Linking a Deep Learning Model for Concussion Classification with Reorganization of Large-Scale Brain Networks in Female Youth
Abstract
1. Introduction
2. Materials and Methods
2.1. Participants
2.2. Concussion Symptom Assessment
2.3. EEG Acquisition
2.4. Data Preprocessing for Deep Learning
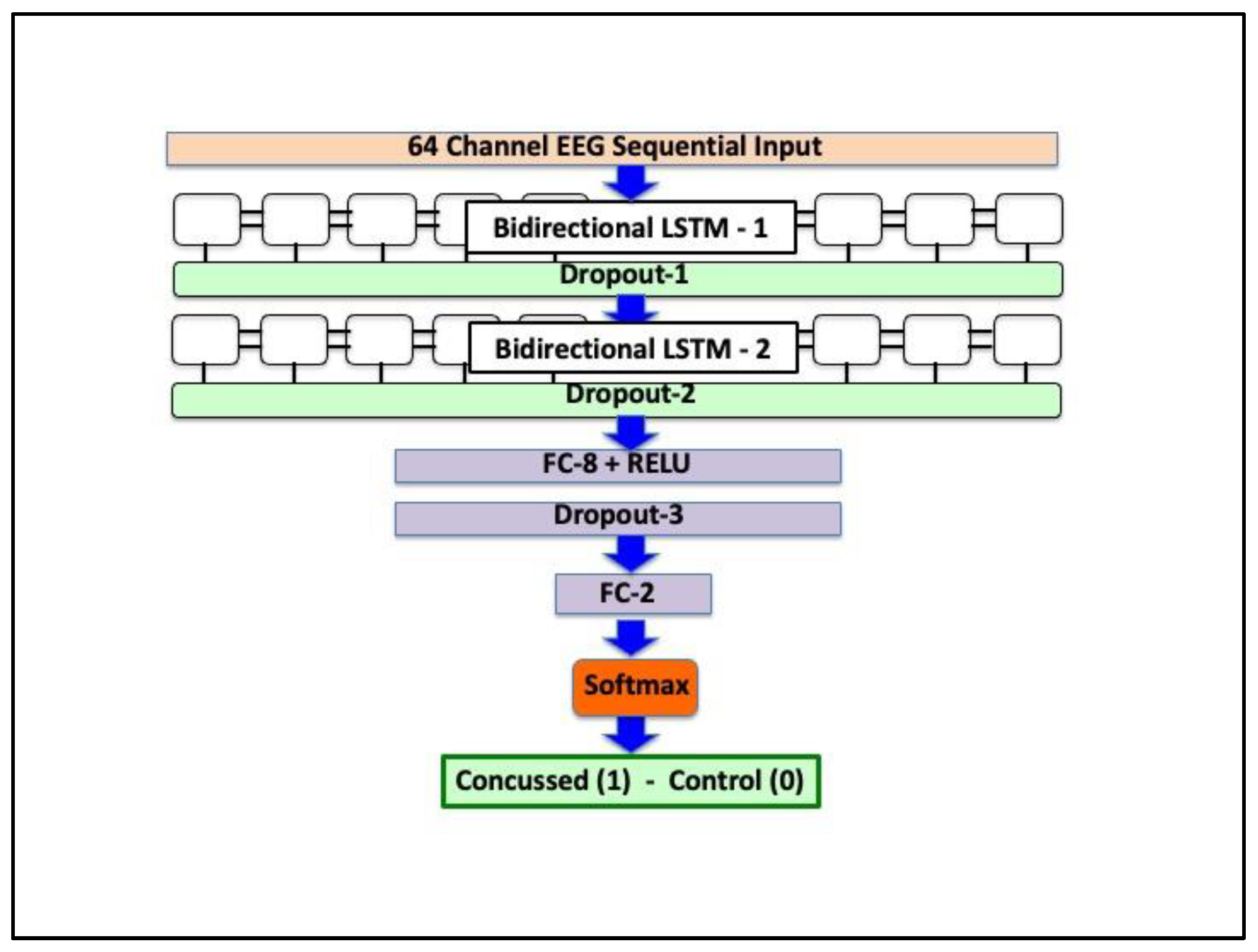
2.5. Deep Neural Network Architecture
2.6. EEG Preprocessing and Source Reconstruction for Causal Connectivity Analysis
2.7. Causal Connectivity
2.8. Degree Assortativity
2.9. Statistical Analyses
3. Results
3.1. Clinical and Demographic Data
3.2. ConcNet
3.3. Causal Connectivity
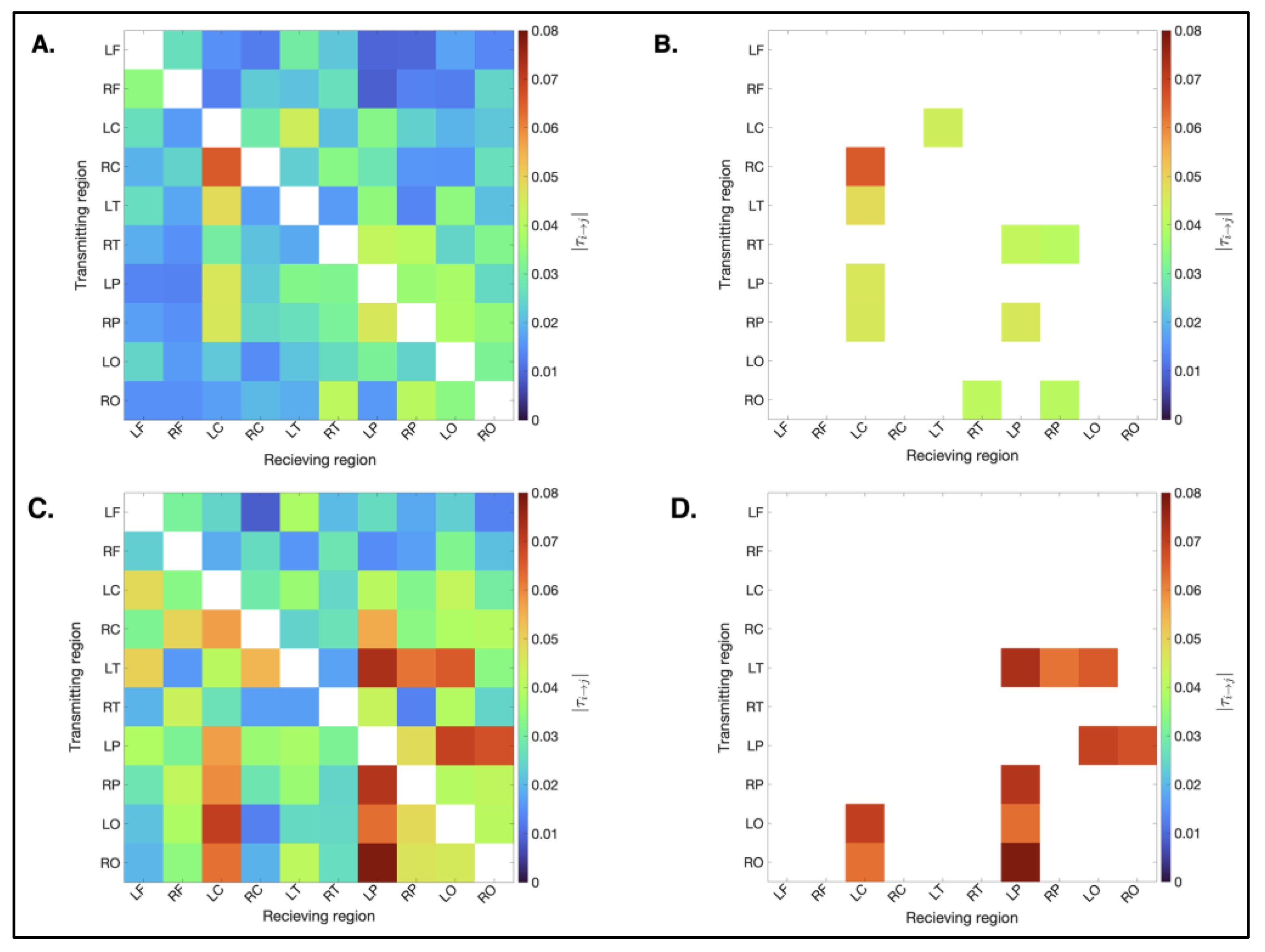
3.3.1. Spatial Distribution
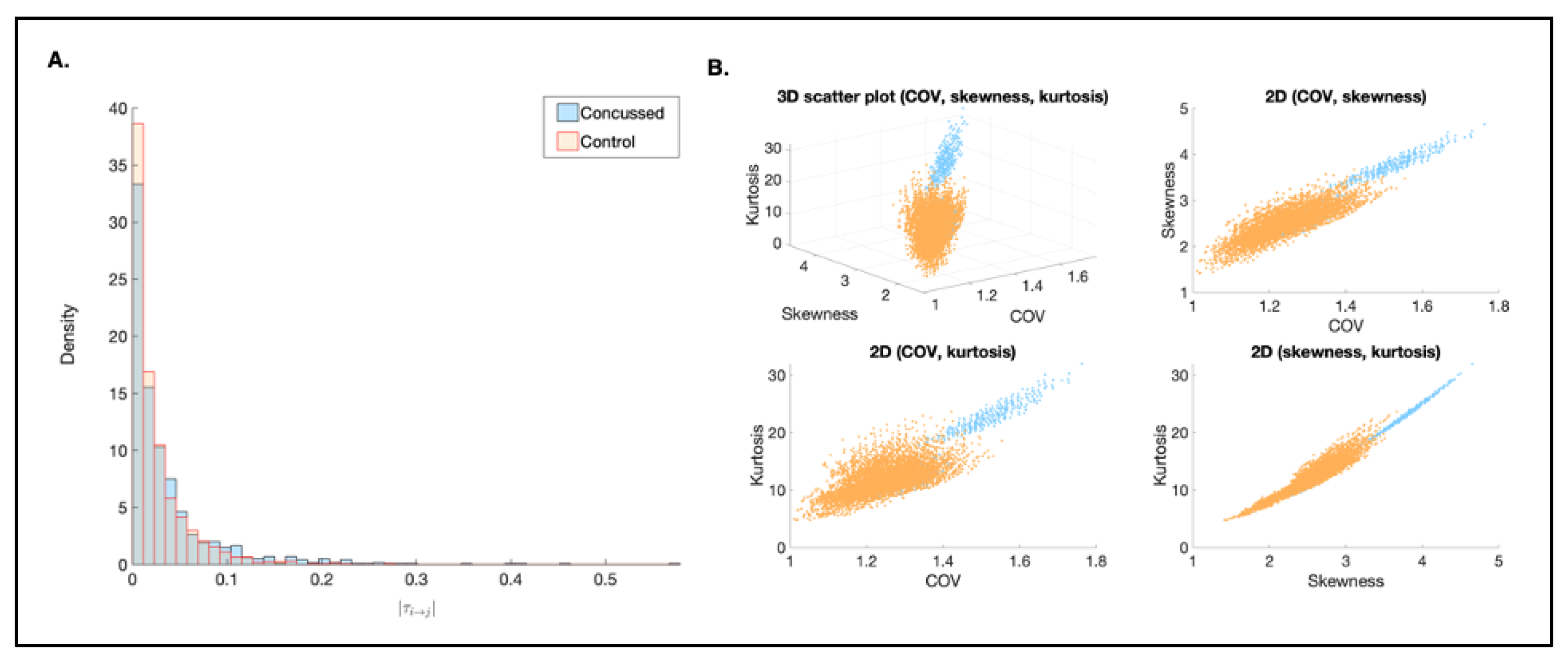
3.3.2. Magnitude
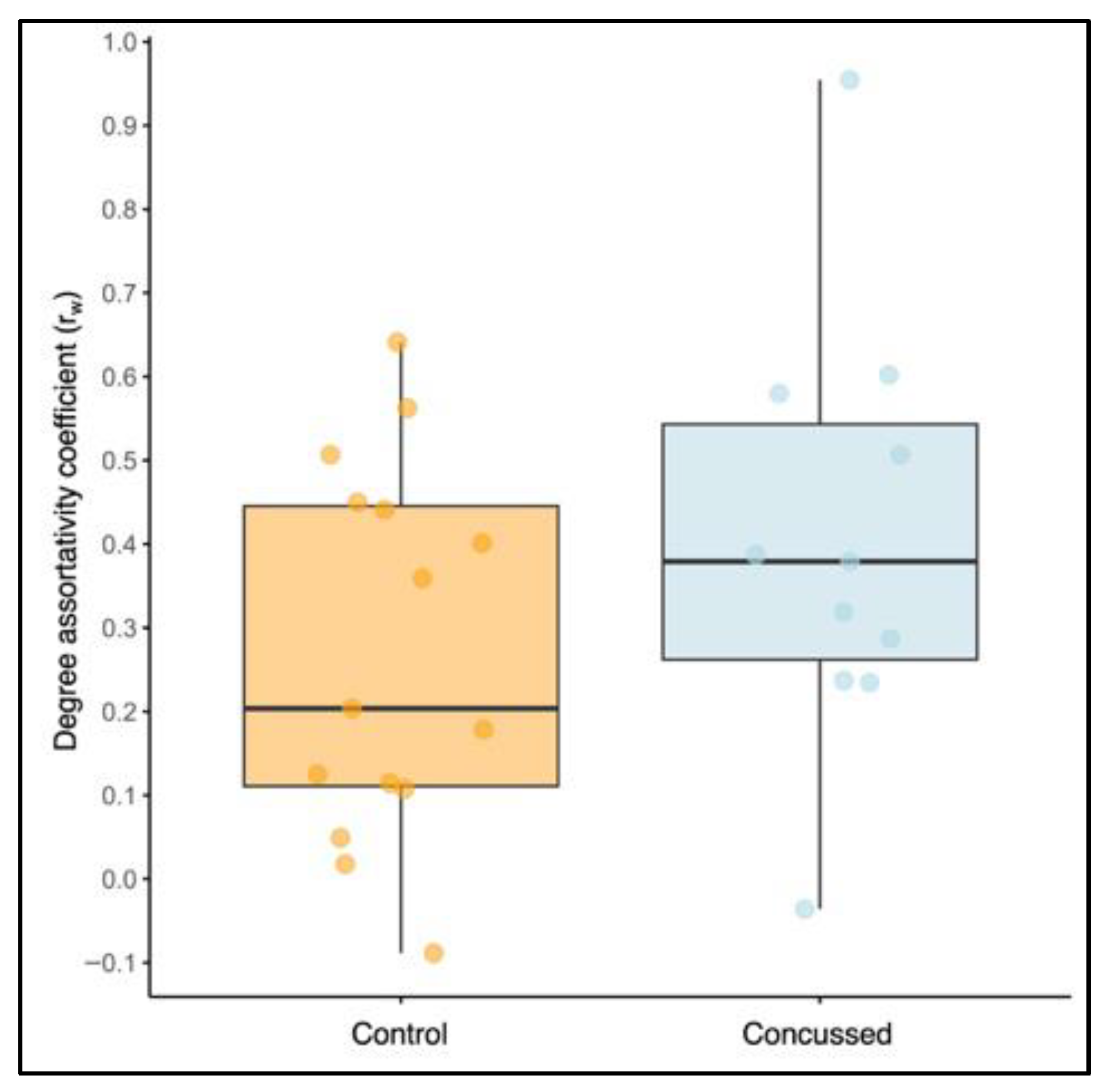
3.3.3. Degree Assortativity
3.3.4. Statistical Analysis for Causal Connectivity
4. Discussion
4.1. Causal Connectivity
4.2. Benefits of EEG Classifiers over Other Neuroimaging Techniques
4.3. Limitations and Future Directions
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Data Availability Statement
Acknowledgments
Conflicts of Interest
Abbreviations
| m(TBI) | Mild traumatic brain injury |
| RS-EEG | Resting-state electroencephalography |
| LSTM | Long short-term memory |
| CM | Confusion matrix |
| AU | Area under the curve |
| ROC | Receiver operating characteristic |
Appendix A
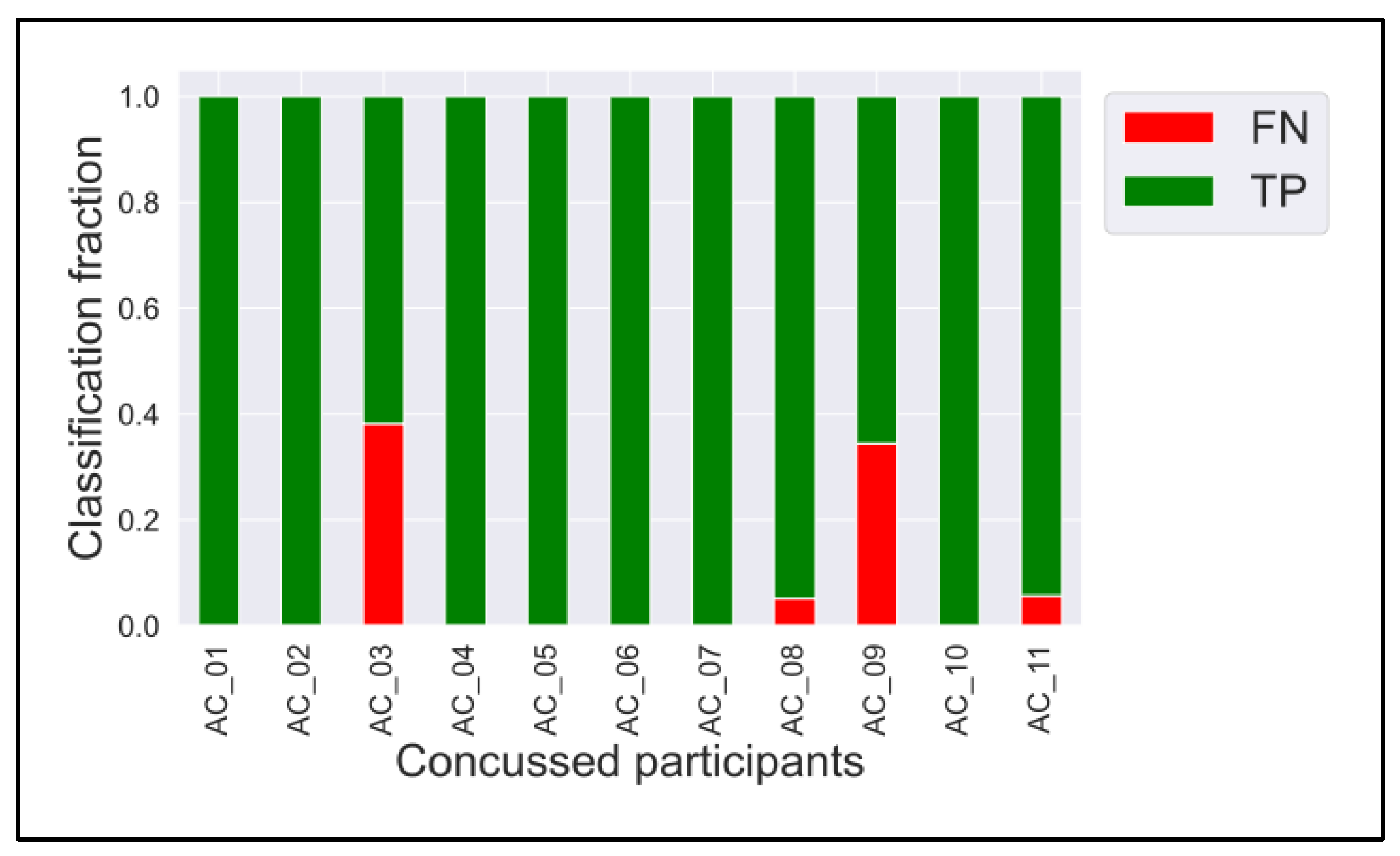
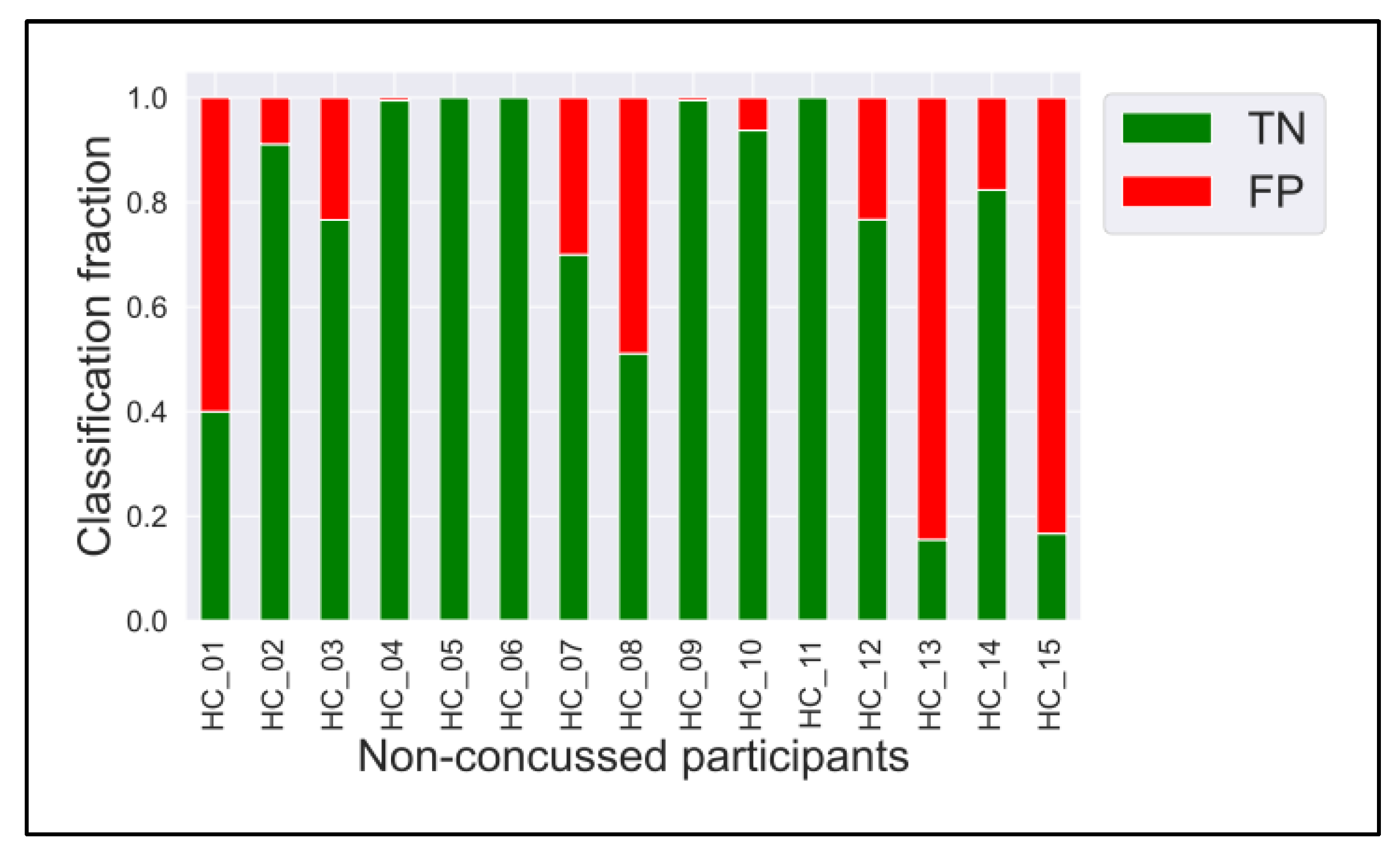


Appendix B
| Control (n = 15) | Concussed (n = 11) | ||||
|---|---|---|---|---|---|
| Transmitter | Receiver | Transmitter | Receiver | ||
| RC | LC | 6.54 × 10−2 | LO | LC | 7.63 × 10−2 |
| LT | LC | 4.83 × 10−2 | RO | LP | 7.25 × 10−2 |
| LP | LC | 4.61 × 10−2 | LT | LP | 7.24 × 10−2 |
| RP | LC | 4.58 × 10−2 | LP | LO | 7.12 × 10−2 |
| RP | LP | 4.58 × 10−2 | LT | LO | 7.07 × 10−2 |
| LC | LT | 4.44 × 10−2 | RP | LP | 6.96 × 10−2 |
| RT | LP | 4.24 × 10−2 | LP | RO | 6.82 × 10−2 |
| RO | RT | 4.10 × 10−2 | RO | LC | 6.69 × 10−2 |
| RO | RP | 4.05 × 10−2 | RP | LC | 6.35 × 10−2 |
| RT | RP | 4.01 × 10−2 | RC | LC | 6.10 × 10−2 |
| RP | LO | 3.84 × 10−2 | LT | RP | 6.02 × 10−2 |
| LP | LO | 3.81 × 10−2 | LP | LC | 5.70 × 10−2 |
| LP | RP | 3.58 × 10−2 | RC | LP | 5.70 × 10−2 |
| RP | RO | 3.47 × 10−2 | LO | LP | 5.60 × 10−2 |
| LT | LP | 3.45 × 10−2 | LT | LF | 5.43 × 10−2 |
| LT | LO | 3.43 × 10−2 | LT | RC | 5.30 × 10−2 |
| RF | LF | 3.42 × 10−2 | LC | LF | 5.27 × 10−2 |
| RO | LO | 3.37 × 10−2 | LP | RP | 5.09 × 10−2 |
| RC | RT | 3.34 × 10−2 | LO | RP | 4.85 × 10−2 |
| LC | LP | 3.28 × 10−2 | RO | RP | 4.81 × 10−2 |
| LP | LT | 3.27 × 10−2 | RC | RF | 4.75 × 10−2 |
| RT | RO | 3.25 × 10−2 | RT | LP | 4.67 × 10−2 |
| LP | RT | 3.19 × 10−2 | RT | RF | 4.65 × 10−2 |
| LO | RO | 3.15 × 10−2 | LC | LO | 4.61 × 10−2 |
| LO | LP | 3.12 × 10−2 | RO | LT | 4.47 × 10−2 |
| RP | RT | 3.08 × 10−2 | RP | RO | 4.30 × 10−2 |
| RT | LC | 2.97 × 10−2 | RT | LO | 4.27 × 10−2 |
| LF | LT | 2.95 × 10−2 | RC | LO | 4.25 × 10−2 |
| LC | RC | 2.88 × 10−2 | LO | RF | 4.22 × 10−2 |
| RC | LP | 2.75 × 10−2 | LP | LF | 4.21 × 10−2 |
| RC | RO | 2.65 × 10−2 | RP | LO | 4.19 × 10−2 |
| RP | LT | 2.64 × 10−2 | RC | RO | 4.16 × 10−2 |
| RF | RT | 2.63 × 10−2 | LF | LT | 4.06 × 10−2 |
| LC | LF | 2.62 × 10−2 | LP | LT | 3.99 × 10−2 |
| LF | RF | 2.60 × 10−2 | RP | RF | 3.98 × 10−2 |
| LT | LF | 2.59 × 10−2 | LO | RO | 3.92 × 10−2 |
| LO | RT | 2.55 × 10−2 | RO | LO | 3.79 × 10−2 |
| LP | RO | 2.53 × 10−2 | LT | LC | 3.78 × 10−2 |
| RP | RC | 2.49 × 10−2 | RO | RF | 3.71 × 10−2 |
| LO | LF | 2.44 × 10−2 | LC | LP | 3.69 × 10−2 |
| RF | RO | 2.43 × 10−2 | LT | RO | 3.63 × 10−2 |
| RT | LO | 2.43 × 10−2 | LC | RF | 3.55 × 10−2 |
| RC | RF | 2.40 × 10−2 | RF | LO | 3.49 × 10−2 |
| LO | RP | 2.39 × 10−2 | RP | LT | 3.48 × 10−2 |
| LC | RP | 2.37 × 10−2 | RC | LF | 3.30 × 10−2 |
| RC | LT | 2.32 × 10−2 | LP | RT | 3.19 × 10−2 |
| LP | RC | 2.27 × 10−2 | LP | RF | 3.19 × 10−2 |
| RF | RC | 2.26 × 10−2 | LC | RP | 3.14 × 10−2 |
| LO | LC | 2.23 × 10−2 | LC | LT | 3.13 × 10−2 |
| LF | RT | 2.22 × 10−2 | RC | RP | 3.05 × 10−2 |
| LC | RO | 2.19 × 10−2 | LC | RO | 3.02 × 10−2 |
| LO | LT | 2.18 × 10−2 | LC | RC | 3.01 × 10−2 |
| RF | LT | 2.15 × 10−2 | RP | LF | 2.98 × 10−2 |
| RT | RC | 2.14 × 10−2 | LF | RF | 2.95 × 10−2 |
| LC | RT | 2.12 × 10−2 | LP | RC | 2.79 × 10−2 |
| LT | RO | 2.10 × 10−2 | RF | RT | 2.77 × 10−2 |
| RO | RC | 2.01 × 10−2 | LF | LP | 2.76 × 10−2 |
| LC | LO | 1.96 × 10−2 | LO | RT | 2.67 × 10−2 |
| RC | LF | 1.93 × 10−2 | LO | LT | 2.66 × 10−2 |
| RO | LT | 1.89 × 10−2 | RT | LC | 2.66 × 10−2 |
| RT | LF | 1.88 × 10−2 | LF | LC | 2.55 × 10−2 |
| RT | LT | 1.82 × 10−2 | RC | RT | 2.55 × 10−2 |
| LT | RF | 1.80 × 10−2 | LF | LO | 2.53 × 10−2 |
| LF | LO | 1.75 × 10−2 | RC | LT | 2.51 × 10−2 |
| RP | LF | 1.69 × 10−2 | RP | RT | 2.49 × 10−2 |
| RO | LC | 1.69 × 10−2 | LC | RT | 2.46 × 10−2 |
| LT | RC | 1.69 × 10−2 | RT | RO | 2.44 × 10−2 |
| LC | RF | 1.65 × 10−2 | RF | RC | 2.43 × 10−2 |
| LO | RF | 1.64 × 10−2 | RO | RT | 2.40 × 10−2 |
| LT | RT | 1.64 × 10−2 | LO | LF | 2.34 × 10−2 |
| RO | LP | 1.61 × 10−2 | RF | RO | 2.29 × 10−2 |
| RC | RP | 1.56 × 10−2 | LF | RT | 2.24 × 10−2 |
| RC | LO | 1.55 × 10−2 | RO | LF | 2.16 × 10−2 |
| RT | RF | 1.53 × 10−2 | RT | LF | 2.12 × 10−2 |
| RP | RF | 1.50 × 10−2 | RF | LF | 2.02 × 10−2 |
| RO | RF | 1.50 × 10−2 | RF | LC | 1.97 × 10−2 |
| RO | LF | 1.50 × 10−2 | LF | RP | 1.94 × 10−2 |
| LF | LC | 1.48 × 10−2 | LT | RT | 1.87 × 10−2 |
| LO | RC | 1.45 × 10−2 | RT | LT | 1.81 × 10−2 |
| LF | RO | 1.38 × 10−2 | RO | RC | 1.80 × 10−2 |
| LP | LF | 1.37 × 10−2 | RT | RC | 1.79 × 10−2 |
| LT | RP | 1.37 × 10−2 | RP | RC | 1.65 × 10−2 |
| RF | RP | 1.34 × 10−2 | RF | LP | 1.58 × 10−2 |
| LP | RF | 1.33 × 10−2 | LT | RF | 1.49 × 10−2 |
| RF | LC | 1.30 × 10−2 | RF | RP | 1.49 × 10−2 |
| RF | LO | 1.28 × 10−2 | LF | RO | 1.40 × 10−2 |
| LF | RC | 1.24 × 10−2 | RT | RP | 1.39 × 10−2 |
| LF | RP | 9.69 × 10−3 | LO | RC | 1.36 × 10−2 |
| LF | LP | 9.67 × 10−3 | RF | LT | 1.34 × 10−2 |
| RF | LP | 8.89 × 10−3 | LF | RC | 9.12 × 10−3 |
References
- Barlow, K.M.; Crawford, S.; Stevenson, A.; Sandhu, S.S.; Belanger, F.; Dewey, D. Epidemiology of postconcussion syndrome in pediatric mild traumatic brain injury. Pediatrics 2010, 126, e374–e381. [Google Scholar] [CrossRef]
- Dalecki, M.; Albines, D.; Macpherson, A.; Sergio, L.E. Prolonged cognitive–motor impairments in children and adolescents with a history of concussion. Concussion 2016, 1, CNC14. [Google Scholar] [CrossRef]
- Mayer, A.R.; Kaushal, M.; Dodd, A.B.; Hanlon, F.M.; Shaff, N.A.; Mannix, R.; Master, C.L.; Leddy, J.J.; Stephenson, D.; Wertz, C.J.; et al. Advanced biomarkers of pediatric mild traumatic brain injury: Progress and perils. Neurosci. Biobehav. Rev. 2018, 94, 149–165. [Google Scholar] [CrossRef] [PubMed]
- Langer, L.; Levy, C.; Bayley, M. Increasing Incidence of Concussion: True Epidemic or Better Recognition? J. Head Trauma Rehabil. 2020, 35, E60–E66. [Google Scholar] [CrossRef]
- Ingram, V.; Fielding, M.; Dunne, L.A.M.; Piantella, S.; Weakley, J.; Johnston, R.D.; McGuckian, T.B. The Incidence of Sports-Related Concussion in Children and Adolescents: A Systematic Review and Meta-Analysis. Sports Med.-Open 2025, 11, 36. [Google Scholar] [CrossRef]
- Fahr, J.; Kraff, O.; Deuschl, C.; Dodel, R. Concussion in Female Athletes of Contact Sports: A Scoping Review. Orthop. J. Sports Med. 2024, 12, 23259671241276447. [Google Scholar] [CrossRef]
- D’Lauro, C.; Jones, E.R.; Swope, L.M.; Anderson, M.N.; Broglio, S.; Schmidt, J.D. Under-representation of female athletes in research informing influential concussion consensus and position statements: An evidence review and synthesis. Br. J. Sports Med. 2022, 56, bjsports-2021-105045. [Google Scholar] [CrossRef] [PubMed]
- Jacobs, E.G. Only 0.5% of neuroscience studies look at women’s health. Here’s how to change that. Nature 2023, 623, 667. [Google Scholar] [CrossRef]
- Saatman, K.E.; Duhaime, A.-C.; Bullock, R.; Maas, A.I.R.; Valadka, A.; Manley, G.T.; Workshop Scientific Team and Advisory Panel Members. Classification of traumatic brain injury for targeted therapies. J. Neurotrauma 2008, 25, 719–738. [Google Scholar] [CrossRef]
- Mayer, A.R.; Ling, J.M.; Yang, Z.; Pena, A.; Yeo, R.A.; Klimaj, S. Diffusion Abnormalities in Pediatric Mild Traumatic Brain Injury. J. Neurosci. 2012, 32, 17961–17969. [Google Scholar] [CrossRef] [PubMed]
- Coyle, H.L.; Bailey, N.W.; Ponsford, J.; Hoy, K.E. A comprehensive characterization of cognitive performance, clinical symptoms, and cortical activity following mild traumatic brain injury (mTBI). Appl. Neuropsychol. Adult 2025, 32, 1430–1446. [Google Scholar] [CrossRef] [PubMed]
- Ledwidge, P.S.; Hartland, L.C.; Huston, C.A.; Jones, C.M.; Neff, E.; Castro, E.; Abt, J.P. Post-concussion changes in the N200 and P300 ERPs are associated with cognitive symptoms and performance. Brain Inj. 2025, 39, 390–399. [Google Scholar] [CrossRef] [PubMed]
- Cao, C.; Slobounov, S. Alteration of cortical functional connectivity as a result of traumatic brain injury revealed by graph theory, ICA, and sLORETA analyses of EEG signals. IEEE Trans. Neural Syst. Rehabil. Eng. 2010, 18, 11–19. [Google Scholar] [CrossRef]
- Liu, S.; Shi, C.; Ma, X.; Zhao, B.; Chen, X.; Tao, L. Cognitive deficits and rehabilitation mechanisms in mild traumatic brain injury patients revealed by EEG connectivity markers. Clin. Neurophysiol. 2021, 132, 554–567. [Google Scholar] [CrossRef]
- Fox, A.J.; Matthews, N.; Qiu, Z.; Filmer, H.L.; Dux, P.E. On the lasting impact of mild traumatic brain injury on working memory: Behavioural and electrophysiological evidence. Neuropsychologia 2024, 204, 109005. [Google Scholar] [CrossRef]
- Borich, M.; Babul, A.-N.; Yuan, P.H.; Boyd, L.; Virji-Babul, N. Alterations in resting-state brain networks in concussed adolescent athletes. J. Neurotrauma 2015, 32, 265–271. [Google Scholar] [CrossRef]
- Raichle, M.E.; Mintun, M.A. Brain work and brain imaging. Annu. Rev. Neurosci. 2006, 29, 449–476. [Google Scholar] [CrossRef]
- Chen, C.; Xu, S.; Zhou, J.; Yi, C.; Yu, L.; Yao, D.; Zhang, Y.; Li, F.; Xu, P. Resting-state EEG network variability predicts individual working memory behavior. NeuroImage 2025, 310, 121120. [Google Scholar] [CrossRef]
- Thanjavur, K.; Babul, A.; Foran, B.; Bielecki, M.; Gilchrist, A.; Hristopulos, D.T.; Brucar, L.R.; Virji-Babul, N. Recurrent neural network-based acute concussion classifier using raw resting state EEG data. Sci. Rep. 2021, 11, 12353. [Google Scholar] [CrossRef]
- Thanjavur, K.; Hristopulos, D.T.; Babul, A.; Yi, K.M.; Virji-Babul, N. Deep Learning Recurrent Neural Network for Concussion Classification in Adolescents Using Raw Electroen-cephalography Signals: Toward a Minimal Number of Sensors. Front. Hum. Neurosci. 2021, 15, 734501. Available online: https://www.readcube.com/articles/10.3389%2Ffnhum.2021.734501 (accessed on 6 February 2023). [CrossRef] [PubMed]
- Daly, A.; Lightbody, G.; Temko, A. Analysis of the impact of deep learning know-how and data in modelling neonatal EEG. Sci. Rep. 2024, 14, 28059. [Google Scholar] [CrossRef]
- Hristopulos, D.T.; Arif, B.; Babul, S.; Brucar, L.R.; Virji-Babul, N. Disrupted Information Flow in Resting-State in Adolescents With Sports Related Concussion. Front. Hum. Neurosci. 2019, 13, 419. [Google Scholar] [CrossRef]
- Reddy, D.D.; Davenport, E.M.; Yu, F.F.; Wagner, B.; Urban, J.E.; Whitlow, C.T.; Stitzel, J.D.; Maldjian, J.A. Alterations in the Magnetoencephalography Default Mode Effective Connectivity following Concussion. AJNR Am. J. Neuroradiol. 2021, 42, 1776–1782. [Google Scholar] [CrossRef]
- Vaughn, K.A.; DeMaster, D.; Kook, J.H.; Vannucci, M.; Ewing-Cobbs, L. Effective connectivity in the default mode network after paediatric traumatic brain injury. Eur. J. Neurosci. 2022, 55, 318–336. [Google Scholar] [CrossRef] [PubMed]
- Li, F.; Lu, L.; Chen, H.; Wang, P.; Chen, Y.-C.; Zhang, H.; Yin, X. Disrupted brain functional hub and causal connectivity in acute mild traumatic brain injury. Aging 2019, 11, 10684–10696. [Google Scholar] [CrossRef]
- Li, F.; Liu, Y.; Lu, L.; Li, H.; Xing, C.; Chen, H.; Yuan, F.; Yin, X.; Chen, Y.-C. Causal interactions with an insular-cortical network in mild traumatic brain injury. Eur. J. Radiol. 2022, 157, 110594. [Google Scholar] [CrossRef] [PubMed]
- Iverson, G.L.; Howell, D.R.; Van Patten, R.; Bloomfield, P.; Gardner, A.J. Sport Concussion Assessment Tool-5th Edition (SCAT5): Normative Reference Values for the National Rugby League Women’s Premiership. Front. Sports Act. Living 2021, 3, 653743. [Google Scholar] [CrossRef]
- Echemendia, R.J.; Meeuwisse, W.; McCrory, P.; Davis, G.A.; Putukian, M.; Leddy, J.; Makdissi, M.; Sullivan, S.J.; Broglio, S.P.; Raftery, M.; et al. The Sport Concussion Assessment Tool 5th Edition (SCAT5): Background and rationale. Br. J. Sports Med. 2017, 51, 851–858. [Google Scholar] [CrossRef]
- Delorme, A.; Makeig, S. EEGLAB: An open source toolbox for analysis of single-trial EEG dynamics including independent component analysis. J. Neurosci. Methods 2004, 134, 9–21. [Google Scholar] [CrossRef]
- Fonov, V.; Evans, A.; McKinstry, R.; Almli, C.; Collins, D. Unbiased nonlinear average age-appropriate brain templates from birth to adulthood. NeuroImage 2009, 47, S102. [Google Scholar] [CrossRef]
- Pascual-Leone, A.; Nguyet, D.; Cohen, L.G.; Brasil-Neto, J.P.; Cammarota, A.; Hallett, M. Modulation of muscle responses evoked by transcranial magnetic stimulation during the acquisition of new fine motor skills. J. Neurophysiol. 1995, 74, 1037–1045. [Google Scholar] [CrossRef]
- Hämäläinen, M.S.; Ilmoniemi, R.J. Interpreting magnetic fields of the brain: Minimum norm estimates. Med. Biol. Eng. Comput. 1994, 32, 35–42. [Google Scholar] [CrossRef]
- Desikan, R.S.; Ségonne, F.; Fischl, B.; Quinn, B.T.; Dickerson, B.C.; Blacker, D.; Buckner, R.L.; Dale, A.M.; Maguire, R.P.; Hyman, B.T.; et al. An automated labeling system for subdividing the human cerebral cortex on MRI scans into gyral based regions of interest. NeuroImage 2006, 31, 968–980. [Google Scholar] [CrossRef]
- Liang, X.S. The Liang-Kleeman Information Flow: Theory and Applications. Entropy 2013, 15, 327–360. [Google Scholar] [CrossRef]
- Liang, X.S. Unraveling the cause-effect relation between time series. Phys. Rev. E 2014, 90, 052150. [Google Scholar] [CrossRef] [PubMed]
- Newman, M.E.J. Assortative Mixing in Networks. Phys. Rev. Lett. 2002, 89, 208701. [Google Scholar] [CrossRef]
- Noldus, R.; Van Mieghem, P. Assortativity in complex networks. J. Complex Netw. 2015, 3, 507–542. [Google Scholar] [CrossRef]
- Rubinov, M.; Sporns, O. Complex network measures of brain connectivity: Uses and interpretations. NeuroImage 2010, 52, 1059–1069. [Google Scholar] [CrossRef]
- Bounova, G. Octave Networks Toolbox [Internet]. Available online: https://github.com/aeolianine/octave-networks-toolbox (accessed on 27 June 2024).
- Covassin, T.; Elbin, R.J.; Bleecker, A.; Lipchik, A.; Kontos, A.P. Are There Differences in Neurocognitive Function and Symptoms Between Male and Female Soccer Players After Concussions? Am. J. Sports Med. 2013, 41, 2890–2895. [Google Scholar] [CrossRef]
- Sattari, S.; Kenny, R.; Liu, C.C.; Hajra, S.G.; Dumont, G.A.; Virji-Babul, N. Blink-related EEG oscillations are neurophysiological indicators of subconcussive head impacts in female soccer players: A preliminary study. Front. Hum. Neurosci. 2023, 17, 1208498. Available online: https://www.frontiersin.org/journals/human-neuroscience/articles/10.3389/fnhum.2023.1208498/full (accessed on 16 August 2025). [CrossRef] [PubMed]
- Michels, L.; Muthuraman, M.; Lüchinger, R.; Martin, E.; Anwar, A.R.; Raethjen, J.; Brandeis, D.; Siniatchkin, M. Developmental changes of functional and directed resting-state connectivities associated with neuronal oscillations in EEG. NeuroImage 2013, 81, 231–242. [Google Scholar] [CrossRef] [PubMed]
- Zhou, Y.; Milham, M.P.; Lui, Y.W.; Miles, L.; Reaume, J.; Sodickson, D.K.; Grossman, R.I.; Ge, Y. Default-Mode Network Disruption in Mild Traumatic Brain Injury. Radiology 2012, 265, 882–892. [Google Scholar] [CrossRef]
- Hillary, F.G.; Grafman, J.H. Injured Brains and Adaptive Networks: The Benefits and Costs of Hyperconnectivity. Trends Cogn. Sci. 2017, 21, 385–401. [Google Scholar] [CrossRef] [PubMed]
- Bouchard, H.C.; Higgins, K.L.; Amadon, G.K.; Laing-Young, J.M.; Maerlender, A.; Al-Momani, S.; Neta, M.; Savage, C.R.; Schultz, D.H. Concussion-Related Disruptions to Hub Connectivity in the Default Mode Network Are Related to Symptoms and Cognition. J. Neurotrauma 2024, 41, 571–586. [Google Scholar] [CrossRef]
- van der Horn, H.J.; Scheenen, M.E.; de Koning, M.E.; Liemburg, E.J.; Spikman, J.M.; van der Naalt, J. The Default Mode Network as a Biomarker of Persistent Complaints after Mild Traumatic Brain Injury: A Longitudinal Functional Magnetic Resonance Imaging Study. J. Neurotrauma 2017, 34, 3262–3269. [Google Scholar] [CrossRef]
- Nathan, D.E.; Oakes, T.R.; Yeh, P.H.; French, L.M.; Harper, J.F.; Liu, W.; Wolfowitz, R.D.; Wang, B.Q.; Graner, J.L.; Riedy, G. Exploring Variations in Functional Connectivity of the Resting State Default Mode Network in Mild Traumatic Brain Injury. Brain Connect. 2015, 5, 102–114. [Google Scholar] [CrossRef]
- Zhang, K.; Johnson, B.; Gay, M.; Horovitz, S.G.; Hallett, M.; Sebastianelli, W.; Slobounov, S. Default Mode Network in Concussed Individuals in Response to the YMCA Physical Stress Test. J. Neurotrauma 2012, 29, 756–765. [Google Scholar] [CrossRef] [PubMed]
- Johnson, B.; Zhang, K.; Gay, M.; Horovitz, S.; Hallett, M.; Sebastianelli, W.; Slobounov, S. Alteration of brain default network in subacute phase of injury in concussed individuals: Resting-state fMRI study. NeuroImage 2012, 59, 511–518. [Google Scholar] [CrossRef]
- Kaushal, M.; España, L.Y.; Nencka, A.S.; Wang, Y.; Nelson, L.D.; McCrea, M.A.; Meier, T.B. Resting-state functional connectivity after concussion is associated with clinical recovery. Hum. Brain Mapp. 2019, 40, 1211–1220. [Google Scholar] [CrossRef]
- Boshra, R.; Ruiter, K.I.; Dhindsa, K.; Sonnadara, R.; Reilly, J.P.; Connolly, J.F. On the time-course of functional connectivity: Theory of a dynamic progression of concussion effects. Brain Commun. 2020, 2, fcaa063. [Google Scholar] [CrossRef]
- Healey, K.; Fang, Z.; Smith, A.; Zemek, R.; Ledoux, A.-A. Adolescents with a concussion have altered brain network functional connectivity one month following injury when compared to adolescents with orthopedic injuries. NeuroImage Clin. 2022, 36, 103211. [Google Scholar] [CrossRef]
- Zhu, D.C.; Covassin, T.; Nogle, S.; Doyle, S.; Russell, D.; Pearson, R.L.; Monroe, J.; Liszewski, C.M.; DeMarco, J.K.; Kaufman, D.I. A Potential Biomarker in Sports-Related Concussion: Brain Functional Connectivity Alteration of the Default-Mode Network Measured with Longitudinal Resting-State fMRI over Thirty Days. J. Neurotrauma 2015, 32, 327–341. [Google Scholar] [CrossRef]
- Hillary, F.G.; Rajtmajer, S.M.; Roman, C.A.; Medaglia, J.D.; Slocomb-Dluzen, J.E.; Calhoun, V.D.; Good, D.C.; Wylie, G.R. The Rich Get Richer: Brain Injury Elicits Hyperconnectivity in Core Subnetworks. PLoS ONE 2014, 9, e104021. [Google Scholar] [CrossRef] [PubMed]
- Iraji, A.; Benson, R.R.; Welch, R.D.; O’Neil, B.J.; Woodard, J.L.; Ayaz, S.I.; Kulek, A.; Mika, V.; Medado, P.; Soltanian-Zadeh, H.; et al. Resting State Functional Connectivity in Mild Traumatic Brain Injury at the Acute Stage: Independent Component and Seed-Based Analyses. J. Neurotrauma 2015, 32, 1031–1045. [Google Scholar] [CrossRef]
- Chamard, E.; Lassonde, M.; Henry, L.; Tremblay, J.; Boulanger, Y.; De Beaumont, L.; Théoret, H. Neurometabolic and microstructural alterations following a sports-related concussion in female athletes. Brain Inj. 2013, 27, 1038–1046. [Google Scholar] [CrossRef]
- Bazarian, J.J.; Zhu, T.; Blyth, B.; Borrino, A.; Zhong, J. Subject-specific changes in brain white matter on diffusion tensor imaging after sports-related concussion. Magn. Reson. Imaging 2012, 30, 171–180. [Google Scholar] [CrossRef]
- Lancaster, M.A.; Olson, D.V.; McCrea, M.A.; Nelson, L.D.; LaRoche, A.A.; Muftuler, L.T. Acute white matter changes following sport-related concussion: A serial diffusion tensor and diffusion kurtosis tensor imaging study. Hum. Brain Mapp. 2016, 37, 3821–3834. [Google Scholar] [CrossRef] [PubMed]
- Zuleger, T.M.; Slutsky-Ganesh, A.B.; Anand, M.; Kim, H.; Warren, S.M.; Grooms, D.R.; Barber Foss, K.D.; Riley, M.A.; Yuan, W.; Gore, R.K.; et al. The effects of sports-related concussion history on female adolescent brain activity and connectivity for bilateral lower extremity knee motor control. Psychophysiology 2023, 60, e14314. [Google Scholar] [CrossRef]
- Amir, J.; Nair, J.K.R.; Del Carpio-O’Donovan, R.; Ptito, A.; Chen, J.-K.; Chankowsky, J.; Tinawi, S.; Lunkova, E.; Saluja, R.S. Atypical resting state functional connectivity in mild traumatic brain injury. Brain Behav. 2021, 11, e2261. [Google Scholar] [CrossRef] [PubMed]
- Wong, J.K.Y.; Churchill, N.W.; Graham, S.J.; Baker, A.J.; Schweizer, T.A. Altered connectivity of default mode and executive control networks among female patients with persistent post-concussion symptoms. Brain Inj. 2023, 37, 147–158. [Google Scholar] [CrossRef]
- Wilde, E.A.; Newsome, M.R.; Ott, S.D.; Hunter, J.V.; Dash, P.; Redell, J.; Spruiell, M.; Diaz, M.; Chu, Z.D.; Goodrich-Hunsaker, N.; et al. Persistent Disruption of Brain Connectivity after Sports-Related Concussion in a Female Athlete. J. Neurotrauma 2019, 36, 3164–3171. [Google Scholar] [CrossRef]
- Shafi, R.; Crawley, A.P.; Tartaglia, M.C.; Tator, C.H.; Green, R.E.; Mikulis, D.J.; Colantonio, A. Sex-specific differences in resting-state functional connectivity of large-scale networks in postconcussion syndrome. Sci. Rep. 2020, 10, 21982. [Google Scholar] [CrossRef] [PubMed]
- Messé, A.; Caplain, S.; Pélégrini-Issac, M.; Blancho, S.; Lévy, R.; Aghakhani, N.; Montreuil, M.; Benali, H.; Lehéricy, S. Specific and Evolving Resting-State Network Alterations in Post-Concussion Syndrome Following Mild Traumatic Brain Injury. PLoS ONE 2013, 8, e65470. [Google Scholar] [CrossRef] [PubMed]
- Churchill, N.; Hutchison, M.G.; Leung, G.; Graham, S.; Schweizer, T.A. Changes in functional connectivity of the brain associated with a history of sport concussion: A preliminary investigation. Brain Inj. 2017, 31, 39–48. [Google Scholar] [CrossRef]
- Newsome, M.R.; Li, X.; Lin, X.; Wilde, E.A.; Ott, S.; Biekman, B.; Hunter, J.V.; Dash, P.K.; Taylor, B.A.; Levin, H.S. Functional Connectivity Is Altered in Concussed Adolescent Athletes Despite Medical Clearance to Return to Play: A Preliminary Report. Front. Neurol. 2016, 7, 116. [Google Scholar] [CrossRef]
- Brett, B.L.; Bryant, A.M.; España, L.Y.; Mayer, A.R.; Meier, T.B. Investigating the overlapping associations of prior concussion, default mode connectivity, and executive function-based symptoms. Brain Imaging Behav. 2022, 16, 1275–1283. [Google Scholar] [CrossRef]
- Plourde, V.; Rohr, C.S.; Virani, S.; Bray, S.; Yeates, K.O.; Brooks, B.L. Default mode network functional connectivity after multiple concussions in children and adolescents. Arch. Clin. Neuropsychol. 2020, 35, 302–311. [Google Scholar] [CrossRef]
- Coenen, J.; van den Bongard, F.; Delling, C.; Reinsberger, C. Functional Connectivity Within the Default Mode Network in Response to Exercise During Return-to-Sport Following Concussion. Neurology 2021, 98, S18. [Google Scholar] [CrossRef]
- Sattari, S.; Damji, S.; McLeod, J.; Mirian, M.S.; Wu, L.C.; Virji-Babul, N. Altered resting state EEG microstate dynamics in acute-phase pediatric mild traumatic brain injury [Internet]. medRxiv 2024. medRxiv:2024.10.26.24316185. Available online: https://www.medrxiv.org/content/10.1101/2024.10.26.24316185v1 (accessed on 20 August 2025).
- Chang, C.; Glover, G.H. Time-frequency dynamics of resting-state brain connectivity measured with fMRI. NeuroImage 2010, 50, 81–98. [Google Scholar] [CrossRef]
- Jones, D.T.; Vemuri, P.; Murphy, M.C.; Gunter, J.L.; Senjem, M.L.; Machulda, M.M.; Przybelski, S.A.; Gregg, B.E.; Kantarci, K.; Knopman, D.S.; et al. Non-Stationarity in the “Resting Brain’s” Modular Architecture. PLoS ONE 2012, 7, e39731. [Google Scholar] [CrossRef]
- Hutchison, R.M.; Womelsdorf, T.; Allen, E.A.; Bandettini, P.A.; Calhoun, V.D.; Corbetta, M.; Della Penna, S.; Duyn, J.H.; Glover, G.H.; Gonzalez-Castillo, J.; et al. Dynamic functional connectivity: Promise, issues, and interpretations. NeuroImage 2013, 80, 360–378. [Google Scholar] [CrossRef]
- Muller, A.M.; Virji-Babul, N. Stuck in a State of Inattention? Functional Hyperconnectivity as an Indicator of Disturbed Intrinsic Brain Dynamics in Adolescents With Concussion: A Pilot Study. ASN Neuro 2018, 10, 1759091417753802. [Google Scholar] [CrossRef] [PubMed]
- Hao, D.; Li, C. The Dichotomy in Degree Correlation of Biological Networks. PLoS ONE 2011, 6, e28322. [Google Scholar] [CrossRef]
- Churchill, N.W.; Hutchison, M.G.; Graham, S.J.; Schweizer, T.A. Long-term changes in the small-world organization of brain networks after concussion. Sci. Rep. 2021, 11, 6862. [Google Scholar] [CrossRef]
- Doshi-Velez, F.; Kim, B. Towards A Rigorous Science of Interpretable Machine Learning. arXiv 2017. [Google Scholar] [CrossRef]
- Brookshire, G.; Pennati, A.; Yoder, K.J.; Tweardy, M.; Quirk, C.; Perkins, M.; Gerrol, S.; Raethel, S.; Nikjou, D.; Nikolova, S.; et al. Measuring electrophysiological changes induced by sub-concussive impacts due to soccer ball heading. Front. Neurol. 2025, 16, 1500796. [Google Scholar] [CrossRef]
- Lee, P.-L.; Chen, S.-H.; Chang, T.-C.; Lee, W.-K.; Hsu, H.-T.; Chang, H.-H. Continual Learning of a Transformer-Based Deep Learning Classifier Using an Initial Model from Action Observation EEG Data to Online Motor Imagery Classification. Bioengineering 2023, 10, 186. [Google Scholar] [CrossRef] [PubMed]
- Peng, Y.; Yang, X.; Li, D.; Ma, Z.; Liu, Z.; Bai, X.; Mao, Z. Predicting flow status of a flexible rectifier using cognitive computing. Expert Syst. Appl. 2025, 264, 125878. [Google Scholar] [CrossRef]
- Alqudah, A.M.; Moussavi, Z. A Review of Deep Learning for Biomedical Signals: Current Applications, Advancements, Future Prospects, Interpretation, and Challenges. Comput. Mater. Contin. 2025, 83, 3753–3841. [Google Scholar] [CrossRef]
- Hidalgo-Lopez, E.; Engman, J.; Poromaa, I.S.; Gingnell, M.; Pletzer, B. Triple network model of brain connectivity changes related to adverse mood effects in an oral contraceptive placebo-controlled trial. Transl. Psychiatry 2023, 13, 209. [Google Scholar] [CrossRef]
- Kozlov, M. How does the brain react to birth control? A researcher scanned herself 75 times to find out. Nature 2024, 634, 1026–1027. [Google Scholar] [CrossRef] [PubMed]
- Petersen, N.; Kilpatrick, L.A.; Goharzad, A.; Cahill, L. Oral contraceptive pill use and menstrual cycle phase are associated with altered resting state functional connectivity. NeuroImage 2014, 90, 24–32. [Google Scholar] [CrossRef] [PubMed]
- Mcleod, J.; Sattari, S.; Chavan, A.; Galea, L.A.M.; Babul, S.; Virji-Babul, N. Menstrual cycle phase modulates causal connectivity in the resting-state brain of healthy females [Internet]. bioRxiv 2024. bioRxiv:2024.06.07.598022. Available online: https://www.biorxiv.org/content/10.1101/2024.06.07.598022v1 (accessed on 8 July 2024).

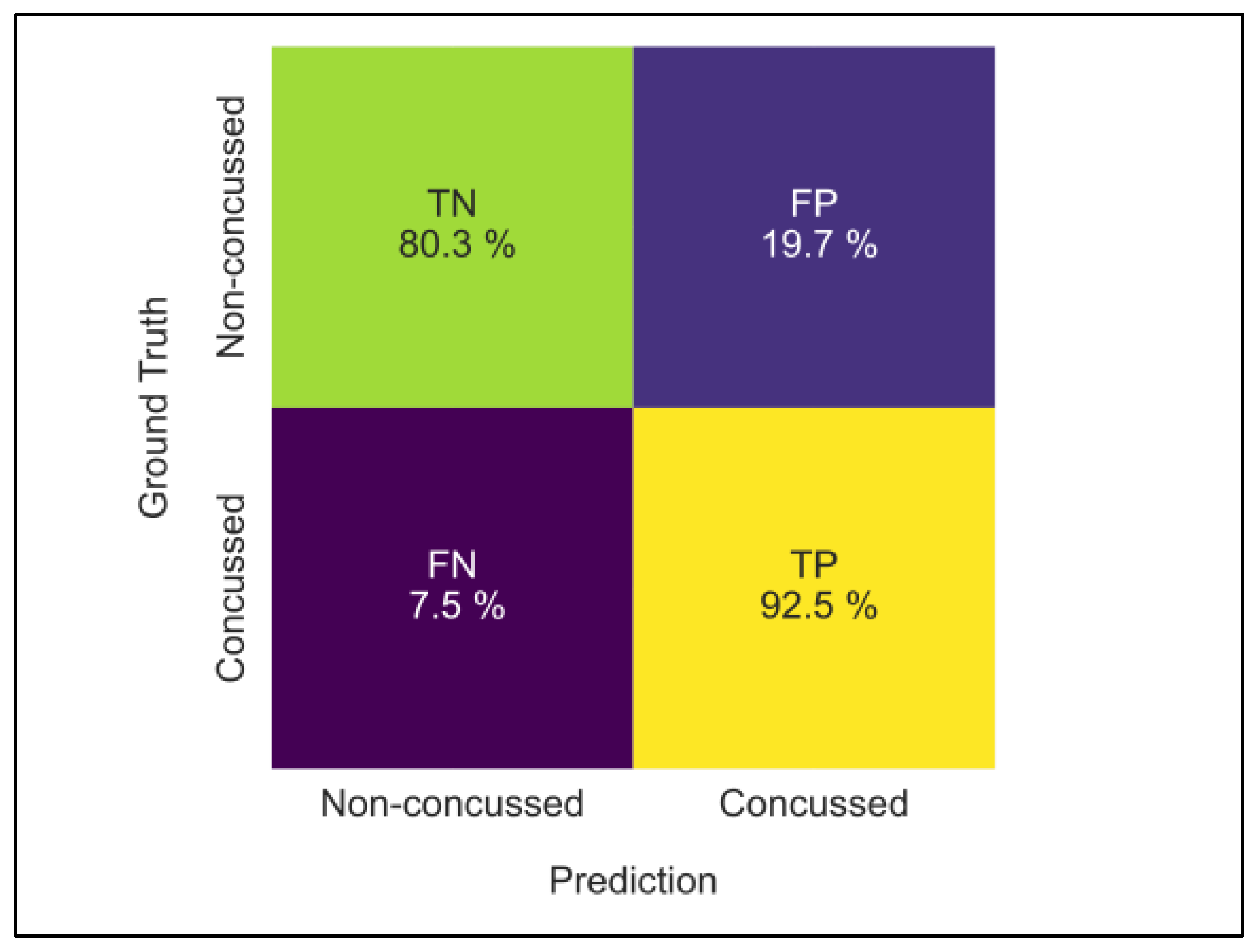


| Non-Concussed (n = 15) | Concussed (n = 11) | |
|---|---|---|
| Age (years) | 21 ± 1.88 | 20 ± 2.46 |
| Time since injury (days) | - | 10 ± 6.85 |
| Previous diagnosed concussions to date | - | 2.25 ± 1.39 |
| SCAT5, total number of symptoms | - | 14.88 ± 5.48 |
| SCAT5, severity of symptoms | - | 34.88 ± 22.53 |
| Metric | Mean | 95% CI |
|---|---|---|
| Accuracy | 84.2% | 81.0%/87.3% |
| Recall | 92.9% | 90.1%/95.6% |
| Specificity | 75.5% | 66.6%/84.4% |
| Precision | 79.6% | 74.4%/84.7% |
| F1 score | 86.2% | 84.4%/88.0% |
| AUC | 0.904 | 0.870/0.915 |
| Transmitter | Receiver | |τi→j| | Transmitter | Receiver | |τi→j| |
|---|---|---|---|---|---|
| RC | LC | 6.54 × 10−2 | LO | LC | 7.63 × 10−2 |
| LT | LC | 4.83 × 10−2 | RO | LP | 7.25 × 10−2 |
| LP | LC | 4.61 × 10−2 | LT | LP | 7.24 × 10−2 |
| RP | LC | 4.58 × 10−2 | LP | LO | 7.12 × 10−2 |
| RP | LP | 4.58 × 10−2 | LT | LO | 7.07 × 10−2 |
| LC | LT | 4.44 × 10−2 | RP | LP | 6.96 × 10−2 |
| RT | LP | 4.24 × 10−2 | LP | RO | 6.82 × 10−2 |
| RO | RT | 4.10 × 10−2 | RO | LC | 6.69 × 10−2 |
| RO | RP | 4.05 × 10−2 | RP | LC | 6.35 × 10−2 |
| RT | RP | 4.01 × 10−2 | RC | LC | 6.10 × 10−2 |
| M | Median | SD | COV | Kurtosis | Skewness | d | |
|---|---|---|---|---|---|---|---|
| Control | 0.0251 | 0.0150 | 0.2920 | 1.1604 | 7.7898 | 1.9275 | 0.0502 |
| Concussed | 0.0364 | 0.0198 | 0.0444 | 1.2270 | 10.4160 | 2.3859 |
Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2025 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
McLeod, J.; Thanjavur, K.; Sattari, S.; Babul, A.; Hristopulos, D.T.; Virji-Babul, N. Linking a Deep Learning Model for Concussion Classification with Reorganization of Large-Scale Brain Networks in Female Youth. Bioengineering 2025, 12, 986. https://doi.org/10.3390/bioengineering12090986
McLeod J, Thanjavur K, Sattari S, Babul A, Hristopulos DT, Virji-Babul N. Linking a Deep Learning Model for Concussion Classification with Reorganization of Large-Scale Brain Networks in Female Youth. Bioengineering. 2025; 12(9):986. https://doi.org/10.3390/bioengineering12090986
Chicago/Turabian StyleMcLeod, Julianne, Karun Thanjavur, Sahar Sattari, Arif Babul, D. T. Hristopulos, and Naznin Virji-Babul. 2025. "Linking a Deep Learning Model for Concussion Classification with Reorganization of Large-Scale Brain Networks in Female Youth" Bioengineering 12, no. 9: 986. https://doi.org/10.3390/bioengineering12090986
APA StyleMcLeod, J., Thanjavur, K., Sattari, S., Babul, A., Hristopulos, D. T., & Virji-Babul, N. (2025). Linking a Deep Learning Model for Concussion Classification with Reorganization of Large-Scale Brain Networks in Female Youth. Bioengineering, 12(9), 986. https://doi.org/10.3390/bioengineering12090986






