Association of Spinopelvic Anatomy with the Level of Lumbar Disc Herniation
Abstract
1. Introduction
2. Materials and Methods
2.1. Study Design
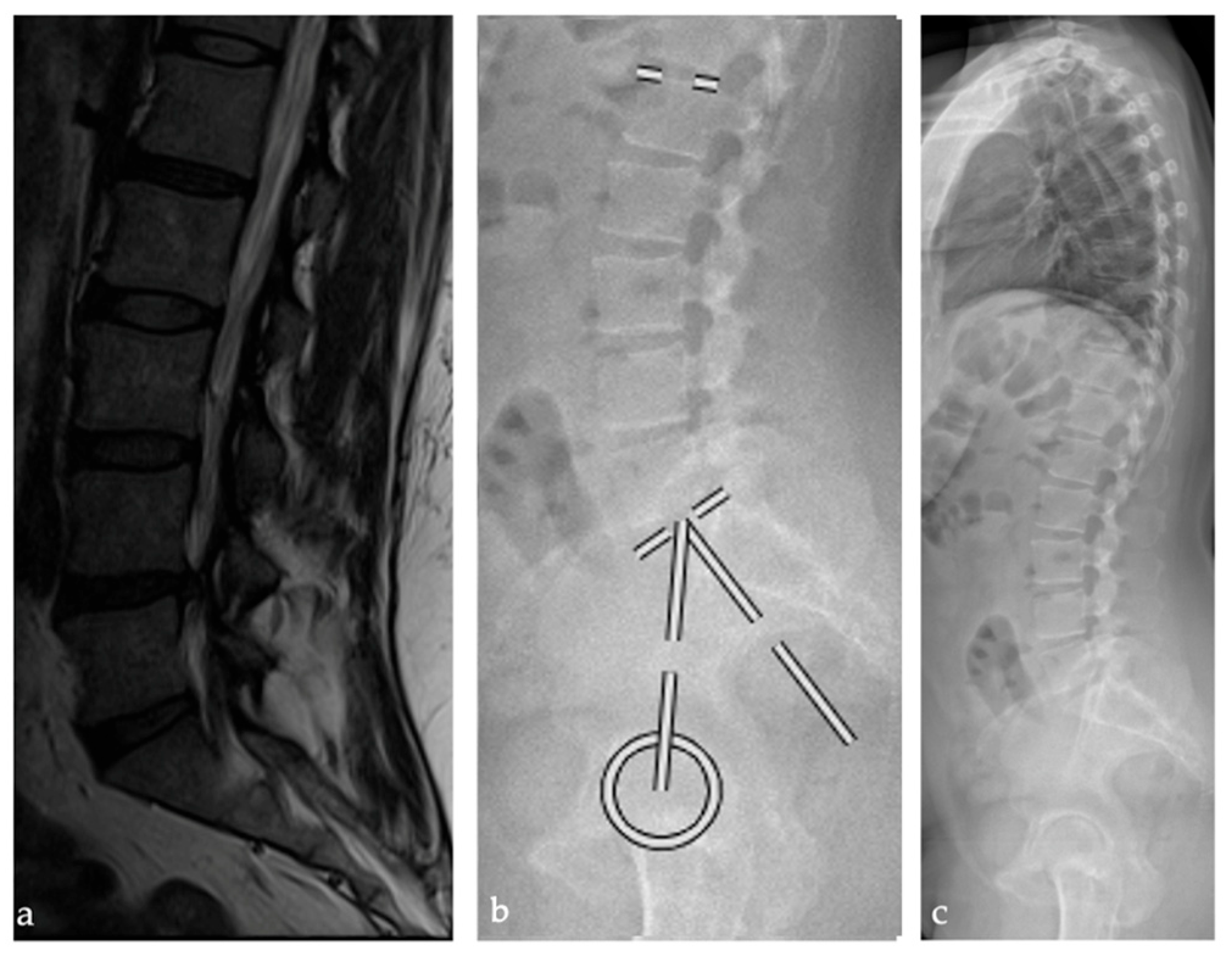
2.2. Radiographic Analysis
2.3. Statistical Analysis
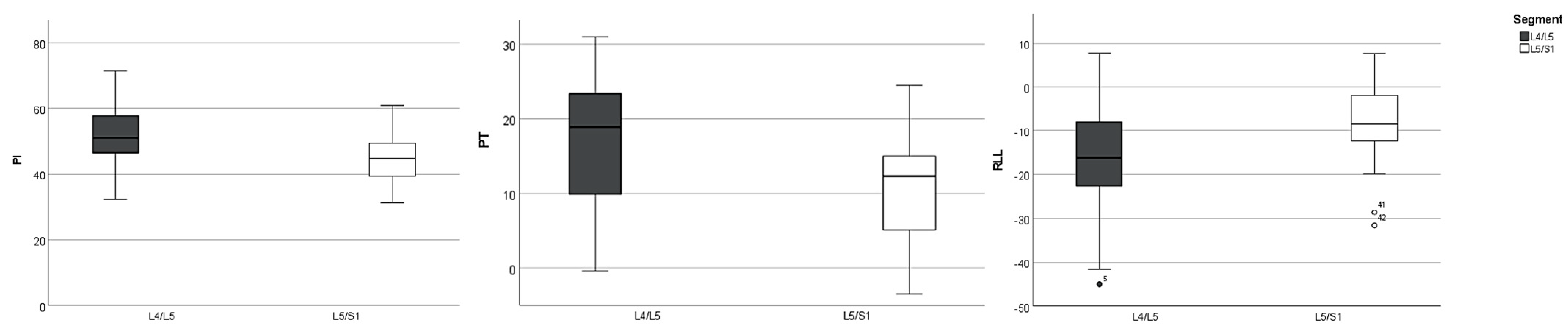
3. Results
4. Discussion
5. Conclusions
Author Contributions
Funding
Informed Consent Statement
Conflicts of Interest
Abbreviations
| PI | Pelvic Incidence |
| PT | Pelvic Tilt |
| LL | Lumbar Lordosis |
| SS | Sacral Slope |
| RLL | Relative Lumbar Lordosis |
| SVA | Sagittal Vertical Axis |
| iPSL | Isthmic spondylolisthesis |
| C7SVA | C7 Sagittal Vertical Axis |
| LDH | Lumbar disc herniation |
References
- Kirnaz, S.; Capadona, C.; Wong, T.; Goldberg, J.L.; Medary, B.; Sommer, F.; McGrath, L.B., Jr.; Härtl, R. Fundamentals of Intervertebral Disc Degeneration. World Neurosurg. 2022, 157, 264–273. [Google Scholar] [CrossRef]
- Fasser, M.R.; Furrer, P.R.; Fisler, L.; Urbanschitz, L.; Snedeker, J.G.; Farshad, M.; Widmer, J. The triadic relationship between spinal posture, loading, and degeneration. Front. Bioeng. Biotechnol. 2025, 13, 1444540. [Google Scholar] [CrossRef]
- Roussouly, P.; Pinheiro-Franco, J.L. Biomechanical analysis of the spino-pelvic organization and adaptation in pathology. Eur. Spine J. 2011, 20, 609. [Google Scholar] [CrossRef]
- Menezes-Reis, R.; Bonugli, G.P.; Dalto, V.F.; da Silva Herrero, C.F.P.; Defino, H.L.A.; Nogueira-Barbosa, M.H. Association Between Lumbar Spine Sagittal Alignment and L4–L5 Disc Degeneration Among Asymptomatic Young Adults. Spine 2016, 41, E1081–E1087. [Google Scholar] [CrossRef]
- Barrey, C.; Jund, J.; Noseda, O.; Roussouly, P. Sagittal balance of the pelvis-spine complex and lumbar degenerative diseases. A comparative study about 85 cases. Eur. Spine J. 2007, 16, 1459–1467. [Google Scholar] [CrossRef] [PubMed]
- Roussouly, P.; Gollogly, S.; Berthonnaud, E.; Dimnet, J. Classification of the normal variation in the sagittal alignment of the human lumbar spine and pelvis in the standing position. Spine 2005, 30, 346–353. [Google Scholar] [CrossRef]
- Strube, P.; Pumberger, M.; Sonnow, L.; Zippelius, T.; Nowack, D.; Zahn, R.K.; Putzier, M. Association Between Lumbar Spinal Degeneration and Anatomic Pelvic Parameters. Clin. Spine Surg. 2018, 31, 263–267. [Google Scholar] [CrossRef] [PubMed]
- Legaye, J.; Duval-Beaupère, G.; Hecquet, J.; Marty, C. Pelvic incidence: A fundamental pelvic parameter for three-dimensional regulation of spinal sagittal curves. Eur. Spine J. 1998, 7, 99–103. [Google Scholar] [CrossRef]
- Sahin, M.S.; Ergün, A.; Aslan, A. The Relationship Between Osteoarthritis of the Lumbar Facet Joints and Lumbosacropelvic Morphology. Spine 2015, 40, E1058–E1062. [Google Scholar] [CrossRef]
- Soydan, Z.; Bayramoglu, E.; Altas, O. The Impact of Spinopelvic Alignment on the Facet Joint Degeneration. Glob. Spine J. 2025, 15, 306–313. [Google Scholar] [CrossRef] [PubMed]
- Bae, J.; Lee, S.H.; Shin, S.H.; Seo, J.S.; Kim, K.H.; Jang, J.S. Radiological analysis of upper lumbar disc herniation and spinopelvic sagittal alignment. Eur. Spine J. 2016, 25, 1382–1388. [Google Scholar] [CrossRef]
- Wei, X.; Gengwu, L.; Chao, C.; Yifan, L.; Shang, S.; Ruixi, H.; Yunhan, J.; Xiaodong, Z.; Zhikun, L. Correlations between the sagittal plane parameters of the spine and pelvis and lumbar disc degeneration. J. Orthop. Surg. Res. 2018, 13, 137. [Google Scholar] [CrossRef] [PubMed]
- Labbus, K.; Bürger, J.; Löchel, J.; Schäfer, F.M.; Putzier, M.; Zahn, R.K. Impact of Individual Spinopelvic Anatomy on the Localization and Severity of Symptomatic Isthmic Spondylolisthesis. Glob. Spine J. 2024, 14, 2311–2316. [Google Scholar] [CrossRef] [PubMed]
- Yokoyama, K.; Tanaka, H.; Ito, Y.; Yamada, M.; Sugie, A.; Wanibuchi, M.; Kawanishi, M. Analgesic Posture and Pelvic Morphology in Patients with Lumbar Disc Herniation. World Neurosurg. 2021, 147, e411–e415. [Google Scholar] [CrossRef]
- Yamato, Y.; Hasegawa, T.; Yoshida, G.; Banno, T.; Arima, H.; Oe, S.; Ide, K.; Yamada, T.; Murakami, Y.; Matsuyama, Y. The effect of pelvic incidence on age-related changes in spinopelvic sagittal alignment in older individuals: A longitudinal study for 10 years. Eur. Spine J. 2025. [Google Scholar] [CrossRef] [PubMed]
- Hadjipavlou, A.G.; Tzermiadianos, M.N.; Bogduk, N.; Zindrick, M.R. The pathophysiology of disc degeneration: A critical review. J. Bone Jt. Surg. Br. 2008, 90, 1261–1270. [Google Scholar] [CrossRef]
- Zukowski, L.A.; Falsetti, A.B.; Tillman, M.D. The influence of sex, age and BMI on the degeneration of the lumbar spine. J. Anat. 2012, 220, 57–66. [Google Scholar] [CrossRef]
- Farshad-Amacker, N.A.; Hughes, A.P.; Aichmair, A.; Herzog, R.J.; Farshad, M. Determinants of evolution of endplate and disc degeneration in the lumbar spine: A multifactorial perspective. Eur. Spine J. 2014, 23, 1863–1868. [Google Scholar] [CrossRef]
- Hedman, T.; Rogers, A. Pathomechanics of Early-Stage Lumbar Intervertebral Disc Degradation Leading to Discogenic Pain—A Narrative Review. Bioengineering 2025, 12, 389. [Google Scholar] [CrossRef]
- Beck, J.; Brisby, H.; Baranto, A.; Westin, O. Low lordosis is a common finding in young lumbar disc herniation patients. J. Exp. Orthop. 2020, 7, 38. [Google Scholar] [CrossRef]
- Dammers, R.; Koehler, P.J. Lumbar disc herniation: Level increases with age. Surg Neurol. 2002, 58, 209–212; discussion 212–213. [Google Scholar] [CrossRef]
- Ould-Slimane, M.; Ferracci, F.X.; Gillibert, A.; Szadkowski, M.; Lesage, C.; Vieira, T.D.; Sacco, R.; d’Astorg, H. Sagittal alignment of the spine and lumbar disc herniation in young adults: A historical, case-control study. Orthop. Traumatol. Surg. Res. 2025, 111, 104219. [Google Scholar] [CrossRef] [PubMed]
- Yilgor, C.; Sogunmez, N.; Yavuz, Y.; Abul, K.; Boissiére, L.; Haddad, S.; Obeid, I.; Kleinstück, F.; Sánchez Pérez-Grueso, F.J.; Acaroğlu, E.; et al. European Spine Study Group. Relative lumbar lordosis and lordosis distribution index: Individualized pelvic incidence-based proportional parameters that quantify lumbar lordosis more precisely than the concept of pelvic incidence minus lumbar lordosis. Neurosurg. Focus. 2017, 43, E5. [Google Scholar] [CrossRef]
- Yilgor, C.; Sogunmez, N.; Boissiere, L.; Yavuz, Y.; Obeid, I.; Kleinstück, F.; Pérez-Grueso, F.J.S.; Acaroglu, E.; Haddad, S.; Mannion, A.F.; et al. European Spine Study Group (ESSG). Global Alignment and Proportion (GAP) Score: Development and Validation of a New Method of Analyzing Spinopelvic Alignment to Predict Mechanical Complications After Adult Spinal Deformity Surgery. J. Bone Joint Surg. Am. 2017, 99, 1661–1672. [Google Scholar] [CrossRef]
- Löchel, J.; Putzier, M.; Dreischarf, M.; Grover, P.; Urinbayev, K.; Abbas, F.; Labbus, K.; Zahn, R. Deep learning algorithm for fully automated measurement of sagittal balance in adult spinal deformity. Eur. Spine J. 2024, 33, 4119–4124. [Google Scholar] [CrossRef]
- Fei, H.; Li, W.S.; Sun, Z.R.; Ma, Q.W.; Chen, Z.Q. Analysis of Spino-pelvic Sagittal Alignment in Young Chinese Patients with Lumbar Disc Herniation. Orthop. Surg. 2017, 9, 271–276. [Google Scholar] [CrossRef] [PubMed]
- Iatridis, J.C.; Nicoll, S.B.; Michalek, A.J.; Walter, B.A.; Gupta, M.S. Role of biomechanics in intervertebral disc degeneration and regenerative therapies: What needs repairing in the disc and what are promising biomaterials for its repair? Spine J. 2013, 13, 243–262. [Google Scholar] [CrossRef]
- Wang, W.; Pei, B.; Wu, S.; Lu, D.; He, P.; Ma, C.; Wu, X. Biomechanical responses of human lumbar spine and pelvis according to the Roussouly classification. PLoS ONE 2022, 17, e0266954. [Google Scholar] [CrossRef]
- Sonne-Holm, S.; Jacobsen, S.; Rovsing, H.C.; Monrad, H.; Gebuhr, P. Lumbar spondylolysis: A life long dynamic condition? A cross sectional survey of 4.151 adults. Eur. Spine J. 2007, 16, 821–828. [Google Scholar] [CrossRef] [PubMed]
- Galbusera, F.; Brayda-Bruno, M.; Costa, F.; Wilke, H.J. Numerical evaluation of the correlation between the normal variation in the sagittal alignment of the lumbar spine and the spinal loads. J. Orthop. Res. 2014, 32, 537–544. [Google Scholar] [CrossRef]
- Jentzsch, T.; Geiger, J.; Bouaicha, S.; Slankamenac, K.; Nguyen-Kim, T.D.; Werner, C.M. Increased pelvic incidence may lead to arthritis and sagittal orientation of the facet joints at the lower lumbar spine. BMC Med. Imaging 2013, 13, 34. [Google Scholar] [CrossRef] [PubMed]
- Filardi, V.; Simona, P.; Cacciola, G.; Bertino, S.; Soliera, L.; Barbanera, A.; Pisani, A.; Milardi, D.; Alessia, B. Finite element analysis of sagittal balance in different morphotype: Forces and resulting strain in pelvis and spine. J. Orthop. 2017, 14, 268–275. [Google Scholar] [CrossRef]
- Vaz, G.; Roussouly, P.; Berthonnaud, E.; Dimnet, J. Sagittal morphology and equilibrium of pelvis and spine. Eur. Spine J. 2002, 11, 80–87. [Google Scholar] [CrossRef]
- Endo, K.; Suzuki, H.; Tanaka, H.; Kang, Y.; Yamamoto, K. Sagittal spinal alignment in patients with lumbar disc herniation. Eur. Spine J. 2010, 19, 435–438. [Google Scholar] [CrossRef] [PubMed]
- Liang, C.; Sun, J.; Cui, X.; Jiang, Z.; Zhang, W.; Li, T. Spinal sagittal imbalance in patients with lumbar disc herniation: Its spinopelvic characteristics, strength changes of the spinal musculature and natural history after lumbar discectomy. BMC Musculoskelet. Disord. 2016, 17, 305. [Google Scholar] [CrossRef] [PubMed]

| Segment | PI [°] | PT [°] | SS [°] | LL [°] | RLL [°] | SVA [mm] | |
|---|---|---|---|---|---|---|---|
| L4/5 (n = 34) | Mean | 52.1 (±9.2) | 17.8 (±8.3) | 34 (±9.7) | 45.8 (±14.4) | −15.5 (±12.4) | 49 (±41.5) |
| L5/S1 (n = 23) | Mean | 44.7 (±8) | 11 (±7.2) | 33.6 (±7.3) | 48.2 (±9.3) | −8.6 (±9.6) | 22.7 (±41.7) |
| p | 0.004 | 0.006 | 0.67 | 0.33 | 0.026 | 0.019 | |
| Mean overall (n = 57) | Mean | 50 (±9.8) | 15.9 (±9.6) | 34.3 (±8.8) | 46.8 (±13) | −13.3 (±12.6) | 39.4 (±43) |
Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2025 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Löchel, J.; Hanisch, M.; Bürger, J.; Labbus, K.; Zahn, R. Association of Spinopelvic Anatomy with the Level of Lumbar Disc Herniation. Bioengineering 2025, 12, 993. https://doi.org/10.3390/bioengineering12090993
Löchel J, Hanisch M, Bürger J, Labbus K, Zahn R. Association of Spinopelvic Anatomy with the Level of Lumbar Disc Herniation. Bioengineering. 2025; 12(9):993. https://doi.org/10.3390/bioengineering12090993
Chicago/Turabian StyleLöchel, Jannis, Moritz Hanisch, Justus Bürger, Kirsten Labbus, and Robert Zahn. 2025. "Association of Spinopelvic Anatomy with the Level of Lumbar Disc Herniation" Bioengineering 12, no. 9: 993. https://doi.org/10.3390/bioengineering12090993
APA StyleLöchel, J., Hanisch, M., Bürger, J., Labbus, K., & Zahn, R. (2025). Association of Spinopelvic Anatomy with the Level of Lumbar Disc Herniation. Bioengineering, 12(9), 993. https://doi.org/10.3390/bioengineering12090993






