscosity, conversion, and flexural strength of experimental resins and composites. Eur. J. Oral Sci. 2009, 117, 442–446. [Google Scholar] [CrossRef]
- Park, C.; Robertson, R.E. Mechanical properties of resin composites with filler particles aligned by an electric field. Dent. Mater. 1998, 14, 385–393. [Google Scholar] [CrossRef] [PubMed]
- Barszczewska-Rybarek, I.M. A Guide through the Dental Dimethacrylate Polymer Network Structural Characterization and Interpretation of Physico-Mechanical Properties. Materials 2019, 12, 4057. [Google Scholar] [CrossRef]
- Rodríguez, H.A.; Kriven, W.M.; Casanova, H. Development of mechanical properties in dental resin composite: Effect of filler size and filler aggregation state. Mater. Sci. Eng. C Mater. Biol. Appl. 2019, 101, 274–282. [Google Scholar] [CrossRef]
- Amaya-Pajares, S.P.; Koi, K.; Watanabe, H.; da Costa, J.B.; Ferracane, J.L. Development and maintenance of surface gloss of dental composites after polishing and brushing: Review of the literature. J. Esthet. Restor. Dent. 2022, 34, 15–41. [Google Scholar] [CrossRef]
- Lemon, M.T.; Jones, M.S.; Stansbury, J.W. Hydrogen bonding interactions in methacrylate monomers and polymers. J. Biomed. Mater. Res. A 2007, 83, 734–746. [Google Scholar] [CrossRef]
- Barszczewska-Rybarek, I.; Jurczyk, S. Comparative Study of Structure-Property Relationships in Polymer Networks Based on Bis-GMA, TEGDMA and Various Urethane-Dimethacrylates. Materials 2015, 8, 1230–1248. [Google Scholar] [CrossRef] [PubMed]
- Polydorou, O.; König, A.; Hellwig, E.; Kümmerer, K. Uthethane dimethacrylate: A molecule that may cause confusion in dental research. J. Biomed. Mater. Res. Part B Appl. Biomater. 2009, 91B, 1–4. [Google Scholar] [CrossRef] [PubMed]
- Bilgili, D.; Dündar, A.; Barutçugil, Ç.; Tayfun, D.; Özyurt, Ö.K. Surface properties and bacterial adhesion of bulk-fill composite resins. J. Dent. 2020, 95, 103317. [Google Scholar] [CrossRef]
- Vogler, E.A. Structure and reactivity of water at biomaterial surfaces. Adv. Colloid Interface Sci. 1998, 74, 69–117. [Google Scholar] [CrossRef] [PubMed]
- Tang, H.; Wang, A.; Liang, X.; Cao, T.; Salley, S.O.; McAllister, J.P.; Simon, K.Y. Effect of surface proteins on Staphylococcus epidermidis adhesion and colonization on silicone. Colloids Surf. B Biointerfaces 2006, 51, 16–24. [Google Scholar] [CrossRef]
- Fan, P.L.; Edahl, A.; Leung, R.L.; Stanford, J.W. Alternative Interpretations of Water Sorption Values of Composite Resins. J. Dent. Res. 1985, 64, 78–80. [Google Scholar] [CrossRef]
- Lodding, D.W. Long-term esthetic provisional restorations in dentistry. Curr. Opin. Cosmet. Dent. 1997, 4, 16–21. [Google Scholar]
- Bowen, R.L.; Rapson, J.E.; Dickson, G. Hardening Shrinkage and Hygroscopic Expansion of Composite Resins. J. Dent. Res. 1982, 61, 654–658. [Google Scholar] [CrossRef] [PubMed]
- Torstenson, B.; Brännström, M. Composite resin contraction gaps measured with a fluorescent resin technique. Dent. Mater. 1988, 4, 238–242. [Google Scholar] [CrossRef] [PubMed]
- Sideridou, I.; Tserki, V.; Papanastasiou, G. Study of water sorption, solubility and modulus of elasticity of light-cured dimethacrylate-based dental resins. Biomaterials 2003, 24, 655–665. [Google Scholar] [CrossRef]
- Blasi, P.; D’Souza, S.S.; Selmin, F.; DeLuca, P.P. Plasticizing effect of water on poly(lactide-co-glycolide). J. Control. Release 2005, 108, 1–9. [Google Scholar] [CrossRef]
- Manzano, M.; Arcos, D.; Delgado, M.R.; Ruiz, E.; Gil, F.J.; Vallet-Regí, M. Bioactive star gels. Chem. Mater. 2006, 18, 5696–5703. [Google Scholar] [CrossRef]
- Brizuela-Velasco, A.; Álvarez-Arenal, Á.; Gil-Mur, F.J.; Gil-Mur, F.J.; Herrero-Climent, M.; Chávarri-Prado, D.; Chento-Valiente, Y.; Dieguez-Pereira, M. Relationship Between Insertion Torque and Resonance Frequency Measurements, Performed by Resonance Frequency Analysis, in Micromobility of Dental Implants: An In Vitro Study. Implant Dent. 2015, 24, 607–611. [Google Scholar] [CrossRef] [PubMed]
- Castellanos, M.I.; Zenses, A.-S.; Grau, A.; Rodríguez-Cabello, J.C.; Gil, F.J.; Manero, J.M.; Pegueroles, M. Biofunctionalization of REDV elastin-like recombinamers improves endothelialization on CoCr alloy surfaces for cardiovascular applications. Colloids Surf. B Biointerfaces 2015, 127, 22–32. [Google Scholar] [CrossRef]
- Salinas, A.J.; Merino, J.M.; Babonneau, F.; Gil, F.J.; Vallet-Regí, M. Microstructure and macroscopic properties of bioactive CaO-SiO2-PDMS hybrids. J. Biomed. Mater. Res. B Appl. Biomater. 2007, 81, 274–282. [Google Scholar] [CrossRef]
- Sideridou, I.; Achilias, D.S.; Spyroudi, C.; Karabela, M. Water sorption characteristics of light-cured dental resins and composites based on Bis-EMA/PCDMA. Biomaterials 2004, 25, 367–376. [Google Scholar] [CrossRef]
- Tsujimoto, A.; Barkmeier, W.W.; Fischer, N.G.; Nojiri, K.; Nagura, Y.; Takamizawa, T.; Latta, M.A.; Miazaki, M. Wear of resin composites: Current insights into underlying mechanisms, evaluation methods and influential factors. Jpn. Dent. Sci. Rev. 2018, 54, 76–87. [Google Scholar] [CrossRef]
- Turssi, C.P.; De Moraes Purquerio, B.; Serra, M.C. Wear of dental resin composites: Insights into underlying processes and assessment methods—A review. J. Biomed. Mater. Res. B Appl. Biomater. 2003, 65, 280–285. [Google Scholar] [CrossRef]
- Pfeifer, C.S.; Silva, L.R.; Kawano, Y.; Braga, R.R. Bis-GMA co-polymerizations: Influence on conversion, flexural properties, fracture toughness and susceptibility to ethanol degradation of experimental composites. Dent. Mater. 2009, 25, 1136–1141. [Google Scholar] [CrossRef]
- Mousavinasab, S.M. Biocompatibility of composite resins. Dent. Res. J. 2011, 8, S21–S29. [Google Scholar]
- Aparicio, C.; Manero, J.M.; Conde, F.; Pegueroles, M.; Planell, J.A.; Vallet-Regí, M.; Gil, F.J. Acceleration of apatite nucleation on microrough bioactive titanium for bone-replacing implants. J. Biomed. Mater. Res. A 2007, 82, 521–529. [Google Scholar] [CrossRef]
- Vila, M.M.; Ginebra, M.P.; Gil, F.J.; Planell, J.A. Effect of porosity and environment on the mechanical behavior of acrylic bone cement modified with acrylonitrile-butadiene-styrene particles: I. Fracture toughness. J. Biomed. Mater. Res. 1999, 48, 121–127. [Google Scholar] [CrossRef]
- Schmalz, G.; Arenholt-Bindslev, D. Biocompatibility of Dental Materials: With 82 Tables; Springer: Berlin/Heidelberg, Germany, 2009. [Google Scholar]
- Gil, F.J.; Espias, A.; Sánchez, L.A.; Planell, J.A. Comparison of the abrasive wear resistance between amalgams, hybrid composite material and different dental cements. Int. Dent. J. 1999, 49, 337–342. [Google Scholar] [CrossRef] [PubMed]
- Cosola, S.; Toti, P.; Babetto, E.; Covani, U.; Peñarrocha-Diago, M.; Peñarrocha-Oltra, D. In-vitro fatigue and fracture performance of three different ferrulized implant connections used in fixed prosthesis. J. Dent. Sci. 2021, 16, 397–403. [Google Scholar] [CrossRef]
- Buxadera-Palomero, J.; Calvo, C.; Torrent-Camarero, S.; Gil, F.J.; Mas-Moruno, C.; Canal, C.; Rodríguez, D. Biofunctional polyethylene glycol coatings on titanium: An in vitro-based comparison of functionalization methods. Colloids Surf. B Biointerfaces 2017, 152, 367–375. [Google Scholar] [CrossRef]
- Mas-Moruno, C.; Garrido, B.; Rodriguez, D.; Ruperez, E.; Gil, F.J. Biofunctionalization strategies on tantalum-based materials for osseointegrative applications. J. Mater. Sci. Mater. Med. 2015, 26, 109. [Google Scholar] [CrossRef] [PubMed]
- Gil, F.J.; Solano, E.; Peña, J.; Engel, E.; Mendoza, A.; Planell, J.A. Microstructural, mechanical and citotoxicity evaluation of different NiTi and NiTiCu shape memory alloys. J. Mater. Sci. Mater. Med. 2004, 15, 1181–1185. [Google Scholar] [CrossRef]
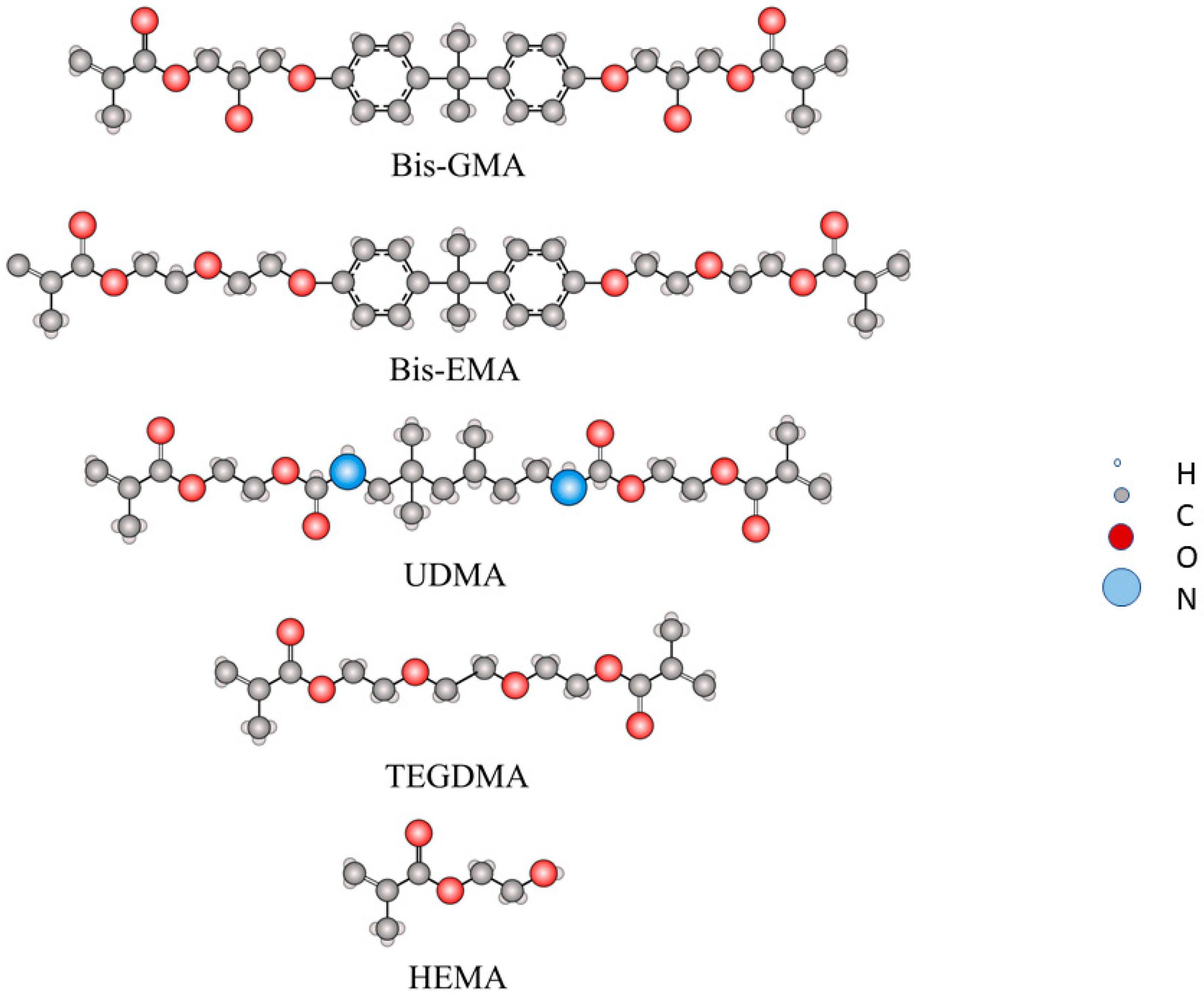



| Monomer | Bis-GMA | Bis-EMA |
|---|---|---|
| Brand | Structur 3 | Visco III |
| Manufacturer | VOCO GmbH Cuxhaven | Anaxdent GmbH D-Stuttgart Germany |
| Dental composite type | Self-curing | Self-curing |
| Composition | Polymers Bis-GMA 5–10% UDMA 10–25% Inorganic Filler Filler (SiO2) 0.9 mm 12–17% Amines Terpenes Benzoyl Peroxide Butylated hydroxytoluene | Polymers Bis-EMA 7–12% UDMA 10–25% 1,4-butanediol dimethacrylate (BDDM) 5–7% Filler (SiO2) 1.7 mm 17–20% Catalyst Silanizer pyrogenic silicic acid |
| Dental Composite | Flexural Strength (MPa) | Modulus of Elasticity (GPa)) | Toughness (mJ) | Displacement Until Fracture (mm) |
|---|---|---|---|---|
| Structur 3 | 127 (16) (*) | 1.92 (0.10) (*) | 36.52 (9.20) (*) | 2.50 (0.38) (*) |
| Visco III | 103 (25) (*) | 1.92 (0.27) (*) | 16.55 (7.55) (**) | 1.72 (0.38) (**) |
| Dental Composite | Roughness (mm) | Contact Angle, θ |
|---|---|---|
| Structur 3 | 0.74 (0.06) (*) | 75.8 (2.6) (*) |
| Visco III | 0.81 (0.07) (*) | 77.3 (5.0) (*) |
| Material | Structur 3 (g/cm3) | Visco III (g/cm3) |
|---|---|---|
| X | 1.324 (*) | 1.592 (**) |
| SD | 0.005 | 0.006 |
| Material | Hardness (HV) |
|---|---|
| Structur 3 | 8.10 (0.76) (*) |
| Visco III | 17.05 (0.93) (**) |
| Material | Water Absorption (µg/mm3) |
|---|---|
| Structur 3 | 15.0 (0.7) (*) |
| Visco III | 11.2 (0.4) (**) |
| Material | |||
|---|---|---|---|
| Property/Units | Result | Structur 3 | Visco III |
| Wear track area/(mm2) | Mean | 0.031 (0.013) (*) | 0.047(0.021) (*) |
| Maximum Friction Coefficient/ad | Mean | 0.740 (0.028) (*) | 0.727(0.071) (*) |
| Average Friction Coefficient/ad | Mean | 0.446(0.043) (*) | 0.416(0.148) (*) |
| Wear Rate/(m3/Nm) × 10−13 | Mean | 1.531(0.563) (*) | 2.342(1.091) (**) |
| Material | Scratch Mark Width, [µm] |
|---|---|
| Structur 3 | 252.4 (3.8) (*) |
| Visco III | 217.0 (3.4) (**) |
| Material | Friction Force, (N) | Dynamic Friction Coef., [a.d] | Penetration Depth, (µm) |
|---|---|---|---|
| Structur 3 | 1.878 (0.386) (*) | 0.259 (0.011) (*) | 38.98 (1.87) (*) |
| Visco III | 2.591 (0.107) (**) | 0.188 (0.039) (**) | 38.34 (5.05) (*) |
Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2025 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Valencia Blanco, O.J.; Fernández-Hernández, S.; de Llanos-Lanchares, H.; Punset Fuste, M.; Delgado García-Menocal, J.A.; Gil Mur, J.; Brizuela Velasco, A. Physicochemical and Mechanical Characterization of Two Self-Curing Composite Resins for Direct Provisional Prostheses. Bioengineering 2025, 12, 996. https://doi.org/10.3390/bioengineering12090996
Valencia Blanco OJ, Fernández-Hernández S, de Llanos-Lanchares H, Punset Fuste M, Delgado García-Menocal JA, Gil Mur J, Brizuela Velasco A. Physicochemical and Mechanical Characterization of Two Self-Curing Composite Resins for Direct Provisional Prostheses. Bioengineering. 2025; 12(9):996. https://doi.org/10.3390/bioengineering12090996
Chicago/Turabian StyleValencia Blanco, Oscar Javier, Saray Fernández-Hernández, Hector de Llanos-Lanchares, Miquel Punset Fuste, José Angel Delgado García-Menocal, Javier Gil Mur, and Aritza Brizuela Velasco. 2025. "Physicochemical and Mechanical Characterization of Two Self-Curing Composite Resins for Direct Provisional Prostheses" Bioengineering 12, no. 9: 996. https://doi.org/10.3390/bioengineering12090996
APA StyleValencia Blanco, O. J., Fernández-Hernández, S., de Llanos-Lanchares, H., Punset Fuste, M., Delgado García-Menocal, J. A., Gil Mur, J., & Brizuela Velasco, A. (2025). Physicochemical and Mechanical Characterization of Two Self-Curing Composite Resins for Direct Provisional Prostheses. Bioengineering, 12(9), 996. https://doi.org/10.3390/bioengineering12090996








